Abstract
Background
Metallo-β-lactamase-producing Pseudomonas aeruginosa (MBL-PA) are important causative agents of nosocomial infections and are associated with significant mortality rates, especially in intensive care units. The timely detection and typing of these strains is essential for surveillance, outbreak prevention and antibiotic therapy optimization. In this study, fifteen VIM-type and fifteen SPM-type MBL-PA strains were selected as strains to establish MALDI-TOF MS SuperSpectra.
Methods
This study was undertaken to evaluate the utility of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) with the VITEK MS plus system in the detection of VIM- and SPM-type MBL-PA isolates. For each species, we increased the reference spectra, and then, a SuperSpectrum was created based on the selection of 39 specific masses. In a second step, we validated the SuperSpectra with the remaining 50 isolates (25 isolates of VIM-type and 25 isolates of SPM-type).
Results
Fifty MBL-PA strains were used as the validation strains, including twenty-five VIM-type and twenty-five SPM-type MBL-PA strains. Complete antimicrobial susceptibility testing and genotypic characterizations were performed for all isolates, which were subsequently identified using the newly created SuperSpectra databases following a previously reported method. The results showed that there was 92% agreement between the MBL profile generated by MALDI-TOF MS and that obtained using gene sequencing analysis methods.
Conclusion
MALDI-TOF MS is a promising, rapid and economical method for detecting VIM- or SPM-type MBL-PA that could be successfully introduced into the routine diagnostic workflow of clinical microbiology laboratories.
Keywords: VIM, SPM, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, SuperSpectra, VITEK MS plus system
Introduction
In recent years, metallo-β-lactamase-producing Pseudomonas aeruginosa (MBL-PA) have become an increasing health care problem worldwide.1–3 MBL-PA are important etiological agents of hospital-acquired infections (HAI), which cause significant mortality, particularly among patients in intensive care units (ICU).4 An increase in drug-resistant P. aeruginosa isolates, especially MBL-PA isolates, has occurred with the widespread use of antibiotics, posing great challenges to clinical anti-infective therapy.5
At present, the imipenem-EDTA double-disk synergy test is primarily used by laboratories to screen for MBL-PA isolates. The limitation of this method is that it can easily lead to false-positive or false-negative results.6 In addition, the gene sequencing analysis method can also be used to detect MBL-PA isolates. However, because this method is time consuming and complex, it is only useful and practical for retrospective studies and not for rapid clinical detection. The timely detection and typing of these strains is essential for surveillance purposes to prevent outbreaks and optimize antibiotic therapy.7 Therefore, there is an urgent need for a new quick and accurate detection method to prevent and control the spread of MBL-PA.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a rapid method for analyzing biological samples that has been widely used for the soft ionization of microbial-specific ribosomal proteins.8–10 Based on a database of specific protein fingerprints constructed from many microorganisms, microorganisms can be rapidly identified by MALDI-TOF MS. Compared with conventional techniques, the advantages of this method include rapidity, cost-effectiveness, accuracy and suitability for the high-throughput identification of bacteria.7,8,10 However, taxonomic and genetic relatedness discrepancies on the species and biovar level complicate the development of detection and identification assays.11
The aim of this study was to use MALDI-TOF MS to distinguish between VIM- and SPM-type MBL-PA clinical isolates. Therefore, SuperSpectra were generated using strains that represent the known genetic VIM- and SPM-type MBL-PA strains according to Saramis software of the VITEK MS RUO (research-use-only) system. Subsequently, the SuperSpectra were evaluated using fifty MBL-PA strains, including twenty-five VIM-type and twenty-five SPM-type MBL-PA strains.
Materials and methods
Bacterial isolates
A total of 80 MBL-PA clinical isolates were collected from Daping Hospital, Army Medical University. A complete antimicrobial susceptibility testing analysis was performed for all isolates using a VITEK-2 Compact (GN-13 card) instrument (bioMerieux, France). Among these isolates, fifteen VIM-type MBL-PA strains and fifteen SPM-type MBL-PA strains were selected as the strains to establish SuperSpectra. The remaining twenty-five VIM-type MBL-PA strains and twenty-five SPM-type MBL-PA strains were used as the validation strains, which had been identified using the MBL phenotype screening methods and commercial identification systems, including the API (API 20E) and VITEK-2 Compact (GN card) systems (bioMerieux, France). All strains were obtained from various clinical patient samples collected from 2014 to 2017. The P. aeruginosa ATCC27853 strain stored in Daping Hospital was used as a negative-control strain. The VIM- and SPM-type MBL-PA strains from the Children’s Hospital of Chongqing Medical University were used as positive-control strains. Culturing and analysis of MBL-PA isolates was performed in a biosafety level two-plus mycobacteriology laboratory in Daping Hospital following biosafety level two-plus precautions.
Identification of isolates by gene sequencing analysis
A genotypic characterization was performed for all isolates. The MBL gene sequencing analysis was used as the gold standard, and all the isolates were used as templates to amplify the MBL gene. The PCR assays were performed to amplify the sequences of the VIM and SPM bla genes as previously described, using the primers described by Dong et al.2,10,12 The PCR products were purified using a 3S spin PCR product purification kit (Shenergy Biocolor, China), and the sequences were searched against the GenBank database using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/blast).
MALDI-TOF MS
Sample preparation
All isolates were inoculated onto sheep blood agar and incubated in 5% CO2 at 37 °C for 24 h. The sample preparation methods, interpretation of the results and quality control strains were previously described.8,9 Briefly, two or three colonies were transferred to a 1.5 mL EPikote tube containing 300 µL water and 900 µL 70% ethanol. After incubating for 30 s, 50 µL of 70% formic acid and 50 µL of acetonitrile were added. The suspensions were centrifuged at 13,000 rpm for 2 min, after which 1.0 µL of each supernatant was applied to a 48-spot polished-steel target plate (bioMerieux, France) and dried. One microliter of a saturated α-cyano-4-hydroxycinnamic acid matrix solution (bioMerieux, France) was applied to each sample, which was allowed to dry before loading into the MALDI-TOF MS instrument.
MALDI-TOF MS acquisition
Protein mass fingerprints were obtained using the MALDI-TOF MS plus mass spectrometer and were within a mass range of 2000 to 20,000 Da with a tolerance of 0.08%. For each target slide, Escherichia coli ATCC8739 was used for instrument calibration according to the protocol of the manufacturer. After the spectrum acquisition, the data were transferred from a VITEK MS acquisition station to the Saramis analysis server. The data are reported as the number of peaks and the highest-level matches compared to those for the Saramis 4.14 RUO database.
Cluster analysis of VIM- and SPM-type MBL-PA using the VITEK MS plus system
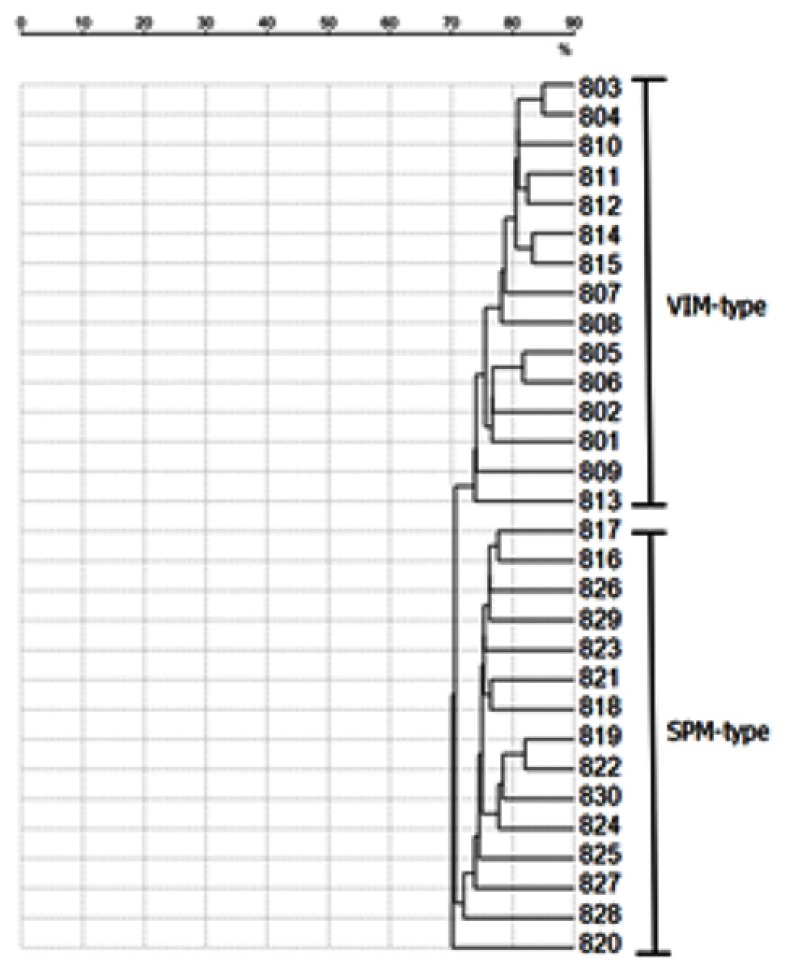
For the first stage of this study, we sought to determine whether there were unique peaks between VIM- and SPM-type MBL-PA as previously described using the VITEK MS plus system. A clustering analysis of VIM- and SPM-type MBL-PA was performed using the Saramis 4.14 RUO database to show the different peaks for each subspecies (Figure 1).
Figure 1.
UPGMA clustering of mass spectra of the established strains (VIM- and SPM-type MBL-producing P. aeruginosa). The VIM- and SPM-type MBL-PA isolates collected from Daping Hospital were identified as two cluster groups by the VITEK MS plus System, which means that there were some different peaks for each subspecies.
Selection of reference spectra
Because the VIM- and SPM-type MBL-PA were not listed in the current RUO Saramis database, we created two new reference spectra for each group. New folders of subspecies were added under the original P. aeruginosa species in the spectral taxonomy tree, and then the imported spectra were pasted into the respective subspecies folders. To be selected, a reference spectrum needed to have a mass number between 100 and 200 and have more than 70% of common masses with other spectra from the same species. Moreover, to be chosen, spectra for the same considered isolate needed to have more than 70% homology. These criteria were recommended by the manufacturer (bioMerieux, France).
Creation of superspectra
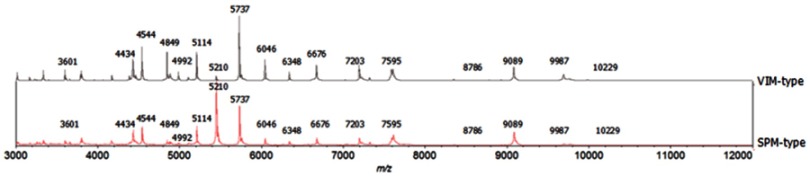
To create the SuperSpectra, we first imported the reference spectra data. Next, the SuperSpectra were created on the basis of the selection of protein masses specific to VIM- and SPM-type MBL-PA. First, we identified the overlapping masses between the VIM- and SPM-type MBL-PA. To avoid overlapping masses, we first created an exclusion list (Figure 2), after which we cluster-analyzed the 30 genetically confirmed MBL-PA isolates collected from Daping Hospital using the VITEK MS plus system. Finally, we chose 39 specific masses to create the SuperSpectra (Table S1) based on comprehensive factors, such as the number of consensus peaks and peak intensity. For each subspecies, SuperSpectra were created on the basis of the selection of the protein masses specific to the considered subspecies, which were compared with all the peaks in the Saramis database. A greater or lesser weight was assigned for each of the selected masses depending on the number of matches for the first non-target subspecies. Finally, the SuperSpectra were activated for subsequent automated identification at the subspecies level.
Figure 2.
Peak profiles of the spectra from VIM- and SPM-type MBL-producing P. aeruginosa. The SuperSpectra of VIM- and SPM-type MBL-PA were generated using the RUO saramis knowledge base database. Among these peaks, 17 peaks overlap, which used as the mass exclusion list (3601 m/z, 4434 m/z, 4544 m/z, 4849 m/z, 4992 m/z, 5114 m/z, 5210 m/z, 5737 m/z, 6046 m/z, 6348 m/z, 6676 m/z, 7203 m/z, 7595 m/z, 8786 m/z, 9089 m/z, 9987 m/z and 10,229 m/z).
Identification of isolates with the VITEK MS plus system
To assess the capability and stability of the newly created SuperSpectra, we chose the remaining 50 isolates (25 isolates of VIM-type and 25 isolates of SPM-type) as the validation strains, which were well identified by MBL gene sequencing. All the validation strains were cultured on sheep blood agar and incubated at 37 °C for 24 h, and each MALDI-TOF MS spectrum was first compared with the SuperSpectra in the Saramis database provided by the manufacturer. When a spectrum matched the SuperSpectra, a confidence value for the best match was given. When no match with the SuperSpectra was observed at a confidence value of at least 75%, the spectrum of the tested isolate was compared with reference spectra in the database. Identification was valid when the confidence value was higher than 75% when compared with the SuperSpectra and higher than 40% when compared with a reference spectrum, as defined by the manufacturer (Saramis Premium User Manual, VITEK MS plus; bioMerieux).
Results
Bacterial identity
A total of 80 MBL-PA isolates were collected from clinical microbiology laboratories of Daping Hospital. Complete antimicrobial susceptibility testing was performed for all isolates, and the results showed that all strains were resistant to imipenem. Subsequently, all the VIM- and SPM-type MBL-PA strains were correctly identified by MBL gene sequencing analysis.
MALDI-TOF MS
MALDI-TOF MS characterization of bacteria is based on differences in the mass-to-charge ratio (m/z) fingerprints of the microorganisms’ proteins, primarily ribosomal proteins, that are most abundantly expressed under all growth conditions. Fifteen VIM-type MBL-PA isolates and fifteen SPM-type MBL-PA isolates were selected as strains to establish SuperSpectra, which was performed via MALDI-TOF MS. Comparing the spectra of each subspecies, 17 overlapping masses and 39 specific masses for VIM- and SPM-type MBL-PA were observed. Peak profiles of the spectra for VIM- and SPM-type MBL-PA were obtained by the VITEK MS plus system and were analyzed with the RUO Saramis Knowledge Base.
Next, all the isolates were grown on sheep blood agar and incubated in a 5% CO2 atmosphere at 37 °C for 24 h, and eight MALDI-TOF MS repeat tests were subsequently performed, giving a total of 120 spectra for VIM-type MBL-PA and 120 spectra for SPM-type MBL-PA. All the spectra were added to the Saramis database as reference spectra. For each subspecies, we selected the specific masses and weighted them by checking the weight table to create the SuperSpectra. Finally, a total of 50 validation strains were tested using the new SuperSpectra implemented in the Saramis database. The characteristics of the isolates are presented in Table 1. The validation results showed that a total of 25 VIM-type MBL-PA isolates were correctly identified. Only four SPM-type MBL-PA isolates were incorrectly identified as VIM-type MBL-PA isolates, which gave conflicting results in repeat testing by the VITEK MS plus system. Altogether, 100% of the VIM-type MBL-PA isolates were correctly identified by the VITEK MS plus system. Eighty-four percent (21 of 25) of the SPM-type MBL-PA isolates were identified using the SuperSpectra, and there was 92% agreement between the MBL profile generated by MALDI TOF MS and that obtained using gene sequencing analysis methods. The SuperSpectra evaluation results are summarized in Table 2. All QC results were within the designated acceptable range, and all the isolates underwent repeat testing using the validation strains.
Table 1.
Characteristics of the fifty isolates used for validation of the SuperSpectra
| Isolate | Patient location | Isolation site | MBL sequencing identification | MALDI-TOF MS identification | Confidence values (%) |
|---|---|---|---|---|---|
| 1 | ICU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 99.9 |
| 2 | ICU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 99.9 |
| 3 | ICU | Secretion | VIM-type MBL-PA | VIM-type MBL-PA | 92.0 |
| 4 | ICU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 92.0 |
| 5 | Surgical ward | Urine | VIM-type MBL-PA | VIM-type MBL-PA | 89.0 |
| 6 | Medical ward | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 85.0 |
| 7 | Pediatrics | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 85.8 |
| 8 | CCU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 99.9 |
| 9 | ICU | Urine | VIM-type MBL-PA | VIM-type MBL-PA | 99.9 |
| 10 | ICU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 81.2 |
| 11 | ICU | Urine | VIM-type MBL-PA | VIM-type MBL-PA | 99.0 |
| 12 | ICU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 99.0 |
| 13 | Surgical ward | Urine | VIM-type MBL-PA | VIM-type MBL-PA | 91.2 |
| 14 | Surgical ward | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 99.9 |
| 15 | Pediatrics | Blood | VIM-type MBL-PA | VIM-type MBL-PA | 99.9 |
| 16 | CCU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 82.5 |
| 17 | ICU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 99.9 |
| 18 | ICU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 99.9 |
| 19 | Medical ward | Urine | VIM-type MBL-PA | VIM-type MBL-PA | 96.0 |
| 20 | Medical ward | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 95.2 |
| 21 | Surgical ward | Drainage | VIM-type MBL-PA | VIM-type MBL-PA | 95.2 |
| 22 | ICU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 90.0 |
| 23 | ICU | BALF | VIM-type MBL-PA | VIM-type MBL-PA | 90.0 |
| 24 | Surgical ward | Pus | VIM-type MBL-PA | VIM-type MBL-PA | 88.0 |
| 25 | CCU | Sputum | VIM-type MBL-PA | VIM-type MBL-PA | 89.0 |
| 26 | Surgical ward | Bile | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 27 | Medical ward | BALF | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 28 | Medical ward | Pharyngeal swab | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 29 | Pediatrics | Sputum | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 30 | CCU | Urine | SPM-type MBL-PA | SPM-type MBL-PA | 88.0 |
| 31 | ICU | Sputum | SPM-type MBL-PA | SPM-type MBL-PA | 91.2 |
| 32 | ICU | Sputum | SPM-type MBL-PA | VIM-type MBL-PA | 85.0 |
| 33 | Surgical ward | Wound | SPM-type MBL-PA | SPM-type MBL-PA | 89.0 |
| 34 | Surgical ward | Catheter | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 35 | Surgical ward | Joint fluid | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 36 | ICU | Sputum | SPM-type MBL-PA | VIM-type MBL-PA | 81.2 |
| 37 | ICU | Sputum | SPM-type MBL-PA | VIM-type MBL-PA | 92.0 |
| 38 | CCU | Sputum | SPM-type MBL-PA | SPM-type MBL-PA | 99.0 |
| 39 | Surgical ward | Secretion | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 40 | Pediatrics | Sputum | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 41 | ICU | Sputum | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 42 | ICU | Urine | SPM-type MBL-PA | SPM-type MBL-PA | 99.0 |
| 43 | Surgical ward | Urine | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 44 | ICU | BALF | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 45 | ICU | Urine | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 46 | ICU | Sputum | SPM-type MBL-PA | SPM-type MBL-PA | 88.0 |
| 47 | ICU | Sputum | SPM-type MBL-PA | SPM-type MBL-PA | 89.0 |
| 48 | CCU | BALF | SPM-type MBL-PA | SPM-type MBL-PA | 99.9 |
| 49 | Surgical ward | Secretion | SPM-type MBL-PA | VIM-type MBL-PA | 82.9 |
| 50 | Medical ward | Sputum | SPM-type MBL-PA | SPM-type MBL-PA | 92.0 |
Table 2.
Results of validation of VITEK MS plus system in identification of VIM- and SPM-type MBL-producing P. aeruginosa isolates using the two SuperSpectra
| Validation strains | No. of strains | Identification by VITEK MS | Confidence values (%) | Percentage |
|---|---|---|---|---|
| VIM-type MBL-PA | 4 | VIM-type MBL-PA | 99.9 | 16.0 |
| 12 | VIM-type MBL-PA | 90.0–99.9 | 48.0 | |
| 9 | VIM-type MBL-PA | 80.0–90.0 | 36.0 | |
| Subtotal | 25 | VIM-type MBL-PA accuracy rate | 100.0 | |
| SPM-type MBL-PA | 5 | SPM-type MBL-PA | 99.9 | 20.0 |
| 8 | SPM-type MBL-PA | 90.0–99.9 | 32.0 | |
| 8 | SPM-type MBL-PA | 80.0–90.0 | 32.0 | |
| 4 | VIM-type MBL-PA | 90.0–99.9 | 16.0 | |
| Subtotal | 25 | SPM-type MBL-PA accuracy rate | 84.0 | |
| Total | 50 | Total accuracy rate | 92.0 |
Note: The validation results showed that all the VIM-type MBL-PA isolates were correctly identified, just four SPM-type MBL-PA isolates were incorrectly identified as VIM-type MBL-PA isolates which got conflicting results in repeat testing by VITEK MS plus system.
Discussion
Traditional molecular epidemiology analyses are unable to control the rapid spread of nosocomial infections caused by pathogenic bacteria.13,14 Therefore, there is an urgent need to develop a new method for typing pathogenic bacteria. As an emerging proteomic tool for microbial identification, MALDI-TOF MS is superior to other methods in saving time and reducing cost. Previous reports showed a greater than 99% accuracy in P. aeruginosa species-level identification, although it remains difficult to distinguish closely related subspecies using this method.10,15,16 The purpose of this study was to assess the accuracy of MALDI-TOF MS in identifying MBL-PA at the subspecies level.
In this study, 30 MBL-PA isolates were selected to establish SuperSpectra after being identified as VIM- or SPM-type MBL-PA using the RUO Saramis Knowledge Base database. In addition, this study is the first to use a mass exclusion list to create the SuperSpectra for detecting bacterial subspecies. We further developed a MALDI-TOF MS protocol and performed a multisite study of the SuperSpectra using the validation strains described in Table 2. Overall, the identification results obtained by the VITEK MS plus system were consistent with those obtained by MBL gene sequencing. However, it should be noted that there are four SPM-type MBL-PA isolates which were incorrectly identified in the experiment, which may be due to changes at genome levels or phenotype levels. In addition, the results obtained using the SuperSpectra for the SPM-type MBL-PA isolates varied from set to set and were not reproducible. This irreproducibility potentially occurred as a function of horizontal gene transfer and genetic background differences that influence the evolution of SPM-type MBL-PA isolates.17–19
Based on these findings, MALDI-TOF MS shows great promise for correctly identifying MBL-PA subspecies. The VITEK MS plus is currently available worldwide and is currently used in our laboratory. Manual calibration is an important step before using the RUO application for bacterial identification.16,20,21 If the calibration is not congruent, the spectra obtained by the MALDI-TOF MS can be of lower quality, resulting in a wrong interpretation.22,23 We validated our databases by combining the results of ten replicates, which demonstrated that the calibration was congruent. Our databases allowed us to correctly identify all the isolates at the species level, and the SuperSpectra that we created had different sensitivities and specificities. This result suggested that a suitable means of improving MBL-PA subspecies identification using the VITEK MS plus is needed without changing the protocol used.16,24 Due to the small sample size of MBL-PA genotypes, such as blaIMP, blaSIM, blaGIM and blaNDM, we have not established any other SuperSpectra, nor have we validated the other genotypes of MBL-PA isolates. In future studies, we will expand our analysis of genotypes of the strains used to establish SuperSpectra and use them to detect of other subspecies.
A primary limitation of this study is that the databases we developed are only applicable in research mode (Saramis) and may not be transposed to the IVD database.20,24 Indeed, the two databases use different algorithms: the Saramis database identifies isolates from SuperSpectra created with inserted reference spectra, while the IVD database identifies isolates after assigning scores according to the number of peaks in a segment of the diagram. Consequently, our results require further improvement to allow the spectra to be integrated into the IVD database.25
Conclusion
MALDI-TOF MS is a promising, rapid and economical method for detecting VIM- and SPM-type MBL-PA that can be successfully introduced into the routine diagnostic workflow of clinical microbiology laboratories. Our study validates the potential ease and accuracy with which MALDI-TOF MS can be incorporated into the IVD system for identifying subspecies of pathogens. MBL-PA identification by MALDI-TOF MS using our databases is a good alternative to molecular biology methods. In addition, the creation and use of SuperSpectra with the VITEK MS plus system potentially provides an efficient on-site method to monitor the spread of antibiotic-resistant bacteria and rapidly detect outbreaks.
Acknowledgment
We give special thanks to Dr. Weiping Lu for kindly providing advice for this study. This study was supported by the Special Project of the “Twelfth Five-year Plan” for Medical Science Development of the PLA (AWS14C003-2).
Abbreviations
MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MBL, metallo-β-lactamase; MBL-PA, metallo-β-lactamase-producing Pseudomonas aeruginosa; VIM, Veronese imipenemase, blaVIM gene was found in Daping Hospital; SPM, Sao Paulo MBL, blaSPM gene was found in Daping Hospital; RUO, research-use-only; EDTA, ethylenediamine tetra-acetic acid; P. aeruginosa, Pseudomonas aeruginosa.
Ethics statement
The apply materials were reviewed and the data conform to ethics document related to human study. It is approved by all members of The Ethics Committee of Daping Hospital of Third Military Medical University.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Khan AU, Maryam L, Zarrilli R. Structure, genetics and worldwide spread of New Delhi Metallo-β-lactamase (NDM): a threat to public health. BMC Microbiol. 2017;17(1):101. doi: 10.1186/s12866-017-1012-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Bij AK, Van Mansfeld R, Peirano G, et al. First outbreak of VIM-2 metallo-β-lactamase-producing Pseudomonas aeruginosa in The Netherlands: microbiology, epidemiology and clinical outcomes. Int J Antimicrob Agents. 2011;37(6):513–518. doi: 10.1016/j.ijantimicag.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 3.Lucena A, Dalla Costa LM, Nogueira KS, et al. Nosocomial infections with metallo-beta-lactamase-producing Pseudomonas aeruginosa: molecular epidemiology, risk factors, clinical features and outcomes. J Hosp Infect. 2014;87(4):234–240. doi: 10.1016/j.jhin.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 4.Cezário RC, Duarte De Morais L, Ferreira JC, Costa-Pinto RM, Da Costa Darini AL, Gontijo-Filho PP. Nosocomial outbreak by imipenem-resistant metallo-beta-lactamase-producing Pseudomonas aeruginosa in an adult intensive care unit in a Brazilian teaching hospital. Enferm Infecc Microbiol Clin. 2009;27(5):269–274. doi: 10.1016/j.eimc.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 5.Valenza G, Joseph B, Elias J, et al. First survey of metallo-beta-lactamases in clinical isolates of Pseudomonas aeruginosa in a German university hospital. Antimicrob Agents Chemother. 2010;54(8):3493–3497. doi: 10.1128/AAC.00080-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafer MM, Al-Agamy MH, El-Mahallawy HA, Amin MA, El Din Ashour S. Dissemination of VIM-2 producing Pseudomonas aeruginosa ST233 at tertiary care hospitals in Egypt. BMC Infect Dis. 2015;15:122. doi: 10.1186/s12879-015-0861-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakarikou C, Ciotti M, Dolfa C, Angeletti S, Favalli C. Rapid detection of carbapenemase-producing Klebsiella pneumoniae strains derived from blood cultures by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS). BMC Microbiol. 2017;17(1):54. doi: 10.1186/s12866-017-0952-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo L, Liu W, Li B, et al. Evaluation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for identification of Mycobacterium abscessus subspecies according to whole-genome sequencing. J Clin Microbiol. 2016;54(12):2982–2989. doi: 10.1128/JCM.01151-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Tang Y, Lu X. Insight into Identification of Acinetobacter Species by Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) in the clinical laboratory. J Am Soc Mass Spectrom. 2018. doi: 10.1007/s13361-018-1911-4 [DOI] [PubMed] [Google Scholar]
- 10.Gautam V, Sharma M, Singhal L, et al. MALDI-TOF mass spectrometry: an emerging tool for unequivocal identification of non-fermenting Gram-negative bacilli. Indian J Med Res. 2017;145(5):665–672. doi: 10.4103/ijmr.IJMR_1105_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lista F, Reubsaet FA, De Santis R, et al. Reliable identification at the species level of Brucella isolates with MALDI-TOF-MS. BMC Microbiol. 2011;11:267. doi: 10.1186/1471-2180-11-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voor IN, ‘t Holt AF, Severin JA, Lesaffre EM, Vos MC. A systematic review and meta-analyses show that carbapenem use and medical devices are the leading risk factors for carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2014;58(5):2626–2637. doi: 10.1128/AAC.01758-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong D, Zou D, Liu H, et al. Rapid detection of Pseudomonas aeruginosa targeting the toxA gene in intensive care unit patients from Beijing, China. Front Microbiol. 2015;6:1100. doi: 10.3389/fmicb.2015.01100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varma-Basil M, Nair D. Molecular epidemiology of tuberculosis: opportunities & challenges in disease control. Indian J Med Res. 2017;146(1):11–14. doi: 10.4103/ijmr.IJMR_941_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fangous MS, Mougari F, Gouriou S, et al. Classification algorithm for subspecies identification within the Mycobacterium abscessus species, based on matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2014;52(9):3362–3369. doi: 10.1128/JCM.00788-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyer C, Gregorowicz G, Mougari F, Raskine L, Cambau E, de Briel D. Comparison of Saramis 4.12 and IVD 3.0 Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Mycobacteria from solid and liquid culture media. J Clin Microbiol. 2017;55(7):2045–2054. doi: 10.1128/JCM.00006-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42(6):1604–1613. doi: 10.1183/09031936.00149212 [DOI] [PubMed] [Google Scholar]
- 18.Sader HS, Reis AO, Silbert S, Gales AC, IMPs V. SPMs: the diversity of metallo-beta-lactamases produced by carbapenem-resistant Pseudomonas aeruginosa in a Brazilian hospital. Clin Microbiol Infect. 2005;11(1):73–76. doi: 10.1111/j.1469-0691.2004.01031.x [DOI] [PubMed] [Google Scholar]
- 19.Kidd TJ, Ritchie SR, Ramsay KA, Grimwood K, Bell SC, Rainey PB. Pseudomonas aeruginosa exhibits frequent recombination, but only a limited association between genotype and ecological setting. PLoS One. 2012;7(9):e44199. doi: 10.1371/journal.pone.0044199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rychert J, Burnham CA, Bythrow M, et al. Multicenter evaluation of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of Gram-positive aerobic bacteria. J Clin Microbiol. 2013;51(7):2225–2231. doi: 10.1128/JCM.00682-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghebremedhin M, Heitkamp R, Yesupriya S, Clay B, Crane NJ. Accurate and rapid differentiation of Acinetobacter baumannii strains by Raman spectroscopy: a comparative study. J Clin Microbiol. 2017;55(8):2480–2490. doi: 10.1128/JCM.01744-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilecen K, Yaman G, Ciftci U, Laleli YR. Performances and reliability of Bruker Microflex LT and VITEK MS MALDI-TOF mass spectrometry systems for the identification of clinical microorganisms. Biomed Res Int. 2015;2015:516410. doi: 10.1155/2015/516410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee W, Kim M, Yong D, Jeong SH, Lee K, Chong Y. Evaluation of VITEK mass spectrometry (MS), a matrix-assisted laser desorption ionization time-of-flight MS system for identification of anaerobic bacteria. Ann Lab Med. 2015;35(1):69–75. doi: 10.3343/alm.2015.35.1.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kassim A, Pflüger V, Premji Z, Daubenberger C, Revathi G. Comparison of biomarker based Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) and conventional methods in the identification of clinically relevant bacteria and yeast. BMC Microbiol. 2017;17(1):128. doi: 10.1186/s12866-017-1037-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng K, Chui H, Domish L, Hernandez D, Wang G. Recent development of mass spectrometry and proteomics applications in identification and typing of bacteria. Proteomics Clin Appl. 2016;10(4):346–357. doi: 10.1002/prca.201500086 [DOI] [PMC free article] [PubMed] [Google Scholar]