Abstract
Ductal carcinoma in situ (DCIS) of the breast is a group of heterogeneous epithelial proliferations confined to the milk ducts that nearly always present in asymptomatic women on breast cancer screening. A stage 0, preinvasive breast cancer, increased detection of DCIS was initially hailed as a means to prevent invasive breast cancer through surgical treatment with adjuvant radiation and/or endocrine therapies. However, controversy in the medical community has emerged in the past two decades that a fraction of DCIS represents overdiagnosis, leading to unnecessary treatments and resulting morbidity. The imaging hallmarks of DCIS include linearly or segmentally distributed calcifications on mammography or nonmass enhancement on breast MRI. Imaging features have been shown to reflect the biological heterogeneity of DCIS lesions, with recent studies indicating MRI may identify a greater fraction of higher-grade lesions than mammography does. There is strong interest in the surgical, imaging, and oncology communities to better align DCIS management with biology, which has resulted in trials of active surveillance and therapy that is less aggressive. However, risk stratification of DCIS remains imperfect, which has limited the development of precision therapy approaches matched to DCIS aggressiveness. Accordingly, there are opportunities for breast imaging radiologists to assist the oncology community by leveraging advanced imaging techniques to identify appropriate patients for the less aggressive DCIS treatments.
Keywords: active surveillance, breast imaging, DCIS, ductal carcinoma in situ, overdiagnosis, overtreatment
Key Messages.
Ductal carcinoma in situ (DCIS) of the breast is a heterogeneous group of intraductal proliferations that are classified as stage 0 breast cancer with a nonobligate potential to progress to invasive disease.
Most cases of DCIS present in asymptomatic women as mammographic calcifications on screening mammography, and most are treated aggressively with surgery, often with adjuvant radiation and/or endocrine therapy.
Because of concerns of overdiagnosis and overtreatment, there is increasing interest in novel approaches in DCIS treatment, and breast imaging radiologists have a unique opportunity to lead active surveillance and precision therapy trials.
Introduction
Ductal carcinoma in situ (DCIS) is a controversial in situ (intraepithelial) neoplasm of the breast with variable and nonobligate potential to progress to invasive breast cancer. Before the widespread implementation of mammography screening programs, DCIS was rarely diagnosed, and it most commonly presented as suspicious bloody nipple discharge, Paget disease of the nipple, or a palpable mass. The large-scale enactment of mammography screening programs in the United States led to a dramatic rise in the rate of DCIS diagnoses since the 1980s, and it now accounts for approximately 25% of screen-detected breast cancers (1).
Opinions regarding the value of detecting DCIS have continuously evolved. Initially, many considered DCIS detection key to the prevention of invasive breast cancer because it was viewed as its direct precursor (2). However, this viewpoint has altered in recent years because only approximately 40% of DCIS lesions progress to invasive breast cancer (3). Accordingly, since some DCIS lesions will never lead to metastatic disease, they will not affect a woman’s lifespan. However, because of a limited ability to identify which DCIS lesions will behave indolently, the vast majority are treated with surgery, often with adjuvant radiation and/or endocrine therapies. Thus, the detection of DCIS has become embroiled in controversies involving breast cancer overdiagnosis and overtreatment.
Given the evolving views and controversies surrounding DCIS, it is essential that breast imaging radiologists are aware of the diagnostic and clinical challenges related to DCIS. As such, this article reviews DCIS biology and pathologic features, typical and uncommon DCIS clinical and imaging presentations, and the clinical impact of diagnosing DCIS. Finally, we discuss current DCIS management trials and opportunities for advanced imaging to help match DCIS management to its variable biology.
Ductal carcinoma in situ (DCIS) biology and pathologic features
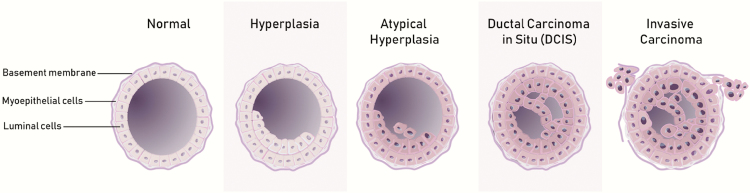
DCIS represents a collection of cells that are morphologically similar to invasive ductal cancer cells (“ductal carcinoma”) but are confined to the milk duct (“in situ”) (4) with an intact basement membrane (5). It represents the most aggressive lesion within a spectrum of intraductal proliferations with an innate, but not obligate, ability to progress to invasive cancer (Figure 1) (6). The time from diagnosis of DCIS to progression of invasive breast cancer cannot be accurately forecasted, and it is likely that some lower-grade lesions diagnosed in older women will never progress to invasive breast cancer in their lifetimes. Furthermore, DCIS does not exist along a predictable, stepwise, linear fashion from atypical ductal hyperplasia (ADH) to low-grade DCIS to high-grade DCIS to invasive carcinoma (7). Current molecular evidence of DCIS progression to invasive carcinoma supports a model where a single founder epithelial cell gives rise to both DCIS and invasive cancer subpopulations within the milk duct, which may occur because of an evolutionary bottleneck or in a multiclonal fashion (8). Recent evidence also indicates that DCIS progression to invasive cancer requires a permissive periductal stromal microenvironment to assist malignant epithelial cells’ invasion through the milk ducts (9).
Figure 1.
Cartoon drawing depicting the spectrum of intraductal proliferations of the breast. The normal milk duct demonstrates a single layer of normal luminal epithelial cells, bound by an intact myoepithelial cell and basement membrane layer. In the case of (usual) ductal hyperplasia, there is epithelial cell proliferation, but these cells retain normal morphology. Atypical (ductal) hyperplasia (ADH) demonstrates both epithelial cell proliferation and low-grade, monomorphic cytological atypia, but the involvement of the duct is generally limited. DCIS may include either more extensive low-grade atypical cells (similar in morphology to ADH) or an intraductal proliferation with greater cytological atypia but an intact basement membrane. Finally, invasive ductal carcinoma includes cytologically atypical cells with a disrupted myoepithelial cellular layer and basement membrane, which allows invasion of the carcinoma cells into the surrounding stroma.
Traditionally, DCIS was classified based on different architectural patterns, including comedo, solid, cribriform, papillary, flat (“clinging”), and micropapillary forms (Figure 2). The comedo pattern is characterized by prominent central necrosis, often with increased mitotic activity and high nuclear grade. In the solid pattern, the cancer cells completely fill the ducts without fenestrations or papillae. The cribriform pattern is represented by fenestrations between the DCIS cells within a breast duct forming lumina and spaces, with the abnormal cells exhibiting relatively small sizes and uniform shapes in low-grade variants. The papillary form refers to the “fern-like” pattern of organization of DCIS cells within the ducts in which the cells are arranged around a fibrovascular core in a radiant or “star burst”–type pattern. Finally, the micropapillary pattern refers to small tufts of cells within the duct, but the tufts lack the fibrovascular core of the papillary type. This morphology is reminiscent of invasive micropapillary carcinoma of the breast. Classification of DCIS lesions based on architectural patterns alone has yielded limited prognostic value, perhaps partially because multiple patterns can be observed in the same lesion (10), and it has generally fallen out of favor.
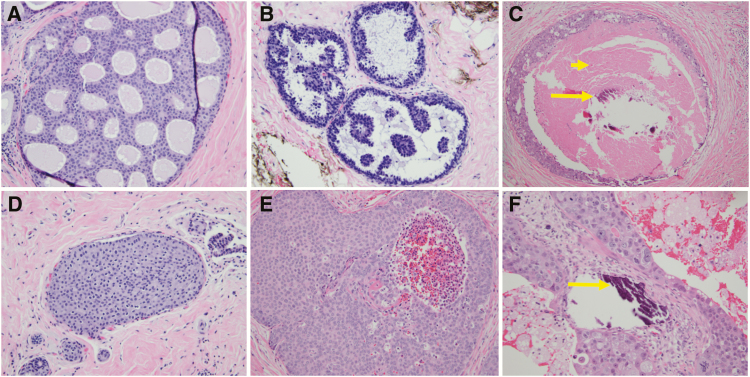
Figure 2.
Examples of traditional architectural (top row) and nuclear grade (bottom row) pathological features of DCIS lesions. Traditional pathological descriptors including cribriform (A), micropapillary (B), and comedo (C) are now less commonly utilized. Instead, most pathologists classify the nuclear grade of DCIS as low (D), intermediate (E), or high (F), and they comment on the presence of comedonecrosis (short arrow), microcalcifications (long arrows), and ER immunoreactivity. Low-grade DCIS (D) has small and monotonous cells with well-defined cell membranes, inconspicuous nucleoli, and sparse mitotic activity. Intermediate-grade DCIS (E) has cells with cytomorphologic features that are in between the low- and high-grade categories. High-grade DCIS (F) has large and pleomorphic cells with prominent nucleoli and numerous mitotic figures.
More recently, the pathology community subdivides the various intraductal proliferative lesions into “noncancerous” (ie, usual ductal hyperplasia (UDH) and ADH) and “cancerous” (ie, DCIS). One challenge with this system is interobserver variability and lack of reproducibility, with one study demonstrating that even when morphologic and histopathologic criteria were standardized, pathologists assigned many of the same lesions to different categories (11). This phenomenon primarily occurs when distinguishing between ADH and low-grade DCIS, as major factors in distinguishing between the two entities are lesion size and quantity of cells (number of foci) rather than distinct morphological features (12, 13). Furthermore, many expert breast pathologists reserve a DCIS designation on core specimens for lesions in which they are certain there is a neoplastic proliferation, whereas lesions that fall short of this interpretation are considered ADH. On a molecular level, some recurrent genetic alterations (eg, losses at 16q and 17p and gains at 1q) occur in both ADH and low-grade DCIS (14, 15), suggesting the distinction between ADH and low-grade DCIS by morphology alone is somewhat arbitrary because these lesions likely do not represent biologically distinct entities. These mutations are unique from those identified in intermediate to high-grade DCIS, and they could create more concrete distinctions among these proliferations moving forward (14–17).
Diagnostic assessment for estrogen receptor (ER) and progesterone receptor (PR) positivity is routinely performed for newly diagnosed DCIS. The majority (75%–80%) of DCIS lesions exhibit ER positivity, which is inversely correlated with DCIS grade (18). ER positivity also correlates with lower recurrence rates and positive response to hormonal therapy. Several other markers commonly obtained for invasive breast cancers, including her2/neu and Ki-67, are not routinely obtained for DCIS, although a few small studies have demonstrated prognostic value (18, 19). Finally, both high p16, another assay infrequently obtained clinically, and high nuclear grade have been found to be associated with future risk of ipsilateral invasive breast cancer diagnosis (20).
Comparison of pathologic assessment of specimens at core needle biopsy (CNB) versus final surgical excision frequently demonstrate discrepancies in nuclear grade and upstaging to invasive cancer, which is likely due to undersampling at CNB. Concordance of nuclear grade between CNB and excision is approximately 75%, but it can range from 59% to 91% (21), with most discrepancies leading to a higher nuclear grade assessment on excision. This is due to the inability to count 10 high power fields for mitosis on CNB specimens, the preference to count mitotic figures on the actively growing tumor at the periphery of surgical excision specimens, and interobserver variability (22, 23). Upstaging to invasive cancer on surgical specimens occurs in approximately 25% of cases (interquartile range: 18.6%–37.2%) (24). Factors associated with a greater likelihood of upstaging can be categorized into the clinical presentation (eg, palpable or symptomatic), imaging appearance (eg, mammographic mass, sonographically visible, or larger size), histopathology results (eg, greater nuclear grade), and biopsy technique (eg, nonstereotactic or smaller gauge biopsy device). These discrepancies can result in subsequent changes in clinical management, particularly in cases of upgrade to invasive disease.
What’s in a name—is DCIS cancer?
The term carcinoma in situ of the breast was coined by Broders in 1932 (25), and these intraductal lesions were further divided into subtypes by Foote and Stewart in 1941 (26). While classic forms of lobular carcinoma in situ (LCIS) subsequently were reclassified as high-risk, “noncancerous” lesions (LCIS itself is controversial and beyond this article’s scope), DCIS is typically grouped with its invasive counterparts as breast cancer (“stage 0” by the National Cancer Comprehensive Network [NCCN]). However, this distinction is not as clear biologically since DCIS lack an essential trait of malignancies: the ability to invade and metastasize (27), and this has led many to question its classification as a cancer.
In response, there is debate about removing the term carcinoma, (4) with the terms ductal intraepithelial neoplasia (DIN) and indolent lesions of epithelial origin (IDLE) proposed. The DIN classification system is currently used for cervical neoplasias. It avoids the term carcinoma and does not distinguish between cancerous and noncancerous lesions. Proponents of the IDLE classification system argue that removal of the term carcinoma from DCIS would promote patient and surgeon willingness to adopt treatments that are less aggressive (28). Other authors have found that women may prefer terms that do not include carcinoma (29), and changing the name could facilitate an increased willingness for patients to accept treatment that is less aggressive (29–31). Nonetheless, changing DCIS’s name has proven challenging, and DCIS remains the prevailing term in the medical community.
Clinical presentations and management
DCIS was rarely diagnosed before the implementation of screening mammography, and it most commonly presented as a palpable lump (Figure 3), nipple discharge, or Paget disease of the nipple. Before the 1980s, DCIS accounted for approximately 2% of all breast cancer diagnoses in the United States (32), with similar rates currently observed in countries where screening mammography has not yet been instituted (33). Screening mammography has increased DCIS incidence from 1.87 (1975) to 32.5(2005) per 100,000, though its diagnosis frequency remains much lower than that of invasive cancers (453.1 per 100,000) (34). Although the absolute number of clinical presentations of DCIS has not declined with screening, the fraction of DCIS cases presenting clinically has decreased by over 75% (35). Like invasive breast cancer, DCIS diagnosis rates are strongly linked to age, although incidence peaks one decade earlier (from 65–69 years to 75–79 years, respectively). The clinical risk factors for developing DCIS are generally similar to those for invasive cancer (eg, BRCA mutation, family history, and mammographic density), although hormone replacement therapy is not linked to DCIS (34).
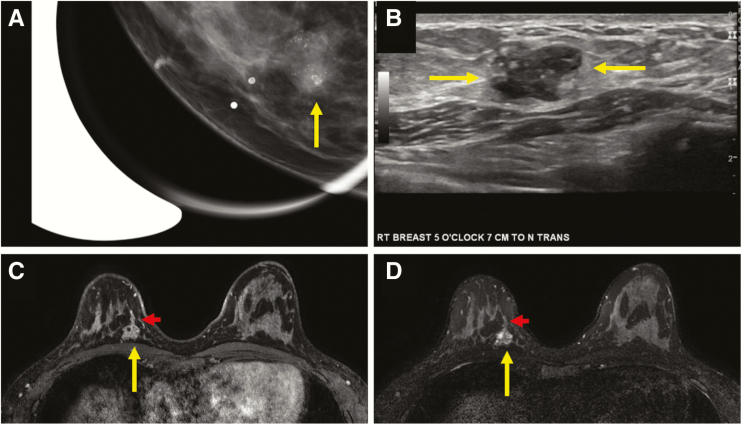
Figure 3.
Multimodality appearance of pure DCIS presenting as a clinically palpable mass in a 39-year-old woman. Craniocaudal (CC) spot-magnification mammographic view of the area of palpable concern in the right breast at 5 o’clock (A) demonstrates an oval-shaped mass with circumscribed margins and fine pleomorphic calcifications within the mass (arrow). A transversely oriented ultrasound image (B) demonstrates an oval-shaped complex solid and cystic mass with circumscribed margins and echogenic foci within consistent with calcifications (arrows). A biopsy was performed under sonographic guidance, and it revealed pure high nuclear grade DCIS. T1-weighted fat-suppressed initial-phase postcontrast MR image from a bilateral breast MRI (C) performed for extent of disease demonstrated the oval-shaped mass at posterior depth at 5 o’clock, with cystic spaces evident on T2-weighted images (D, long arrow) and ductal extension that was sonographically and mammographically occult (short arrow). Pathology remained pure high nuclear grade DCIS on surgical excision.
DCIS management is generally uniform and independent of clinical and pathology factors. Outside of a few trials studying the effectiveness of DCIS observation (discussed below), almost all women with DCIS undergo surgery, usually wide local excision (WLE). Approximately one in three women undergo mastectomy, and this decision is multifactorial and in part dependent on disease extent, patient education and socioeconomic status, and surgeon recommendation (36). In general, radiation therapy is offered as standard treatment after WLE because multiple randomized controlled trials have demonstrated it reduces local recurrence risk by 43% (37). To date, prospective trials to identify candidates for whom adjuvant radiation therapy is unnecessary have not been successful. For example, the Radiation Therapy Oncology Group (RTOG) 9804 trial randomly assigned women considered to be at low risk (mammographically detected DCIS, low-to-intermediate grade, less than 25 mm, and surgical margins greater than 3mm) to WLE with or without adjuvant radiotherapy, but found local failure rates to be higher in those who did not receive radiation (6.7% vs. 0.9%) at approximately seven years of median follow-up (38). Endocrine therapy in ER-positive tumors also has been shown to decrease the risk of recurrence (39); however, its effect is diminished when radiation therapy is also administered. Accordingly, the NCCN generally recommends adjuvant radiation therapy and/or hormone therapy for women undergoing WLE (40).
Several models incorporating pathologic and clinical features to guide DCIS treatment have been published, but they are used sparingly across institutions. The best-known models are the Memorial Sloan Kettering Nomogram (41) and the USC/Van Nuys Prognostic Index (42), which combine pathologic span, nuclear grade, and comedonecrosis with patient age, family history, and margin status to determine the risk of recurrence. A major barrier to their widespread use has been limited validation of efficacy of these clinical models across different sites. A 12-gene molecular assay (Oncotype DX DCIS score) recently has been introduced to provide a 10-year risk of local recurrence after treatment with the intent to guide radiation therapy decisions (43). However, its high cost, reliance on common proliferation genes, and lack of validation across a broad range of DCIS grades and sizes have limited use to date. Further validation of this assay may come from a recently completed prospective trial studying the use of MRI in conjunction with the Oncotype DX DCIS score (ECOG-ACRIN 4112 trial) (44).
Although pure DCIS itself is generally considered to be nonlethal, the risk of developing an invasive breast cancer after DCIS diagnosis is four times higher than in the general population (45), and a portion of such women eventually die of breast cancer. Several clinical features have been identified to be associated with future invasive breast cancer diagnosis, including younger age, premenopausal status, black race, elevated body mass index, and detection by palpation (20, 46). Furthermore, Surveillance, Epidemiology, and End Results (SEER) data indicate breast cancer–specific mortality following a DCIS diagnosis is 3.3%, and that young age (younger than 35 years at diagnosis) and black race were independent mortality risk factors (47). Interestingly, these rates were independent from rates of ipsilateral invasive breast cancer after treatment, suggesting mortality is a relevant clinical endpoint for DCIS lesions.
DCIS features on mammography
Since the advent of screening mammography, DCIS most commonly presents as suspicious microcalcifications without associated mass, asymmetry, or distortion, accounting for up to 75% of cases presenting on mammography (48). The primary forms of calcifications identified on mammography are calcium oxalate, which are often not visible on pathology without polarized light and nearly always reflect benign, non-DCIS pathology, and calcium phosphate, which can represent either malignant (especially calcium hydroxyapatite forms) or benign pathologies (49). Unfortunately, these calcification subtypes cannot readily be distinguished on the basis of mammographic appearance alone. Furthermore, the exact reason DCIS and other breast cancers produce calcifications is unclear, though it is likely a combination of passive (ie, degenerative/dystrophic) and active (ie, secretory and activation of bone matrix proteins) processes (50).
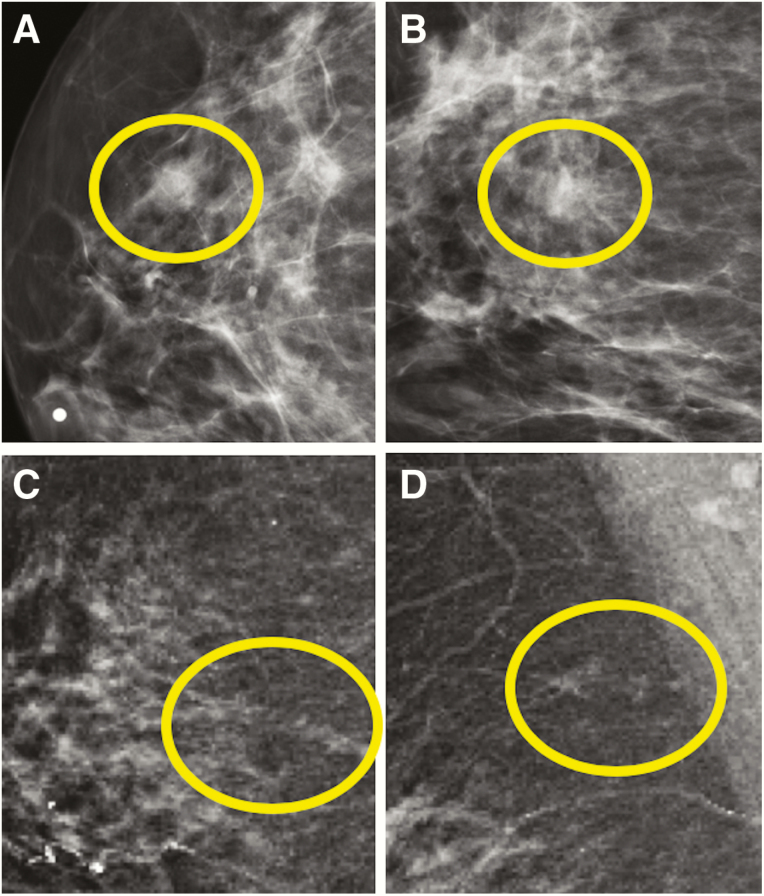
The most specific calcification morphologies are fine linear branching (positive predictive value [PPV] = 70%) and fine pleomorphic (PPV = 29%), although the less suspicious morphologies, including amorphous (PPV = 29%) and coarse heterogeneous (PPV = 15%), can also represent DCIS (Figure 4) (51). Fine pleomorphic, fine linear branching, “casting,” and “crushed stone” (the latter two being non–Breast Imaging-Reporting and Data System terms) (52, 53) have been reported to be associated with higher-grade DCIS or comedonecrosis (54–56) (Table 1), although these correlations are not particularly reliable (57). Calcification morphology accounts for only a part of a lesion’s malignant predictive value, and even typically benign calcification morphologies (eg, round/punctate) can reflect DCIS if distributed in a suspicious manner (eg, linearly/segmental distribution). Furthermore, some distributions, such as linear (58), have independently correlated with histopathologic features (55). Finally, approximately 10% of pure DCIS lesions can present as a dominant mass or asymmetry (Figure 5), which more often reflects low-grade DCIS, whereas architectural distortion can be present in 7%–13% of pure DCIS cases, often in association with sclerosing adenosis or radial scars (55).
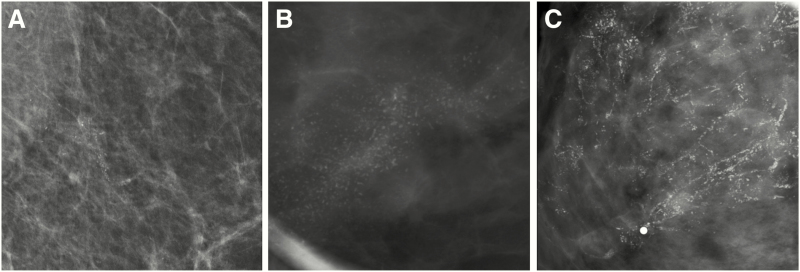
Figure 4.
Examples of various mammographic presentations of high nuclear grade DCIS, including asymptomatic linearly distributed amorphous calcifications on mediolateral 90-degree spot-magnification view (A), asymptomatic segmentally distributed fine pleomorphic calcifications on craniocaudal spot-magnification view (B), and diffusely distributed fine linear branching calcifications on craniocaudal spot-magnification view involving a clinically palpable, imaging occult mass (C). Microinvasion was present in both (A) and (C) on final surgical excision. Core needle biopsy (CNB)–diagnosed DCIS upgrades to invasive disease on surgical excision in approximately one quarter of cases. Risk factors for upgrade include clinical symptoms, mass on ultrasound or mammography, larger span, high nuclear grade, and CNB performed with a smaller gauge biopsy device.
Table 1.
Mammographic and Sonographic Features Reported to Correlate with Basic DCIS Histopathologic Features
| DCIS Pathology Feature | Reported Associated Mammographic Feature | Reported Associated Sonographic Feature |
| High Nuclear Grade | 1. Fine linear branching or fine pleomorphic morphology (48) 2. “Casting-type” morphology (52) |
Detected on mammogram ± ultrasound (59, 61) |
| Non–High Nuclear Grade | 1. Mammographic mass or asymmetry without calcifications (48, 56, 58) 2. Round/punctate calcifications (58) |
Detected at ultrasound alone (59, 61) |
| Comedonecrosis | 1. Branching, “rod-shaped,” ductal distribution (58) 2. “Casting-type” morphology (52) 3. Fine linear branching or fine pleomorphic morphology (48) |
Not detected on ultrasound (59) |
Figure 5.
Examples of low-grade DCIS presenting mammographically. In the first example (A and B), a round mass with obscured margins (circles) was identified in the upper outer quadrant of the right breast at anterior depth on 2D screening mammogram views (BB marker denotes the nipple on the craniocaudal view). Pathological evaluation of this mass yielded low-grade DCIS without comedonecrosis arising in association with an intraductal papilloma. In the second example (C and D), a developing focal asymmetry in the right breast was identified on synthetic 2D screening mammogram views in the right breast at 12 o’clock, posterior depth (circles). Pathology at this site revealed low-grade DCIS without comedonecrosis.
Ultrasound features of DCIS
While the visibility of DCIS on ultrasound has a wide range in the literature (8%–50%) (59), it is generally regarded as the least valuable imaging modality for DCIS detection and depiction. However, ultrasound can be useful to further evaluate mammographic findings that are not pure calcifications (eg, mass, asymmetry, or distortion) and to facilitate ultrasound-guided biopsy. The appearance of DCIS on ultrasound is variable (Figure 6), and it include benign-appearing masses, complex solid and cystic masses, masses with a “pseudomicrocystic” appearance (60), hypoechoic irregular masses, and “nonmass” presentations such as echogenic foci and dilated ducts (60). In general, DCIS lesions visible only on ultrasound are lower grade (59, 61), with a lower likelihood of comedonecrosis or her2/neu amplification than that of mammographically detected lesions (59), possibly because they reflect a more indolent growth pattern or are associated with coexisting benign pathologies (eg, intraductal papillomas).
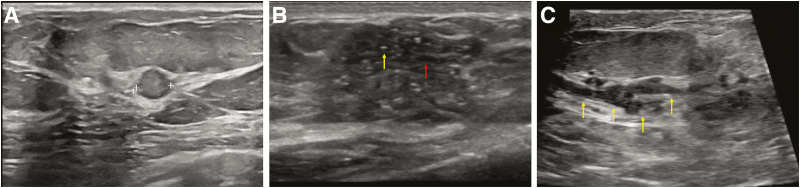
Figure 6.
Examples of DCIS presenting on ultrasound in three patients. In the first example (A), a round-shaped mass with circumscribed margins and heterogeneous echogenicity (calipers) revealed low-grade DCIS on pathology. In the second example (B), an irregular-shaped mass with angular and indistinct margins with associated microcalcifications (white arrow) and “pseudomicrocysts” (gray arrow) is present at the site of a clinically palpable mass. Biopsy revealed high-grade DCIS, and mammography confirmed the presence of fine pleomorphic calcifications within the mass. In the third example (C), there is duct ectasia (arrows) with low-level echoes within the ducts, which corresponded to a new mammographic focal asymmetry. Biopsy of this finding revealed papillary DCIS with a focus of microinvasion.
“Nonmass” presentations of DCIS on ultrasound are likely under recognized because of their overlap in appearance with benign duct ectasia (62). In a series of over 700 DCIS lesions, Watanabe et al found over 60% manifested as nonmass abnormalities, most commonly hypoechoic areas, followed by abnormalities of the ducts (62). Echogenic foci–representing calcifications also were an uncommon sonographic manifestation of DCIS in this series. However, it is important to note that malignant calcifications are often more readily detected by ultrasound as compared with benign calcifications, and calcifications are more common in higher-grade DCIS (60).
MRI features of DCIS
Early studies demonstrated that MRI had a high false-negative rate for DCIS detection attributable to an inability to identify calcifications. However, improved spatial resolution of breast MRI in subsequent years has led to recognition of nonmass enhancement (NME), which is DCIS’s commonest MRI presentation. Subsequently, multiple studies have demonstrated that MRI is superior to mammography for DCIS detection, especially for high nuclear grade subtypes (63, 64). Jansen and colleagues helped determine that DCIS’s unique enhancement pattern is partly due to gadolinium penetrating the basement membrane and collecting into milk ducts (65), providing clues as to why higher-grade DCIS lesions are preferentially visible on MRI (66).
The hallmark of DCIS on MRI is segmentally distributed NME with clumped internal enhancement and variable kinetic features (Figure 7). NMEs account for the majority of DCIS lesions identified on MRI (60%–81%) (67–69), and they generally reflect a pathology growth pattern that extends along the milk ducts. As such, “segmental” (triangular/wedge shape, with the apex toward the nipple), “focal area” (a small portion of a breast quadrant), and “linear” (includes “ductal” or “branching”) represent a larger fraction of DCIS lesions than they do “regional” or “diffuse” descriptors, which reflect pathology spanning multiple ductal systems. Among internal enhancement patterns, “clumped” is most specific for DCIS, and it accounts for approximately half of DCIS lesions. “Clustered ring” internal enhancement, a newer BI-RADS term, is believed to be specific for DCIS because it likely represents gadolinium accumulation in periductal and intraductal spaces (70).
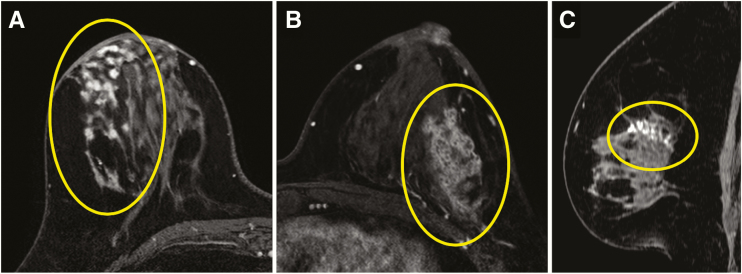
Figure 7.
Three examples of high nuclear grade DCIS presenting on breast MRI on images from the axially acquired T1-weighted fat-suppressed first postcontrast portion of a dynamic contrast-enhanced series, including a focal area of clumped nonmass enhancement (NME) in the right breast (circle) (A), segmentally distributed clustered ring NME in the left breast (circle) (B), and linearly distributed clumped NME (circle) on a sagittal reconstruction of the left breast (C).
DCIS lesions can also present as masses or foci. Such rarer presentations may represent a DCIS growth pattern that primarily expands rather than spreads along the milk ducts. Interestingly, several studies have found that foci (71) and masses (72, 73) presenting as DCIS were more often low grade than NMEs, supporting a more indolent growth pattern. Regardless of morphology, DCIS exhibits variable semiquantitative kinetic features, generally peaking later than invasive cancers do and often resulting in a medium initial phase and/or a delayed phase persistent or plateau (74).
In addition to being the most sensitive modality for DCIS detection, MRI also has been shown to be more accurate than mammography is at determining its full extent. In one study, MRI was able to estimate accurately the pathologic extent of DCIS within 5 mm in 60% of cases, compared with 38% with mammography (75). The overall sensitivity of MRI to determine disease extent accurately is reported to reach almost 89%, compared with 55% with mammography alone (69, 76). Furthermore, recent studies have shown that higher spatial resolution techniques using three tesla magnets can provide incremental benefit in disease-extent determination (77, 78). Despite these promising results, the practical surgical benefit of this improved depiction of DCIS is less clear, with a recent meta-analysis demonstrating no reduction in reoperation rate with preoperative MRI, though it was noted that variable approaches across sites limited the analysis (79). In contrast, a recent multicenter study demonstrated that when MRI approach and management are standardized, the successful WLE rate is very high (96.1%), with 78.5% of such women undergoing a single WLE (without reexcision needed) (44).
Overdiagnosis and overtreatment
Despite the dramatic rise in DCIS diagnosis rates with imaging, the corresponding influence on reducing invasive cancer rates has not been linear. A population-based study from the National Health Service demonstrated that for every three cases of screen-detected DCIS that were treated, there was one fewer invasive cancer in the next three years (80). These findings have led to increasing concerns regarding overdiagnosis of DCIS, defined as diagnosis of disease that will never become symptomatic or life threatening. Overdiagnosis in turn leads to overtreatment, increased health care expenditures, and increased patient anxiety (81).
Although concerns regarding overdiagnosis and breast cancer screening overall are frequently overstated, the case for overdiagnosis of screen-detected (particularly lower-risk) DCIS is more compelling based on several modeling studies of patient survival (82). Sagara et al demonstrated that among women with low-grade DCIS, there was no significant difference in breast cancer survival between women who underwent surgical excision (98.8%) and those who did not (98.6%) after 10 years of follow-up (83). Similarly, Ryser et al evaluated outcomes in a low-risk cohort (older than age 40 years, non-high grade, and ER/PR positive) of DCIS patients who did not undergo locoregional care (84). The risk of ipsilateral breast cancer was 5.9% at 7.5 years, but the all-cause risk of death was 28.2%. Since it is the standard of care for all women with DCIS to undergo excision, the true natural history of the disease is unknown, and further prospective work is needed to develop estimates of overdiagnosis that are more definitive.
Active surveillance
Active surveillance is an alternative management strategy for DCIS that avoids surgical excision in favor of imaging follow-up and possible chemoprevention (85). Similar to the evolution of treatment of early stage prostate cancer, active surveillance seeks to deescalate treatment in order to provide therapies that are more personalized for patients, while addressing overtreatment concerns (86, 87). The success of active surveillance as an alternative management strategy is dependent on two primary factors: excluding women with CNB-occult invasive disease and identifying women at low risk for future progression to invasive disease. Although overall upstaging rates to invasive disease are approximately 25%, the application of demographic and pathologic factors, most notably high nuclear grade DCIS and DCIS with associated masses, can reduce upstaging rates to 10% or less (24, 88, 89). Furthermore, both human and computer-derived imaging features have shown moderate success at predicting upstaging (90–92).
Predicting which patients will progress to invasive disease in the future is more challenging. Data from actual active surveillance patients are limited by small samples sizes, short follow-up intervals, and heterogeneous pathology profiles (93, 94). Small longitudinal follow-up studies of untreated women for whom DCIS was missed on initial pathology show that 39% to 46% will progress to invasive disease over very long follow- up periods of up to 42 years (95, 96). Finally, recurrence-free survival among women with untreated positive margins following DCIS excision demonstrates survival rates of 13%–35%, depending on nuclear grade (97). Although these noncontrolled, retrospective series demonstrate that some women with DCIS will progress to invasive disease, they also imply that a subgroup of women may be safe to avoid surgical excision.
To more definitively answer whether it is safe to undergo active surveillance for DCIS, there are three prospective trials in progress: The Comparison of Operative versus Medical Endocrine Therapy for Low Risk DCIS trial (COMET) in the United States (98), the Low Risk DCIS trial (LORIS) in the United Kingdom (99), and the Management of Low-Risk DCIS trial (LORD) in the Netherlands (100) (Table 2). For all three studies, enrolled participants are randomly assigned to either surgery with standard of care radiation and/or hormonal therapy or nonsurgical active monitoring. There are notable differences in the primary endpoints, inclusion criteria, and exclusion criteria between the trials. In brief, both the COMET and LORD trials have a primary outcome of ipsilateral IDC incidence, whereas the LORIS trial’s endpoint is IDC-specific survival. For the LORD and LORIS trials, patients are followed with yearly mammograms, whereas the COMET trial patients undergo semiannual mammograms. MRI and ultrasound are not components of the surveillance strategy. The LORD trial is the most conservative of the three trials because it includes only low-grade DCIS and excludes high-risk women, whereas the COMET trial is the most inclusive because it allows all non–high-grade DCIS, high-risk patients, and patients with bilateral DCIS. These trials are still in recruitment and will be collecting information until at least 2024.
Table 2.
Summary of Current DCIS Active Surveillance Trials
| COMET | LORIS | LORD | |
| Primary Outcome | Ipsilateral IDC incidence | Ipsilateral IDC survival | Ipsilateral IDC incidence |
| Target enrollment | 1,200 | 932 | 1,240 |
| Randomization | 1:1 | 1:1 | 1:1 |
| Year initiated | 2017 | 2014 | 2017 |
| Recruitment duration | 4 years | 6 years | 4 years |
| Follow-up interval | 5–7 years | 10 years | 10 years |
| Imaging surveillance | Biannual mammogram | Annual mammogram | Annual mammogram |
| Treatment | May get endocrine therapy | May get endocrine therapy | None |
| Inclusion criteria | |||
| Age (years) | ≥40 | ≥46 | ≥45 |
| Nuclear grade | Low and intermediate | Low risk* | Low |
| Appearance | Calcifications only | Calcifications only | Calcifications only |
| Receptor status | ER/PR+ and HER2- | N/A | N/A |
| Biopsy technique | VACB or surgical biopsy | VACB and/or surgical biopsy | VACB: 6 cores with 8/9 gauge or 12 cores with 10/11 gauge |
| Exclusion criteria | |||
| History of cancer | Exclude if IDC or DCIS | Exclude if IDC or ipsilateral DCIS | Exclude all prior cancers except cervical in situ or basal carcinoma |
| Symptomatic | Exclude | Exclude | Exclude |
| Comedonecrosis | Exclude | Exclude | N/A |
| Synchronous IDC | Exclude | Exclude | Exclude |
| Bilateral DCIS | Include | Include | Exclude |
| High risk | Include | Exclude if high risk per NICE guidelines | Exclude if family with BRCA 1/2 mutation |
Abbreviations: IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; VACB, vacuum-assisted core biopsy; NICE, National Institute for Health Care and Excellent (United Kingdom)
* The LORIS trial uses a central pathology review and stratifies DCIS into low and high risk. Low-risk DCIS includes low nuclear grade and the bottom half of intermediate nuclear grade.
The role of imaging to support evolving DCIS management
As prognostic differences among breast cancer subtypes are increasingly recognized, there is an exciting opportunity for breast imaging radiologists to support novel therapy approaches matched to personal risk. Advances in imaging biomarker identification, artificial intelligence, and radiomics all hold promise to assist with DCIS management and to address overdiagnosis (101). Perhaps most promising, multiparametric MRI combining dynamic contrast-enhanced and diffusion-weighted imaging approaches can probe vascularity and cellularity to provide unique imaging-based assays with potential to reduce overdiagnosis and overtreatment of low-risk DCIS.
Preliminary data support the potential of MRI to decrease the number of less aggressive DCIS diagnoses. Because the PPV of mammographically detected calcifications is less than 30% (102) and it identifies a greater proportion of low-grade DCIS, as compared with MRI, there is interest in using MRI to triage biopsies of calcifications (103). A study by Strobel et al examined 78 BI-RADS category 4 “pure clustered calcifications” and found that MRI had a 100% negative predictive value (NPV) for excluding invasive or microinvasive disease (104). Furthermore, the three false negatives on MRI (out of 21 total malignancies) were low-grade DCIS less than 12 mm in size. Baltzer et al similarly found that MRI had a 94% NPV for excluding malignancy in 152 mammographically detected suspicious calcifications (both false negatives were non–high-grade DCIS) and a 100% NPV for excluding invasive breast cancer (105). If validated in future prospective trials, MRI’s high NPV to exclude high-grade DCIS and invasive disease could assist with reducing overdiagnosis of the less important forms of DCIS.
Compared with other modalities such as mammography or ultrasound, multiparametric breast MRI is better able to capture the biological nature of DCIS (71). Prior studies have suggested MRI features can correlate with basic pathologic features, such as nuclear grade, comedonecrosis, and ER positivity. Specifically, lower-grade DCIS lesions are less likely to exhibit suspicious enhancement (63), and, when visible, they tend to be smaller with high contrast-to-noise ratios on DWI (106, 107). Furthermore, a pilot study suggested that low-grade DCIS lesions generally exhibited higher ADC values than high-grade lesions did (108), whereas a radiomics study demonstrated that quantitative heterogeneity parameters could predict nuclear grade and her2/neu amplification (77).
Finally, MRI features also may directly predict recurrence risk after treatment. Kim et al recently found that higher amounts of background parenchymal enhancement (BPE) surrounding DCIS lesions correlated with recurrence (109), whereas Luo et al found that higher DCIS signal enhancement ratio, larger DCIS functional tumor volume, and greater ipsilateral breast BPE also were associated with recurrence (110). Given these promising findings, it is probable that radiomics-based assays derived from even larger databases of breast MRIs could lead to a readily available DCIS imaging test to assist with precision therapy.
Conclusion
Despite its recognition over one century ago, DCIS remains a controversial breast pathology with a relatively homogeneous treatment approach. Most DCIS lesions are identified on imaging in asymptomatic women, and DCIS is the primary source of rising concerns regarding breast cancer overdiagnosis. Over the past two decades, a demand has developed for trials that aim to decrease overtreatment through the selection of patients who can avoid radiation therapy or even undergo active surveillance in lieu of any treatment. Promising data have emerged suggesting imaging features may be captured as independent biological assays that can complement molecular, clinical, and pathologic features to create improved DCIS risk profiles. As the medical community is at the cusp of an artificial intelligence revolution, breast imaging radiologists are uniquely positioned to be leaders in the next era of DCIS management.
Acknowledgments
This work was supported through a grant from the National Cancer Institute (NCI R01-CA203883) and a Patient-Centered Outcomes Research Institute (PCORI) Award (PCS-1505–30497) as part of the COMET study (Alliance Foundation Trials, https://alliancefoundationtrials.org).
Conflict of interest statement
None declared.
References
- 1. Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 2010; 102(3):170–178. [DOI] [PubMed] [Google Scholar]
- 2. Cady B. How to prevent invasive breast cancer: detect and excise duct carcinoma in situ. J Surg Oncol 1998;69(2):60–62. [DOI] [PubMed] [Google Scholar]
- 3. Cowell CF, Weigelt B, Sakr RA, et al. . Progression from ductal carcinoma in situ to invasive breast cancer: revisited. Mol Oncol 2013;7(5):859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graff S. Ductal carcinoma in situ: should the name be changed? J Natl Cancer Inst 2010;102(1):6–8. [DOI] [PubMed] [Google Scholar]
- 5. Allegra CJ, Aberle DR, Ganschow P, et al. . National Institutes of Health State-of-the-Science conference statement: diagnosis and management of ductal carcinoma in situ September 22–24, 2009. J Natl Cancer Inst 2010;102(3):161–169. [DOI] [PubMed] [Google Scholar]
- 6. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de Vijver MJ.. WHO Classification of Tumours of the Breast. 4th ed. Lyon, France: WHO Press; and LYON IARC Press; 2012. [Google Scholar]
- 7. Costarelli L, Campagna D, Mauri M, Fortunato L. Intraductal proliferative lesions of the breast-terminology and biology matter: premalignant lesions or preinvasive cancer? Int J Surg Oncol 2012;2012:501904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casasent AK, Edgerton M, Navin NE. Genome evolution in ductal carcinoma in situ: invasion of the clones. J Pathol 2017; 241(2):208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nelson AC, Machado HL, Schwertfeger KL. Breaking through to the other side: microenvironment contributions to DCIS initiation and progression. J Mammary Gland Biol Neoplasia 2018;23(4):207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quinn CM, Ostrowski JL. Cytological and architectural heterogeneity in ductal carcinoma in situ of the breast. J Clin Pathol 1997;50(7):596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elmore JG, Longton GM, Carney PA, et al. . Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 2015;313(11):1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 1985;55(11):2698–2708. [DOI] [PubMed] [Google Scholar]
- 13. Tavassoli FA, Norris HJ. A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer 1990; 65(3):518–529. [DOI] [PubMed] [Google Scholar]
- 14. Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol 2011;223(2):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchió C, Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology 2010;57(2):171–192. [DOI] [PubMed] [Google Scholar]
- 16. Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol 2005;205(2):248–254. [DOI] [PubMed] [Google Scholar]
- 17. Pang JM, Gorringe KL, Wong SQ, Dobrovic A, Campbell IG, Fox SB. Appraisal of the technologies and review of the genomic landscape of ductal carcinoma in situ of the breast. Breast Cancer Res 2015;17:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lester SC, Bose S, Chen YY, et al. ; Members of the Cancer Committee, College of American Pathologists Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch Pathol Lab Med 2009;133(1):15–25. [DOI] [PubMed] [Google Scholar]
- 19. Kerlikowske K, Molinaro AM, Gauthier ML, et al. . Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst 2010;102(9):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visser LL, Groen EJ, van Leeuwen FE, Lips EH, Schmidt MK, Wesseling J. Predictors of an invasive breast cancer recurrence after dcis: a systematic review and meta-analyses. Cancer Epidemiol Biomarkers Prev 2019;28(5):835–845. [DOI] [PubMed] [Google Scholar]
- 21. Rakha EA, Ellis IO. An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol 2007;60(12):1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monticciolo DL. Histologic grading at breast core needle biopsy: comparison with results from the excised breast specimen. Breast J 2005;11(1):9–14. [DOI] [PubMed] [Google Scholar]
- 23. Dahlstrom JE, Sutton S, Jain S. Histological precision of stereotactic core biopsy in diagnosis of malignant and premalignant breast lesions. Histopathology 1996; 28(6):537–541. [DOI] [PubMed] [Google Scholar]
- 24. Brennan ME, Turner RM, Ciatto S, et al. . Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology 2011;260(1):119–128. [DOI] [PubMed] [Google Scholar]
- 25. Broders AC. Carcinoma in situ contrasted with benign penetrating epithelium. J Am Med Assoc 1932;99(20):1670–1674. [Google Scholar]
- 26. Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol 1941;17(4):491– 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 28. Esserman LJ, Thompson IM, Reid B, et al. . Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 2014; 15(6):e234–e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nickel B, Barratt A, Hersch J, Moynihan R, Irwig L, McCaffery K. How different terminology for ductal carcinoma in situ (DCIS) impacts women’s concern and management preferences: a qualitative study. Breast 2015;24(5):673–679. [DOI] [PubMed] [Google Scholar]
- 30. McCaffery K, Nickel B, Moynihan R, et al. . How different terminology for ductal carcinoma in situ impacts women’s concern and treatment preferences: a randomised comparison within a national community survey. BMJ Open 2015;5(11):e008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Omer ZB, Hwang ES, Esserman LJ, Howe R, Ozanne EM. Impact of ductal carcinoma in situ terminology on patient treatment preferences. JAMA Intern Med 2013;173(19):1830–1831. [DOI] [PubMed] [Google Scholar]
- 32. Rosner D, Bedwani RN, Vana J, Baker HW, Murphy GP. Noninvasive breast carcinoma: results of a national survey by the American College of Surgeons. Ann Surg 1980;192(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mutebi M, Simonds H. Breast ductal carcinoma in situ in an unscreened population: presentation, diagnosis and management at a single tertiary centre. S Afr J Surg 2017;55(1):4–9. [PubMed] [Google Scholar]
- 34. Virnig BA, Wang SY, Shamilyan T, Kane RL, Tuttle TM. Ductal carcinoma in situ: risk factors and impact of screening. J Natl Cancer Inst Monogr 2010;2010(41):113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pandya S, Mackarem G, Lee AKC, McLellan R, Heatley GJ, Hughes K.. Ductal carcinoma in situ: the impact of screening on clinical presentation and pathologic features. Breast J 1998;4(3):146–51. [Google Scholar]
- 36. Rutter CE, Park HS, Killelea BK, Evans SB. Growing use of mastectomy for ductal carcinoma in situ of the breast among young women in the United States. Ann Surg Oncol 2015;22(7):2378–2386. [DOI] [PubMed] [Google Scholar]
- 37. Fisher B, Costantino J, Redmond C, et al. . Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med 1993;328(22):1581–1586. [DOI] [PubMed] [Google Scholar]
- 38. McCormick B, Winter K, Hudis C, et al. . RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol 2015;33(7):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cuzick J, Sestak I, Pinder SE, et al. . Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 2011;12(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gradishar WJ, Anderson BO, Balassanian R, et al. . Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14(3):324–354. [DOI] [PubMed] [Google Scholar]
- 41. Rudloff U, Jacks LM, Goldberg JI, et al. . Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol 2010;28(23):3762–3769. [DOI] [PubMed] [Google Scholar]
- 42. Silverstein MJ. The University of Southern California/Van Nuys Prognostic Index for Ductal Carcinoma in Situ of the Breast. Am J Surg 2003;186(4):337–343. [DOI] [PubMed] [Google Scholar]
- 43. Solin LJ, Gray R, Baehner FL, et al. . A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst 2013;105(10):701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lehman CD, Gatsonis C, Romanoff J, et al. . Association of magnetic resonance imaging and a 12-gene expression assay with breast ductal carcinoma in situ treatment. JAMA Oncology 2019. doi:10.1001/jamaoncol.2018.6269. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wärnberg F, Yuen J, Holmberg L. Risk of subsequent invasive breast cancer after breast carcinoma in situ. Lancet 2000; 355(9205):724–725. [DOI] [PubMed] [Google Scholar]
- 46. Shamliyan T, Wang SY, Virnig BA, Tuttle TM, Kane RL. Association between patient and tumor characteristics with clinical outcomes in women with ductal carcinoma in situ. J Natl Cancer Inst Monogr 2010;2010(41):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 2015;1(7):888–896. [DOI] [PubMed] [Google Scholar]
- 48. Barreau B, de Mascarel I, Feuga C, et al. . Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations. Eur J Radiol 2005;54(1):55–61. [DOI] [PubMed] [Google Scholar]
- 49. O’Grady S, Morgan MP. Microcalcifications in breast cancer: from pathophysiology to diagnosis and prognosis. Biochim Biophys Acta Rev Cancer 2018;1869(2):310–320. [DOI] [PubMed] [Google Scholar]
- 50. Cox RF, Morgan MP. Microcalcifications in breast cancer: lessons from physiological mineralization. Bone 2013;53(2):437–450. [DOI] [PubMed] [Google Scholar]
- 51. D’Orsi CJ SE, Mendelson EB, Morris EA, et al. . ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 52. Holmberg L, Wong YN, Tabár L, et al. . Mammography casting-type calcification and risk of local recurrence in DCIS: analyses from a randomised study. Br J Cancer 2013;108(4):812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tabar L, Dean PB. Thirty years of experience with mammography screening: a new approach to the diagnosis and treatment of breast cancer. Breast Cancer Res 2008;10(Suppl 4):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rauch GM, Hobbs BP, Kuerer HM, et al. . Microcalcifications in 1657 patients with pure ductal carcinoma in situ of the breast: correlation with clinical, histopathologic, biologic features, and local recurrence. Ann Surg Oncol 2016;23(2):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamada T, Mori N, Watanabe M, et al. . Radiologic-pathologic correlation of ductal carcinoma in situ. Radiographics 2010;30(5):1183–1198. [DOI] [PubMed] [Google Scholar]
- 56. Yang WT, Tse GM. Sonographic, mammographic, and histopathologic correlation of symptomatic ductal carcinoma in situ. AJR Am J Roentgenol 2004;182(1):101–110. [DOI] [PubMed] [Google Scholar]
- 57. Slanetz PJ, Giardino AA, Oyama T, et al. . Mammographic appearance of ductal carcinoma in situ does not reliably predict histologic subtype. Breast J 2001; 7(6):417–421. [DOI] [PubMed] [Google Scholar]
- 58. Evans A, Pinder S, Wilson R, et al. . Ductal carcinoma in situ of the breast: correlation between mammographic and pathologic findings. AJR Am J Roentgenol 1994;162(6):1307–1311. [DOI] [PubMed] [Google Scholar]
- 59. Moon HJ, Kim EK, Kim MJ, Yoon JH, Park VY. Comparison of clinical and pathologic characteristics of ductal carcinoma in situ detected on mammography versus ultrasound only in asymptomatic patients. Ultrasound Med Biol 2019;45(1):68–77. [DOI] [PubMed] [Google Scholar]
- 60. Wang LC, Sullivan M, Du H, Feldman MI, Mendelson EB. US appearance of ductal carcinoma in situ. Radiographics 2013;33(1):213–228. [DOI] [PubMed] [Google Scholar]
- 61. Izumori A, Takebe K, Sato A. Ultrasound findings and histological features of ductal carcinoma in situ detected by ultrasound examination alone. Breast Cancer 2010; 17(2):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Watanabe T, Yamaguchi T, Tsunoda H, et al. . Ultrasound image classification of ductal carcinoma in situ (DCIS) of the breast: analysis of 705 DCIS lesions. Ultrasound Med Biol 2017;43(5):918–925. [DOI] [PubMed] [Google Scholar]
- 63. Kuhl CK, Schrading S, Bieling HB, et al. . MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 2007;370(9586):485–492. [DOI] [PubMed] [Google Scholar]
- 64. Lehman CD, Gatsonis C, Kuhl CK, et al. ; ACRIN Trial 6667 Investigators Group MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007;356(13):1295–1303. [DOI] [PubMed] [Google Scholar]
- 65. Jansen SA, Paunesku T, Fan X, et al. . Ductal carcinoma in situ: X-ray fluorescence microscopy and dynamic contrast-enhanced MR imaging reveals gadolinium uptake within neoplastic mammary ducts in a murine model. Radiology 2009;253(2):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kuhl CK. Why do purely intraductal cancers enhance on breast MR images? Radiology 2009;253(2):281–283. [DOI] [PubMed] [Google Scholar]
- 67. Rosen EL, Smith-Foley SA, DeMartini WB, Eby PR, Peacock S, Lehman CD. BI-RADS MRI enhancement characteristics of ductal carcinoma in situ. Breast J 2007;13(6):545–550. [DOI] [PubMed] [Google Scholar]
- 68. Chan S, Chen JH, Agrawal G, et al. . Characterization of pure ductal carcinoma in situ on dynamic contrast-enhanced MR imaging: do nonhigh grade and high grade show different imaging features? J Oncol 2010; 2010. pii: 431341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jansen SA, Newstead GM, Abe H, Shimauchi A, Schmidt RA, Karczmar GS. Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade. Radiology 2007;245(3):684–691. [DOI] [PubMed] [Google Scholar]
- 70. Tozaki M, Igarashi T, Fukuda K. Breast MRI using the VIBE sequence: clustered ring enhancement in the differential diagnosis of lesions showing non-masslike enhancement. AJR Am J Roentgenol 2006;187(2):313–321. [DOI] [PubMed] [Google Scholar]
- 71. Esserman LJ, Kumar AS, Herrera AF, et al. . Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol 2006;24(28):4603–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu H, Peng W. MRI morphological classification of ductal carcinoma in situ (DCIS) correlating with different biological behavior. Eur J Radiol 2012;81(2):214–217. [DOI] [PubMed] [Google Scholar]
- 73. Baur A, Bahrs SD, Speck S, et al. . Breast MRI of pure ductal carcinoma in situ: sensitivity of diagnosis and influence of lesion characteristics. Eur J Radiol 2013;82(10):1731–1737. [DOI] [PubMed] [Google Scholar]
- 74. Newstead GM. MR imaging of ductal carcinoma in situ. Magn Reson Imaging Clin N Am 2010;18(2):225–240, viii. [DOI] [PubMed] [Google Scholar]
- 75. Marcotte-Bloch C, Balu-Maestro C, Chamorey E, et al. . MRI for the size assessment of pure ductal carcinoma in situ (DCIS): a prospective study of 33 patients. Eur J Radiol 2011;77(3):462–467. [DOI] [PubMed] [Google Scholar]
- 76. Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J 2005;11(6):382–390. [DOI] [PubMed] [Google Scholar]
- 77. Chou SS, Gombos EC, Chikarmane SA, Giess CS, Jayender J. Computer-aided heterogeneity analysis in breast MR imaging assessment of ductal carcinoma in situ: correlating histologic grade and receptor status. J Magn Reson Imaging 2017;46(6):1748–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pickles MD, Gibbs P, Hubbard A, Rahman A, Wieczorek J, Turnbull LW. Comparison of 3.0 T magnetic resonance imaging and X-ray mammography in the measurement of ductal carcinoma in situ: a comparison with histopathology. Eur J Radiol 2015;84(4):603–610. [DOI] [PubMed] [Google Scholar]
- 79. Fancellu A, Turner RM, Dixon JM, Pinna A, Cottu P, Houssami N. Meta-analysis of the effect of preoperative breast MRI on the surgical management of ductal carcinoma in situ. Br J Surg 2015;102(8):883–893. [DOI] [PubMed] [Google Scholar]
- 80. Duffy SW, Dibden A, Michalopoulos D, et al. . Screen detection of ductal carcinoma in situ and subsequent incidence of invasive interval breast cancers: a retrospective population-based study. Lancet Oncol 2016;17(1):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Groen EJ, Elshof LE, Visser LL, et al. . Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast 2017;31:274–283. [DOI] [PubMed] [Google Scholar]
- 82. Kopans DB. Point: the New England Journal of Medicine article suggesting overdiagnosis from mammography screening is scientifically incorrect and should be withdrawn. J Am Coll Radiol 2016;13(11S):R50–R52. [DOI] [PubMed] [Google Scholar]
- 83. Sagara Y, Mallory MA, Wong S, et al. . Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg 2015;150(8):739–745. [DOI] [PubMed] [Google Scholar]
- 84. Ryser MD, Weaver DL, Zhao F, et al. . Cancer outcomes in DCIS patients without locoregional treatment. J Natl Cancer Inst 2019. doi:10.1093/jnci/djy220. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kuerer HM. Ductal carcinoma in situ: treatment or active surveillance? Expert Rev Anticancer Ther 2015;15(7):777–785. [DOI] [PubMed] [Google Scholar]
- 86. Grimm LJ, Shelley Hwang E. Active surveillance for DCIS: the importance of selection criteria and monitoring. Ann Surg Oncol 2016;23(13):4134–4136. [DOI] [PubMed] [Google Scholar]
- 87. Matulewicz RS, Weiner AB, Schaeffer EM. Active surveillance for prostate cancer. JAMA 2017;318(21):2152. [DOI] [PubMed] [Google Scholar]
- 88. Grimm LJ, Ryser MD, Partridge AH, et al. . Surgical upstaging rates for vacuum assisted biopsy proven DCIS: implications for active surveillance trials. Ann Surg Oncol 2017;24(12):3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Soumian S, Verghese ET, Booth M, et al. . Concordance between vacuum assisted biopsy and postoperative histology: implications for the proposed Low Risk DCIS Trial (LORIS). Eur J Surg Oncol 2013;39(12):1337–1340. [DOI] [PubMed] [Google Scholar]
- 90. Shi B, Grimm LJ, Mazurowski MA, et al. . Prediction of occult invasive disease in ductal carcinoma in situ using deep learning features. J Am Coll Radiol 2018;15(3 Pt B):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shi B, Grimm LJ, Mazurowski MA, et al. . Can occult invasive disease in ductal carcinoma in situ be predicted using computer-extracted mammographic features? Acad Radiol 2017;24(9):1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Harowicz MR, Saha A, Grimm LJ, et al. . Can algorithmically assessed MRI features predict which patients with a preoperative diagnosis of ductal carcinoma in situ are upstaged to invasive breast cancer? J Magn Reson Imaging 2017;46(5):1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Grimm LJ, Ghate SV, Hwang ES, Soo MS. Imaging features of patients undergoing active surveillance for ductal carcinoma in situ. Acad Radiol 2017;24(11):1364–1371. [DOI] [PubMed] [Google Scholar]
- 94. Meyerson AF, Lessing JN, Itakura K, et al. . Outcome of long term active surveillance for estrogen receptor-positive ductal carcinoma in situ. Breast 2011;20(6):529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 2005;103(12):2481–2484. [DOI] [PubMed] [Google Scholar]
- 96. Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses’ Health Study. Cancer 2005;103(9):1778–1784. [DOI] [PubMed] [Google Scholar]
- 97. Khan S, Epstein M, Lagios MD, Silverstein MJ. Are we overtreating ductal carcinoma in situ (DCIS)? Ann Surg Oncol 2017;24(1):59–63. [DOI] [PubMed] [Google Scholar]
- 98. Comparison of Operative versus Medical Endocrine Therapy for Low Risk DCIS: The COMET Trial Patient-Centered Outcomes Research Institute; [8 August 2016]; Available from: https://www.pcori.org/research-results/2016/comparing-treatment-options-women-low-risk-ductal-carcinoma-situ-dcis---comet. Accessed 15 July 2019. [Google Scholar]
- 99. LORIS A phase III trial of surgery versus active monitoring for Low Risk Ductal Carcinoma in situ (DCIS). University of Birmingham; [19 November 2016]; Available from: http://www.birmingham.ac.uk/research/activity/mds/trials/crctu/trials/loris/index.aspx. Accessed 15 July 2019. [Google Scholar]
- 100. Management of low-risk DCIS (LORD). The Netherlands Cancer Institute; [19 November 2016]; Available from: https://clinicaltrials.gov/ct2/show/NCT02492607. Accessed 15 July 2019. [Google Scholar]
- 101. Rahbar H, McDonald ES, Lee JM, Partridge SC, Lee CI. How can advanced imaging be used to mitigate potential breast cancer overdiagnosis? Acad Radiol 2016;23(6):768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kettritz U, Rotter K, Schreer I, et al. . Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer 2004;100(2):245–251. [DOI] [PubMed] [Google Scholar]
- 103. Wells CJ, O’Donoghue C, Ojeda-Fournier H, Retallack HE, Esserman LJ. Evolving paradigm for imaging, diagnosis, and management of DCIS. J Am Coll Radiol 2013;10(12):918–923. [DOI] [PubMed] [Google Scholar]
- 104. Strobel K, Schrading S, Hansen NL, Barabasch A, Kuhl CK. Assessment of BI-RADS category 4 lesions detected with screening mammography and screening US: utility of MR imaging. Radiology 2015;274(2):343–351. [DOI] [PubMed] [Google Scholar]
- 105. Baltzer PAT, Bennani-Baiti B, Stöttinger A, Bumberger A, Kapetas P, Clauser P. Is breast MRI a helpful additional diagnostic test in suspicious mammographic microcalcifications? Magn Reson Imaging 2018; 46:70–74. [DOI] [PubMed] [Google Scholar]
- 106. Rahbar H, Parsian S, Lam DL, et al. . Can MRI biomarkers at 3 T identify low-risk ductal carcinoma in situ? Clin Imaging 2016;40(1):125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rahbar H, Partridge SC, Demartini WB, et al. . In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology 2012; 263(2):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Iima M, Le Bihan D, Okumura R, et al. . Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study. Radiology 2011;260(2):364–372. [DOI] [PubMed] [Google Scholar]
- 109. Kim SA, Cho N, Ryu EB, et al. . Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery. Radiology 2014;270(3):699–707. [DOI] [PubMed] [Google Scholar]
- 110. Luo J, Johnston BS, Kitsch AE, et al. . Ductal carcinoma in situ: quantitative preoperative breast MR imaging features associated with recurrence after treatment. Radiology 2017;285(3):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]