Abstract
Background
True population-based clinical and outcomes data are lacking for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated glomerulonephritis (AAGN). Therefore we aimed to estimate the incidence, prevalence and mortality of AAGN, as well as the relationship between the grade of chronic renal damage at presentation and renal and non-renal outcomes.
Methods
Patients with AAGN were identified among a population-based incident cohort of 57 Olmsted County residents diagnosed with ANCA-associated vasculitis (AAV) in 1996–2015. Incidence rates were age and sex adjusted to the 2010 US white population. Age- and sex-adjusted prevalence was calculated for 1 January 2015. Survival rates were compared with expected rates in the Minnesota population. Chronic renal damage was assessed by chronicity score (CS) on biopsies performed at diagnosis.
Results
Thirty-four (60%) patients had AAGN. Of these, 65% had microscopic polyangiitis (MPA) and 74% were myeloperoxidase (MPO)-ANCA positive. The annual incidence of AAGN was 2.0/100 000 population [95% confidence interval (CI) 1.3–2.7] and the overall prevalence was 35/100 000 (95% CI 24–47). Mortality for AAGN was increased (P < 0.001), whereas mortality for AAV without glomerulonephritis did not differ from the general population. Minimal to mild CS predicted recovery of renal function at 1 year; clinical diagnosis (granulomatosis with polyangiitis versus MPA) and ANCA specificity (proteinase 3 versus MPO) did not. This observation was replicated in an independent cohort of 38 newly diagnosed AAGN patients seen at our centre over the 1999–2014 period.
Conclusions
The annual incidence and prevalence of AAGN in Minnesota are 2.0/100 000 and 35/100 000, respectively. Mortality is worse compared with AAV patients without glomerulonephritis. More advanced renal damage at diagnosis predicts less renal recovery.
Keywords: ANCA-associated vasculitis, glomerulonephritis, granulomatosis with polyangiitis, nephritis, renal insufficiency
INTRODUCTION
Glomerulonephritis is a common disease manifestation of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) and often presents with severe kidney function impairment, affecting both renal and overall patient survival [1–4]. The annual reported incidence rate of ANCA-associated glomerulonephritis (AAGN) in Europe is between 1.2 and 1.3 [95% confidence interval (CI) 1.0–1.6] per 100 000 inhabitants ≥18 years of age [5–8], and a similar incidence has been observed in Japan [9]. We have recently reported the annual incidence of AAV in a well-defined region of the USA at ∼ 3.3 per 100 000, with ∼60% of this population having renal involvement at diagnosis [3]. Population-based data specifically addressing the epidemiology of AAGN and associated outcomes in the USA are lacking.
The renal prognosis of AAGN differs greatly between individual patients. Several attempts to classify patients by ANCA specificity rather than by clinical diagnosis showed that renal function at baseline and renal survival are inferior in myeloperoxidase (MPO)-AAV compared with proteinase 3 (PR3)-AAV [6, 10]. Histological findings on renal biopsy also have a strong prognostic value. The International Pathology Classification comprises four categories of renal lesions, each associated with a different prognosis, and MPO-AAV patients are more likely to have renal pathology classified as mixed or sclerotic, usually associated with a successively worse outcome [11, 12]. Others have proposed renal scarring at presentation and renal function at 6 months as predictors of renal response to treatment [13]. Overall, these findings suggest that the grade of chronic damage in the kidney at presentation seems to be one of the principal predictors for the renal prognosis and overall patient survival.
The International Pathology Classification is currently the only validated histological classification for renal AAV. However, its prognostic value has not been confirmed in all independent cohorts [14, 15]. A uniform, semi-quantitative approach called the chronicity score (CS), specifically assessing chronic changes in all kidney biopsies regardless of underlying disease, has been proposed by an international group, but has not yet been utilized to score chronicity in AAGN [16].
The aim of this study was to report the annual incidence, prevalence and mortality of AAGN during the past 20 years in a population-based cohort of Olmsted County, Minnesota (USA) residents and to evaluate the effect of renal damage at presentation on the recovery of renal function at 1 year after diagnosis using the recently developed CS.
MATERIALS AND METHODS
Data sources
Through the resources of the Rochester Epidemiology Project (REP), the population of Olmsted County is well suited for investigation of the epidemiology of AAV because comprehensive medical records for all residents seeking medical care are available. The record linkage system allows ready access to medical records for inpatients and outpatients from all health care providers for the local population, including the Mayo Clinic, the Olmsted Medical Center and their affiliated hospitals, local nursing homes and the few private practitioners. Furthermore, the Mayo Clinic was the only regional centre in Olmsted County that performed and read renal biopsies in the study period. The potential of this data system for use in population-based studies has previously been described [17, 18]. The system also allows complete ascertainment of the outcomes of interest (i.e. renal function and clinical data) for AAV cases among the residents of Olmsted County.
Case ascertainment
Olmsted County residents with incident AAV from 1 January 1996 to 31 December 2015 were previously identified by medical record review [3]. Briefly, patients were selected based on diagnostic codes for granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), AAV, ANCA arteritis and renal vasculitis. To ensure complete detection of all potential cases, the laboratory database was searched for positive ANCA test results detected by enzyme-linked immunosorbent assay and immunofluorescence. AAV cases were included if they fulfilled the criteria of at least one of the following classification schemes: American College of Rheumatology (ACR) classification criteria or the modified ACR criteria for GPA [19, 20], ACR criteria for EGPA [21], Chapel Hill Consensus Conference (CHCC) definition [22] or European Medical Agency (EMA) algorithm [23]. Patients were defined as having AAGN if they had biopsy-proven AAGN and/or an AAV-related increase in creatinine >30% and/or a decrease in creatinine clearance >25% and/or if they had haematuria or red cell casts in the urinary sediment.
Clinical and histological data ascertainment
All medical records from identified patients were reviewed. Information on patient demographics, clinical manifestations, laboratory findings, histopathology, radiology and disease activity at baseline and during follow-up were abstracted. Data, required to compute the Birmingham Vasculitis Activity Score for Wegener granulomatosis (BVAS/WG) [24] at AAV diagnosis were also abstracted from the medical records.
All clinically obtained creatinine values were collected. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate the estimated glomerular filtration rate (eGFR) [25, 26]; kidney function was scored according to the Kidney Disease Outcomes Quality Initiative (KDOQI) for staging chronic kidney disease (CKD) classification [27]. End-stage renal disease was defined as a sustained eGFR <15 mL/min/1.73 m2 or the need renal replacement therapy (dialysis or transplantation).
Only renal specimens obtained at the time of diagnosis were considered for CS calculation. Biopsies were independently scored by two physicians, blinded to the clinical data, according to the previously standardized definitions. Differences in scoring between the two physicians were resolved by re-reviewing the biopsies and coming to a consensus.
Follow-up was continued until death, migration or 31 December 2016. The study was approved by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center.
Independent replication cohort
To validate the prognostic value of the CS for recovery of renal function at 1 year in patients with AAGN, an independent cohort of consecutive US patients followed at Mayo Clinic Rochester between 1 January 1999 and 1 January 2014 was identified, fulfilling the same inclusion and exclusion criteria as the Olmsted County patients. Only newly diagnosed patients with biopsy-confirmed AAGN (with either a diagnosis of MPA or GPA), with available creatinine data from the time of renal biopsy and 12 months were included. Patients already part of the Olmsted County incidence cohort or patients who underwent renal transplantation during the observation period were excluded.
Statistical analysis
Comparisons between groups were performed using chi-squared and rank sum tests. Age- and sex-specific annual incidence rates of AAGN were calculated using the number of incidence cases as the numerator and population estimates for adults (age ≥18 years) based on decennial census counts as the denominator, with linear interpolation to estimate population size for intercensal years. Prevalence rates on 1 January 2015 were calculated using the number of prevalent cases as the numerator and the population estimates for adults (age ≥18 years) from the census as the denominator. Overall incidence and prevalence rates were age and sex adjusted to the 2010 US white population.
Survival following the diagnosis of AAV among those with and without AAGN and by level of CS was estimated using Kaplan–Meier methods. Observed and expected survival rates were compared using the log-rank test, where expected survival for persons of the same age, sex and calendar year was estimated using Minnesota population life tables. The ratio of the observed number of deaths to the expected number, the standardized mortality ratio (SMR), was estimated. Ninety-five percent confidence intervals (CIs) were computed for the SMR assuming that the expected rates are fixed and the observed number of deaths follows a Poisson distribution. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographic, clinical and laboratory findings of the incidence cohort at diagnosis
The demographic and clinical characteristics of the 57 patients diagnosed with AAV (28 MPA, 23 GPA and 6 EGPA) in Olmsted County between 1996 and 2015 are summarized in Table 1. Thirty-four patients had renal involvement at diagnosis.
Table 1.
Features of patients with incident AAV diagnosed in Olmsted County, Minnesota, 1996–2015, according to the presence of AAGN
| Characteristic | AAGN (n = 34) | No AAGN (n = 23) | Total (N = 57) | P-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 66.0 (15.3) | 54.8 (15.9) | 61.5 (16.4) | 0.008 |
| Sex (female), n (%) | 15 (44) | 13 (57) | 28 (49) | 0.358 |
| Ethnicity (Caucasian)a, n (%) | 33 (97) | 23 (100) | 56 (98) | 0.407 |
| Clinical diagnosis, n (%) | 0.015 | |||
| GPA | 9 (26) | 14 (61) | 23 (40) | |
| MPA | 22 (65) | 6 (26) | 28 (49) | |
| EGPA | 3 (9) | 3 (13) | 6 (11) | |
| ANCA groups, n (%) | 0.004 | |||
| ANCA negative | 0 (0) | 5 (22) | 5 (9) | – |
| p-ANCA/MPO-ANCA | 25 (74) | 9 (39) | 34 (60) | – |
| c-ANCA/PR3-ANCA | 9 (26) | 9 (39) | 18 (32) | – |
| Renal involvement as a first symptom, n (%) | 9 (26) | – | 9 (16) | – |
| Diagnostic delay (months), median (IQR) | 2.1 (0.9–7.9) | 7.4 (3.1–23.0) | 3.6 (1.0–12.5) | 0.030 |
| Renal biopsy (positive), n (%) | 24 (69) | – | – | – |
| BVAS/WG, median (IQR) | 7.0 (6.0–9.0) | 3.0 (3.0–6.0) | 6.0 (4.0–8.0) | <0.001 |
| Generalb, n (%) | 17 (50) | 12 (52) | 29 (51) | 0. 872 |
| Cutaneousb, n (%) | 6 (18) | 7 (30) | 13 (23) | 0.259 |
| Mucous membrane/eyesb, n (%) | 2 (6) | 0 (0) | 2 (4) | 0.236 |
| Ear, nose and throatb, n (%) | 8 (24) | 14 (61) | 22 (39) | 0. 004 |
| Pulmonaryb, n (%) | 15 (44) | 15 (65) | 30 (53) | 0.118 |
| Gastrointestinalb, n (%) | – | – | – | – |
| Renalb, n (%) | 34 (100) | – | – | – |
| Cardiovascularb, n (%) | 0 (0) | 1 (4) | 1 (2) | 0.220 |
| Nervous systemb, n (%) | 8 (24) | 5 (22) | 13 (23) | 0.874 |
| Haemoglobinc (g/dL), mean (SD) | 10.5 (2.0) | 12.0 (1.8) | 11.1 (2.0) | <0.001 |
| WBCc (×109/L), mean (SD) | 11.4 (5.0) | 10.4 (5.0) | 11.0 (5.0) | 0.441 |
| ESRc (mm/1 h), median (IQR) | 50 (25–81) | 24 (10–64) | 43 (16.5–72.5) | 0.111 |
| CRPc (mg/L), median (IQR) | 19.9 (6.9–45.0) | 8.4 (3.0–66.2) | 12.6 (4.5– 60.0) | 0.189 |
| Creatininec (mg/dL), median (IQR) | 2.2 (1.3–3.8) | 1.0 (0.8–1.2) | 1.3 (0.9–2.6) | <0.001 |
| eGFRc (mL/min/1.73 m2), median (IQR) | 27 (15–44) | 78 (65–88) | 43 (21.5–82) | <0.001 |
All but one patient (a Native American) were Caucasian.
System involvement was detailed according to the BVAS items.
General laboratory data not available in all patients (haemoglobin, WBC, platelets: missing in 2 patients without AAGN; ESR: missing in 5 patients with and 4 patients without AAGN; CRP: missing in 15 patients with and 4 patients without AAGN).
c-ANCA, cytoplasmic ANCA; p-ANCA, perinuclear ANCA; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell. Bold values in the P-values column, highlights significant p values from the non significant ones.
Patients with renal involvement were > 10 years older on average than those without (66.0 ± 15.3 versus 54.8 ± 15.9 years; P = 0.008). Sixty-five percent of the patients with AAGN had a clinical diagnosis of MPA and 74% were MPO-ANCA positive compared with 26% with MPA and 39% with MPO-ANCA in the group of AAV patients without glomerulonephritis. The BVAS/WG was significantly higher at diagnosis in the group of patients with AAGN [7.0 (95% CI 6.0, 9.0) versus 3.0 (3.0, 6.0); P < 0.001], who had a shorter diagnostic delay (from the time of the first symptom or sign until the time of diagnosis of AAV; median 2.1 versus 7.4 months; P = 0.030).
Among the 34 patients with AAGN, 9 had GPA, 22 MPA and 3 EGPA; 25 had MPO-AAV and 9 PR3-AAV. When AAGN patients were stratified for clinical diagnosis or ANCA specificity, no statistically significant differences in age, sex, total BVAS/WG and laboratory exams were found between the groups (P > 0.05 in all comparisons). The eGFR was not different between AAGN patients with GPA, MPA and EGPA {GPA, median 31 [interquartile range (IQR) 15–44], MPA 26.5 (12–42) and EGPA 82 (26–100) mL/min/1.73 m2; P = 0.28} and between PR3-AAV and MPO-AAV [PR3-AAV, median 31 (IQR 15–61), MPO-AAV 27 (17–42) mL/min/1.73 m2; P = 0.98].
A total of 15 patients with AAGN experienced 31 relapses, and 13 patients without AAGN at diagnosis experienced 25 relapses during 232 and 166 person-years of follow-up, respectively. Relapse rates were similar between patients with and without AAGN; the rates of any relapse were 13.4 and 15.1 per 100 person-years, respectively, and the rates of any severe relapse (BVAS/WG ≥3) were 6.5 and 8.4 per 100 person-years, respectively (P > 0.05 for both comparisons).
The annual incidence, mortality and prevalence rates
The annual age- and sex-adjusted incidence rate of AAGN was 2.0 (95% CI 1.3–2.7) per 100 000 population (Table 2). The overall age-adjusted annual incidence rate was 1.5 (95% CI 0.7–2.3) per 100 000 in adult women and 2.5 (95% CI 1.4–3.6) in adult men. The age- and sex-adjusted annual incidence rate for adults was 1.3 (95% CI 0.7–1.8) per 100 000 population for MPA glomerulonephritis, 0.5 (95% CI 0.2–0.9) for GPA glomerulonephritis and 0.2 (95% CI 0.0–0.4) for EGPA glomerulonephritis. Annual incidence rates were then calculated based on ANCA subtype. The age- and sex-adjusted annual incidence rate of PR3-AAGN for the adult population was 0.5 (95% CI 0.2–0.9) per 100 000 population and 1.5 (95% CI 0.9–2.1) for MPO-AAGN.
Table 2.
Age- and sex-adjusted annual incidence of AAGN among residents of Olmsted County, Minnesota, 1996–2015, per 100 000 population age ≥18 years and age- and sex-adjusted prevalence of AAGN among residents of Olmsted County, Minnesota on 1 January 2015 by per 100 000 population age ≥18 years
| Group | Incidence |
Prevalence |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female |
Male |
Total |
Female |
Male |
Total |
|||||||
| n | Age-adjusted rate (95% CI) | n | Age-adjusted rate (95% CI) | N | Age- and sex-adjusted rate (95% CI) | n | Age-adjusted rate (95% CI) | n | Age-adjusted rate (95% CI) | N | Age- and sex-adjusted rate (95% CI) | |
| Overall | 15 | 1.5 (0.7–2.3) | 19 | 2.5 (1.4–3.6) | 34 | 2.0 (1.3–2.7) | 21 | 36 (21–52) | 16 | 34 (17–52) | 37 | 35 (24–47) |
| GPA | 3 | 0.3 (0.0–0.6) | 6 | 0.7 (0.1–1.3) | 9 | 0.5 (0.2–0.9) | 7 | 12 (3–21) | 9 | 19 (6–31) | 16 | 15 (8–22) |
| MPA | 12 | 1.2 (0.5–1.9) | 10 | 1.4 (0.5–2.2) | 22 | 1.3 (0.7–1.8) | 13 | 23 (10–35) | 5 | 11 (1–22) | 18 | 17 (9–25) |
| EGPA | 0 | – | 3 | 0.4 (0.0–0.8) | 3 | 0.2 (0.0–0.4) | 1 | 2 (0–5) | 2 | 4 (0–10) | 3 | 3 (0–6) |
| ANCA neg. | 0 | – | 0 | – | 0 | – | 3 | 5 (0–11) | 0 | – | 3 | 3 (0–6) |
| MPO-AAV | 12 | 1.2 (0.5–1.9) | 13 | 1.8 (0.8–2.7) | 25 | 1.5 (0.9–2.1) | 12 | 21 (9–33) | 7 | 15 (4–27) | 19 | 18 (10–26) |
| PR3-AAV | 3 | 0.3 (0.0–0.6) | 6 | 0.7 (0.1–1.3) | 9 | 0.5 (0.2–0.9) | 6 | 10 (2–18) | 8 | 17 (5–29) | 14 | 13 (6–20) |
Rates per 100 000 population, adjusted to the US White 2010 population.
ANCA, anti-neutrophil cytoplasmic antibody; CI, confidence interval; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PR3, proteinase-3; neg., negative.
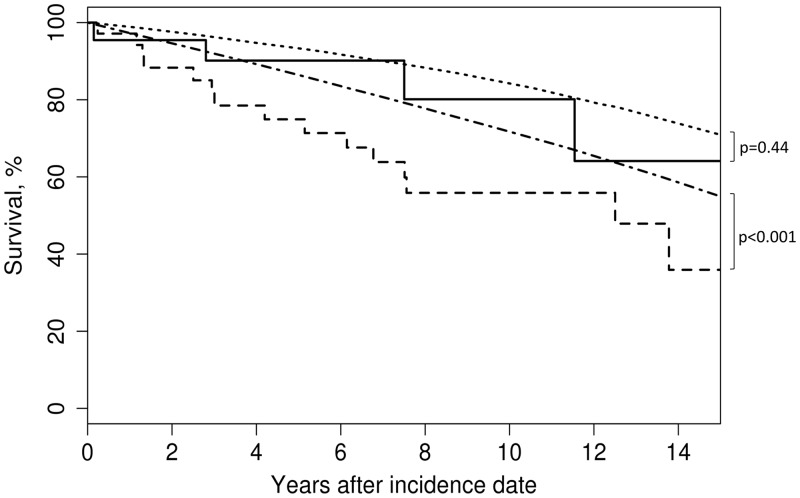
There were 15 deaths among the 34 patients with AAGN and 4 deaths among the 23 patients without (Table 3). Age- and sex-adjusted mortality was compared with the general population with and without glomerulonephritis over a median follow-up of 6.1 years (IQR 2.8, 11.0). With 7.0 expected deaths, the SMR for AAGN was 2.1 (95% CI 1.2–3.5). However, survival of patients without AAGN was not significantly different than that of the general population [2.7 expected deaths; SMR 1.5 (95% CI 0.4–3.8)]. The 5- and 10-year survival rates were 74% (95% CI 60–92%) and 54% (95% CI 38–77%), respectively, among patients with AAGN and 90% (95% CI 78–100%) and 80% (95% CI 61–100%), respectively, among patients without AAGN (Figure 1).
Table 3.
Mortality and survival rates for Olmsted County residents with incident AAGN by renal involvement in 1996–2015
| Measure | AAGN | No AAGN |
|---|---|---|
| Number of patients | 34 | 23 |
| Number of deaths | 15 | 4 |
| Expected number of deaths | 7.0 | 2.7 |
| SMR (95% CI) | 2.1 (1.2–3.5) | 1.5 (0.4–3.8) |
| 1-sample log-rank test P-value | <0.001 | 0.44 |
| 2-year survival rate (95% CI) | 88 (78–100) | 96 (87–100) |
| 5-year survival rate (95% CI) | 74 (60–92) | 90 (78–100) |
| 10-year survival rate (95% CI) | 54 (38–77) | 80 (61–100) |
AAGN, ANCA associated-glomerulonephritis; CI, confidence interval.
FIGURE 1.
Patient survival in AAV without AAGN (n = 23; solid line) compared with patients with AAGN (n = 34; dashed line) at AAV incidence and expected age-, sex-, and calendar year–adjusted mortality in Minnesota for those without AAGN (dotted line) and for those with AAGN (dashed/dotted line).
The age- and sex-adjusted prevalence of AAGN in adults (age ≥18 years) on 1 January 2015 was 35 (95% CI 24–47) per 100 000 population, similar for females and males. The age- and sex-adjusted prevalence of AAGN by AAV type is shown in Table 2.
Chronic damage assessment by CS in baseline renal biopsies
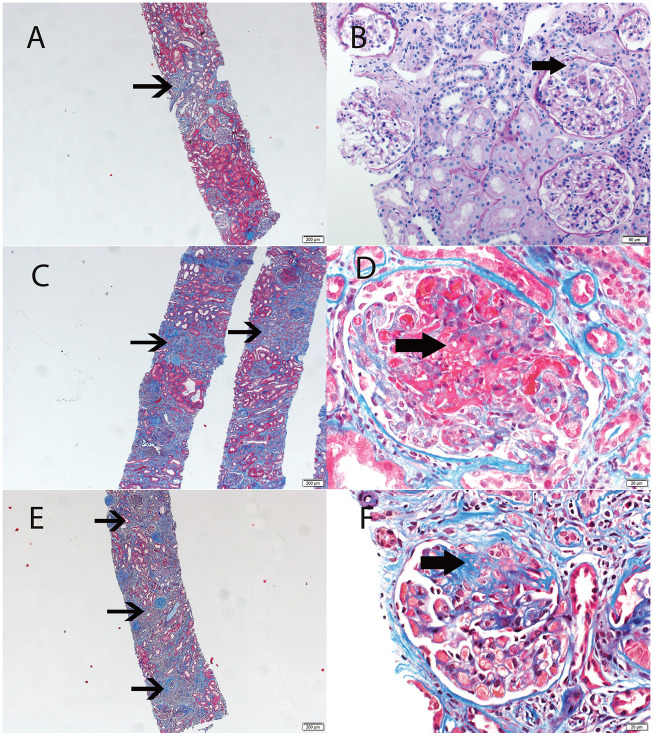
Renal biopsies were performed in 24 of 34 patients with 22 of the biopsies occurring at AAV diagnosis. The analysis of chronic damage was based on these 22 biopsies. Using the CS (Figure 2) [5], 14 patients were identified in the minimal/mild chronic changes class (4 minimal and 10 mild) and 8 patients in the moderate/severe chronic changes class (6 moderate and 2 severe) (Supplementary data, Table S1). The clinical and laboratory data of patients were divided into two groups according to their CS and are presented in Table 4. No significant differences were found in these two groups for age at onset, sex, diagnostic delay, clinical diagnosis, ANCA-specificity, BVAS/WG, eGFR and clinical and laboratory findings at index.
FIGURE 2.
Light microscopy showing grades of chronicity in renal biopsies. Each panel is one patient biopsy. (A and B) Mild chronic changes, (C and D) moderate chronic changes and (E and F) severe chronic changes. Minimal chronic changes were not represented since changes are absent or hardly perceptible. All stains are Masson trichrome, except B, which is a periodic acid–Schiff stain. Thin arrows point to areas of tubular atrophy and interstitial fibrosis (A, C, E). Thick arrows point to glomerular lesions: small cellular crescent (B), large area of segmental fibrinoid necrosis (necrotizing lesion) (D) and segmental sclerosis/scar (F).
Table 4.
Comparison of baseline clinical and laboratory features of patients with incident biopsy-proven AAGN by CS
| Characteristic | Minimal/mild (n = 14) | Moderate/severe (n = 8) | P-value |
|---|---|---|---|
| Age (years), mean (SD) | 63.8 (14.7) | 69.1 (14.3) | 0.453 |
| Sex (female), n (%) | 7 (50) | 4 (50) | 1.000 |
| Ethnicity (Caucasiana), n (%) | 13 (93) | 8 (100) | 0.439 |
| Clinical diagnosis, n (%) | 0.176 | ||
| GPA | 6 (43) | 1 (13) | |
| MPA | 8 (57) | 6 (75) | |
| EGPA | 0 (0) | 1 (13) | |
| ANCA groups, n (%) | 0.240 | ||
| p-ANCA/MPO-ANCA | 9 (64) | 7 (88) | |
| c-ANCA/PR3-ANCA | 5 (36) | 1 (13) | |
| Diagnostic delay (months), meadian (IQR) | 2.9 (1.3–12.4) | 1.0 (0.6–4.8) | 0.219 |
| BVAS/WG, median (IQR) | 8.5 (6.0–11.0) | 6.5 (6.0–8.0) | 0.605 |
| Generalb, n (%) | 9 (64) | 2 (25) | 0.076 |
| Cutaneousb, n (%) | 2 (14) | 2 (25) | 0.531 |
| Mucous membrane/eyesb, n (%) | 2 (14) | 0 (0) | 0.262 |
| Ear, nose and throatb, n (%) | 5 (36) | 0 (0) | 0.054 |
| Pulmonaryb, n (%) | 7 (50) | 3 (38) | 0.571 |
| Gastrointestinalb, n (%) | – | – | – |
| Renalb, n (%) | 14 (100) | 8 (100) | 1.000 |
| Cardiovascularb, n (%) | – | – | – |
| Nervous systemb, n (%) | 2 (14) | 3 (38) | 0.211 |
| Haemoglobin (g/dL), mean (SD) | 10.5 (2.8) | 10.3 (0.7) | 0.891 |
| WBC (×109/L), mean (SD) | 10.9 (5.0) | 11.7 (4.8) | 0.682 |
| ESR (mm/1 hr), median (IQR)c | 50 (25–72) | 47 (19–99) | 0.910 |
| CRP (mg/L), median (IQR)c | 11.7 (5.0–22.3) | 87.8 (19.3–225.3) | 0.123 |
| Creatinine (mg/dL), median (IQR) | 2.2 (1.6–3.8) | 3.3 (2.3–4.1) | 0.339 |
| eGFR (mL/min/1.73 m2), median (IQR) | 26 (12–42) | 17 (13.5–26.5) | 0.245 |
| 0–29d, n (%) | 8 (57) | 7 (88) | 0.300 |
| 30–60, n (%) | 4 (29) | 1 (13) | |
| >60, n (%) | 2 (14) | 0 (0) | |
| UP:UCc, median (IQR) | 1.4 (1.1–2.9) | 0.8 (0.7–1.9) | 0.149 |
All but one patient (a Native American) were Caucasian.
System involvement was detailed according to the BVAS items.
General laboratory data not available in all patients (ESR: missing in one patient with minimal/mild and four patients with moderate/severe chronicity index; CRP missing in five patients with minimal/mild and four patients with moderate/severe chronicity index; UP:UC at diagnosis was not available for five patients with minimal/mild chronicity index).
No patients required renal replacement therapy at diagnosis.
c-ANCA, cytoplasmic ANCA; p-ANCA, perinuclear ANCA; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cells; ESRD, end-stage renal disease; UP:UC, urinary protein:urinary creatinine ratio.
Induction treatment approaches were similar in patients of the two groups (P > 0.05 in all comparisons): oral and/or intravenous glucocorticoids were used in all patients but one; oral cyclophosphamide was used in 10 (71%) patients in the minimal/mild group and in 4 (50%) in the moderate/severe group; rituximab was used in 2 patients in each group (14% and 25% respectively); mycophenolate mofetil in 1 patient in the moderate/severe group; plasmapheresis was added in 2 patients in the minimal/mild group. One patient in the minimal/mild group received rituximab as a maintenance therapy, and oral glucocorticoids, azathioprine, mycophenolate mofetil and methotrexate were similarly used in both groups as maintenance treatment (P > 0.05 in all comparisons).
The median CS was 3.0 for minimal/mild classes and 6.5 for moderate/severe classes. There was no direct concordance between CS and the International Pathology Classification, although 64% of minimal/mild class patients had a focal class of the International Pathology Classification and 87.5% of moderate/severe patients had a mixed or sclerotic class of the International Pathology Classification. In some patients, the results of the two scores were extremely different (Supplementary data, Table S1). For instance, patient 21 had a focal class by the International Pathology Classification but a severe class by CS, with the CS being a better predictor of renal prognosis in this patient (eGFR at 0, 6 and 12 months was 15.0, 15.8 and 16.8 mL/min/1.73 m2, respectively).
Association of chronic renal damage at baseline and AAV outcomes
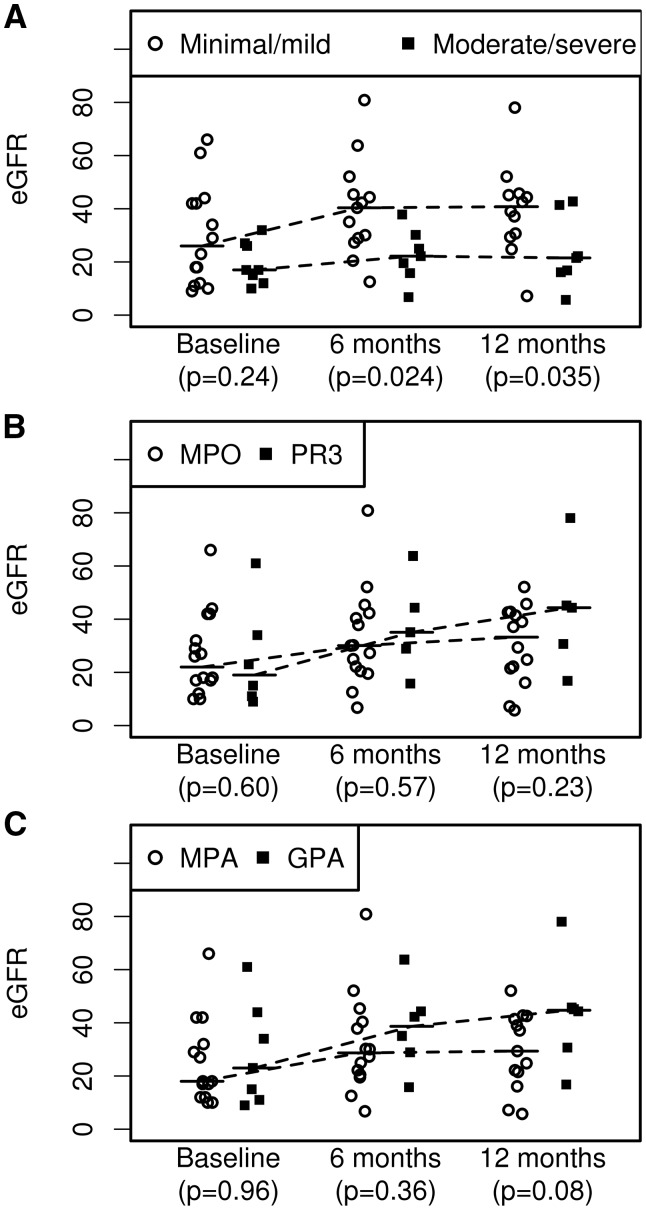
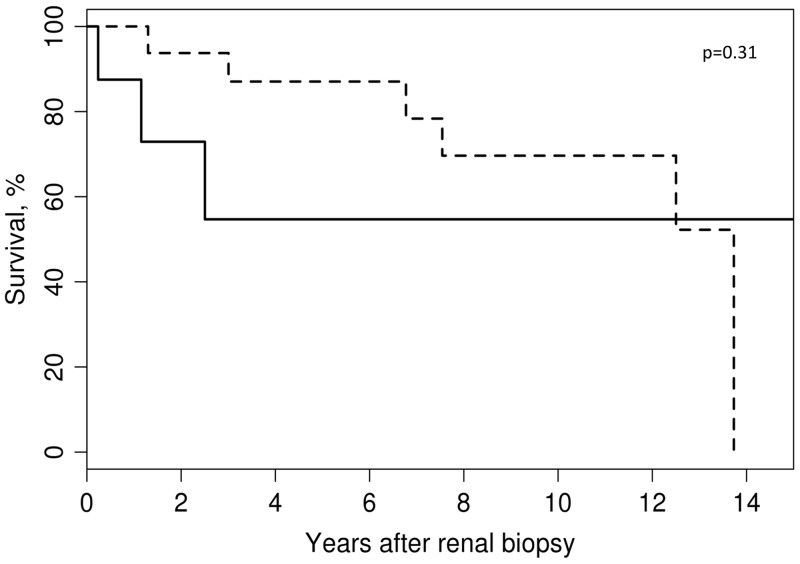
Although eGFR at baseline was not statistically different between patients with moderate/severe CS [median eGFR 17.0 (IQR 13.5–26.5) mL/min/1.73 m2] compared with patients with minimal/mild CS [median eGFR 26.0 (IQR 12.0–42.0) mL/min/1.73 m2], there was a significant difference at 6 months and 1 year of follow-up between these two groups (Figure 3A) [6 months: minimal/mild, median 40.4 (IQR 28.9–45.4) mL/min/1.73 m2; moderate/severe, median 22.2 (IQR 15.8–30.2) mL/min/1.73 m2, P = 0.024; 12 months: minimal/mild, median 40.8 (IQR 30.0–45.4) mL/min/1.73 m2; moderate/severe, median 21.5 (IQR 16.1–41.4) mL/min/1.73 m2, P = 0.035, respectively]. When patients were stratified by clinical diagnosis (GPA versus MPA) (Figure 3B) or ANCA serology (PR3-AAV versus MPO-AAV) (Figure 3C), renal function recovery was not significantly different either at 6 or 12 months (Supplementary data, Table S2). The presence of the moderate/severe class at renal biopsy portended a worse overall prognosis, although it was not significantly associated with patient survival after adjustment for age and sex [hazard ratio (HR) 2.30 (95% CI 0.45–11.70); P = 0.31] (Figure 4).
FIGURE 3.
(A) Chronic renal damage at diagnosis, as assessed by CS in renal biopsies, was significantly associated with renal recovery at 6 months and 12 months (based on eGFR) (A), whereas (B) ANCA serology (PR3-AAV versus MPO-AAV) and (C) clinical diagnosis (GPA versus MPA) were not.
FIGURE 4.
Age- and sex-adjusted patient survival by CS class: minimal/mild (n = 14; dashed line) versus moderate/severe (n = 8; solid line) [HR 2.30 (95% CI 0.45–11.70); P = 0.31 adjusted for age and sex].
Since our analysis of the Olmsted County patients who underwent a renal biopsy at the time of diagnosis represents the first application of the CS specifically to AAGN, we also analysed a comparable control cohort that fulfilled the same inclusion and exclusion criteria. Clinical and demographic data as well as the renal outcomes categorized by CS of this replication cohort comprising 38 patients are summarized in Supplementary data, Table S3. The eGFR values of these patients grouped by CS mirrored the ones observed in the biopsy-proven subset from the Olmsted County incidence cohort (Supplementary data, Table S3).
DISCUSSION
The current study is the first true population-based study to describe the annual incidence, prevalence and mortality rates of AAGN in a geographically well-defined area in the USA. The overall annual incidence rate of AAGN was 2.0 per 100 000 inhabitants, which is higher than those reported in Europe and Japan [5–7, 9, 28]. Prevalence was estimated at ∼ 35 per 100 000 and the overall mortality rates for patients with AAGN were significantly higher than the general population after adjustment for age and sex, in contrast with mortality rates of AAV patients without glomerulonephritis.
Although the incidence we reported in Olmsted County is higher than other population-based studies [5–7, 9, 29], it should be considered that in some of these studies, particularly those from northern Europe, the annual incidence was estimated for biopsy-proven AAGN. The annual incidence of biopsy-proven AAGN in the current population-based cohort (n = 24, instead of the total n = 34 used to calculate the incidence of AAGN here) is similar to that observed in some northern European cohorts [8], although still higher compared with others [29]. As indications and contraindications for renal biopsies are not interpreted and applied uniformly between individual patients, physicians and medical centres, we decided to focus on the overall annual incidence of AAGN instead of limiting our analysis to biopsy-proven AAGN. Indeed, we believe that our estimate better reflects how AAGN is actually diagnosed in routine practice, and hence is more generalizable. Finally, in our base population, the overall incidence of MPA was similar to that of GPA [3], which differs from previous reports in populations with similar genetic background [6, 8, 30–32], thus probably contributing, at least in part, to the higher incidence of AAGN.
Renal involvement in AAV affects patient survival when compared with general population life tables. In our cohort, patients with AAGN were indeed older, more frequently had MPA and were MPO-ANCA positive. Few studies analysed if the stratification of patients by clinical diagnosis, ANCA specificity and the presence of renal involvement may affect overall survival [2, 3, 5, 33]. Furthermore, age naturally represents a risk factor for death and has been shown to contribute to the stratification of AAV patient survival [34]. Overall, the difference in survival rate between MPA and GPA has been shown to be likely explained by the variation in age and renal function at entry, representing two independent risk factors of death [5]. Our data further integrate the current knowledge, supporting the concept that glomerulonephritis in the context of AAV negatively affects life expectancy even after correction for age and sex. Chronic renal damage as measured by the CS at diagnosis provided better stratification of renal prognosis than clinical diagnosis (GPA versus MPA) or ANCA-serology (PR3-ANCA versus MPO-ANCA). We confirmed the role of CS in the stratification of renal function recovery in an independent replication cohort. Patients classified in the moderate/severe CS class on renal biopsy at diagnosis had worse renal function recovery, likely due to advanced chronic damage by AAV at presentation. Our results are consistent with a previous hospital-based cohort study showing that severe renal scarring and renal function at 6 months was associated with a lower response of renal function to treatment [13]. However, our findings do not confirm cohort and population-based studies that found renal survival to be significantly worse in MPO-AAV patients than in PR3-AAV patients [2, 10, 11]. We acknowledge that our study may be underpowered to detect prognostic differences between small subsets defined by clinical diagnosis, ANCA specificity and CS. However, despite this limited statistical power, CS gave a positive signal of effect to predict recovery of renal function both in the Olmsted County incidence cohort and the replication cohort. As expected, patients with less chronic damage (mild and moderate CS categories) improve renal function much better than patients with more chronic renal damage. The CS identifies patients that cannot recover renal function with great accuracy, probably because it integrates both diffuse glomerular and extraglomerular chronic damage, thus supporting its clinical utility in AAGN.
The serum creatinine levels of our incidence cohort were lower than those reported in other series [5–8], even though the Olmsted County incidence cohort has a higher proportion of patients with MPO-ANCA, a factor usually associated with worse renal function at presentation [3]. One possible explanation is a shorter delay in diagnosis and immediate treatment at an expert referral centre of all patients.
Baseline serum creatinine is one of the main predictors of renal function during follow-up in hospital-based cohorts [2, 6, 35–40]. This detail also highlights the importance of CS in predicting the recovery of renal function, showing that CS does not simply reflect the renal function at baseline, hence might be even more helpful than creatinine to stratify at baseline the future renal outcome.
The International Pathology Classification is currently the only validated histological classification for AAGN. Although most papers systematically reviewed in a recent meta-analysis supported its clinical utility [41], not all attempts to validate the International Pathology Classification in independent cohorts succeeded in demonstrating its prognostic value [14, 15, 42, 43], raising questions about its ability to predict the prognosis of AAV for individual patients. A possible explanation is that only glomeruli are taken into consideration in this classification, potentially overlooking the amount of damage in non-glomerular renal tissue. Conversely, the CS specifically assesses chronic renal changes, including the degree of interstitial fibrosis, tubular atrophy and arteriosclerosis, which might improve the ability to predict renal outcome in AAGN by providing complementary information derived from biopsies in addition to the International Pathology Classification [43, 44].
The strengths of this population-based patient cohort study include utilization of the REP, a comprehensive record linkage system that allows the capture of all the clinically recognized cases of AAV in Olmsted County. Furthermore, AAV and AAGN diagnoses were verified by medical record review, minimizing the likelihood of overdiagnosis or physician-based AAV diagnosis not fulfilling international classification criteria, a common concern in coding-based studies [3, 45, 46]. Moreover, this is the first study to assess the utility of the CS in AAV, and to evaluate its contribution to predict renal and patient prognosis.
The main limitation of this study was the relatively small number in our base population as well as the even smaller number of patients that underwent a renal biopsy, reducing the statistical power of some analyses. In addition, given the retrospective design, patient clinical information was not systematically obtained by the physicians following an established protocol. Finally, we cannot derive any conclusion for chronic renal damage in ANCA-negative patients, since they were not biopsied coincidentally. However, ANCA-negative glomerulonephritis seems to represent an independent disease entity from ANCA-positive vasculitis [47, 48].
CONCLUSIONS
The incidence of AAGN in Olmsted County, USA, was higher than previously reported for other countries in the world. Survival was reduced in patients with AAGN compared with the general population and to AAV patients without glomerulonephritis. Chronic renal damage at diagnosis as assessed by CS, but not clinical diagnosis and ANCA serology, allowed the stratification of patients to predict renal function recovery at 6 and 12 months and potentially predicted overall survival.
FUNDING
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under award number R01AG034676 and Clinical and Translational Science Awards grant number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICT OF INTEREST STATEMENT
The authors have no financial or non-financial potential conflicts of interest to declare related to this project. D.C. received fellowship grants from the French Society of Rheumatology and from Brest University Hospital, Brest, France. E.C.-L.G.’s salary was funded by an American Society of Nephrology fellowship.
(See related article by Kronbichler and Jayne. Estimating the epidemiology of anti-neutrophil cytoplasm antibody-associated renal vasculitis and the role of histologic chronicity in predicting renal outcomes. Nephrol Dial Transplant 2019; 34: 1429--1432)
Supplementary Material
REFERENCES
- 1. Sinico RA, Di Toma L, Radice A.. Renal involvement in anti-neutrophil cytoplasmic autoantibody associated vasculitis. Autoimmun Rev 2013; 12: 477–482 [DOI] [PubMed] [Google Scholar]
- 2. de Joode AA, Sanders JS, Stegeman CA.. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol 2013; 8: 1709–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berti A, Cornec D, Crowson CS. et al. The epidemiology of ANCA associated vasculitis in Olmsted County, Minnesota (USA): a 20 year population-based study. Arthritis Rheumatol 2017; 69: 2338–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cornec D, Cornec-Le Gall E, Specks U.. Clinical trials in antineutrophil cytoplasmic antibody-associated vasculitis: what we have learnt so far, and what we still have to learn. Nephrol Dial Transplant 2017; 32(Suppl 1): i37–i47 [DOI] [PubMed] [Google Scholar]
- 5. Mohammad AJ, Jacobsson LT, Westman KW. et al. Incidence and survival rates in Wegener’s granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and polyarteritis nodosa. Rheumatology (Oxford) 2009; 48: 1560–1565 [DOI] [PubMed] [Google Scholar]
- 6. Mohammad AJ, Segelmark M.. A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol 2014; 41: 1366–1373 [DOI] [PubMed] [Google Scholar]
- 7. Watts RA, Scott DG, Jayne DR. et al. Renal vasculitis in Japan and the UK–are there differences in epidemiology and clinical phenotype? Nephrol Dial Transplant 2008; 23: 3928–3931 [DOI] [PubMed] [Google Scholar]
- 8. Mohammad AJ, Weiner M, Sjowall C. et al. Incidence and disease severity of anti-neutrophil cytoplasmic antibody-associated nephritis are higher than in lupus nephritis in Sweden. Nephrol Dial Transplant 2015; 30: i23–i30 [DOI] [PubMed] [Google Scholar]
- 9. Fujimoto S, Uezono S, Hisanaga S. et al. Incidence of ANCA-associated primary renal vasculitis in the Miyazaki Prefecture: the first population-based, retrospective, epidemiologic survey in Japan. Clin J Am Soc Nephrol 2006; 1: 1016–1022 [DOI] [PubMed] [Google Scholar]
- 10. Hilhorst M, van Paassen P, Tervaert JW.. Proteinase 3-ANCA vasculitis versus myeloperoxidase-ANCA vasculitis. J Am Soc Nephrol 2015; 26: 2314–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quintana LF, Perez NS, De Sousa E. et al. ANCA serotype and histopathological classification for the prediction of renal outcome in ANCA-associated glomerulonephritis. Nephrol Dial Transplant 2014; 29: 1764–1769 [DOI] [PubMed] [Google Scholar]
- 12. Berden AE, Ferrario F, Hagen EC. et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010; 21: 1628–1636. [DOI] [PubMed] [Google Scholar]
- 13. Lee T, Gasim A, Derebail VK. et al. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol 2014; 9: 905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kristensen T, Gregersen JW, Krag SR. et al. The relation between histopathological classification and renal outcome, ANCA subtype and treatment regimens in ANCA-associated vasculitis. Clin Exp Rheumatol 2016; 34(3 Suppl 97): S105–S110 [PubMed] [Google Scholar]
- 15. Moroni G, Binda V, Leoni A. et al. Predictors of renal survival in ANCA-associated vasculitis. Validation of a histopatological classification schema and review of the literature. Clin Exp Rheumatol 2015; 33: 56–63 [PubMed] [Google Scholar]
- 16. Sethi S, D’Agati VD, Nast CC. et al. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 2017; 91: 787–789 [DOI] [PubMed] [Google Scholar]
- 17. St Sauver JL, Grossardt BR, Yawn BP. et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011; 173: 1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. St Sauver JL, Grossardt BR, Leibson CL. et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012; 87: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leavitt RY, Fauci AS, Bloch DA. et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum 1990; 33: 1101–1107 [DOI] [PubMed] [Google Scholar]
- 20. WGET Research Group. Design of the Wegener’s Granulomatosis Etanercept Trial (WGET). Control Clin Trials 2002; 23: 450–468 [DOI] [PubMed] [Google Scholar]
- 21. Masi AT, Hunder GG, Lie JT. et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990; 33: 1094–1100 [DOI] [PubMed] [Google Scholar]
- 22. Jennette JC, Falk RJ, Bacon PA. et al. 2012 Revised International Chapel Hill Consensus conference nomenclature of vasculitides. Arthritis Rheum 2013; 65: 1–11 [DOI] [PubMed] [Google Scholar]
- 23. Watts R, Lane S, Hanslik T. et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007; 66: 222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stone JH, Hoffman GS, Merkel PA. et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001; 44: 912–920 [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Coresh J, Greene T. et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254 [DOI] [PubMed] [Google Scholar]
- 26. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1): S1–S266 [PubMed] [Google Scholar]
- 28. Watts RA, Mahr A, Mohammad AJ. et al. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol Dial Transplant 2015; 30: i14–i22 [DOI] [PubMed] [Google Scholar]
- 29. Hedger N, Stevens J, Drey N. et al. Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: a 10-year retrospective study. Nephrol Dial Transplant 2000; 15: 1593–1599 [DOI] [PubMed] [Google Scholar]
- 30. Fujimoto S, Watts RA, Kobayashi S. et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the UK. Rheumatology (Oxford) 2011; 50: 1916–1920 [DOI] [PubMed] [Google Scholar]
- 31. Watts RA, Al-Taiar A, Scott DG. et al. Prevalence and incidence of Wegener's granulomatosis in the UK general practice research database. Arthritis Rheum 2009; 61: 1412–1416 [DOI] [PubMed] [Google Scholar]
- 32. Watts RA, Gonzalez-Gay MA, Lane SE. et al. Geoepidemiology of systemic vasculitis: comparison of the incidence in two regions of Europe. Ann Rheum Dis 2001; 60: 170–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Booth AD, Almond MK, Burns A. et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41: 776–784 [DOI] [PubMed] [Google Scholar]
- 34. Guillevin L, Pagnoux C, Seror R. et al. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011; 90: 19–27 [DOI] [PubMed] [Google Scholar]
- 35. de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R. et al. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: a prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 2006; 17: 2264–2274 [DOI] [PubMed] [Google Scholar]
- 36. Hogan SL, Falk RJ, Chin H. et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 2005; 143: 621–631 [DOI] [PubMed] [Google Scholar]
- 37. Chen SF, Wang H, Huang YM. et al. Clinicopathologic characteristics and outcomes of renal thrombotic microangiopathy in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis. Clin J Am Soc Nephrol 2015; 10: 750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Day CJ, Howie AJ, Nightingale P. et al. Prediction of ESRD in pauci-immune necrotizing glomerulonephritis: quantitative histomorphometric assessment and serum creatinine. Am J Kidney Dis 2010; 55: 250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manenti L, Vaglio A, Gnappi E. et al. Association of serum C3 concentration and histologic signs of thrombotic microangiopathy with outcomes among patients with ANCA-associated renal vasculitis. Clin J Am Soc Nephrol 2015; 10: 2143–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hauer HA, Bajema IM, Van Houwelingen HC. et al. Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int 2002; 62: 1732–1742 [DOI] [PubMed] [Google Scholar]
- 41. Chen YX, Xu J, Pan XX. et al. Histopathological classification and renal outcome in patients with antineutrophil cytoplasmic antibodies-associated renal vasculitis: a study of 186 patients and metaanalysis. J Rheumatol 2017; 44: 304–313 [DOI] [PubMed] [Google Scholar]
- 42. Bjorneklett R, Sriskandarajah S, Bostad L.. Prognostic value of histologic classification of ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol 2016; 11: 2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ford SL, Polkinghorne KR, Longano A. et al. Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidney Dis 2014; 63: 227–235 [DOI] [PubMed] [Google Scholar]
- 44. Berden AE, Jones RB, Erasmus DD. et al. Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol 2012; 23: 313–321 [DOI] [PubMed] [Google Scholar]
- 45. Finkielman JD, Merkel PA, Schroeder D. et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147: 611–619 [DOI] [PubMed] [Google Scholar]
- 46. Sreih AG, Annapureddy N, Springer J. et al. Development and validation of case-finding algorithms for the identification of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis in large healthcare administrative databases. Pharmacoepidemiol Drug Saf 2016; 25: 1368–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen M, Yu F, Wang SX. et al. Antineutrophil cytoplasmic autoantibody-negative pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol 2007; 18: 599–605 [DOI] [PubMed] [Google Scholar]
- 48. Sethi S, Zand L, De Vriese AS. et al. Complement activation in pauci-immune necrotizing and crescentic glomerulonephritis: results of a proteomic analysis. Nephrol Dial Transplant 2017; 32: i139–ii45 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.