Abstract
Patient: Female, 91
Final Diagnosis: Flecainide toxicity
Symptoms: Bradycardia
Medication: Flecainide
Clinical Procedure: —
Specialty: Cardiology
Objective:
Unusual clinical course
Background:
Flecainide is a class Ic antiarrhythmic agent used in the treatment of supraventricular and ventricular arrhythmias. It is associated with a potent adverse effect profile; however, the effects of flecainide toxicity in the setting of a pacemaker have not been well described. We describe a unique case of flecainide toxicity secondary to acute kidney injury in the setting of a dual-chamber pacemaker, resulting in ventricular capture latency and intermittent failure to capture.
Case Report:
The patient was a 91-year-old female with a history of atrial fibrillation maintained in sinus rhythm on flecainide, who presented complaining of purple visual disturbances and syncope. She was found to be hypotensive and bradycardic, with a heart rate between 30 to 40 beats per minute. Lab work was notable for creatinine at 2.12 mg/dL. A 12-lead ECG demonstrated atrial and ventricular pacing with severely widened QRS complex and a significant latency between the pacemaker ventricular spike and the ventricular capture. The pacemaker was interrogated, revealing a significant increase in ventricular threshold from 0.75 V at 0.5 ms at baseline to 5.0 V at 1 ms to obtain consistent capture. After multiple boluses of IV sodium bicarbonate, the QRS acutely narrowed, latency interval improved, and consistent pacing capture was achieved. The flecainide level drawn on arrival was 3.09 mcg/mL.
Conclusions:
Flecainide increases the ventricular capture threshold for pacemakers. Toxicity in these patients may present with pacemaker ventricular capture latency or failure to capture.
MeSH Keywords: Acute Kidney Injury; Drug-Related Side Effects and Adverse Reactions; Flecainide; Pacemaker, Artificia
Background
Flecainide is a Vaughan-Williams class Ic antiarrhythmic agent used most commonly in the treatment of atrial fibrillation, atrial flutter, and supraventricular and ventricular arrhythmias in patients without known structural or ischemic cardiac disease [1,2]. As a class Ic agent, its primary mechanism of action is blockade of fast sodium channels, resulting in a reduction in myocardial conduction velocity and a negative inotropic effect on the myocardium. Its primary action in atrial tissue is to prolong the effective refractory period (ERP), demonstrating use-dependence, resulting in a more pronounced effect as the heart rate increases [3]. Flecainide has a narrow therapeutic window and a highly variable plasma half-life in the setting of renal dysfunction. A mortality rate of 10–25% has been reported with class Ic antiarrhythmic agent toxicity [4]. Although flecainide toxicity is described in the literature on acute kidney injury, there is a paucity of literature on the acute presentation of flecainide toxicity in the setting of a cardiac pacemaker [5]. We describe a case of flecainide toxicity secondary to acute kidney injury in the setting of dual-chamber pacing, resulting in ventricular capture latency and intermittent failure to capture [6].
Case Report
The patient was a 91-year-old female with a history of paroxysmal atrial fibrillation maintained in normal sinus rhythm on flecainide for more than 3 years. She also had sick sinus syndrome, with a dual-chamber pacemaker placed many years prior, COPD, and heart failure with preserved ejection fraction. The patient presented for the first time to the Emergency Department (ED) complaining of nausea, lightheadedness, and generalized weakness. The patient’s medication list was notable for the use of flecainide 100 mg BID and metoprolol tartrate 50 mg BID. A recent transthoracic echocardiogram had been performed a few months prior and had demonstrated a normal ejection fraction, concentric left ventricular hypertrophy, and biatrial enlargement. The patient had recently undergone a total right knee replacement for degenerative joint disease 4 weeks prior, and post-operatively the patient had developed these symptoms along with poor oral intake. The patient’s creatinine was noted to be elevated at 2.09 compared to her baseline normal renal function. The patient was briefly admitted for acute kidney injury, re-hydrated with intravenous fluids, and discharged home without change in medications.
A few days later, the patient developed worsening fatigue and lightheadedness with several episodes of syncope. She also reported new visual disturbances with purple discoloration. She presented again to the ED and was found to be hypotensive, tachypneic, mildly encephalopathic, and bradycardic with a heart rate of 30–40 beat per minute (bpm). A 12-lead ECG demonstrated atrial and ventricular pacing with severely widened QRS complex (QRS duration >200 ms) with a significantly latency between the pacemaker ventricular spike and the ventricular capture. Metabolic studies were remarkable for an ongoing elevation in creatinine at 2.12 with a GFR of 23 mL/min per 1.73 m2 and a lactate of 3.8 mmol/L with a potassium level at the upper limit of normal.
The patient’s dual-chamber pacemaker was emergently interrogated and found to be intermittently capturing in the ventricle. The ventricular threshold during device interrogation indeed revealed a significant increase from 0.75 V at 0.5 ms at baseline to 5.0 V at 1 ms to obtain consistent capture, even though a significant latency persisted, as noted earlier (Figure 1A). The patient was in complete heart block with no ventricular escape, as demonstrated by the pacemaker intracardiac electrograms (Figure 2). Due to concern for suspected flecainide toxicity, she was given IV sodium bicarbonate and a norepinephrine drip was started for initial hemodynamic support. After multiple boluses of IV sodium bicarbonate, the QRS acutely narrowed, pacemaker latency interval improved, and consistent pacing capture was achieved, although the paced QRS remained abnormally prolonged (Figure 1B). The patient’s cardiogenic shock secondary to bradycardia rapidly resolved. Two days later, the paced QRS complex duration became narrower, similar to baseline (Figure 1C).
Figure 1.
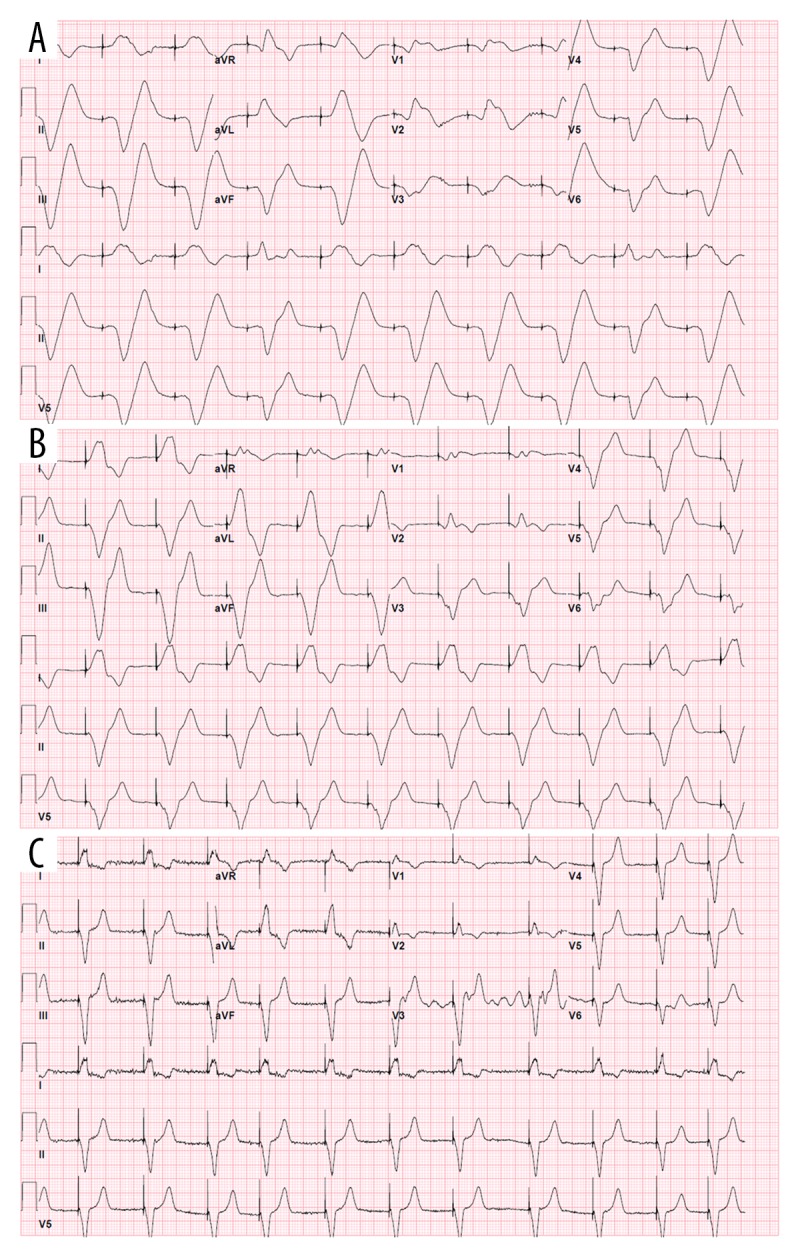
(A) 12-lead ECG performed after the pacemaker was switched into VVI mode at a pacing threshold of 5 mV at 1 ms. Note the impressive latency between the ventricular spike and the ventricular depolarization and the QRS duration above 200 ms. (B) 12-lead ECG performed after treatment with intravenous sodium bicarbonate, demonstrating improvement of the QRS duration and disappearance of the latency interval. (C) 12-lead ECG performed a day later, demonstrating a ventricular paced rhythm with a QRS narrower than that before ECG.
Figure 2.
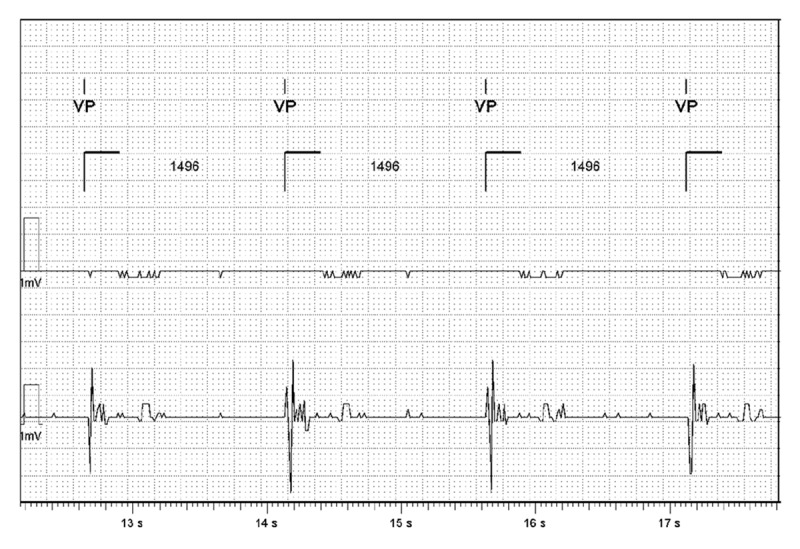
Intracardiac electrograms from the initial pacemaker interrogation, demonstrating no ventricular escape at 40 bpm.
The flecainide level drawn on arrival was 3.09 mcg/mL (reference range: 0.20–0.99 mcg/mL). A few days later, the intrinsic atrioventricular conduction resumed. The electrolytes remained in the normal range throughout the hospitalization. The patient’s acute kidney injury resolved and she was discharged home. Flecainide was discontinued and the patient was continued on metoprolol tartrate for control of her atrial fibrillation.
Discussion
We describe a case of a patient with a pacemaker who presented with flecainide toxicity, in cardiogenic shock, with unstable bradycardia, and increased ventricular threshold and latency, leading to intermittent ventricular capture. Flecainide has a potent adverse effect profile. The medication has a steep dose-response curve and has been recognized as having a narrow therapeutic index [7]. Flecainide toxicity can result in nausea, vomiting, visual disturbances, and seizure [8]. In line with this, our patient presented with nausea, fatigue, pre-syncope, and visual disturbances. Like other antiarrhythmic agents, flecainide has pro-arrhythmic properties, particularly at serum levels greater than >1 mcg/mL and in patients with structural or ischemic cardiac disease [9]. Along with dose-dependent prolongation of the PR interval and widening of the QRS complex, it has been reported that ventricular tachyarrhythmia, high-grade AV block, and bundle branch block are associated with flecainide toxicity [8].
In our patient, the QRS complex was found to be severely widened on presentation, with a duration greater than 200 ms and intermittent ventricular capture due to increased threshold. Flecainide toxicity results in rate-dependent QRS complex widening by competitive inhibition of the Nav1.5 cardiac sodium channels, which results in a slowing of the phase 0 portion of the cardiac action potential [10].
A unique feature of this case is a dual-chamber pacemaker patient becoming pacemaker-dependent, with acute flecainide toxicity, and subsequently developing syncope due to ventricular capture latency and intermittent failure to capture. It has been demonstrated that flecainide can increase the ventricular capture threshold for pacemakers, and patients at therapeutic doses may require pacemaker setting adjustment when initiating flecainide [11]. However, pacemaker failure to capture in the setting of acute flecainide toxicity has seldom been reported in the literature [5]. Our case also demonstrates a prolonged pacemaker latency interval in the setting of flecainide toxicity and subsequent rapid improvement in latency interval after treatment with intravenous sodium bicarbonate, which has not been previously reported. Along with elevated capture thresholds, the ECG findings in this case were the result of the device attempting to pace while the myocardium was in an extended refractory period secondary to flecainide. The intrinsic function of the device itself was normal. Adjustment of the pacemaker settings to optimize pacing capture and prompt recognition that this was not “device failure” were important in rapid resolution and minimization of morbidity in this patient.
Sodium bicarbonate is a well described therapy for flecainide toxicity. It has been demonstrated that both alkalization and increased sodium concentration reverse flecainide effects on Purkinje fibers in animal models. This may be caused by accelerating flecainide molecule dissociation from the sodium channel receptor and by competitive inhibition, but the mechanism is not fully understood [12]. In our patient, prompt sodium bicarbonate administration resulted in rapid resolution of the patient’s device failure to capture and cardiogenic shock. Cardiac pacing has previously been described as a treatment for flecainide toxicity [13]. While it may be helpful when patients present with complete heart block, our case and other cases described in the literature suggest that cardiac pacing may be of minimal benefit when the predominant issue is QRS widening [14,15]. Acidification of the urine has been shown to increase urinary excretion of flecainide, but there is little data available on its clinical role in the setting of flecainide toxicity, and it was not pursued in the treatment of this patient [16].
In the patient described, the etiology of flecainide toxicity was an acute kidney injury, which has been recognized as a common trigger. Flecainide is eliminated from the body by renal excretion and the hepatic cytochrome P450 CYP2D6 enzyme. The primary elimination mechanism is renal excretion and, in the setting of normal renal function, 80–90% of the drug is excreted into the urine [17]. In a patient with intact renal and hepatic function, the medication’s plasma half-life is approximately 12–26 hours. However, studies have demonstrated that the medication has a highly variable plasma half-life in the setting of renal dysfunction, and its usage requires caution and dose adjustment in these circumstances [18]. An initial maximum oral dosage of 100 mg daily is typically recommended in patients with moderate to severe renal failure (creatinine clearance <35 ml/min) [19]. Importantly, flecainide cannot be removed with the use of hemodialysis. Furthermore, lower doses should be considered in elderly patients, as in our case. Some authors recommend altogether avoiding class I antiarrhythmic agents in very elderly patients due to their adverse effect profile [20]. Flecainide particularly must be used with caution in these patients due to the high probability of undiagnosed coronary artery disease. Flecainide also has a known negative inotropic effect and has been associated with increased mortality in heart failure patients, particularly among patients with heart failure with reduced ejection fraction [21]. Although data are scarce regarding the use of flecainide in patients with heart failure with preserved ejection fraction, we believe flecainide should be used with caution in these patients as well.
Conclusions
Flecainide is a class Ic antiarrhythmic agent with a narrow therapeutic index and a potent adverse effect profile. Our report describes a unique case of flecainide toxicity in a pacemaker-dependent patient. This case illustrates several important points regarding the treatment of patients with flecainide toxicity. When treating patients that have renal dysfunction and older patients with flecainide, physicians should exercise caution. Patients with acute kidney injury should have their medication discontinued or at least reduced until resolution. Particular care should be practiced when treating patients on flecainide, even when they are not pacemaker-dependent, as it is associated with the risk of iatrogenic complete heart block and risk of pacemaker failure to capture due to elevated myocardial capture thresholds. This could be more dramatic for patients that are pacemaker-dependent. Sodium bicarbonate is an effective therapy for flecainide toxicity.
References:
- 1.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias – executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Supraventricular Arrhythmias) Circulation. 2003;108(15):1871–909. doi: 10.1161/01.CIR.0000091380.04100.84. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 3.Saksena S, Camm AJ, Boyden PA. Electrophysiological disorders of the heart. 2012.
- 4.Winkelmann BR, Leinberger H. Life-threatening flecainide toxicity. A pharmacodynamic approach. Ann Intern Med. 1987;106(6):807–14. doi: 10.7326/0003-4819-106-6-807. [DOI] [PubMed] [Google Scholar]
- 5.Apps A, Miller CP, Fellows S, Jones M. Cardiac devices with class 1C antiarrhythmics: A potentially toxic combination. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-210598. pii: bcr2015210598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suffredini JM, Rutland J, Akpunonu P, et al. Flecainide toxicity resulting in pacemaker latency and intermittent failure to capture. Heart Rhythm. 2019;5(Suppl.):519. doi: 10.12659/AJCR.916370. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamargo J, Le Heuzey JY, Mabo P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur J Clin Pharmacol. 2015;71(5):549–67. doi: 10.1007/s00228-015-1832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolecki PF, Curry SC. Poisoning by sodium channel blocking agents. Crit Care Clin. 1997;13(4):829–48. doi: 10.1016/s0749-0704(05)70371-7. [DOI] [PubMed] [Google Scholar]
- 9.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324(12):781–88. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 10.Ramos E, O’Leary ME. State-dependent trapping of flecainide in the cardiac sodium channel. J Physiol. 2004;560(Pt 1):37–49. doi: 10.1113/jphysiol.2004.065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellestrand KJ, Burnett PJ, Milne JR, et al. Effect of the antiarrhythmic agent flecainide acetate on acute and chronic pacing thresholds. Pacing Clin Electrophysiol. 1983;6(5 Pt 1):892–99. doi: 10.1111/j.1540-8159.1983.tb04410.x. [DOI] [PubMed] [Google Scholar]
- 12.Bou-Abboud E, Nattel S. Relative role of alkalosis and sodium ions in reversal of class I antiarrhythmic drug-induced sodium channel blockade by sodium bicarbonate. Circulation. 1996;94(8):1954–61. doi: 10.1161/01.cir.94.8.1954. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd T, Zimmerman J, Griffin GD. Irreversible third-degree heart block and pacemaker implant in a case of flecainide toxicity. Am J Emerg Med. 2013;31(9):1418e1411–12. doi: 10.1016/j.ajem.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Auzinger GM, Scheinkestel CD. Successful extracorporeal life support in a case of severe flecainide intoxication. Crit Care Med. 2001;29(4):887–90. doi: 10.1097/00003246-200104000-00041. [DOI] [PubMed] [Google Scholar]
- 15.Corkeron MA, van Heerden PV, Newman SM, Dusci L. Extracorporeal circulatory support in near-fatal flecainide overdose. Anaesth Intensive Care. 1999;27(4):405–8. doi: 10.1177/0310057X9902700413. [DOI] [PubMed] [Google Scholar]
- 16.Muhiddin KA, Johnston A, Turner P. The influence of urinary pH on flecainide excretion and its serum pharmacokinetics. Br J Clin Pharmacol. 1984;17(4):447–51. doi: 10.1111/j.1365-2125.1984.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conard GJ, Ober RE. Metabolism of flecainide. Am J Cardiol. 1984;53(5):41B–51B. doi: 10.1016/0002-9149(84)90501-0. [DOI] [PubMed] [Google Scholar]
- 18.Aliot E, Capucci A, Crijns HJ, et al. Twenty-five years in the making: Flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13(2):161–73. doi: 10.1093/europace/euq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams AJ, McQuinn RL, Walls J. Pharmacokinetics of flecainide acetate in patients with severe renal impairment. Clin Pharmacol Ther. 1988;43(4):449–55. doi: 10.1038/clpt.1988.57. [DOI] [PubMed] [Google Scholar]
- 20.Karamichalakis N, Letsas KP, Vlachos K, et al. Managing atrial fibrillation in the very elderly patient: Challenges and solutions. Vasc Health Risk Manag. 2015;11:555–62. doi: 10.2147/VHRM.S83664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaker GC, Blackshear JL, McBride R, et al. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 1992;20(3):527–32. doi: 10.1016/0735-1097(92)90003-6. [DOI] [PubMed] [Google Scholar]


