Abstract
Purpose
To elucidate the relationship between disorganization of retinal inner layers (DRILs) and retinal function in diabetic patients without diabetic retinopathy (DR) and with nonproliferative DR, but without diabetic macular edema (DME).
Methods
Fifty-seven participants with diabetes mellitus (DM) and 18 healthy controls underwent comprehensive ophthalmic examination, fundus photography, and spectral-domain optical coherence tomography. Scans of the fovea were evaluated for the presence of DRIL. Retinal function was evaluated using Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity, the quick contrast sensitivity function (qCSF) on the AST Sentio Platform, short-wavelength automated perimetry (SWAP), standard automated perimetry (SAP), and frequency doubling perimetry (FDP). ANOVA and Kruskal-Wallis were used to compare retinal function in subjects with and without DRIL. Tukey-Kramer test and Wilcoxon were used for post hoc analysis.
Results
DRIL was identified in 9 of 57 diabetic subjects. DRIL subjects had higher body mass index and longer diabetes duration compared to diabetic subjects without DRIL (P = 0.03 and P = 0.009, respectively). Subjects with DRIL had reduced ETDRS visual acuity (P = 0.003), contrast sensitivity function (P = 0.0003), and SAP performance (PSD, P < 0.0001) compared to controls and diabetic subjects without DRIL. Structural analysis revealed inner retinal thinning, and some outer retinal thinning, associated with DRIL.
Conclusions
Diabetic subjects with DRIL have reduced retinal function compared to those without DRIL, and defective retinal lamination may be an early cellular consequence of diabetes responsible for this in some patients. Following further longitudinal studies, DRIL may be a readily available and reliable structural biomarker for reduced retinal function in early diabetic neuroretinal disease.
Keywords: diabetic retinopathy, DRIL, OCT, contrast sensitivity, perimetry
Diabetic retinopathy (DR), a well-studied complication of diabetes mellitus (DM), affects approximately 35% of diabetic patients worldwide.1 DR affects the entire neurovascular unit of the retina,2,3 and recent studies have identified retinal dysfunction and structural changes resulting from neuroretinal disruption, sometimes occurring prior to the characteristic microvascular clinical findings of DR.4–7 With the prevalence of DM predicted to double by 2030,8 there is a crucial need to clarify the cellular mechanisms of neurovascular unit disruption, and to identify reliable endpoints based on these cellular defects. These steps may lead to improved means to develop treatments to prevent vision loss from DM.9
Until recently, the search for a structural marker of visual function in diabetic patients has been unsuccessful, because commonly measured parameters, such as optical coherence tomography (OCT) retinal thickness,10,11 are of limited predictive value for current and future visual acuity. However, investigators have used spectral-domain optical coherence tomography (SD-OCT) to explore disorganization of the retinal inner layers (DRILs) as a new potential marker.12–16 Retinal tissue has a very organized structure, and disturbance of its layers, especially in the macular region, can reduce visual function. Sun et al.12 showed that the presence of foveal DRIL was associated with worse baseline visual acuity in patients with diabetic macular edema (DME) and predicted visual acuity at 8 months. The presence of foveal DRIL was also associated with reduced visual acuity in patients with resolved DME.13,17 Nicholson et al.14 found that the presence of DRIL was correlated with macular capillary nonperfusion, a metric of maculopathy, in severe nonproliferative DR (NPDR) and proliferative DR. Also, Balaratnasingam et al.15 showed that the extent of DRIL correlated with the size of the foveal avascular zone, another metric of maculopathy, in patients with all grades of DR, without DME. Most recently, Das et al.16 found the presence of DRIL was associated with increasing severity of DR, specifically proliferative DR. They also found that DRIL was associated with structural disruption in the outer retina, specifically the ellipsoid zone (EZ) and the external limiting membrane (ELM).16 Taken together, DRIL appears to be a readily available and reliable predictive tool for visual acuity, capillary perfusion, and other morphological changes in severe DR, proliferative DR, and DME.
Nevertheless, DRIL has not been studied in diabetic patients without clinical evidence of DR and with early-stage DR, and its role in early diabetic neuroretinal impairment remains unclear. Furthermore, its ability to serve as a biomarker for other relevant aspects of visual function, such as visual fields and contrast sensitivity, has not been determined. Thus, the purpose of this research was to serve as a pilot study to elucidate the effects of DRIL in early neuroretinal disease. Specifically, we were interested in determining the effects of DRIL on several aspects of visual function in patients without clinical evidence of DR and with DR, without DME or proliferative changes.
Methods
This study was conducted at the University of Michigan W. K. Kellogg Eye Center. The protocol was approved by the Institutional Review Board of the University of Michigan Medical School (HUM 99155). Patients were recruited from the University of Michigan clinics and University of Michigan Health Research website between March 2016 and January 2017. Written informed consent was obtained from all patients before participation in the study.
Subject Enrollment and Evaluation
The study protocol and cohort were described previously.4 Subjects were enrolled into four groups: healthy subjects (control group), diabetics without retinopathy (no DR group), diabetics with mild NPDR (mild NPDR group), and diabetics with moderate to very severe NPDR (moderate NPDR group). Inclusion criteria for the control group were: (1) age ≥ 18 years, (2) no clinical diagnosis of diabetes, and (3) Early Treatment Diabetic Retinopathy Study (ETDRS) DR severity level of 10—no detectable retinopathy. Inclusion criteria for the diabetic group were: (1) age ≥ 18 years and (2) diabetes as defined by the American Diabetes Association criteria for diagnosis.18 The mild NPDR group included patients with ETDRS DR grade 20 to 35 and the moderate NPDR group included patients with ETDRS DR grade 43 to 53.
Exclusion criteria for all the groups were: any neurological or systemic disease (other than DM), any drug intake that could impair vision, Snellen or equivalent best corrected visual acuity (BCVA) worse than 20/40, spherical equivalent more than ± 6.0 diopters (D), proliferative DR, DME as judged by fundus photographs or OCT, pregnancy or nursing, and inability to give informed consent or to complete testing.
All subjects underwent a comprehensive ophthalmologic examination in the Kellogg Clinic Research Center, including slit lamp examination, applanation tonometry, measurement of BCVA with the electronic visual acuity (EVA) tester using the e-ETDRS protocol, color fundus photography, SD-OCT, contrast sensitivity using quick contrast sensitivity function (qCSF) method on the Sentio Platform (Adaptive Sensory Technology, San Diego, CA, USA),19 and three methods of perimetry testing: short-wavelength automated perimetry (SWAP), standard automated perimetry (SAP), and frequency doubling perimetry (FDP).
Fundus Photography
Color fundus photographs were taken using nonsimultaneous stereoscopic, on-axis, nonsteered, 200° ultrawide field (UWF) imaging (Optos 200TX, Optos plc, Dunfermile, UK), and the images were magnified to the equivalent field dimensions of 7-standard fields of the ETDRS scale. DR grade was determined based on the images and clinical evaluation by VMC and TWG.
Spectral-Domain Optical Coherence Tomography
SD-OCT (Spectralis HRA+OCT, Heidelberg Engineering, Inc., Heidelberg, Germany) was performed using the following scan acquisition parameters: macular scan volume, 37 B-scans, each spaced 120 μm, 15° × 15°, automatic real-time (ART) mean of 12 in high resolution (HR) mode. For quality control, all OCT scans were performed in a masked fashion by VMC and KAJ, with the same technique and parameters used for cases and controls.
Three SD-OCT scans above and below the foveal scan line were evaluated for the presence of DRIL in each subject by two reviewers (KAJ and CS) under masked conditions. DRIL was identified when the boundaries of the ganglion cell, inner plexiform layer, inner nuclear layer, and outer plexiform layer could not be identified and demarcated.12 The horizontal extent of DRIL was measured on each scan within the 6 mm ring of the ETDRS grid centered at the fovea. Pearson correlation coefficients were used to determine agreement of DRIL measurements among the two reviewers. On scans with r < 0.8, the DRIL horizontal length was re-measured until the calculations were agreeable. The total extent of DRIL for each subject was then calculated by taking the average measurements from both reviewers across all seven scans (foveal scan with three scans above and below). The retinal layers on each SD-OCT scan were also segmented semiautomatically using the built-in software of the Heidelberg Spectralis. The boundaries of all segmented layers were carefully reviewed by two reviewers (VMC and KAJ) and adjusted when necessary. Retinal thickness was analyzed using the ETDRS grid, which included the 1 mm central fovea, 3 mm inner ring, and 6 mm outer ring. The inner and outer rings were sectioned into superior, inferior, temporal, and nasal quadrants. The retinal thickness was recorded for the total retina, total inner retina, and total outer retina (between the ELM and RPE). The total inner retina thickness was used because we could not accurately segment the individual retinal layers in eyes with DRIL.
ETDRS Visual Acuity
Visual acuity was evaluated using the EVA Tester (Jaeb Center for Health Research, Tampa, FL, USA) with E-ETDRS protocol. One eye of each subject was selected for the study; if both eyes met eligibility criteria, the eye with more severe retinopathy was chosen. If both eyes were eligible for the same retinopathy group, then the eye with the better visual acuity was selected.
Contrast Sensitivity
Contrast sensitivity was evaluated using the Quick Contrast Sensitivity (qCSF)19 method on the AST Sentio Platform (Adaptive Sensory Technology, San Diego, CA, USA),20 a computerized method for evaluating the contrast thresholds over a wider range of contrast (0.002% to 100%) and spatial frequency (approximately 1 to 27 cycles per degree).21 All participants were tested monocularly following measurement of BCVA, while the untested eye was covered with a patch. The area under the logCSF (AULCSF), integrated from 1.5 to 18 cpd, served as a metric of contrast sensitivity function and was used for statistical analysis.
Visual Fields
SWAP and SAP
SWAP was performed using the 24-2 SITA-SWAP strategy (version 4.1) on the Humphrey Field Analyzer II. Each narrow-band blue (440 nm wavelength) Goldmann size V target was presented for 200 ms on a 100 cd/m2 yellow background. SAP was performed using 24-2 SITA-standard strategy (version 4.1) on the Humphrey Field Analyzer II. Each white light stimulus was a Goldmann size III target, which was presented for 200 ms on a white background illuminated to 10 cd/m2. Fifty-two test locations (54 minus the two locations at the blind spot) were evaluated for both SWAP and SAP. Lens correction was automatically calculated by built-in technology of the Humphrey Field Analyzer II. Foveal threshold (FT), mean deviation (MD), and pattern standard deviation (PSD) were recorded and used for statistical analysis. FT is the absolute threshold sensitivity at the fovea, MD is a global index of the age adjusted average deviation from the mean across all test locations, and PSD is a global index of the uniformity of the deviation compared to age-matched controls. Subjects with reduced retinal function have more depressed MD values (negative) and higher PSD values (positive).
Frequency Doubling Perimetry
The FDP 24-2 strategy was performed on the Humphrey Matrix 715 Visual Field Analyzer. The stimulus was a 0.25 cpd monochrome sinusoidal grating of vertical gray stripes that was phase reversed at 18 Hz. The minimum contrast threshold of the 5° diameter stimulus was measured at each of 55 test locations. The subjects wore their own prescription glasses. FT, MD, and PSD were recorded.
Results were considered reliable when fixation losses, false-positive errors, and false-negative errors were less than 33%. Only subjects with two reliable tests for each strategy were included. The second of these two reliable tests was used for statistical analysis to minimize practice and learning effects.
Statistical Analysis
Demographics data were summarized as means ± SDs for continuous variables, and frequencies for categorical variables. The data distribution was assessed for normality graphically and with the Shapiro-Wilk test. ANOVA was used for parametric variables and the Kruskal-Wallis test was used to compare continuous nonparametric variables. The Tukey-Kramer HSD (parametric) and Wilcoxon (nonparametric) were used for post hoc analysis. The Bonferroni correction for multiple comparisons was applied to functional analyses such that the statistical significance would occur at P ≤ 0.005. For other tests, P ≤ 0.05 was considered statistically significant. Statistical analysis was performed with JMP, Version Pro 13 (SAS Institute, Inc., Cary, NC, 1989–2007).
Results
Demographics
Table 1 describes the demographics of the 75 participants included in the study. ANOVA and Kruskal-Wallis tests were used to determine if there were differences among the groups. The cohort consisted of 68.4% males; the control group only had 27.8% males while the moderate NPDR group had 80% males (P = 0.0007 among all groups). Among the diabetic subjects, 78.9% had type 2 diabetes. Subjects with mild and moderate NPDR had a longer duration of diabetes than subjects with no DR (P = 0.002 and P = 0.016, respectively). Diabetic subjects had higher body mass index (BMI) and hemoglobin A1C (HbA1C) than control subjects (P = 0.002 for both). There was no difference in age or type of diabetes among the groups.
Table 1.
Subject Demographics
|
Control (n
= 18) |
No DR (n
= 23) |
Mild NPDR (n
= 19) |
Moderate NPDR (n
= 15) |
P
Value |
|
| Age, y (SD) | 51.7 (14.3) | 53.7 (12.2) | 57.4 (12.7) | 59.9 (11.6) | 0.25 |
| Sex, male (%) | 27.8 | 56.5 | 73.7 | 80 | 0.007* |
| Diabetes type, type 2 (%) | NA | 78.4 | 68.4 | 93.3 | 0.21 |
| Diabetes duration, y (SD) | NA | 9.35 (8.67) | 20.8 (12.5) | 17.5 (11.4) | 0.003* |
| BMI (SD) | 26.2 (4.4) | 32.9 (7.9) | 30.4 (5.1) | 35.7 (6.29) | 0.001* |
| HbA1c, % (SD) | 5.45 (0.26) | 7.75 (1.99) | 8.05 (1.0) | 7.87 (1.54) | 0.0005* |
Statistically significant result.
Presence of DRIL
The presence of DRIL was identified in SD-OCT scans from 9 of 57 (16%) of diabetic subjects and in none of the control subjects. Table 2 shows the demographic characteristics among all subjects based on the presence of DRIL. DRIL was identified in one diabetic subject without clinical evidence of DR, in four subjects with mild NPDR, and in four subjects with moderate-severe NPDR. The Figure shows an example of DRIL in a subject with moderate NPDR. T-tests and Fisher's exact tests were used to determine if there were differences among the two groups in each retinopathy group. We found no significant differences in age, sex, diabetes type, diabetes duration, BMI, and HbA1C among subjects with and without DRIL within each retinopathy group. When we did the same analysis, comparing subjects with and without DRIL, among all diabetic subjects, we found that subjects with DRIL had higher BMI and longer diabetes duration than subjects without DRIL (P = 0.03 and P = 0.009, respectively).
Table 2.
Subject Demographics Based on DRIL Status
|
Control (n
= 18) |
No DR (n
= 23) |
Mild NPDR (n
= 19) |
Moderate NPDR (n
= 15) |
P
Value, No DRIL/DRIL |
|||||
| DRIL status no DRIL/DRIL | 18 | 0 | 22 | 1 | 15 | 4 | 11 | 4 | 0.13 |
| Age, y (SD) | 54.3 (12.2) | 41.0 | 55.2 (13.3) | 65.5 (5.2) | 62.1 (9.8) | 54.0 (15.6) | 0.79 | ||
| Sex, male (%) | 54.6 | 100 | 66.7 | 100 | 81.8 | 88.9 | 0.15 | ||
| Diabetes type, type 2 (%) | 77.3 | 100 | 60.0 | 100 | 100 | 75.0 | 0.42 | ||
| Diabetes duration, y (SD) | 8.3 (7.3) | 23.0 | 19.6 (13.2) | 25.3 (9.2) | 15.4 (10.8) | 23.0 (12.8) | 0.009* | ||
| BMI (SD) | 32.9 (7.9) | 31.8 | 29.8 (5.1) | 35.1 (3.7) | 35.3 (7.3) | 36.6 (2.9) | 0.03* | ||
| HbA1c, % (SD) | 7.4 (2.0) | 7.4 | 8.8 (0.9) | 8.8 (1.4) | 7.9 (1.6) | 7.9 (1.7) | 0.52 | ||
Statistically significant result.
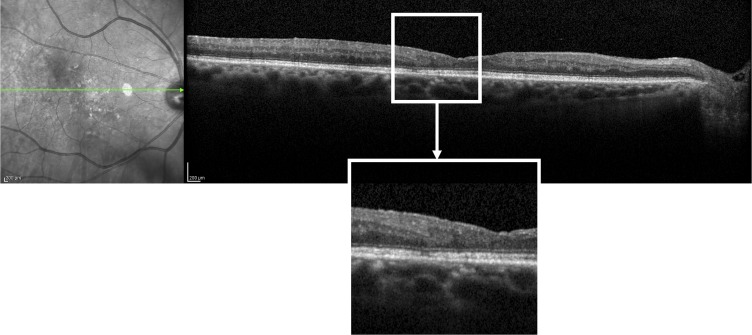
Figure.
SD-OCT image of DRIL found in 73-year-old male with type 2 DM for 18 years and moderate NPDR. DRIL extent was measured to be 731 μm. Functionally, visual acuity was 20/32 and AULCSF was 0.60.
Horizontal Extent of DRIL
The overall Pearson correlation coefficient for agreeable DRIL measurements was 0.98 between the two reviewers. The DRIL extent among the nine subjects ranged from 123.5 μm to 731.0 μm in the macular region, within the 6 mm ring of the ETDRS grid centered at the fovea. The least DRIL was identified in a 38-year-old female with 18 years of type 2 diabetes, and moderate NPDR. The greatest extent of DRIL was identified in a 73-year-old male, also with 18 years of type 2 diabetes and moderate NPDR (Fig.). The subject with DRIL but no clinical evidence of DR was a 41-year-old male with 12 years of type 2 diabetes. We compared the four subjects with < 275 μm of DRIL (median extent of DRIL) to the five subjects with > 300 μm of DRIL using Student's t-tests and found no differences in age, diabetes duration, HbA1C, visual acuity, contrast sensitivity function, and perimetry performance. DRIL was identified in only one female subject (<275 μm DRIL) and only one subject with type 1 diabetes (>275 μm DRIL); the rest were type 2 diabetic males (n = 7).
Retinal Function and DRIL
Retinal function was evaluated using ETDRS visual acuity, the Quick Contrast Sensitivity method, SWAP, SAP, and FDP. Results are depicted in Table 3. ANOVA and Kruskal-Wallis tests were used to determine if there were differences among the groups and Bonferroni correction for multiple comparisons was applied such that statistical significance would occur at P = 0.005. Post hoc analysis was used to determine if there were differences among diabetic subjects with DRIL and without.
Table 3.
Comparison of Retinal Function With DRIL
|
Control (n
= 18) |
No DRIL (n
= 48) |
DRIL (n
= 9) |
P
Value All Groups Corrected |
P
Value No DRIL, DRIL |
|
| ETDRS logMAR, (Snellen equivalent) | −0.11 (20/16) | 0.00 (20/20) | 0.03 (20/20) | 0.003* | 0.27 |
| AULCSF (SD) | 1.60 (0.13) | 1.33 (0.27) | 1.20 (0.33) | 0.0003* | 0.34 |
| SWAP 24-2 | |||||
| MD (SD) | −2.14 (1.91) | −4.81 (4.59) | −7.75 (5.83) | 0.03 | 0.15 |
| PSD (SD) | 2.40 (0.47) | 2.92 (1.23) | 4.00 (2.29) | 0.008 | 0.03 |
| FT (SD) | 24.16 (3.65) | 21.75 (5.18) | 17.78 (6.50) | 0.04 | 0.11 |
| SAP 24-2 | |||||
| MD (SD) | −0.56 (0.72) | −2.37 (3.26) | 2.67 (3.02) | 0.03 | 0.71 |
| PSD (SD) | 1.38 (0.24) | 2.47 (2.28) | 3.24 (1.65) | <0.0001* | 0.009* |
| FT (SD) | 34.6 (1.46) | 34.0 (2.06) | 32.8 (2.68) | 0.09 | 0.19 |
| FDP 24-2 | |||||
| MD (SD) | 0.61 (1.95) | −1.19 (3.92) | −1.22 (3.09) | 0.11 | 0.61 |
| PSD (SD) | 2.53 (0.36) | 3.17 (1.07) | 3.65 (0.96) | 0.02 | 0.23 |
| FT (SD) | 31.7 (2.34) | 28.6 (4.99) | 27.4 (4.16) | 0.008 | 0.36 |
Statistically significant result.
All retinal function tests used in this study previously showed reduced performance in diabetic subjects compared to healthy controls.4 Among controls and diabetic subjects with and without DRIL, we found statistically significant differences using ETDRS visual acuity, AULCSF (a metric of the Quick Contrast Sensitivity method), and SAP. The results of SWAP and FDP, relatively selective tests of inner retinal function,22,23 were not significantly different among groups despite a tendency of reduced performance in DRIL subjects. SAP, a nonselective test of visual pathways, was the only test that detected statistically significant dysfunction in diabetic subjects with DRIL compared to those without DRIL in PSD but not in MD or FT (P = 0.009). Thus, it appears that DRIL is associated with a quantifiable degree of retinal dysfunction even in early stages of diabetic neuroretinal impairment.
OCT Thickness and DRIL
Macular SD-OCT scans were obtained and segmented semiautomatically using the Heidelberg Spectralis software and two masked reviewers. The total retinal thickness, inner retinal thickness, and outer retinal thickness were compared among controls, diabetic subjects without DRIL, and diabetic subjects with DRIL. The Figure shows the result of the OCT analysis in the center, inner ring, and outer ring of the ETDRS grid. Diabetic subjects with DRIL had a 6% reduction in total retinal thickness compared to diabetic subjects without DRIL and control subjects both in the inferior and nasal quadrants of the inner ETDRS ring (P = 0.03 for both). In the same areas, diabetic subjects with DRIL had a 7% reduction in inner retinal thickness compared to diabetic subjects without DRIL and control subjects (P = 0.05 for both). This suggests that the thinning of the inner retina may be resulting in a significant thinning of the total retina. In the outer retina, diabetic subjects with DRIL had a 2.5% reduction in retinal thickness compared to diabetic subjects without DRIL and a 2% reduction compared to control subjects (P = 0.05) in the superior segment of the outer ETDRS grid. Taken together, DRIL appears to be associated to retinal thinning mostly in the inner retina, but also in the outer retina.
Discussion
The identification of secondary tests and endpoints for screening and disease management is imperative for the development of new treatments to prevent the progressive vision loss associated with diabetic neuroretinal disease. Previous studies have shown that disruption of the inner retina, identified on SD-OCT as DRIL, can predict visual acuity in patients with vision threatening DR. Therefore, this study evaluated the presence of this structural disruption of the inner retina in early stages of diabetic neuroretinal disease and its effect on multiple aspects of visual function. Previous studies have described the presence of DRIL in cases of severe NPDR, PDR, current and resolved DME,12,15–17 retinal vein occlusion,15 uveitic cystoid macular edema,24 closed globe trauma,25 retinitis pigmentosa,26 and acute retinal necrosis.27 This study evaluated DRIL in diabetes before the appearance of vascular changes and with DR, before DME and PDR. This study is the first to compare functional performance in subjects with and without DRIL using an automated contrast sensitivity method and three visual field testing strategies, in addition to ETDRS visual acuity testing. Additionally, this study evaluated retinal thickness in subjects with DRIL compared to subjects without DRIL.
Visual function depends on intact organization of the cellular pathways in the retina. All tests of retinal function used in this study have previously revealed neuroretinal impairment in diabetic patients with early DR.4 In this study, ETDRS visual acuity, contrast sensitivity tested using the Quick Contrast Sensitivity method, and SAP detected significant functional differences among controls, diabetic subjects without DRIL, and diabetic subjects with DRIL. SAP was particularly useful in detecting subtle retinal dysfunction in diabetic subjects with DRIL compared to diabetic subjects without DRIL. The significant difference was detected in PSD, but not in MD or FT, suggesting that DRIL may be associated with increased variability across the visual field. Since ETDRS visual acuity, contrast sensitivity, and SAP are nonselective tests of retinal function, it appears that the dysfunction associated with DRIL is also nonspecific. Testing with FDP and SWAP, selective tests of inner retinal function,22,23 did not detect significant differences among groups; however, future studies with a larger sample size may be able to detect differences.
The development of DRIL in multiple retinal diseases suggests it is not specific to DR but a common response to retinal stress. Recent studies have revealed the cellular basis of retinal lamination during normal fetal development, resulting in distinct layers of cell bodies and synapses.28 The OCT findings of DRIL include loss of definition of the boundaries of the layers,12 consistent with prior reports of loss of inner retinal neurons and thinning of the plexiform layers, particularly of the inner retina.29,30 In this study, we found inner retinal thinning in the inferior and nasal quadrants of the of the ETDRS grid, suggestive of neuronal atrophy associated with DRIL. We also detected outer retinal thinning, to a lesser degree, in the outer superior quadrant of the ETDRS grid. These findings provide insight into the loss of structural integrity associated with diabetes, and future studies can focus on how to best objectively evaluate structural disruption on a cellular level. Also, the relationship of structural changes to metabolic defects, such as loss of anabolic signaling31 and/or excessive inflammatory mediators, will be important to define the basis of DRIL and to prevent or reverse its course.
The current study found an association between higher BMI and longer diabetes duration in persons with DRIL, and a greater likelihood of DRIL in subjects with mild to moderate NPDR than in persons without visible DR. However, additional work is needed to better understand the factors that lead to or prevent the presence of DRIL.
The limitations of this study include its cross-sectional nature and modest sample size. Analysis of a larger sample size with similar distribution of males and females in the control group and retinopathy groups will be important to rule out any influence that sex has on retinal structure and function. Furthermore, higher density SD-OCT volume scans may provide better sensitivity for detection of DRIL. Thus, DRIL may be detected in more patients at risk of neuroretinal impairment. The extent of DRIL identified in our patients is difficult to compare to the extent of DRIL described in previous studies because we examined a larger area (6 mm ring of the ETDRS grid centered at the fovea) versus other studies that used the 1 mm ring12,13 and the 3 mm ring.15 This difference needs to be considered when designing future protocols for DRIL in early DR, where it may not be as prevalent as in DME and PDR. The use of 6 mm SD-OCT scans likely contributed to the detection of DRIL in eyes with no or minimal vascular retinopathy. With more advanced technology, it may also be possible to evaluate for DRIL outside the macular area, which may provide further insight into the pathogenesis of diabetic neurodegeneration. Finally, additional longitudinal analysis is necessary to determine whether changes in DRIL predict changes in multiple aspects of visual function long-term.
The findings of this study further emphasize a correlation between neuroretinal structure and function. DRIL could potentially serve as reliable and readily available tool for screening and monitoring neuroretinal impairment in diabetes.
Conclusions
This pilot analysis strengthens the potential utility of DRIL as a clinical marker of visual function to test potential novel therapies for diabetic patients with early stages of neuroretinal impairment. Future studies should include a larger cohort and a longitudinal analysis to determine whether DRIL will be useful tool in the detection, monitoring, and management of neuroretinal impairment in DR.
Acknowledgments
Supported by Research to Prevent Blindness, the Taubman Medical Research Institute, R01 EY20582 and R24 DK082841 (TWG).
Disclosure: K.A. Joltikov, None; C.A. Sesi, None; V.M. de Castro, None; J.R. Davila, None; R. Anand, None; S.M. Khan, None; N. Farbman, None; G.R. Jackson, None; C.A. Johnson, None; T.W. Gardner, NovoNordisk (C), Zebra Biologics (C)
References
- 1.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Experiment Ophthalmol. 2016;44:260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Gardner TW, Davila JR. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:1–6. doi: 10.1007/s00417-016-3548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joltikov KA, de Castro VM, Davila JR, et al. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:BIO277–BIO290. doi: 10.1167/iovs.17-21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113:E2655–E2664. doi: 10.1073/pnas.1522014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson GR, Scott IU, Quillen DA, Walter LE, Gardner TW. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:699–703. doi: 10.1136/bjophthalmol-2011-300467. [DOI] [PubMed] [Google Scholar]
- 7.Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cell alterations. J Diabetes Res. 2013;2013:905058. doi: 10.1155/2013/905058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20:6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair P, Aiello LP, Gardner TW, Jampol LM, Ferris FLI. Report From the NEI/FDA Diabetic Retinopathy Clinical Trial Design and Endpoints Workshop. Invest Opthalmology Vis Sci. 2016;57:5127–5142. doi: 10.1167/iovs.16-20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browning DJ, Glassman AR, Aiello LP, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou WC, Brown DM, Payne JF, Wykoff CC. Relationship between visual acuity and retinal thickness during anti-vascular endothelial growth factor therapy for retinal diseases. Am J Ophthalmol. 2017;180:8–17. doi: 10.1016/j.ajo.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. 2014;132:1309–1316. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 13.Sun JK, Radwan SH, Soliman AZ, et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. 2015;64:2560–2570. doi: 10.2337/db14-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson L, Ramu J, Triantafyllopoulou I, et al. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin Experiment Ophthalmol. 2015;43:735–741. doi: 10.1111/ceo.12557. [DOI] [PubMed] [Google Scholar]
- 15.Balaratnasingam C, Inoue M, Ahn S, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123:2352–2367. doi: 10.1016/j.ophtha.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136:202–208. doi: 10.1001/jamaophthalmol.2017.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radwan SH, Soliman AZ, Tokarev J, Zhang L, van Kuijk FJ, Koozekanani DD. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol. 2015;133:820–825. doi: 10.1001/jamaophthalmol.2015.0972. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Executive Summary: Standards of Medical Care in Diabetes—2014. Diabetes Care. 2013;37(suppl 1):S5–S13. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 19.Lesmes L, Lu Z, Baek J, Albright T. Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method. J Vis. 2010;10(3):17. doi: 10.1167/10.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorr M, Wille M, Viulet T, Sanchez E, Bex PJ, Lu Z. Next-generation vision testing: the quick CSF. Curr Dir Biomed Eng. 2015;1:1–4. [Google Scholar]
- 21.Hou F, Lesmes LA, Kim W, et al. Evaluating the performance of the quick CSF method in detecting contrast sensitivity function changes. J Vis. 2016;16(6):18. doi: 10.1167/16.6.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racette L, Sample PA. Short-wavelength automated perimetry. Ophthalmol Clin North Am. 2003;16:227–236. doi: 10.1016/s0896-1549(03)00010-5. vi–vii. [DOI] [PubMed] [Google Scholar]
- 23.Anderson AJ, Johnson CA. Mechanisms isolated by frequency-doubling technology perimetry. Invest Ophthalmol Vis Sci. 2002;43:398–401. [PubMed] [Google Scholar]
- 24.Grewal DS, O'Sullivan ML, Kron M, et al. Association of disorganization of retinal inner layers with visual acuity in eyes with uveitic cystoid macular edema. Am J Ophthalmol. 2017;177:116–125. doi: 10.1016/j.ajo.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Lu Y, Huang H, Zheng J, Hou P, Chen W. Prediction of visual prognosis with spectral-domain optical coherence tomography in outer retinal atrophy secondary to closed globe trauma. Retina. 2013;33:1258–1262. doi: 10.1097/IAE.0b013e31827b63ba. [DOI] [PubMed] [Google Scholar]
- 26.Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150:149–165. doi: 10.1016/j.exer.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki J, Goto H, Minoda H, Iwasaki T, Sakai J, Usui M. Analysis of retinal findings of acute retinal necrosis using optical coherence tomography. Ocul Immunol Inflamm. 2006;14:165–170. doi: 10.1080/09273940600672198. [DOI] [PubMed] [Google Scholar]
- 28.Amini R, Rocha-martins M, Norden C. Neuronal migration and lamination in the vertebrate retina. Front Neurosci. 2017;11:742. doi: 10.3389/fnins.2017.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dijk HW, Kok PHB, Garvin M, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stem MS, Dunbar GE, Jackson GR, Farsiu S, Pop-Busui R, Gardner TW. Glucose variability and inner retinal sensory neuropathy in persons with type 1 diabetes mellitus. Eye (Lond) 2016;30:825–832. doi: 10.1038/eye.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fort PE, Losiewicz MK, Pennathur S, et al. mTORC1-independent reduction of retinal protein synthesis in type 1 diabetes. Diabetes. 2014;63:3077–3090. doi: 10.2337/db14-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]