Abstract
Flame retardants (FRs) such as polybrominated diphenyl ethers and organophosphate FR (OPFR) persist in the environment and interact with multiple nuclear receptors involved in homeostasis, including estrogen receptors (ERs). However, little is known about the effects of FR, especially OPFR, on mammalian neuroendocrine functions. Therefore, we investigated if exposure to FR alters hypothalamic gene expression and whole-animal physiology in adult wild-type (WT) and ERα KO mice. Intact WT and KO males and ovariectomized WT and KO females were orally dosed daily with vehicle (oil), 17α-ethynylestradiol (2.5 μg/kg), 2,2’, 4,4-tetrabromodiphenyl ether (BDE-47, 1 or 10 mg/kg), or an OPFR mixture {1 or 10 mg/kg of tris(1, 3-dichloro-2-propyl)phosphate, triphenyl phosphate, and tricresyl phosphate each} for 28 days. Body weight, food intake, body composition, glucose and insulin tolerance, plasma hormone levels, and hypothalamic and liver gene expression were measured. Expression of neuropeptides, receptors, and cation channels was differentially altered between WT males and females. OPFR suppressed body weight and energy intake in males. FR increased fasting glucose levels in males, and BDE-47 augmented glucose clearance in females. Liver gene expression indicated FXR activation by BDE-47 and PXR and CAR activation by OPFR. In males, OPFR increased ghrelin but decreased leptin and insulin independent of body weight. The loss of ERα reduced the effects of both FR on hypothalamic and liver gene expression and plasma hormone levels. The physiological implications are that males are more sensitive than ovariectomized females to OPFR exposure and that these effects are mediated, in part, by ERα.
Keywords: flame retardants, hypothalamus, energy homeostasis, estrogen receptors
Because polybrominated diphenyl ethers (PBDE) have been phased out of use in the United States since 2004 and subsequently concentrations in humans have declined (Zota et al., 2013), many products now employ nonbrominated flame retardants (FRs) such as organophosphate FRs (OPFRs) (Hoffman et al., 2017; van der Veen and de Boer, 2012). Therefore, environmental concentrations of OPFR are increasing and are detectable in significant concentrations in women’s breast milk and urine (Cequier et al., 2014; Hoffman et al., 2014, 2017; Kim et al., 2014; Sundkvist et al., 2010). A primary source of OPFR is dust in the home and work environments. Indeed, OPFR are found in low μg/g concentration in house dust (3-month geometric mean for Tris (1,3-dichloro-2-propyl)phosphate (TDCPP) = 1.58 μg/g and for TPP = 6.8 μg/g) (Meeker et al., 2013) and in dust from offices (6.06 μg/g) and vehicles (12.5 μg/g) (Carignan et al., 2013) as well as drinking water (Li et al., 2014), and air in offices and aircrafts (Yang et al., 2014). OPFR are known to interact with a range of nuclear receptors in vitro. Indeed, triphenyl phosphate (TPP) and tricresyl phosphate (TCP) activate human estrogen receptor (ER)α/β transactivation assays, although with lower potency than 17β-estradiol (E2) (Kojima et al., 2013; Liu et al., 2012; Pillai et al., 2014). TDCPP is a potential ER antagonist (Liu et al., 2012) and also upregulates ERα target genes (Liu et al., 2013a,b).
Little is known about the effects of adult OPFR exposure on the neuroendocrine control of energy homeostasis in mammalian models. In chicks, TDCPP-induced cholestatic liver and biliary fibrosis, decreased plasma cholesterol, disrupted lipid and steroid metabolism, induced CYP3A37 and CYP2H1 expression, and altered ApoE, hepatocyte nuclear factor 4α, and peroxisome proliferator activated receptor (PPARα) expression (Farhat et al., 2014). In zebrafish, TDCPP and TPP decreased fecundity, increased plasma E2, and upregulated steroid hormone receptors and reproductive genes in the hypothalamus and pituitary in a sex-dependent manner (Liu et al., 2013a,b). These studies demonstrate that selected OPFR can interact with nuclear receptors to elicit complex interactions impacting neural growth, steroidogenesis, lipid and glucose homeostasis, and hypothalamic functions.
Although a number of brain regions play a role in body weight homeostasis (Berthoud, 2002), the hypothalamus is regarded as the key regulator of energy homeostasis, especially the arcuate nucleus (ARC) (Saper et al., 2002). The ARC is a heterogeneous nucleus containing neurons involved in energy homeostasis, growth, and reproduction including proopiomelanocortin (POMC), neuropeptide Y (NPY)/agouti-related peptide (AgRP), growth hormone-releasing hormone (GHRH), and kisspeptin-neurokinin B (Tac2)-dynorphin (KNDy) neurons (Bosch et al., 2012; Gottsch et al., 2011; Proudan et al., 2015). These neurons are in a unique position because of their proximity to a “leaky” region of blood-brain barrier and subsequently receive information reflecting the body’s energy status (Schwartz et al., 2000).
A wide range of hormones produced by the gonads, fat, pancreas, gastrointestinal tract, and liver modulate ARC neurons (Woods, 2009). E2 modulates energy intake and expenditure and controls glucose homeostasis through actions of ERα in the hypothalamus (Mauvais-Jarvis et al., 2013). ERα knockout (KO) females are phenotypically obese, glucose intolerant, and resistant to the effects of E2 as full-grown adults (Geary et al., 2001; Yasrebi et al., 2017). Leptin and insulin, peripheral hormones from fat and pancreas, differentially depolarize and hyperpolarize POMC and NPY neurons (Baquero et al., 2014; Qiu et al., 2010, 2014). These hormones activate TRPC channels (TRPC5) to excite POMC neurons and activate KATP channels to suppress NPY neurons through their respective receptors, LepR and InsR (Baquero et al., 2014; Elias et al., 1999; Mirshamsi et al., 2004; Qiu et al., 2010, 2014). Ghrelin is secreted by the stomach to drive hunger and increase feeding through the growth hormone secretagogue receptor (GHSR). GHSR is expressed in NPY/AgRP neurons and increases NPY neuronal excitability (Andrews, 2011; Nogueiras et al., 2010;). GHSR is also highly expressed in KNDy neurons and is upregulated by estradiol through ERα (Yang et al., 2016a,b). GHSR stimulation activates a Gq-coupled signaling pathway that inhibits KCNQ channel activity to increase neuronal excitability (Shi et al., 2013; Yasrebi et al., 2016). The KCNQ family of potassium channels produces the neuronal M-current, a noninactivating outward potassium current under the control of E2 in the ARC (Roepke et al., 2011).
As ERα is highly expressed in the ARC (Roepke et al., 2007), there is potential for FR to disrupt ERα-mediated pathways involved in energy homeostasis in the ARC. Although PBDE have been phased out in the United States and Europe, it is crucial to identify the impacts of their replacement compounds, OPFR, to determine if these compounds are also harmful. We chose to use TPP, TCP, and TDCPP as a mixture due to their potential interactions with steroid receptors and their detection in human samples. Furthermore, these OPFR disrupt neural, reproductive, and homeostatic gene expression and function in a sex-dependent manner in nonmammalian models. Therefore, we hypothesized that OPFR treatment will differentially impinge on homeostatic ARC genes between male and female mice. Because FRs may interact with nuclear steroid receptors, in particular ERα, we also hypothesize that their effects would be reduced in mice lacking the functional expression of ERα (ERα KOs).
MATERIALS AND METHODS
Animal care
All animal procedures were completed in compliance with institutional guidelines based on National Institutes of Health standards and were performed with Institutional Animal Care and Use Committee approval at Rutgers University. Wild-type (WT) C57/BL6J mice and Ex3a ERα KO transgenic mice (provided by Dr Ken Korach, NIEHS) (Hewitt et al., 2010) were bred in-house and maintained under controlled temperature (25 °C) and 12/12-h light/dark cycle. Mice were fed ad libitum a chow diet (LabDiet PicoLab Verified 5v75 IF, <75 ppm phytoestrogens) and given free access to water. To eliminate the need to track and characterize the estrous cycle before sample collection and reduce the impact of estrogens on ER-mediated transcription, all adult females were bilaterally ovariectomized (OVX) under isoflurane anesthesia using sterile no-touch technique according to the NIH Guidelines for Survival Rodent Surgery. Animals were given a dose of analgesia (4 mg/kg carprofen [Rimadyl]) 1 day following surgery for pain management. Animals typically lost 1–2 g of weight within 24 h after surgery.
Chemicals
17α-ethynylestradiol (EE2), TPP (CAS no. 115-86-6; purity = 99%), and TDCPP (CAS no. 13674-87-8; purity = 95.6%) were purchased from Sigma-Aldrich (St. Louis, Missouri). 2,2′, 4,4′-tetrabromodiphenyl ether (BDE-47) was purchased from Matrix Scientific (CAS no. 5436-43-1; purity = 95+%; Elgin, South Carolina), and TCP (CAS no. 1330-78-5; purity = 99%) was purchased from AccuStandard (New Haven, Connecticut). For the stock solution, 100 mg of the BDE-47 or 100 mg of each OPFR were dissolved in 1 ml of acetone. For the working stock, 100 μl of the acetone: FR mixture was added to 10 ml of sesame oil and mixed over a stir plate for 48 h with venting. Ultra residue-analyzed toluene (CAS no. 108-88-3; purity = 99.7%) was purchased from ThermoFisher Scientific (Waltham, Massachusetts). TPP D15 (CAS no. 1173020-30-8; purity = 98%) was purchased from Cambridge Isotope Laboratories (Tewksbury, Massachusetts). 13C12 BDE-47 (IUPAC no. 47 L; purity = 99%) was purchased from Wellington Laboratories (Guelph, Ontario, Canada). 1 μg/ml TPP D15 and 1 μg/ml 13C12 BDE-47 stock solutions were made in toluene.
Experiment no. 1: WT brain and liver tissue collection
Intact male and OVX female WT mice were divided into 6 endocrine disrupting compound (EDC) treatment groups (n = 8/treatment/sex): Oil (negative control), EE2 (positive estrogenic control; 2.5 μg/kg/d), 2 doses of BDE-47 (1 or 10 mg/kg/d), and 2 doses of OPFR mixture (TCP, TPP, and TDCPP at 1 or 10 mg/kg/d of each OPFR). BDE-47 was included in these experiments to compare effects between the groups of FR and because BDE-47 and its metabolites potentially have estrogenic activity (Lu et al., 2014). Age range at the start of dosing for males was 12–16 weeks, and date of ovariectomy for females was 12–16 weeks. Animals were dosed orally using peanut butter as the carrier. Untreated peanut butter was given to the mice to acclimate the mice for 4 days before FR dosing. For females, acclimation occurred 3 days before surgery and FR dosing began immediately after surgery. Dosing consisted of mixing 100–150 mg of all-natural peanut butter with the respective sesame oil mixture (blank [oil], EE2, BDE-47, and OPFR) at a volume determined by weight (25 μl for a 25-g mouse). Dosing continued every morning (1000 h) for 4 weeks. After the dosing periods, all mice were fasted for 1 h and decapitated after sedation with ketamine (100 µl of 100 mg/ml, IP; Henry Schein [Melville, New York]) at 1000 h.
The brain was immediately extracted from the skull and rinsed in ice-cold Sorensen’s buffer for 30 s. The brain was cut using a brain matrix (Ted Pella, Redding, California) into 1-mm thick coronal rostral and caudal blocks corresponding to plates 42–53, respectively, from The Mouse Brain in Stereotaxic Coordinates (Paxinos and Franklin, 2008). Blocks of the basal hypothalamus (BH) were transferred to RNALater (Life Technologies, Grand Island, New York) and stored overnight at 4 °C. The rostral and caudal parts of the ARC were dissected from slices using a dissecting microscope. Dissected tissue was stored in RNALater at –80 °C. The abdominal cavity was dissected for liver tissue (secondary lobe). Liver tissue was fixed in RNALater and stored at –80 °C. Liver RNA was extracted using a standard TRIzol extraction (Life Technologies) coupled with Macherey-Nagel NucleoSpin RNA extraction kit with rDNase digestion (Bethlehem, Pennsylvania). Total ARC RNA was extracted from the combined rostral and caudal ARC using Ambion RNAqueous-Micro Kits (Life Technologies) as per the manufacturer’s protocol. Total RNA was treated with DNase I using the extraction kit protocol at 37 °C for 30 min to minimize any genomic DNA contamination. Liver and arcuate RNA quantity and quality were determined using a NanoDrop ND-2000 spectrophotometer (ThermoFisher, Waltham, Massachusetts) and an Agilent 2100 Bioanalyzer and RNA Nano Chips (Agilent Technologies, Santa Clara, California). Only samples with RNA Integrity Number (RIN) > 8 were used.
Experiment no. 2: WT energy and glucose homeostasis
A second group of WT intact male and OVX female mice (n = 8 per group) were separated into 4 groups (Oil, EE2, 1 mg/kg BDE-47, and 1 mg/kg of OPFR mixture) and dosed for 4 weeks. Because we found similar effects on gene expression between the 2 doses of OPFR, we eliminated the 10 mg/kg dose for experiment nos. 2 and 3. Age ranges at the start of dosing for males was 10–12 weeks, and date of ovariectomy for females was 10–12 weeks. At the end of the 4 weeks, a small rodent MRI (EchoMRI, Houston, Texas) was used to determine body composition. For a glucose tolerance test (GTT), each mouse was IP-injected with a bolus of glucose (2 g/kg) after a 5 h fast. Glucose was measured in tail blood using an AlphaTrak glucometer (Zoetis, Parsippany, NJ). Glucose measurements were taken every 0, 15, 30, 60, 90, and 120 min after injection. For the insulin tolerance test (ITT), mice were IP-injected with insulin (0.75 U/kg body weight in sterile saline) after a 4 h fast. Glucose measurements were taken at 0, 15, 30, 60, 90, and 120 min after insulin injection.
After sufficient recovery from the ITT (approximately 1 week) during which dosing continued, all mice were fasted for 1 h and decapitated after sedation with ketamine at 1000 h. Trunk blood was collected in a K+ EDTA collection tube. Plasma was prepared for peptide hormone analysis by adding a protease inhibitor, 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (1 mg/ml, Sigma-Aldrich), to each collection tube. Samples were maintained on ice until centrifugation at 1, 100 rcf for 15 min at 4 °C. Plasma was stored at –80 °C until analysis. Plasma insulin, leptin, and ghrelin levels were determined by multiplex assay (MMHMAG-44 K, EMD Millipore, Billerica, Massachusetts).
Experiment no. 3: ERα KO exposure and tissue collection
Intact male and OVX female ERα KO mice were divided into 4 treatment groups (n = 6/treatment/sex): Oil, EE2, BDE-47 (1 mg/kg), and the OPFR mixture (1 mg/kg). Age ranges at the start of dosing for males was 10–12 weeks, and date of ovariectomy for females was 10–12 weeks. KOs were dosed for 4 weeks. After dosing, all mice were sedated, decapitated, and prepared for brain, liver, and plasma collection as described above. ARC and liver RNA was prepared for analysis of gene expression, and plasma was prepared and stored for analysis of peptide hormone levels.
Reverse transcription and quantitative real-time PCR
Analysis of gene expression used standard protocols for quantitative real-time PCR (qPCR) as previously published (Mamounis et al., 2014). Briefly, for both ARC and liver RNA, complementary DNA (cDNA) was synthesized using a standard Superscript III reverse transcriptase (Life Technologies) protocol: 5 min at 25 °C, 60 min at 50 °C, and 15 min at 70 °C. All primers were designed to span exon-exon junctions and synthesized by Life Technologies, using Clone Manager 5 software (Sci Ed Software, Cary, North Carolina). See Supplementary Table 1 for a list of all primer sequences. qPCR amplification followed standard protocols for either PowerSYBR Green (Life Technologies) or Sso Advanced SYBR Green (BioRad, Hercules, CA) master mixes on CFX-Connect Real-time PCR instrument (BioRad). All efficiencies were between 90% and 110%. The relative mRNA expression was calculated using the ΔΔCT method and a calibrator of diluted (1:20) cDNA from liver or medioBH of an untreated male. The geometric mean of the reference genes β-actin (Actb), hypoxanthine guanine phosphoribosyl transferase 1 (Hprt), and glyceraldehyde 3-phosphate (Gapdh) was used to calculate δCq values. Quantification values were generated only from samples showing a single product at the expected melting point. All gene expression data were expressed as an n-fold difference relative to the calibrator (Schmittgen and Livak, 2008).
Dosing and serum sample analytical methods
Serum samples from mice treated with either 10 mg/kg/d of BDE-47 or the OPFR mixture were spiked with 1 μg/ml of the internal standard (13C12 BDE-47 and TPP D15, respectively) for recovery calculations, having a final concentration of 50 ng/ml. After spiking, the samples were vortexed for 1 min followed by the addition of toluene. Samples were then vortexed for 2 min and allowed to stand for 5 min. Finally, samples were centrifuged at 6500 rpm at 4 °C for 5 min, and the organic layer was collected for gas chromatography-mass spectrometry (GC-MS) analysis. Serum sample extracts were analyzed using an Agilent 7890B GC/240 ion trap MS. Chromatographic separation of the analytes was achieved using an Agilent DB-XLB microcapillary column (30 m × 180 μm i.d. × 0.18 μm film thickness; Santa Clara, California). Two microliter of serum extract were injected into a septum programmable injector in splitless mode. The septum programmable injector temperature program was held at 150 °C for 0.5 min, then ramped up to 280 °C at a rate of 150 °C/min and held for 12 min, before dropping to 150 °C at a rate of 8 °C/min. The GC oven temperature program was held at 90 °C for 2 min followed by a temperature ramp of 18 °C/min to 200 °C, and a final temperature ramp of 5 °C/min to 300 °C with a 4.89-min hold. Serum extracts were analyzed under electron impact ionization using selected ion storage of the most abundant ion. These ions were used for quantitation of each compound: TDCPP (99 m/z), TPP (326 m/z), TPP D15 (341 m/z), BDE-47 (487 m/z), 13C12 BDE-47 (498 m/z), and TCP (366 m/z). Analyte responses were used to quantify each analyte against an external calibration curve in serum and recovery of internal standards (TPP D15 for TDCPP, TPP, and TCP; 13C12 BDE-47 for BDE-47) was calculated. The BDE-47 dosing solution (1 mg/ml dose) had a concentration of 0.98 ± 0.22 mg/ml, and the OPFR dosing solution (1 mg/ml dose) had a concentration of 0.99 ± 0.18 mg/ml (TDCPP), 0.98 ± 0.33 mg/ml (TPP), and 1.0 ± 0.24 mg/ml (TCP). The limits of detection for TDCPP, TPP, TCP, and PBDE-47 were 0.09, 0.02, 0.03, and 0.01 ng/ml, respectively.
Data analysis
All RNA extractions, reverse transcriptions, and qPCR analyses were conducted as a group for the experiment 1 ARC and liver samples. All the KO gene expression studies in experiment 3 were prepared and analyzed together. All WT physiology data were from experiment 2, except for the addition of the body weight data from experiment 1. All metabolic hormone data from WT (experiment 2) and KO (experiment 3) mice were analyzed in the same batch of multiplex plates.
All the data are expressed as mean ± SEM. All physiology data were analyzed using GraphPad Prism software (GraphPad Software, La Jolla, California) by a 2-way ANOVA (EDC and genotype) with a post hoc Bonferroni’s multiple comparisons test except for the GTT and ITT, which were analyzed by a repeated-measures ANOVA (EDC and time). All gene expression data were analyzed by a 1-way ANOVA within sex. All GC-MS data were analyzed by a 1-way ANOVA with a post hoc Tukey’s multiple comparison test. We did not analyze for sex differences because sexes were not of similar condition (intact vs gonadectomized). In all experiments, effects were considered significant at an α ≤ 0.05.
RESULTS
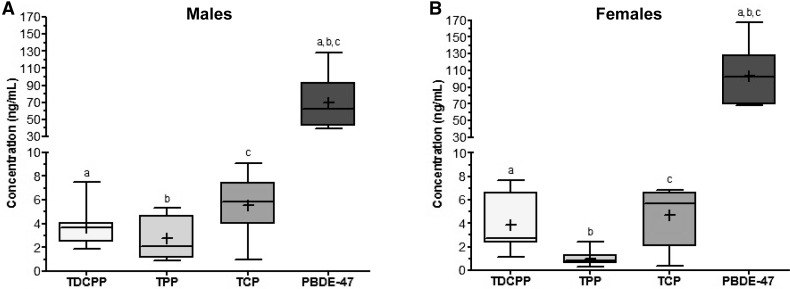
Concentrations of BDE-47 and OPFR in Serum Samples
Quantitative analysis of the serum extracts from WT mice treated with BDE-47 (10 mg/kg/d, n = 8 for each sex) or OPFR mixture (TPP, TCP, and TDCPP; 10 mg/kg/d of each OPFR, n = 8 for each sex) were performed using GC-MS (Figure 1) . Serum extracts were injected and analyzed in triplicate. Within intact WT males, the serum concentration of BDE-47 (70 ± 11 ng/ml) was significantly higher (p < .05) than that of TPP (2.8 ± 0.63 ng/ml), TCP (5.6 ± 0.89 ng/ml), and TDCPP (3.7 ± 0.61 ng/ml). Similarly, within OVX WT females, the serum concentration of BDE-47 (103 ± 12.4 ng/ml) was also significantly higher (p < .05) than that of TPP (1.0 ± 0.23 ng/ml), TCP (4.7 ± 0.91 ng/ml), and TDCPP (3.9 ± 0.86 ng/ml).
Figure 1.
Serum concentrations of TDCPP, TPP, TCP, and PBDE-47 in (A) intact WT males and (B) OVX WT females dosed with 10 mg/kg/d of PBDE-47 (BDE-10) or of the OPFR (OP-10) mixture (n = 8 for each group [sex + FR]). Data were analyzed by a 1-way ANOVA with post hoc Tukey’s multiple comparison test. Letters denote comparison between analytes where a–c are p < .05. Data are presented as mean ± SE.
Effects of the FR on Body Weight, Energy Intake, and Glucose Homeostasis
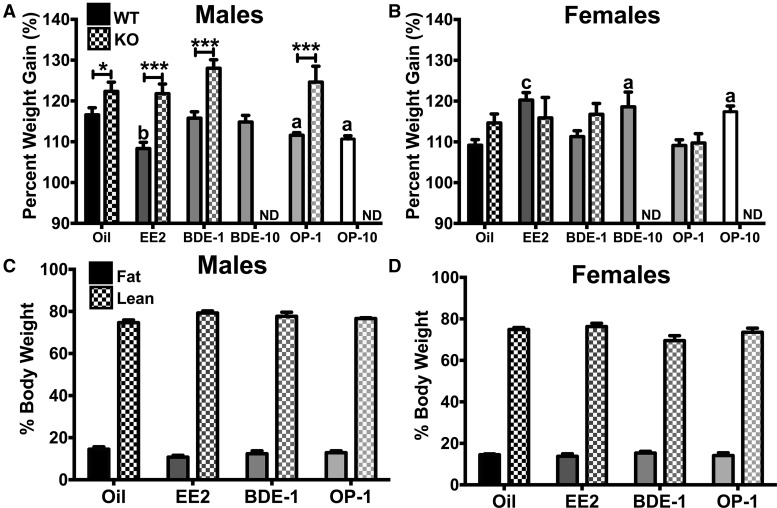
Body weight gain was determined by calculating percent body weight gain ([week 4 body weight ÷ week 0 body weight] × 100; Figs. 2A and B). Data from experiments 1 and 2 were combined for oil, EE2, BDE-1, and OP-1 for the WT and presented with data from experiment 3 (KO). Within intact WT males, there was an effect of EDC (F[5, 74] = 5.361, p < .001). Within KO males, there was no effect of EDC on percent body weight gain. However, when comparing oil, EE2, BDE-1, and OP-1 doses between WT and KO, body weight gain was determined by both genotype (F[1, 81] = 60.85, p < .0001) and EDC (F[3, 81] = 4.128, p < .01). Specifically, EE2 (p < .01), OP-1 (p < .05), and OP-10 (p < .05) reduced WT male body weights, and KO males in all treatment groups gained more weight than their WT counterparts. In OVX WT females, body weight gain was augmented by EE2 (p < .001), BDE-10 (p < .05), and OP-10 (p < .05) compared with oil-treated females (F[5, 73] = 8.513, p < .0001). When comparing treatment groups between WT and KO females, only EDC affected body weight gain (F[3, 79] = 5.384, p < .01), not genotype, unlike in males. There was no effect of EDC on body composition, ie, fat and lean mass, in intact males (Figure 2C) or OVX females (Figure 2D). Because we only measured body composition at week 4, we cannot state with any certainty that the changes in body weight involved changes in adiposity.
Figure 2.
A, Body weight gain of intact WT (oil, EE2, BDE-1, OP-1: n = 16; BDE-10, OP-10: n = 8) and ERα KO (n = 6) males. B, Body weight gain of OVX WT (oil, EE2, BDE-1, OP-1: n = 16; BDE-10, OP-10: n = 8) and ERα KO (n = 6) females. C, Percent fat mass and percent lean mass in intact WT males. D, Percent fat mass and percent lean mass in OVX WT females. Data were analyzed by a 2-way ANOVA with post hoc Bonferroni’s multiple comparisons test. ND, no data. Letters denote comparison to oil and asterisks denote comparison between genotypes: *a = p < .05; **b = p < .01; ***c = p < .001. Data are presented as mean ± SE.
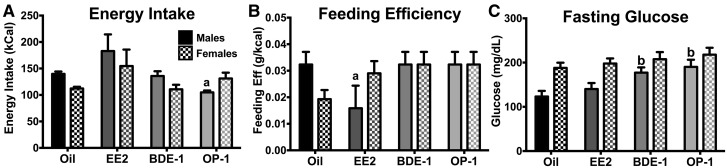
In addition to gaining less mass, OP-1-treated WT males consumed less (p < .05) than oil-treated WT males (F[3, 12] = 3.757, p < .05; Figure 3A). Feeding efficiency in intact WT males was reduced by EE2 compared with oil-treated males (p < .05; F[3, 12] = 2.983, p < .05; Figure 3B). There was no effect of EDC on energy intake or feeding efficiency in OVX WT females. Both BDE-1 and OP-1 induced hyperglycemia in fasted (5 h) WT males (BDE-1: p < .01; OP-1: p < .01; F[3, 28] = 5.191, p < .01; Figure 3C). There was no effect of EDC on fasting glucose levels in OVX WT females.
Figure 3.
(A) Weekly energy intake, (B) weekly feeding efficiency, and C: Fasting (5 h) glucose levels in WT males and females. Data (n = 4 for A and B, n = 8 for C) were analyzed by a 2-way ANOVA with post hoc Bonferroni’s multiple comparisons test. Letters denote comparison to oil: a = p < .05; b = p < 0.01. Data are presented as mean ± SE.
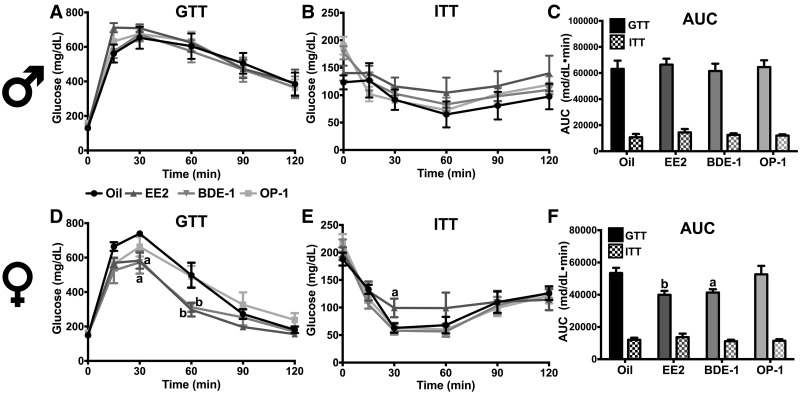
EDC had no effect on glucose clearance or insulin tolerance in males (Figs. 4A–C). Conversely, EDC increased glucose clearance in OVX WT females (F[3, 27] = 3.753, p < .05; Figure 4D). Specifically, EE2 and BDE-1 increased glucose clearance at 30 min (p < .05 and < .05, respectively) and 60 min (p < .01 and < .01, respectively). There was no effect of EDC on insulin tolerance in OVX WT females, although EE2 at 30 min (p < .05) reduced glucose clearance, indicating a slower glucose uptake compared with oil-treated females (Figure 4E). Analysis of area under the curve illustrated the increase in glucose clearance by EE2 (p < .01) and BDE-1 (p < .05) (EDC: F[3, 27] = 4.137, p < .05; Figure 4F).
Figure 4.
Glucose tolerance of WT males (A) and females (D). Insulin tolerance of WT males (B) and females (E). (C) AUC analysis of (A and B). (F) AUC analysis of (D) and (E). Data were analyzed by a 2-way ANOVA with post hoc Bonferroni’s multiple comparisons test. Letters denote comparison to oil: *a = p < .05; **b = p < .01. Data (n = 8) are presented as mean ± SE.
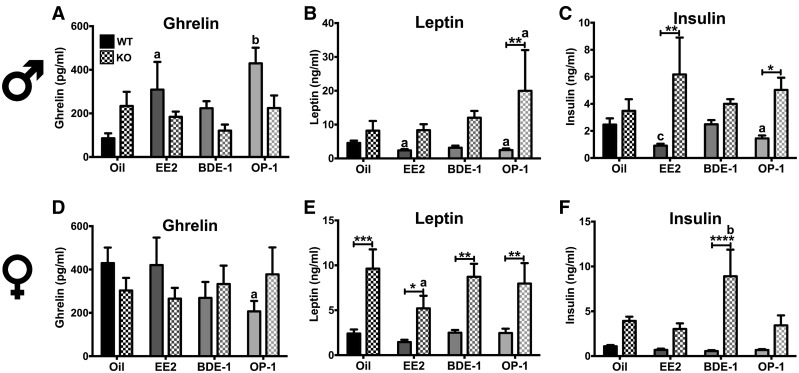
Plasma peptide hormone levels were measured in males and females from experiment 2 (WT) and experiment 3 (KO). In males, ghrelin was impacted by EDC (F[3, 48] = 3.662, p < .05), but not by genotype. Specifically, EE2 (p < .05) and OP-1 (p < .01) induced hyperghrelinemia in WT males, increasing plasma ghrelin by 4-fold (Figure 5A). Plasma leptin in males was differentially expressed between the genotypes (F[1, 48] = 10.92, p < .01) and EDC (F[3, 48] = 3.557, p < .05; Figure 5B). Leptin was reduced by EE2 (p <.05) and OP-1 (p < .05) in WT (F[3, 48] = 3.557, p < .5) and increased by OP-1 (p < .05) in KO. The differential effect of OP-1 on insulin between WT and KO was significant (p < .01). Plasma insulin levels were also affected by genotype (F[1, 48] = 18.09, p < .0001) and EDC (F[3, 48] = 6.405, p < .001; Figure 5C). EE2 (p < .001) and OP-1 (p < .05) reduced plasma insulin levels in WT. Although there was no effect of EDC in KO males, KO males treated with EE2 (p < .01) and OP-1 (p < .05) expressed higher insulin levels than WT counterparts.
Figure 5.
Plasma levels of peptide hormone ghrelin (A, D), leptin (B, E), and insulin (C, F), in male and female WT and KO mice. Data (n = 8 for WT, n = 6 for KO) were analyzed by a 2-way ANOVA with post hoc Bonferroni’s multiple comparisons test. Letters denote comparison to oil and asterisks denote comparison between genotypes: *a = p < .05; **b = p < .01; ***c = p < .001. Data are presented as mean ± SE.
In OVX WT and KO females, there was no effect of EDC or genotype on plasma ghrelin, although OP-1 reduced ghrelin in WT females compared with oil-treated WT (p < .05; Figure 5D). Plasma leptin levels were not altered by EDC but were differentially expressed between the genotypes (KO ≫ WT) (F[1, 47] = 46.04, p < .0001; Figure 5E). Plasma insulin levels were affected by EDC (F[3, 47] = 3.475, p < .05) and genotype (F[1, 47] = 32.67, p < .0001) with a significant interaction between the 2 factors (F[3, 47] = 4.075, p < .05; Figure 5F). BDE-1 increased plasma insulin only in KO females (p < .01) producing a difference between WT and KO treated with BDE-1 (p < .0001).
E2 replacement is known to induce uterine hypertrophy through an ERα-mediated mechanism (Mamounis et al., 2014). In our study, only EE2 increased uterine weight (2.2 ± 0.5 g, p < .01) compared with oil (0.6 ± 0.5 g) in WT females. There were no significant effects of FR treatment on uterine weight at any dose in WT or KO (data not shown).
ARC Gene Expression in WT and ERKO Mice
We selected E2-responsive ARC genes primarily involved in reproduction, energy homeostasis, and neuronal excitability (Qiu et al., 2006; Roepke et al., 2007; Yang et al., 2016a,b; Yasrebi et al., 2016). Genes were grouped based on function as neuropeptides, hormone and nuclear receptors, and cation channels. Differential ARC gene expression by FR in intact male and OVX female WT and KO mice is reported in Tables 1 and 2. The first set of ARC genes are the neuropeptides involved in energy homeostasis including POMC, cocaine- and amphetamine-regulated transcript (CART), NPY, and AgRP. These neuropeptides were differentially regulated by FR in males. Pomc expression was affected by EDC (F[5, 40] = 4.92, p < .01) and was increased approximately 2-fold by OP-1 (p < .001) and OP-10 (p < .001). In females, OP-10 reduced Pomc expression by approximately 30% (p < .05). In males, all EDC reduced Cart expression by approximately 50%–70% (all: p < .0001; (F[5, 40] = 16.79, p < .0001), and in females, BDE-1 (p < .05) and OP-10 (p < .05) treatment reduced Cart expression by approximately 30% (F[5, 40] = 2.67, p < .05). Npy expression was affected by EDC only in males (F[5, 40] = 32.16, p < .0001), in which all EDC reduced Npy expression by approximately 30%–70% (all: P < 0.001). Agrp expression was increased approximately 2-fold after OP-10 treatment (p < .001) in males (F[5, 40] = 6.24, p < .001) while no EDC had any affect in OVX females. Another ARC neuropeptide under the control of E2 is kisspeptin. Kiss1 expression was increased approximately 2-fold by both FR in males (F[5, 40] = 2.54, p < .05) and females (F[5, 42] = 4.26, p < .01). In KO mice, there were no effects of any EDC on Pomc, Npy, and Agrp expression. Cart was reduced by OP-1 treatment (p < .05; F[5, 20] = 3.94, p < .05), and Kiss1 was reduced by EE2 (p < .05) and BDE-47 (p < .05; F[5, 20] = 3.66, p < .05) in males.
Table 1.
Arcuate Expression of Neuropeptides and Hormone Receptors From WT and ERKO Male and Females
| Males |
Females |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Geno-type | EE2 | BDE-1 | BDE-10 | OP-1 | OP-10 | EE2 | BDE-1 | BDE-10 | OP-1 | OP-10 |
| Agrp | WT | n.s. | 1.5 ± 0.2a | 1.2 ± 0.2 | n.s. | 1.9 ± 0.2d | n.s. | n.s. | n.s. | n.s. | n.s. |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Cart | WT | 0.5 ± 0.1d | 0.4 ± 0.0d | 0.3 ± 0.0d | 0.4 ± 0.0d | 0.5 ± 0.1d | n.s. | 0.7 ± 0.1a | n.s. | n.s. | 0.7 ± 0.1a |
| KO | n.s. | n.s. | NA | 0.7 ± 0.1a | NA | n.s. | n.s. | NA | n.s. | NA | |
| Esr1 | WT | 0.4 ± 0.0d | 0.4 ± 0.0d | 0.3 ± 0.0d | 0.4 ± 0.0d | 0.4 ± 0.0d | n.s. | n.s. | n.s. | n.s. | n.s. |
| KO | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Ghsr | WT | 6.5 ± 0.4d | 5.5 ± 0.5d | 4.6 ± 0.2d | 4.9 ± 0.1d | 5.4 ± 0.5d | n.s. | n.s. | 0.8 ± 0.0b | 0.8 ± 0.0a | 0.8 ± 0.0a |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Insr | WT | 8.1 ± 0.6d | 7.2 ± 1.4c | 5.9 ± 0.5d | 7.3 ± 0.4d | 7.2 ± 0.6d | 1.9 ± 0.1a | 2.7 ± 0.2d | 2.6 ± 0.1d | 2.6 ± 0.2d | 2.5 ± 0.2d |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Kiss1 | WT | n.s. | n.s. | 2.0 ± 0.2a | n.s. | 2.4 ± 0.5a | n.s. | n.s. | 2.6 ± 0.4b | 2.1 ± 0.3a | 2.0 ± 0.2a |
| KO | n.s. | 0.6 ± 0.1a | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Lepr | WT | 4.4 ± 0.4d | 4.5 ± 0.6d | 3.3 ± 0.2c | 3.9 ± 0.3d | 4.1 ± 0.2d | n.s. | n.s. | n.s. | n.s. | n.s. |
| KO | 0.5 ± 0.1d | 0.4 ± 0.0d | NA | 0.3 ± 0.1d | NA | n.s. | n.s. | NA | n.s. | NA | |
| Npy | WT | 0.6 ± 0.0d | 0.4 ± 0.0d | 0.3 ± 0.0d | 0.3 ± 0.0d | 0.5 ± 0.0d | n.s. | n.s. | n.s. | n.s. | n.s. |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Pomc | WT | n.s. | n.s. | n.s. | n.s. | 2.4 ± 0.4c | n.s. | n.s. | n.s. | n.s. | 0.7 ± 0.1a |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Pparg | WT | 3.4 ± 0.6d | 2.8 ± 0.7b | 2.6 ± 0.5a | n.s. | 2.4 ± 0.3a | 2.1 ± 0.3a | n.s. | n.s. | 2.1 ± 0.4a | n.s. |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | 2.9 ± 0.4b | NA | |
All data were normalized to oil-treated within each sex: a = p < .05; b = p < .01; c = p < .001; d = p < .0001. n = 7–8 for WT and 5–6 for KO. NA = not applicable; n.s., not significant.
Table 2.
Arcuate Expression of Cation Channels From WT and ERKO Male and Females
| Males |
Females |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Geno-type | EE2 | BDE-1 | BDE-10 | OP-1 | OP-10 | EE2 | BDE-1 | BDE-10 | OP-1 | OP-10 |
| Cacna1g | WT | 5.0 ± 0.5d | 3.2 ± 0.5a | 3.5 ± 0.3b | 4.1 ± 0.6b | 4.0 ± 0.6b | 1.9 ± 0.2 | 4.0 ± 0.3d | 4.6 ± 0.4d | 5.4 ± 0.4d | 4.3 ± 0.8d |
| KO | n.s. | 0.9 ± 0.1 | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Cacna1h | WT | 4.0 ± 0.3d | 2.4 ± 0.2 | 3.1 ± 0.3b | 3.2 ± 0.3b | 3.8 ± 0.6d | 1.4 ± 0.1a | n.s. | n.s. | 0.9 ± 0.1 | n.s. |
| KO | 0.7 ± 0.1a | 0.7 ± 0.1a | NA | 0.6 ± 0.1b | NA | n.s. | n.s. | NA | 1.5 ± 0.2 | NA | |
| Cacna1i | WT | 2.5 ± 0.2d | 1.8 ± 0.2 | 2.7 ± 0.2d | 2.9 ± 0.2d | 2.8 ± 0.3d | 1.7 ± 0.1a | n.s. | n.s. | n.s. | n.s. |
| KO | 1.8 ± 0.2a | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Kcnq2 | WT | 2.7 ± 0.1d | 1.8 ± 0.2 | 2.0 ± 0.1b | 2.8 ± 0.2d | 2.8 ± 0.4d | 1.8 ± 0.1d | n.s. | n.s. | n.s. | n.s. |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Kcnq3 | WT | 2.6 ± 0.1d | 1.8 ± 0.1a | 2.3 ± 0.2d | 2.8 ± 0.2d | 2.7 ± 0.2d | 1.8 ± 0.1c | n.s. | n.s. | n.s. | n.s. |
| KO | 1.8 ± 0.4 | 1.9 ± 0.3 | NA | n.s. | NA | n.s. | n.s. | NA | 0.4 ± 0.1a | NA | |
| Kcnq5 | WT | 2.3 ± 0.1d | n.s. | 2.1 ± 0.2b | 2.5 ± 0.2d | 2.3 ± 0.3d | 2.4 ± 0.2d | n.s. | n.s. | n.s. | n.s. |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | |
| Trpc5 | WT | 5.0 ± 0.3d | 3.1 ± 0.3c | 3.5 ± 0.2d | 3.7 ± 0.3d | 4.0 ± 0.4d | 0.7 ± 0.1d | n.s. | n.s. | n.s. | n.s. |
| KO | n.s. | n.s. | NA | n.s. | NA | 2.1 ± 0.3a | n.s. | NA | n.s | NA | |
All data were normalized to oil-treated within each sex: a = p < .05; b = p < .01; c = p < .001; d = p < .0001. n = 7–8 for WT and 5–6 for KO. NA, not applicable; n.s., not significant.
Hormone and nuclear receptors for E2 (ERα/Esr1), ghrelin (GHSR), insulin (InsR), leptin (LepR), and fatty acids (PPARγ) modulate energy balance through actions in the ARC (Long et al., 2014; Qiu et al., 2010, 2014; Yasrebi et al., 2016, 2017). Esr1 expression was reduced by approximately 50%–60% by all EDC in males (F[5, 40] = 31.87, p < .0001) but not in females. Insr expression was increased by all EDC 6- to 8-fold in males (all: p < .0001 except BDE-10: p < .001; F[5, 40] = 11.88, p < .0001) and 2- to 3-fold in females (all: p < .0001 except EE2: p < .05; F[5, 42] = 11.22, p < .0001). Lepr expression was increased approximately 4-fold by all EDC in males (all: p < .0001 except BDE-10: p < .001; F[5, 40] = 11.31, p < .0001) but not in females. Ghsr expression was also augmented 4- to 6-fold by EDC in males (F[5, 40] = 25.2, p < .0001; all: p < .0001) but reduced approximately 10%–20% in females (F[5, 42] = 4.24, p < .01; BDE-10: p < .01; OP-1 and OP-10: p < .05). Pparγ expression in males was augmented 2- to 3-fold by all EDC except for OP-1 (EE2: p < .0001; BDE-1: p < .01; BDE-10: p < .05; OP-10: p < .05; F[5, 40] = 2.63, p < .05), and Pparγ expression in females was augmented approximately 2-fold by EE2 (p < .05) and OP-1 (p < .05; F[5, 42] = 3.39, p < .05). In KO mice, Ghsr and Insr expression was not altered by EDC in males or females. Conversely, Lepr expression was decreased by EE2 (p < .05), BDE-1 (p < .01), and OP-1 (p < .001) in males (F[3, 18] = 41.80, p < .0001). As in the WT, Pparγ expression was augmented by OP-1 (p < .05) in KO females (F[3, 18] = 7.816, p < .01).
We also examined cation channel subunits that are involved in neuroendocrine functions including the potassium channel KCNQ subunits (KCNQ2, -3, -5) (Roepke et al., 2011, 2012), T-type calcium channel subunits (Cav3.1, Cav3.2, Cav3.3) (Bosch et al., 2009; Qiu et al., 2006), and nonselective cation current canonical transient receptor potential 5 (TRPC5) (Qiu et al., 2010, 2014) (see Table 2). Kcnq2 expression was not changed by FR in WT females but was increased by EE2 (p < .0001; F[5, 42] = 5.64, p < .001). In WT males, EE2 (p < .0001), BDE-10 (p < .01), OP-1 (p < .0001), and OP-10 (p < .0001) increased Kcnq2 expression 2- to 3-fold (F[5, 40] = 11.9, p < .0001). As with Kcnq2, Kcnq3 expression in females was not altered by EDC except for EE2 (p < .001; F[5, 42] = 12.04, p < .0001). Kcnq3 expression in males was increased approximately 2- to 3-fold by EE2 (p < .0001), BDE-1 (p < .05), BDE-10 (p < .0001), OP-1 (p < .0001), and OP-10 (p < .0001; F[5, 40] = 13.15, p < .0001). Kcnq5 expression in females was also not changed by EDC except for EE2 (p < .0001; F[5, 42] = 12.29, p < .0001) and was increased 2- to 3-fold in males by EE2 (p < .001), BDE-10 (p < .01), OP-1 (p < .0001) and OP-10 (p < .001; F[5, 40] = 8.42, p < .0001). In KO mice, Kcnq2 and Kcnq5 expression was not altered by EDC in males or females. However, Kcnq3 expression was decreased by OP-1 (p < .05) only in females (F[3, 18] = 4.34, p < .05).
ARC expression of the T-type calcium channel subunits was also regulated by EDC exposure. Cav3.1 (Cacna1g) expression was increased 3- to 5-fold by EDC in males (EE2: p < .0001; BDE-1: p < .05; BDE-10: p < .01; OP-1: p < .01; OP-10: p < .01; F[5, 40] = 6.25, p < .001) and females (all: p < .0001; F[5, 42] = 25.06, p < .0001). Cav3.2 (Cacna1h) expression was increased 2- to 3-fold by all EDC, except BDE-1 in males (F[5, 40] = 8.23, p < .0001), and only by EE2 (p < .05) in females (F[5, 42] = 4.9, p < .01). Similarly, Cav3.3 (Cacna1i) expression was increased 2- to 3-fold by all EDC in males (F[5, 40] = 11.53, p < .0001) except for BDE-1 and only by EE2 in females (p < .01; F[5, 42] = 4.75, p < .01). All EDC exposures increased Trpc5 expression in males 3- to 5-fold (F[5, 40] = 17.71, p < .0001; all: p < .0001 except BDE-1: p < .001) with no effect in females except a reduction by EE2 (p < .05; F[5, 42] = 2.97, p < .05). In KO mice, Cav3.2 expression was reduced by EDC in males (F[3, 18] = 5.52, p < .01; EE2: p < .05, BDE-1: p < .05; OP-1: p < .01). EDC had no effect on Cav3.1, Cav3.3, or Trpc5 in the KO.
Regulation of Xenobiotic Receptor Target Genes in Livers from WT and ERKO Mice
PBDE are known to activate xenobiotic receptors (pregnane X receptor [PXR], PPARα, constitutive androstane receptor [CAR]) in the liver and subsequently modulate receptor target genes (Pacyniak et al., 2007; Sueyoshi et al., 2014). In WT males, the target genes regulated by EDC were Abcb11 (Bsep) (F[5, 40] = 84.16, p < .0001), Cd36 (F[5, 40] = 4.93, p < .01), Cyp2b10 (F[5, 40] = 10.89, p < .0001), Cyp3a11 (F[5, 40] = 19.27, p < .0001), Cyp4a10 (F[5, 40] = 2.66, p < .05), Slc51b (Ostβ) (F[5, 40] = 6.49, p < .001), and Nr0b2 (Shp) (F[5, 40] = 6.66, p < .001) (see Table 3). In WT females, the target genes regulated by EDC were Bsep (F[5, 41] = 65.91, p < .0001), Cd36 (F[5, 41] = 7.90, p < .0001), Cyp2b10 (F[5, 41] = 17.89, p < .0001), Cyp3a11 (F[5, 41] = 15.0, p < .0001), Cyp4a10 (F[5, 41] = 3.37, p < .05), Cyp7a1 (F[5, 41] = 5.64, p < .001), Ostβ (F[5, 41] = 6.43, p < .001), and Shp (F[5, 41] = 17.91, p < .0001). PBDE treatment increased expression of Bsep, Ostβ, Cyp3a11, and Cyp2b10 in males and females. OPFR treatment only increased expression of Cyp3a11 and Cyp2b10 in males. PBDE and OPFR suppressed Cd36 in both males and females, and OPFR suppressed Shp in males and females. In KO mice, the only target gene in the liver increased by FR (BDE-1) was Cd36 (p < .05) in males (F[3, 19] = 3.81, p < .05).
Table 3.
Liver Expression of Xenobiotic Receptor Target Genes From WT and ERKO Male and Females
| Males |
Females |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Geno-type | EE2 | BDE-1 | BDE-10 | OP-1 | OP-10 | EE2 | BDE-1 | BDE-10 | OP-1 | OP-10 | |
| Bsep | WT | 6.6 ± 0.5d | 7.4 ± 0.5d | 6.5 ± 0.3d | n.s. | n.s. | 11.5 ± 0.8d | 8.1 ±0.9d | 9.7 ± 0.8d | n.s. | n.s. | |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | ||
| Cd36 | WT | n.s. | n.s. | 0.6 ± 0.1a | n.s. | n.s. | 0.4 ± 0.0c | 0.4 ±0.1c | n.s. | 0.5 ± 0.1b | n.s. | |
| KO | n.s. | 3.2 ± 0.8a | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | ||
| Cyp2b10 | WT | n.s. | 5.2 ± 1.0d | 3.5 ± 0.6b | n.s. | 3.7 ± 0.4b | n.s. | n.s. | 5.0 ± 0.8d | n.s. | n.s. | |
| KO | n.s. | 2.1 ± 0.6d | NA | v | NA | n.s. | n.s. | NA | n.s. | NA | ||
| Cyp3a11 | WT | 3.7 ± 0.5d | 4.8 ± 0.5d | 2.8 ± 0.2c | n.s. | 2.8 ± 0.2c | 1.9 ± 0.2a | n.s. | 2.8 ± 0.4d | n.s. | n.s. | |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | ||
| Cyp4a10 | WT | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.6 ± 0.1b | n.s. | |
| KO | n.s. | n.s. | NA | n.s. | NA | v | n.s. | NA | n.s. | NA | ||
| Cyp7a1 | WT | n.s. | n.s. | n.s. | n.s. | n.s. | 0.2 ± 0.0b | n.s. | n.s. | n.s. | n.s. | |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | ||
| Ostβ | WT | n.s. | 3.3 ± 0.8b | 2.1 ± 0.3a | n.s. | n.s. | n.s. | 2.7 ± 0.4a | 2.9 ± 0.4a | n.s. | n.s. | |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | ||
| Shp | WT | n.s. | n.s. | n.s. | 0.4 ± 0.1a | n.s. | 5.3 ± 0.7d | n.s. | n.s. | n.s. | n.s. | |
| KO | n.s. | n.s. | NA | n.s. | NA | n.s. | n.s. | NA | n.s. | NA | ||
All qPCR data are normalized to oil-treated within sex: a = p < .05; b = p < .01; c = p < .001; d = p < .0001. n = 7–8 for WT and 5–6 for KO. NA, not applicable; n.s., not significant.
DISCUSSION
Our characterization of the effects of adult FR exposure on the neuroendocrine control of energy homeostasis began with measurements of the FR in mouse serum. These serum concentrations (low ng/ml) correlate to the concentrations found in human serum, hair, nail, and urine samples and supports the environmental relevance of our dosing concentrations. In human serum collected in the United States, TDCPP and TPP were not detected in serum, although BDE-47 was detected in 94% of the samples with a geomean concentration of 17 ng/g lipid (Liu et al., 2016). However, in the same study, both TPP and TDCPP were detected in hair, fingernail, and toenail samples at concentrations ranging from 280 to 1980 ng/g for TPP and 230–390 ng/g TDCPP. In another study from China, TPP was measured at concentrations of 30–40 ng/g lipid in human serum with TPP consisting of approximately 5% of the total OPFR concentration (Ma et al., 2017). The metabolites for TDCPP (bis[1, 3-dichloro-2-propyl[ phosphate), and TPP (diphenyl phosphate [DPP]) are detectable in urine samples from adults and children in the range of 0.1–1000 ng/ml, but most commonly between 1 and 10 ng/ml (Butt et al., 2014; Hoffman et al., 2017; Meeker et al., 2013). Interestingly, TPP was not detected in serum from rat dams dosed to 1 mg/kg TPP for 10 days, while DPP was detected at approximately 700 ng/ml in the urine (Phillips et al., 2016). The differences in the serum concentrations between the OPFR compounds and PBDE-47 were likely due to the detoxification and clearance rates of each compound. Indeed, the whole-body half-life (t1/2α) of BDE-47 is 1.5 days and 1.1 days in blood, while the terminal half-life (t1/2β) is 23 and 13 days, respectively (Staskal et al., 2005). The half-life of the OPFR range from 0.63 h (t1/2α) and 13.9 days (t1/2β) for TPP (Carrington et al., 1988) to 46 h (t1/2β) for TCP (Abou-Donia et al., 1990) and <5 days for TDCPP in rats (Lynn, 1980). Despite the higher clearance rates, these 3 OPFR, or their metabolites, may accumulate with repeated dosing sufficiently to impact to the neuroendocrine axis. Further investigation into the partition and deposition of these 3 OPFR in the adult mouse model especially in the hypothalamus and whole brain is warranted.
There are few in vivo studies examining the effects of OPFR treatment on the hypothalamic control of energy balance in adult male or female rodents. Due to the ability of BDE-47, TDCPP, TPP, and TCP (or their metabolites) to interact with ER, our objective was to determine if the selected FR alter ARC gene expression of known E2-regulated genes in a sex-dependent manner using the mouse (Kojima et al., 2013; Liu et al., 2012). We demonstrated that FR, especially OPFR, differentially regulate the expression of ARC genes in adult, intact males and OVX females. In WT males, OPFR increased Pomc and decreased Npy, producing an anorectic neuropeptide gene profile. Consequently, OPFR-exposed WT males consumed less chow (a decrease in energy intake) than the oil-treated WT males, which reduced weight gain over the 4 weeks of dosing. As expected, KO males weighed more than their WT counterparts, regardless of EDC treatment, and were not susceptible to the effects of EE2 and OPFR on weight gain. Conversely, WT females exposed to the high-dose of OPFR gained more weight than the oil-treated females, which correlated with a reduction in anorexigenic (Pomc/Cart) gene expression. As expected, OVX KO females did not gain more weight than their WT counterparts and were not susceptible to EDC treatment like KO males. These novel findings indicate that the effects of adulthood OPFR exposure are potentially dependent on the differential actions of ERα within each sex, although we cannot state this with certainty since the female mice were OVX. Alternatively, OPFR exposure may sensitize females to an obesogenic diet while reducing sensitivity in males, especially during a longer duration of oral dosing.
These effects on neuropeptide gene expression may be partially dependent on ERα, as only Cart was downregulated in KO males similar to WT males. Furthermore, FR (and EE2) treatment suppressed male ARC Esr1 gene expression, which is a similar response to ligand exposure (E2 treatment) in female mice (Yang et al., 2016a,b). However, female Esr1 gene expression was unaffected. It is well established that ovariectomy promotes hyperphagia and body weight gain, which can be prevented by E2 replacement acting through ERα (Asarian and Geary, 2002). Therefore, OPFR may not be acting directly on ERα but via regulation of the Esr1 gene. FR also interact with multiple steroid and nuclear receptors in vitro such as PXR, thyroid receptors, PPARα/γ, androgen receptors (ARs), and mineralocorticoid and glucocorticoid receptors (Belcher et al., 2014; Hu et al., 2014; Kojima et al., 2013). Interestingly, PPARγ expression is elevated by FR in males and females and may mediate, in part, the effects of these compounds on ARC gene expression. PPARγ is a known modulator of POMC neuronal activity and mediates the impact of high-fat diets on the hypothalamus (Long et al., 2014). However, hypothalamic PPARγ activation augments Npy and Agrp expression in the ARC (Garretson et al., 2015), but these findings do not align with our results. Further investigation is required to determine which nuclear or steroid receptors are interacting with FR either directly or indirectly to control ARC gene expression; those studies should utilize global or brain-specific ERα/β, PPARγ, or other nuclear receptor KO models.
Whether interacting with ERα or PPARγ, FR upregulated ARC expression of peptide hormone receptors Ghsr, Insr, and Lepr in males and Insr in females. InsR is a tyrosine kinase receptor activated by insulin to control glucose metabolism and suppress appetite (Hill et al., 2010). ARC insulin signaling activates POMC neurons and inhibits NPY neurons (Qiu et al., 2014). The adipokine leptin also activates POMC neurons and inhibits NPY neurons through its receptor (Qiu et al., 2010). Both insulin and leptin receptor activation targets nonselective canonical transient receptor potential (TRPC5) channels in POMC and KNDy neurons (Qiu et al., 2010, 2014). Activation of these channels causes depolarization in the neurosecretory neurons, leading to suppressed food intake and other physiological outcomes. Therefore, an increase in Insr or Lepr expression in POMC neurons or an increase of Trpc5 expression would increase their sensitivity to insulin and leptin, leading to decreased food intake.
OPFR also affected leptin and insulin plasma levels. Leptin production from adipose tissue and insulin production from the pancreas were reduced by OPFR in WT males but were augmented in KO males. The differences between the genotypes indicate that the peripheral actions of OPFR may not be mediated by ERα but rather by other nuclear receptors including PPARγ (Kim et al., 2013; Kubota et al., 1999; Tung et al., 2017). Consequently, the increase in leptin and insulin receptor expression in the ARC may be offset by the OPFR-induced hypoinsulinemia and hypoleptinemia in WT males. Furthermore, the increase in receptor may be due to the decrease in ligand as many hormone receptors are downregulated or undergo desensitization when ligand concentrations are elevated (Zabeau et al., 2003). In females, leptin production was not modulated by FR. However, leptin production in KO females was higher compared with WT females. The elevated leptin in KO has been previously reported and is indicative of greater adiposity in KO females (Yasrebi et al., 2017). Interestingly, insulin was elevated by BDE-47 (1 mg/kg) in KO females compared with both oil-treated KO and BDE-47–treated WT females. This unexpected finding suggests that ERα protects against BDE-47–induced insulin production in OVX females or that the lack of ERα disrupts the interactions of FR with the pancreatic β-cells.
GHSR is a G-protein–coupled receptor that is activated by ghrelin, a hormone secreted by the stomach to promote hunger (Andrews, 2011). In the ARC, GHSR is primarily found in NPY/AgRP, KNDy, and GHRH neurons (Yang et al., 2016a,b; Yasrebi et al., 2016). In our study, GHSR expression was upregulated by FR in males but not in females. Interestingly, E2 upregulates Ghsr in the ARC through ERα (Yang et al., 2016a,b) primarily in KNDy neurons (Yang et al., 2016a,b) and not in NPY/AgRP neurons (Yasrebi et al., 2016). Considering the fact that males consumed less energy with OPFR, we hypothesize that the increase in GHSR expression is occurring in KNDy neurons from WT males and potentially impacts the effects of ghrelin on the negative feedback of gonadal steroids on luteinizing hormone pulse frequency. This may be especially true in WT males due to OPFR-induced hyperghrelinemia, which is potentially mediated by ERα.
Activation of the GHSR in NPY neurons suppresses the KCNQ-mediated M-current (Yasrebi et al., 2016). When stimulated by depolarization, KCNQ (Kv.7) channels facilitate an outward potassium current (M-current) to stabilize membrane potential and reduce action potential frequency (Roepke et al., 2011). KCNQ channel subunits Kcnq2, Kcnq3, and Kcnq5 are highly expressed in the ARC POMC and NPY neurons (Roepke et al., 2007), and expression of these subunits are controlled in NPY neurons by fasting in males and females (Roepke et al., 2011). In our study, KCNQ expression was increased in males by FR but only by EE2 in females, which is similar to the effects of E2 in females (Roepke et al., 2011). An increase in KCNQ expression and subsequent M-current activity would lead to a suppression of neuronal excitability. We hypothesize that these effects are occurring primarily in NPY neurons because such an increase in M-current activity would lead to a decrease in food intake.
T-type calcium channels associated with the Cav3.1 (Cacna1g), Cav3.2 (Cacna1h), and Cav3.3 (Cacna1i) subunits are highly expressed in ARC POMC and KNDy neurons and produce the low-voltage–activated calcium currents responsible for neuronal burst firing and neurotransmitter release (Bosch et al., 2013; Qiu et al., 2006). In female mice, E2 increases Cacna1g in the ARC through an ERE-dependent mechanism (Bosch et al., 2009; Yang et al., 2016a,b), increases Cacna1h expression by both ERα and ERβ activation (Bosch et al., 2009), and increases Cacna1i in ARC KNDy neurons (Gottsch et al., 2011). It is unknown whether E2 or androgens control Cav3.x channels in the ARC of males. In our study, FR, along with EE2, increased expression of all 3 subunits in males, but only Cav3.1 in females. Potentially, an increase in Cav 3.x channel expression results in more burst firing in neurosecretory neurons, leading to more frequent secretion of their respective neuropeptides (α-melanocyte-stimulating hormone (MSH), β-endorphin, kisspeptin, neurokinin B, etc.) and controlling downstream homeostatic functions.
One of these downstream homeostatic functions is glucose homeostasis and, in particular, hepatic glucose production (Lin et al., 2010). Both BDE-47 and OPFR elevated fasting glucose levels in males, indicating an increase in hepatic glucose production either directly by altering liver glucose metabolism or indirectly by altering the neuroendocrine control of glucose production. FR did not have an effect in females, most likely due to the disruptive effects of ovariectomy (loss of E2) on glucose metabolism. Indeed, glucose clearance was augmented by EE2 and BDE-47 in females, recapitulating the effects of E2 replacement in OVX female rodents (Yasrebi et al., 2017). The activation of ERα increases glucose tolerance by regulating the glucose transporter type 4 (GLUT4) expression and activity in skeletal muscle (Gorres et al., 2011). Thus, ERα KO females exhibit impairments to glucose clearance (Yasrebi et al., 2017) and were not examined in this study.
FR, especially PBDE, can induce xenobiotic receptor signaling in the liver. In our in vivo study, BDE-47 activated farnesoid X receptor (FXR), PXR, and CAR target genes in males and females and OPFR activated PXR and CAR only in males. In human hepatic cells, BDE-47 enhances PXR and CAR activation (Hu et al., 2014; Sueyoshi et al., 2014;). In fact, in vivo, PBDE (BDE-47, -09, and -209) induce Cyp3a11 and Cyp2b10 gene expression by activating PXR in rat livers (Pacyniak et al., 2007). Whether BDE-47 is a FXR modulator needs detailed study in the future. Little is known about the impacts of in vivo OPFR exposure on xenobiotics receptors and their targets genes in the liver. In transfected human liver cells, TPP activates mouse and human PXR and CAR (Honkakoski et al., 2004). Surprisingly, in the ERα KO, there were no significant effects on target gene expression except for an increase in Cd36 by BDE-47, indicating activation of PPARα (Gao et al., 2013). The lack of target gene regulation in the KO suggests that these mechanisms are dependent on interaction with ERα or that the loss of ERα in the liver disrupts normal xenobiotic receptor activity or expression.
In summary, FR, or their metabolites, alter hypothalamic and liver gene expression of intact male and OVX female mice and impact food intake and glucose homeostasis in a sex-dependent manner. If ARC neurons, especially POMC and NPY (which express ERα and PPARγ) are found to be more sensitive to ghrelin, insulin, or leptin after FR exposure, then the downstream control of energy homeostasis (feeding behavior, energy expenditure) could be altered (see Figure 6). Furthermore, if these neurons exhibit elevated cation channel activation, their intrinsic activity and response to these hormones or other neurotransmitters would be changed. Because FR augment both hormone receptors and cation channel subunits in the heterogeneous ARC, future experiments will characterize cell type-specific gene expression using standard whole-cell patch clamp electrophysiology coupled with single-cell qPCR after FR treatment. Results from the current study provide insight into the effects of adult exposure to FR in a part of the rodent brain that controls energy homeostasis. Although not “obesogens”, FR, especially OPFR, do disrupt energy homeostasis and may sensitize the animal to further assaults through either diet or concurrent EDC exposure.
Figure 6.
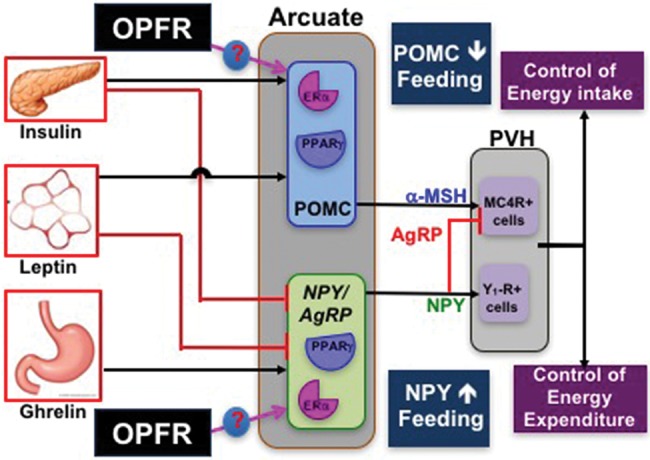
An illustration of our hypothesis that OPFR interact with ERα and/or PPARγ in arcuate POMC and NPY/AgRP neurons to control expression of hormone receptors and cation channels to alter release of α-MSH, NPY, and AgRP on downstream targets in the paraventricular hypothalamus, leading to a decrease in food intake or an increase in energy expenditure.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
U.S. Department of Agriculture-National Institute of Food and Agriculture (NJ06107) and the National Institutes of Health (R00DK083457, R00DK083457-S1, and R21ES027119 to T.A.R.; R01GM104037 to G.L.G; P30ES005022 to T.A.R., G.L.G., S.M.M., B.T.B.). T.S.T. was funded, in part, by (R25ES020721) and S.M.M. was funded by (R01ES021800).
Supplementary Material
ACKNOWLEDGMENTS
The authors must thank Dr Sara Campbell for the use of the EMD Millipore MAGPIX Multiplex System, Dr Judy Storch for the use of the EchoMRI Body Composition Analyzer, and for the many undergraduate students who assisted in dosing the mice.
REFERENCES
- Abou-Donia M. B., Nomeir A. A., Bower J. H., Makkawy H. A. (1990). Absorption, distribution, excretion and metabolism of a single oral dose of [14C]tri-o-cresyl phosphate (TOCP) in the male rat. Toxicology 65, 61–74. [DOI] [PubMed] [Google Scholar]
- Andrews Z. B. (2011). Central mechanisms involved in the orexigenic actions of ghrelin. Peptides 32, 2248–2255. 10.1016/j.peptides.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Asarian L., Geary N. (2002). Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 42, 461–471. 10.1006/hbeh.2002.1835 [DOI] [PubMed] [Google Scholar]
- Baquero A. F., de Solis A. J., Lindsley S. R., Kirigiti M. A., Smith M. S., Cowley M. A., Zeltser L. M., Grove K. L. (2014). Developmental switch of leptin signaling in arcuate nucleus neurons. J. Neurosci. 34, 9982–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher S. M., Cookman C. J., Patisaul H. B., Stapleton H. M. (2014). In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol. Lett. 228, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H. R. (2002). Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 26, 393–428. 10.1016/S0149-7634(02)00014-3 [DOI] [PubMed] [Google Scholar]
- Bosch M. A., Hou J., Fang Y., Kelly M. J., Rønnekleiv O. K. (2009). 17Beta-estradiol regulation of the mRNA expression of T-type calcium channel subunits: Role of estrogen receptor alpha and estrogen receptor beta. J. Comp. Neurol. 512, 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M. A., Tonsfeldt K. J., Rønnekleiv O. K. (2013). mRNA expression of ion channels in GnRH neurons: Subtype-specific regulation by 17β-estradiol. Mol. Cell Endocrinol. 367, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M. A., Xue C., Rønnekleiv O. K. (2012). Kisspeptin expression in guinea pig hypothalamus: Effects of 17β-estradiol. J. Comp. Neurol. 520, 2143–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt C. M., Congleton J., Hoffman K., Fang M., Stapleton H. M. (2014). Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 48, 10432–10438. [DOI] [PubMed] [Google Scholar]
- Carignan C. C., McClean M. D., Cooper E. M., Watkins D. J., Fraser A. J., Heiger-Bernays W., Stapleton H. M., Webster T. F. (2013). Predictors of tris(1, 3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ. Int. 55, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington C., Burt C., Abou-Donia M. (1988). In vivo31P nuclear magnetic resonance studies on the absorption of triphenyl phosphite and tri-o-cresyl phosphate following subcutaneous administration in hens. Drug Metab. Dispos. 16, 104–109. [PubMed] [Google Scholar]
- Cequier E., Marcé R. M., Becher G., Thomsen C. (2014). A high-throughput method for determination of metabolites of organophosphate flame retardants in urine by ultra performance liquid chromatography-high resolution mass spectrometry. Anal. Chim. Acta 845, 98–104. [DOI] [PubMed] [Google Scholar]
- Elias C. F., Aschkenasi C., Lee C., Kelly J., Ahima R. S., Bjorbæk C., Flier J. S., Saper C. B., Elmquist J. K. (1999). Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23, 775–786. [DOI] [PubMed] [Google Scholar]
- Farhat A., Buick J. K., Williams A., Yauk C. L., O'Brien J. M., Crump D., Williams K. L., Chiu S., Kennedy S. W. (2014). Tris(1, 3-dichloro-2-propyl) phosphate perturbs the expression of genes involved in immune response and lipid and steroid metabolism in chicken embryos. Toxicol. Appl. Pharmacol. 275, 104–112. [DOI] [PubMed] [Google Scholar]
- Gao M., Bu L., Ma Y., Liu D., Blachier F. (2013). Concurrent activation of liver X receptor and peroxisome proliferator-activated receptor alpha exacerbates hepatic steatosis in high fat diet-induced obese mice. PLoS One 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garretson X. J. T., Teubner B. J. W., Grove K. L., Vazdarjanova A., Ryu X., Bartness T. J. (2015). Peroxisome proliferator-activated receptor g controls ingestive behavior, Agouti-related Protein, and Neuropeptide Y mRNA in the arcuate hypothalamus. J. Neurosci. 35, 4571–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N., Asarian L., Korach K. S., Pfaff D. W., Ogawa S. (2001). Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-a null mice. Endocrinology 142, 4751–4757. 10.1210/endo.142.11.8504 [DOI] [PubMed] [Google Scholar]
- Gorres B. K., Bomhoff G. L., Morris J. K., Geiger P. C. (2011). In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J. Physiol. 589, 2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch M. L., Popa S. M., Lawhorn J. K., Qiu J., Tonsfeldt K. J., Bosch M. A., Kelly M. J., Rønnekleiv O. K., Sanz E., McKnight G. S., et al. (2011). Molecular properties of kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152, 4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt S. C., Kissling G. E., Fieselman K. E., Jayes F. L., Gerrish K. E., Korach K. S. (2010). Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. faseb J. 24, 4660–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. W., Elias C. F., Fukuda M., Williams K. W., Berglund E. D., Holland W. L., Cho Y. R., Chuang J. C., Xu Y., Choi M., et al. (2010). Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 11, 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., Butt C. M., Webster T. F., Preston E. V., Hammel S. C., Makey C., Lorenzo A. M., Cooper E. M., Carignan C., Meeker J. D., et al. (2017). Temporal trends in exposure to organophosphate flame retardants in the United States. Environ. Sci. Technol. Lett. 4, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., Daniels J. L., Stapleton H. M. (2014). Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ. Int. 63, 169–172. 10.1016/j.envint.2013.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkakoski P., Palvimo J. J., Penttilä L., Vepsäläinen J., Auriola S. (2004). Effects of triaryl phosphates on mouse and human nuclear receptors. Biochem. Pharmacol. 67, 97–106. [DOI] [PubMed] [Google Scholar]
- Hu X. N., Zhang J. Q., Jiang Y. S., Lei Y. X., Lu L. G., Zhou J., Huang H. Y., Fang D., Tao G. H. (2014). Effect on metabolic enzymes and thyroid receptors induced by BDE-47 by activation the pregnane X receptor in HepG2, a human hepatoma cell line. Toxicol. In Vitro 28, 1377–1385. [DOI] [PubMed] [Google Scholar]
- Kim H.-S., Hwang Y.-C., Koo S.-H., Park K. S., Lee M.-S., Kim K.-W., Lee M.-K., Sesti G. (2013). PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-Cells. PLoS One 8, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-W. W., Isobe T., Muto M., Tue N. M., Katsura K., Malarvannan G., Sudaryanto A., Chang K.-H. H., Prudente M., Viet P. H., et al. (2014). Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 116, 91–97. [DOI] [PubMed] [Google Scholar]
- Kojima H., Takeuchi S., Itoh T., Iida M., Kobayashi S., Yoshida T. (2013). In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 314, 76–83. [DOI] [PubMed] [Google Scholar]
- Kubota N., Terauchi Y., Miki H., Tamemoto H., Yamauchi T., Komeda K., Satoh S., Nakano R., Ishii C., Sugiyama T., et al. (1999). PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4, 597–609. [DOI] [PubMed] [Google Scholar]
- Li J., Yu N., Zhang B., Jin L., Li M., Hu M., Zhang X., Wei S., Yu H. (2014). Occurrence of organophosphate flame retardants in drinking water from China. Water Res. 54, 53–61. [DOI] [PubMed] [Google Scholar]
- Lin H. V., Plum L., Ono H., Gutiérrez-Juárez R., Shanabrough M., Borok E., Horvath T. L., Rossetti L., Accili D. (2010). Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 59, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Wang Q., Liang K., Liu J., Zhou B., Zhang X., Liu H., Giesy J. P., Yu H. (2013). Effects of tris(1, 3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquat. Toxicol. 128–129, 147–157. [DOI] [PubMed] [Google Scholar]
- Liu L. Y., He K., Hites R. A., Salamova A. (2016). Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ. Sci. Technol. 50, 3065–3073. 10.1021/acs.est.5b05073 [DOI] [PubMed] [Google Scholar]
- Liu X., Ji K., Choi K. (2012). Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat. Toxicol. 114–115, 173–181. [DOI] [PubMed] [Google Scholar]
- Liu X., Ji K., Jo A., Moon H. B., Choi K. (2013). Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat. Toxicol. 134–135, 104–111. [DOI] [PubMed] [Google Scholar]
- Long L., Toda C., Jeong J. K., Horvath T. L., Diano S. (2014). PPARγ ablation sensitizes proopiomelanocortin neurons to leptin during high-fat feeding. J. Clin. Invest. 124, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Cai Z., Fu J., Luo S., Liu C., Li X., Zhao D. (2014). Molecular docking and molecular dynamics studies on the interactions of hydroxylated polybrominated diphenyl ethers to estrogen receptor alpha. Ecotoxicol. Environ. Saf. 101, 83–89. 10.1016/j.ecoenv.2013.12.018 [DOI] [PubMed] [Google Scholar]
- Lynn R. (1980). Diester metabolites of the flame retardant chemicals, tris(1, 3-dichloro-2- propyl)phosphate and tris(2, 3-dibromopropyl) phosphate in the rat: Identification and quantification. Res. Commun. Chem. Pathol. Pharmacol. 45, 5323–5331. [PubMed] [Google Scholar]
- Ma Y., Jin J., Li P., Xu M., Sun Y., Wang Y., Yuan H. (2017). Organophosphate ester flame retardant concentrations and distributions in serum from inhabitants of Shandong, China, and changes between 2011 and 2015. Environ. Toxicol. Chem. 36, 414–421. 10.1002/etc.3554 [DOI] [PubMed] [Google Scholar]
- Mamounis K. J., Yang J. A., Yasrebi A., Roepke T. A. (2014). Estrogen response element-independent signaling partially restores post-ovariectomy body weight gain but is not sufficient for 17b-estradiol’s control of energy homeostasis. Steroids 81, 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F., Clegg D. J., Hevener A. L. (2013). The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 34, 309–338. 10.1210/er.2012-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Cooper E. M., Stapleton H. M., Hauser R. (2013). Urinary metabolites of organophosphate flame retardants: Temporal variability and correlations with house dust concentrations. Environ. Health Perspect. 121, 580–585. 10.1289/ehp.1205907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirshamsi S., Laidlaw H. a., Ning K., Anderson E., Burgess L. a., Gray A., Sutherland C., Ashford M. L. J. (2004). Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 5, 54.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R., Williams L. M., Dieguez C. (2010). Ghrelin: New molecular pathways modulating appetite and adiposity. Obes. Facts 3, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyniak E. K., Cheng X., Cunningham M. L., Crofton K., Klaassen C. D., Guo G. L. (2007). The flame retardants, polybrominated diphenyl ethers, are pregnane X receptor activators. Toxicol. Sci. 97, 94–102. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K. B. J. (2008). The Mouse Brain in Stereotaxic Coordinates, Compact, Third Edition: The Coronal Plates and Diagrams, 3rd ed.Academic Press, London, UK. [Google Scholar]
- Phillips A. L., Chen A., Rock K. D., Horman B., Patisaul H. B., Stapleton H. M. (2016). Transplacental and lactational transfer of Firemaster® 550 components in dosed Wistar rats. Toxicol. Sci. 153, 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai H. K., Fang M., Beglov D., Kozakov D., Vajda S., Stapleton H. M., Webster T. F., Schlezinger J. J. (2014). Ligand binding and activation of PPARγ by Firemaster 550: Effects on adipogenesis and osteogenesis in vitro. Environ. Health Perspect. 122, 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudan N., Peroski M., Grignol G., Merchenthaler I., Dudas B. (2015). Juxtapositions between the somatostatinergic and growth hormone-releasing hormone (GHRH) neurons in the human hypothalamus. Neuroscience 297, 205–210. [DOI] [PubMed] [Google Scholar]
- Qiu J., Bosch M. A., Jamali K., Xue C., Kelly M. J., Rønnekleiv O. K. (2006). Estrogen upregulates T-type calcium channels in the hypothalamus and pituitary. J. Neurosci. 26, 11072–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Fang Y., Rønnekleiv O. K., Kelly M. J. (2010). Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J. Neurosci. 30, 1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Zhang C., Borgquist A., Nestor C. C., Smith A. W., Bosch M. A., Ku S., Wagner E. J., Rønnekleiv O. K., Kelly M. J. (2014). Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 19, 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke T. A., Malyala A., Bosch M. A., Kelly M. J., Rønnekleiv O. K. (2007). Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology 148, 4937–4951. [DOI] [PubMed] [Google Scholar]
- Roepke T. A., Qiu J., Smith A. W., Rønnekleiv O. K., Kelly M. J. (2011). Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J. Neurosci. 31, 11825–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke T. A., Smith A. W., Ronnekleiv O. K., Kelly M. J. (2012). Serotonin 5-HT2C receptor-mediated inhibition of the M-current in hypothalamic POMC neurons. AJP Endocrinol Metab 302, E1399–E1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C. B., Chou T. C., Elmquist J. K. (2002). The need to feed. Neuron 36, 199–211. 10.1016/S0896-6273(02)00969-8 [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Woods S. C. Jr, Seeley D.P., R. J., Baskin D. G. (2000). Control of food intake. Nature, 404, 661–671. [DOI] [PubMed] [Google Scholar]
- Shi L., Bian X., Qu Z., Ma Z., Zhou Y., Wang K., Jiang H., Xie J. (2013). Peptide hormone ghrelin enhances neuronal excitability by inhibition of Kv7/KCNQ channels. Nat. Commun. 4, 1435.. [DOI] [PubMed] [Google Scholar]
- Staskal D. F., Diliberto J. J., De Vito M. J., Birnbaum L. S. (2005). Toxicokinetics of BDE 47 in female mice: Effect of dose, route of exposure, and time. Toxicol. Sci. 83, 215–223. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T., Li L., Wang H., Moore R., Kodavanti P. R. S., Lehmler H. J., Negishi M., Birnbaum L. S. (2014). Flame retardant BDE-47 effectively activates nuclear receptor CAR in human primary hepatocytes. Toxicol. Sci. 137, 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist A. M., Olofsson U., Haglund P. (2010). Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 12, 943–951. 10.1039/b921910b [DOI] [PubMed] [Google Scholar]
- Tung E. W. Y., Ahmed S., Peshdary V., Atlas E., Óvilo C. (2017). Firemaster® 550 and its components isopropylated triphenyl phosphate and triphenyl phosphate enhance adipogenesis and transcriptional activity of peroxisome proliferator activated receptor (Pparγ) on the adipocyte protein 2 (aP2) promoter. PLoS One 12, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I., de Boer J. (2012). Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. 10.1016/j.chemosphere.2012.03.067 [DOI] [PubMed] [Google Scholar]
- Woods S. C. (2009). The Control of food intake: Behavioral versus molecular perspectives. Cell Metab. 9, 489–498. 10.1016/j.cmet.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Ding J., Huang W., Xie W., Liu W. (2014). Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air particulate matter. Environ. Sci. Technol. 48, 63–70. 10.1021/es403186z [DOI] [PubMed] [Google Scholar]
- Yang J., Mamounis K., Yasrebi A., Roepke T. (2016). Regulation of gene expression by 17β-estradiol in the arcuate nucleus of the mouse through ERE-dependent and ERE-independent mechanisms. Steroids 107, 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Yasrebi A., Snyder M., Roepke T. (2016). The interaction of fasting, caloric restriction, and diet-induced obesity with 17β-estradiol on the expression of KNDy neuropeptides and their receptors in the female mouse. Mol. Cell Endocrinol. 437, 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasrebi A., Hsieh A., Mamounis K. J., Krumm E. A., Yang J. A., Magby J., Hu P., Roepke T. A. (2016). Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17β-estradiol. Mol. Cell Endocrinol. 422, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasrebi A., Rivera J. A., Krumm E. A., Yang J. A., Roepke T. A. (2017). Activation of estrogen response element-independent ERα signaling protects female mice from diet-induced obesity. Endocrinology 158, 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau L., Lavens D., Peelman F., Eyckerman S., Vandekerckhove J., Tavernier J. (2003). The ins and outs of leptin receptor activation. FEBS Lett. 546, 45–50. [DOI] [PubMed] [Google Scholar]
- Zota A. R., Linderholm L., Park J. S., Petreas M., Guo T., Privalsky M. L., Zoeller R. T., Woodruff T. J. (2013). Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ. Sci. Technol. 47, 11776–11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.