Abstract
Background
Corresponding with improved survival among patients with breast cancer, the awareness of the long-term effects of cancer treatments has increased. CANcer TOxicities (CANTO) aims to identify predictors of development and persistence of long-term toxicities in patients treated for stages I–III breast cancer and to characterise their incidence, as well their impact. In this paper, we describe the methodology used in this study and provide a first characterisation of the study population.
Methods
CANTO (NCT01993498) is a French prospective, longitudinal cohort study enrolling patients with invasive cT0-cT3cN0-3M0 breast cancer of 26 French cancer centres. Patients are assessed at diagnosis, 3–6 (M0), 12 (M12), 36 (M36) and 60 (M60) months after completion of primary surgery, chemotherapy or radiotherapy whichever comes last. CANTO collects clinical, treatment, toxicity data, an extensive list of validated patient-reported outcomes (focusing on quality of life, psychological and behavioural questionnaires) and ad hoc socioeconomic questionnaires. Blood collection is performed at diagnosis, M0, M12, M36 and M60. Biologic sub-studies are ongoing (eg, microbiotic and cognitive sub-study).
Results
Enrolment started in 2012; by October 2018, 12 012 patients had been enrolled. Data collected have a low missing completion rate (<5% for key clinical variables, <20% for patient-reported outcomes). Blood, serum and plasma samples are stored in over 96% of patients. Among the first 5801 patients enrolled in CANTO, 76.7% of patients had hormone receptor positive and human epidermal growth factor 2 negative tumours; 73.1% of patients had breast conserving surgery; 90.4% received adjuvant radiotherapy, 53.4% (neo) adjuvant chemotherapy, 11.3% adjuvant trastuzumab and 80.3% adjuvant hormonotherapy.
Conclusions
CANTO represents a unique opportunity to explore important medical, biological and psychosocial outcomes on breast cancer survivor population.
Keywords: cohort study, breast cancer, survivorship, toxicity, quality of life
Background
Due to improvements in early detection and treatment, nearly 80% of women diagnosed with breast cancer in developed countries can expect long-term disease-free survival. Currently, almost 2 million women in Europe have a history of breast cancer and it is projected that this number will continue to increase.1 2 Corresponding with improved survival, an awareness of survivorship care challenges has increased. Central to survivorship is the identification and management of the long-term effects of the cancer and its treatment.1–3
Although prior studies showed that most of the patients with breast cancer can return to a comparable health status to that of patients without breast cancer, a substantial portion of breast cancer survivors face long-term treatment-related toxicities (including, but not limited to fatigue, pain, emotional disorders, cognitive impairment, sexual dysfunction, lymphoedema, cardiotoxicity, infertility) with important psychological, functional and social impact. In addition, there is some evidence that calls into question substantial underdiagnoses and undermanagement of treatment-related toxicities.4
A major barrier to identifying factors associated with the development and persistence of long-term toxicities and potential targets for impactful intervention is the absence of longitudinal and well-powered data sets assessing treatment-related toxicities with detailed information on demographic, lifestyle, social, medical, psychological, molecular and biological factors that can allow us to understand how these factors interact and impact treatment-related toxicities and quality of life.5
Most of the available data are focused on the most acute effects of cancer treatments and are limited by small study size and absence of national efforts. In addition, most of the studies are cross-sectional and do not capture the long-term complexity and variability of symptoms over time, which can be intermittent and fluctuate from years to decades, while having a major impact on patients’ day-to-day function.6 Finally, most of the data resources do not have biospecimens for biomarker and molecular measures.
Therefore, associated with a call for a focus on survivorship is intrinsically associated with a need of research studies that overcome the limitations of prior studies and advance the understanding of long-term toxicities among breast cancer survivors. In the USA, after the ‘Institute of Medicine Report, From Cancer patient to Cancer Survivor: Lost in translation’, several initiatives including the American Cancer Society’s Studies of Cancer Survivors have started.7 8 In Europe, similar initiatives started to take place.9 10 In the same direction, from 2009 to 2013, one of the axis of the French national plan against cancer was to reduce treatment-related toxicity in breast cancer management, and one of the biggest research initiatives was to support data collection on those living after cancer.11
CANcer TOxicities (CANTO) (NCT01993498, UNICANCER 0140/1103, 2011-A01095-36 (‘study of chronic toxicity of treatment of patients with localised breast cancer’) is a French prospective cohort study with the primary objective of identifying factors predictive of chronic toxicity in patients treated for a stage I–III breast cancer. The secondary objectives include (1) to describe long-term chronic toxicities, their incidence and psychological, social and economic impact of chronic toxicities at the societal level and (2) build a multidisciplinary network that will be leveraged on the data set findings.
In this article, we will summarise CANTO protocol and describe the rational, methodology and implementation of this prospective cohort study.
Methods
Study design and organisational structure
Study design
CANTO is a French longitudinal prospective multicentre cohort study of women with localised, stage I–IIIA breast cancer receiving their primary breast cancer care at one of the 26 participating French cancer centres. CANTO recruiting centres include 20 comprehensive cancer centres, two university hospitals in the greater Paris area, two public non-teaching hospitals and two private hospitals. Enrolment started in 20 March 2012; by October 2018, 12 012 patients were enrolled.
Organisational structure
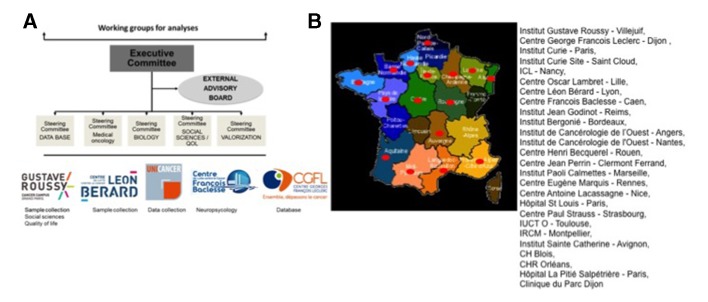
CANTO is run and coordinated by UNICANCER (National French Cooperative Breast Cancer Inter Group) uniting a group of oncologists, sociologists, physiologists and psychiatrists, biologists, epidemiologists and statisticians from different French institutions. In addition an external multidisciplinary, international advisor board is also assembled (figure 1).
Figure 1.
(A) CANTO organisational structure. (B) CANTO participating centres. CH, Centre Hospitalier; CHR, Centre Hospitalier Régional; ICL, Institut de Cancerologie de Lorraine; IUCT O, Institut Universitaire du Cancer de Toulouse Oncopole; IRCM, Institut de Recherche en Cancérologie; QoL, quality of life.
All patients enrolled in the study provide written informed consent. Patients who are enrolled in ancillary studies signed a separate consent form.
CANTO is registered in public repositories, such as clinical trials.org as NCT0199349812 and the French epidemiology portal.13
Study population
Patients with the following characteristics are eligible for inclusion: (1) have a proven invasive breast cancer, (2) cT0-cT3, cN0-3 tumour by American Joint Committee on Cancer (AJCC) seventh edition,14 (3) 18 years old or older at diagnosis, (4) untreated at the time of inclusion (including surgery), (5) fluent in French and (6) able to provide written, informed consent. Patients with the following characteristics are excluded: (1) T4 and stage IV breast cancer by AJCC seventh edition,14 (2) evidence of local or distant recurrence, (3) history of other cancer within 5 years prior to entry into the study, with the exception of basal cell carcinoma of the skin or carcinoma in situ of the cervix and (4) receipt of any breast cancer treatment prior to study entry.
Study measures and collection procedures
Main procedures
Table 1 summarises data measures and schedule of data collection.
Table 1.
Data measures and schedule of data collection
| Visits | Inclusion | Follow-up after M0 | ||||
| Visit at M0 3–6 months after treatment |
Visit at M12 | Via telephone at M24 | Visit at M36 | Visit at M60 | ||
| Inclusion/non-inclusion criteria | x | |||||
| Signed informed consent | x | |||||
| Inclusion | x | |||||
| Clinical examination | ||||||
| Patient medical history | x | |||||
| Clinical examination | x | x | x | x | X | |
| Size, weight, performance status | x | x | x | x | x | |
| Vital signs | x | x | x | x | x | |
| Toxicity evaluation | x | x | x | x | ||
| Concomitant treatments | x | x | x | x | x | |
| Blood tests | ||||||
| Complete blood count | x | x | x | x | ||
| Hepatic function/ionogram | x | x | x | x | ||
| Glycaemia/creatinaemia/lipid panel | x | x | x | x | ||
| 25-hydroxycholecalciferol D3/troponine/brain natriuretic peptide | x | x | x | x | ||
| FSH, LH, oestradiolaemia* | x | x | x | |||
| Paraclinical examination† | ||||||
| Osteodensitometry‡ | x‡ | x‡ | x‡ | |||
| Left ventricular ejection fraction§ | x | x | x | |||
| Questionnaires | ||||||
| Quality of life | HADS, LOT, BDI-SF, QLQC30-BR23 FA13/12, SF12, GPAQ16 |
HADS, IOCv2 QLQC30-BR23, FA13/12, GPAQ16 |
HADS, IOCv2 QLQC30-BR23, FA13/12, GPAQ16, SF12 |
HADS, QLQC30-BR23, FA13/12, GPAQ16 IOCv2, SF12 |
HADS, IOCv2 QLQC30-BR23, FA13/12, GPAQ16, SF12 |
|
| Questionnaires social impact/economy | Social situation | Social impact | Professional impact¶ | Social impact | Social impact | |
| Patient follow-up booklet | x | x | x | x | x | |
| Biological sample collection | ||||||
| Mandatory blood sample | x | x | x | |||
*To be performed in non-menopausal patients.
†Mandatory examinations to be performed in case of clinical signs of chronic toxicity: echography or myocardial scintigraphy in case of dyspnoea or other signs indicating the possibility of cardiac impairment, ECG in case of palpitations, pelvic echography and if needed hysteroscopy in case of metrorrhagia.
‡For postmenopausal patients at diagnosis and regardless of the hormone receptor positivity status at M0.
§In case of treatment with antiaromatase agents, for the visits at 3 years and 5 years. Mandatory examination: at inclusion and at M0, in case of treatment with antracyclines/trastuzumab/radiotherapy to the left breast and/or IMC. During follow-up: examination to be prescribed according to the good clinical practices. In case of treatment with trastuzumab, echography or myocardial scintigraphy every 3 months for 1 year, and thereafter at 5 years if no anomaly. In case of treatment with anthracyclines, radiotherapy to the left breast and/or IMC: echography or myocardial scintigraphy at 5 years.
¶ Questionnaires on professional impact, only for patients with professional activity at M12.
BDI, Beck Depression Inventory; EORTC-QLQ, European Organization for Research and Treatment-Quality of Life Questionnaire; FSH, follicle stimulating hormone; GPAQ, Global Physical Activity Questionnaire; HADS, Hospital Anxiety and Depression Scale; IMC, intramammary chain; IOCv2, Impact of Cancer Questionnaire; LH, luteinizing hormone; LOT-R, Life Orientation Questionnaire de Scheier et Carver Revised; M0, month 0 of surveillance, 3–6 after treatment completion; M12, month 12 of surveillance, 12 months after M0; M36, month 36 of surveillance, 36 months after M0; M60, month 60 of surveillance, 60 months after M0; SF-12, 12-Item Short Form Survey.
All patients are followed for a minimum of 5 years. Patients are assessed at diagnosis (baseline), 3–6 after treatment completion (month 0 of surveillance or M0), 12 (M12), 36 (M36) and 60 (M60) months after M0. Treatment completion is defined as completion of primary treatment, defined as surgery, chemotherapy or radiotherapy, whichever comes last. Adjuvant trastuzumab, adjuvant endocrine therapy or participation in clinical trials can be ongoing after this time point, as well as any reconstructive or prophylactic surgery. Up to M60, follow-up visits take place at the participating investigation centre and conducted by a clinical research nurse (CRN). Patients have a patient booklet focused on toxicity experienced that mimics the electronic case report forms (eCRFs). This booklet is handled to the CRN in each visit, who reviews the information and grades toxicity described using the common toxicity criteria (CTC) adverse events scale—European Organisation for Research and Treatment of Cancer (EORTC), V.4.15 Data collection is interrupted in case of metastatic recurrence or second cancer.
Structured and secured eCRFs were developed for the study. All patients are assigned to a study identification number. Identifiers are removed from the data. eCRFs are filled online in every centre and stored at the Centre George Francois Leclerc (Dijon, France). Complete blood sample is stored at Centre Léon Bérard (Lyon, France) for genomic studies. Serum and plasma samples are stored at Institut Gustave Roussy (Villejuif, France).
Quality of procedures
Rigorous data quality assurance processes includes initial and follow-up data management training; online edit checking during web-based data entry; programmed logic checks against the pooled data repository and remote and on-site monitorings of a random sample of source documents against the submitted data throughout the data collection process to ensure the accuracy of the data used.
Measures
Clinical and toxicity measures
Clinical data collection, including detailed patients’ demographics, medical history and tumour classification, physical examination, weight, height, performance status and toxicity data collection using cCTC adverse events—EORTC, V.4,15is performed by a CRN. At every visit, the CRN reviews the patient booklet and after a patient interview annotates the clinical data accordingly. Data collected not only include concomitant medications, general information such as smoke, alcohol, hospitalisation, medical or paramedical consultations, paraclinical data (such as left ventricular ejection fraction (LVEF), bone densitometry information, haematological and biochemical standard evaluation) but also detailed toxicity information on arm and breast morbidity and focused on specific areas of interest such as gynaecology, rheumatology, haematology, cardiology, pneumology, gastroenterology and dermatology. In case of an abnormality detected during one of these visits, the CRN is responsible for alerting the patient’s referent physician.
Paraclinical measures
Blood test and physiological evaluation including complete blood count, platelets, liver function assessment, ionogram, creatinaemia, glycaemia, cholesterolaemia, triglyceride level determination are performed at inclusion, M0, M36 and M60.
In premenopausal women, evaluation of the ovary function and follicle reserve: follicle-stimulating hormone, luteinizing hormone, oestradiolaemia are performed at M0 and M36.
Bone densitometry is performed in postmenopausal patients at diagnosis regardless of the hormone receptor status, as well as in all patients treated by aromatase inhibitors at inclusion, M36 and M60.
Myocardial echography and/or LVEF radionuclide measurement is performed by cardiac echography and/or radionuclide measurement of LVEF, as follow: (1) at inclusion and at M0, in patients treated with anthracyclines/trastuzumab/and/or radiotherapy on the left breast and/or chain mammary intern, (2) at M60 in patients treated with anthracyclines, radiotherapy on breast/left wall and/or radiotherapy of the internal mammary chain, (3) every 3 months for 1 year and at M60, for patients treated with trastuzumab.
Other mandatory examinations to be performed in case of clinical signs of chronic toxicity: echography or myocardial scintigraphy in case of dyspnoea or other signs indicating the possibility of cardiac impairment, ECG in case of palpitations, pelvic echography and if needed hysteroscopy in case of metrorrhagia.
Additional examinations are performed according to the standard recommendations followed by each of the participating institution at providers’ discretion.
Social and psychological impact and quality of life patient-reported outcome measures
Self-administrated questionnaires include Hospital Anxiety and Depression Scale (HADS),16 Life orientation Questionnaire de Scheier et Carver revised (LOT-R),17 Beck Depression Inventory (BDI-SF),18 19 EORTC QLQC30-BR23,20 21 EORTC-F13/12,22 2312-Item Short Form Survey (SF) 12,24 Global Physical Activity Questionnaire (GPAQ)16,25 Impact of Cancer Questionnaire (IOCv2)26 and detailed economic and social evaluation are performed. In addition, a work questionnaire is performed 24 months after end of treatment (M24). Table 1 details schedule of these patient-reported outcome measures.
Biologic measures
At inclusion and M36, 30 mL of blood is collected (10 mL peripheral blood sample collected in a dry tube, 10 mL in heparinised tube, 2×5 mL in an EDTA tube). At M12, 20 mL of blood is collected (10 mL peripheral blood sample in a dry tube and 10 mL in heparinised tube).
Specimens are collected on fasting. After collection, samples are kept at room temperature (22°C) for 60 min after collection, then centrifuged, and once centrifuged, the samples are immediately frozen at −80°C and stored at −80°C for 3 months prior to transfer to liquid nitrogen.
Ancillary substudies
In addition, CANTO represents a framework for several substudies. Examples of biological substudies ongoing in CANTO include (1) microbiota that aims to determine the role of the intestinal flora (microbiota) in response to chemotherapy and (2) CANTO-COG that aims to evaluate the cognitive impact of treatment-related toxicity. For all substudies, a specific approval from regulatory entities and ethics committee is required and separate written informed consent.
Power consideration and data analysis
The study is expected to have enough power to examine associations with treatment-related toxicities. For example, a total of 2330 patients with complete EORTC QLQC30-BR23 data at any specific time point will allow to detect a standardised effect size as small as 0.25 for a binary baseline covariate of 10% prevalence with a 85% power at a two-sided significance level of 0.01 (to allow for some multiple testing).27 For covariates with higher prevalence, the power will be higher.
Data quality metrics
Enrolment started in 2012, and by October 2018, 12 012 patients were enrolled. A first data lock was performed after the 5801 patients were enrolled and have reached M12. For clinical data, an average of <5% missing data per variable is present and for patient-reported outcomes, an average of <20% of missing data. At inclusion, 96.8% of patients have blood, plasma and serum samples available and among these >97% have DNA extracted.
Population clinical and treatment characteristics
Table 2 includes selected study population characteristics of the first 5801 women enrolled in CANTO (intermediate cohort). The vast majority of patients had hormone receptor-positive tumours (87.2%) and 14.3% had human epidermal growth factor 2 (HER2)-positive tumours; 49.3% of patients had stage I disease; 73.1% of patients were treated with breast conserving surgery; 90.4% received adjuvant radiation therapy, 53.4% (neo) adjuvant chemotherapy, 11.3% adjuvant trastuzumab and 80.3% adjuvant hormonotherapy.
Table 2.
Patient’s clinical and treatment characteristics, intermediate cohort
| Patient clinical and treatment characteristics | |
| N, % | |
| 5801, 100 | |
| Patient clinical characteristics | |
| Age | |
| <50 | 1.698 (29.3) |
| 50–64 | 2.503 (43.1) |
| ≥65 | 1.600 (27.6) |
| AJCC stage* | |
| Stage I | 2.843 (49.3) |
| Stage II | 2.368 (41.0) |
| Stage III | 558 (9.7) |
| Missing | 32 |
| Molecular subtype† | |
| HR+ HER2+ | 603 (10.5) |
| HR+ HER2− | 4.409 (76.7) |
| HR− HER2+ | 222 (3.8) |
| HR− HER2− | 516 (9.0) |
| Missing | 51 |
| Grade | |
| 1 | 1.050 (18.3) |
| 2 | 3.015 (52.4) |
| 3 | 1.682 (29.3) |
| Missing | 54 |
| Treatment characteristics | |
| Surgery | |
| None | 22 (0.4) |
| Breast conserving surgery | 4.223 (73.1) |
| Mastectomy | 1.532 (26.5) |
| Missing | 22 |
| Axillary lymph node dissection | |
| Sentinel lymph node biopsy | 3.443 (59.6) |
| Lymph node dissection | 2.334 (40.4) |
| Missing | 24 |
| Adjuvant radiotherapy | |
| No | 555 (9.6) |
| Yes | 5.212 (90.4) |
| Missing | 34 |
| Neo/adjuvant chemotherapy | |
| No | 2.691 (46.6) |
| Yes | 3.079 (53.4) |
| Missing | 31 |
| Neo/adjuvant endocrine therapy | |
| No | 1.134 (19.7) |
| Yes | 4.608 (80.3) |
| Missing | 59 |
| Neo/adjuvant trastuzumab | |
| No | 5.110 (88.7) |
| Yes | 650 (11.3) |
| Missing | 41 |
*According to AJCC (version 7),14 for patients treated with neoadjuvant therapy, we considered clinical stage; for all other patients, we considered pathological stage.
†HR and HER2 status, as abstracted from pathology reports. HR is considered positive if the estrogen receptor and/or progesterone receptor are positive. For HER2 classification, the fluorescence in situ hybridisation or IHC result was used. A positive result was considered if HER2 was amplified or IHC 3+.
AJCC, American Joint Committee on Cancer; HER2, human epidermal growth factor 2; HR, hormone receptor; IHC, immunohistochemistry.
Discussion
By October 2018, almost 12 012 French patients with breast cancer were recruited and will be followed in the CANTO cohort for a minimum of 5 years. There is a minimal missing clinical data collection and high rate of patient-reported outcome completion rate. Blood, plasma and serum samples of more than 96% of patients are stored.
Millions of women are breast cancer survivors and a substantial proportion suffers from long-term treatment-related toxicities.3 Although there is a global call to focus on the management of these patients, so far only limited focused research has been done in this setting. Most of the cohorts of breast cancer survivors are not representative of the overall population (eg, single institutional studies, studies that only include patients covered by specific insurance programme) and are lacking in comprehensiveness and completeness (ie, lacking detailed clinical, treatment information, patient-reported outcomes (including psychological, functional and social impact) and biological samples). CANTO tried to address these limitations. CANTO participants are from 26 centres distributed across France that will be followed for a minimum of 5 years with longitudinal data collection. CANTO followed standard methodological quality criteria for observational studies.28 It has an independent steering committee involved in the definition of the study methodology, study implementation and analytic strategy. It is registered in public repositories. It implemented data quality procedures. It has few missing data for measures of interest; the patient population has well-described inclusion and exclusion criteria; the outcomes of interest are well characterised, the length of observation has sufficient duration to capture treatment-related toxicity and the sample size was calculated based on defined hypotheses.28
In addition, CANTO consists of a dedicated and engaged national network, and therefore, the CANTO sites can subsequently put in place practical solutions devised for this patient population.
We acknowledge some limitations. First, CANTO focuses on women diagnosed and followed in France with early breast cancer and therefore may not be generalisable to all women including women in other countries as well as women with advanced disease. Although it covers a substantial proportion of French cancer centres, it is not a population-based sample. Second, we do not have a healthy comparison population, but we will be able to explore the range of toxicities severity in patients with cancer across a variety of treatment intensities, using instruments that were validated among patients with cancer. Third, as with all prospective studies with quality of life endpoints, there is a risk of biased statistical inference with missing data and we will adhere closely to standards put forth by the US National Research Council.29
Conclusions
The CANTO cohort, in addition with recent assembled survivorship cohorts,7–10 represent a major opportunity to better understand long-term treatment-related toxicity on cancer survivors, having the statistical power needed to make potential new discoveries about causes, mediators and moderators of treatment-related toxicities and providing light on avenues to diminish the impact of toxicities among survivors.
Footnotes
FA and PA contributed equally.
Contributors: Al authors contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript or revising it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The study was supported by the Agence Nationale pour la Recherche (ANR-10-COHO-0004).
Competing interests: IVL: Honoraria: AstraZeneca, Novartis, Kephren, outside the submitted work. PC: Honoraria: AstraZeneca, Nanostring Technologies, Novartis, Pfizer, Roche; Consulting or advisory role: Novartis, Pfizer; Research funding: AstraZeneca (Institutional), Novartis, Pfizer, Pierre Fabre (Institutional); Travel and accommodation expenses: Novartis, Pfizer, Roche, outside the submitted work. OT: Honoraria: Roche, MSD, Novartis, Lilly, Astra Zeneca; Grants: Roche MSD, BMS, outside the submitted work. Other authors: no relationships to disclose.
Patient consent for publication: Not required.
Ethics approval: The study was approved by French regulatory authorities (14 September 2011) and French ethics committee (14 October 2011).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Otto SJ, Fracheboud J, Verbeek ALM, et al. Mammography screening and risk of breast cancer death: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2012;21:66–73. 10.1158/1055-9965.EPI-11-0476 [DOI] [PubMed] [Google Scholar]
- 3.Runowicz CD, Leach CR, Henry NL, et al. American cancer Society/American Society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol 2016;34:611–35. 10.1200/JCO.2015.64.3809 [DOI] [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 2000;18:743–53. 10.1200/JCO.2000.18.4.743 [DOI] [PubMed] [Google Scholar]
- 5.Elena JW, Travis LB, Simonds NI, et al. Leveraging epidemiology and clinical studies of cancer outcomes: recommendations and opportunities for translational research. J Natl Cancer Inst 2013;105:85–94. 10.1093/jnci/djs473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Council NR, Medicine Io . From cancer patient to cancer survivor: lost in transition (2006), 2006. [Google Scholar]
- 8.Smith T, Stein KD, Mehta CC, et al. The rationale, design, and implementation of the American cancer Society's studies of cancer survivors. Cancer 2007;109:1–12. 10.1002/cncr.22387 [DOI] [PubMed] [Google Scholar]
- 9.van de Poll-Franse LV, Horevoorts N, van Eenbergen M, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer 2011;47:2188–94. 10.1016/j.ejca.2011.04.034 [DOI] [PubMed] [Google Scholar]
- 10.Bouhnik A-D, Bendiane M-K, Cortaredona S, et al. The labour market, psychosocial outcomes and health conditions in cancer survivors: protocol for a nationwide longitudinal survey 2 and 5 years after cancer diagnosis (the VICAN survey). BMJ Open 2015;5:e005971 10.1136/bmjopen-2014-005971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Available: https://www.e-cancer.fr/Plan-cancer/Les-Plans-cancer-de-2003-a-2013/Le-Plan-cancer-2009-2013 [Accessed 30 Jul 2019].
- 12.Available: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01993498&cntry=&state=&city=&dist= [Accessed 30 Jul 2019].
- 13.Available: https://epidemiologie-france.aviesan.fr/en/ezfind/research?search=canto+&facet=catalogue&sort=score%7Cdesc&hsearch= [Accessed 30 Jul 2019].
- 14.Available: https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%207th%20Ed%20Cancer%20Staging%20Manual.pdf [Accessed 30 Jul 2019].
- 15.Available: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf [Accessed 30 Jul 2019].
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 17.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the life orientation test. J Pers Soc Psychol 1994;67:1063–78. 10.1037/0022-3514.67.6.1063 [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Guth D, Steer RA, et al. Screening for major depression disorders in medical inpatients with the Beck depression inventory for primary care. Behav Res Ther 1997;35:785–91. 10.1016/S0005-7967(97)00025-9 [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Steer RA. Internal consistencies of the original and revised Beck depression inventory. J Clin Psychol 1984;40:1365–7. [DOI] [PubMed] [Google Scholar]
- 20.Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 21.Sprangers MA, Groenvold M, Arraras JI, et al. The European organization for research and treatment of cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 1996;14:2756–68. 10.1200/JCO.1996.14.10.2756 [DOI] [PubMed] [Google Scholar]
- 22.Weis J, Arraras JI, Conroy T, et al. Development of an EORTC quality of life phase III module measuring cancer-related fatigue (EORTC QLQ-FA13). Psychooncology 2013;22:1002–7. 10.1002/pon.3092 [DOI] [PubMed] [Google Scholar]
- 23.Weis J, Tomaszewski KA, Hammerlid E, et al. International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12). J Natl Cancer Inst 2017;109. doi: 10.1093/jnci/djw273. [Epub ahead of print: 01 May 2017]. [DOI] [PubMed] [Google Scholar]
- 24.Ware J, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 25.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 2009;6:790–804. 10.1123/jpah.6.6.790 [DOI] [PubMed] [Google Scholar]
- 26.Zebrack BJ, Ganz PA, Bernaards CA, et al. Assessing the impact of cancer: development of a new instrument for long-term survivors. Psychooncology 2006;15:407–21. 10.1002/pon.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cocks K, King MT, Velikova G, et al. Evidence-Based guidelines for determination of sample size and interpretation of the European organisation for the research and treatment of cancer quality of life questionnaire core 30. J Clin Oncol 2011;29:89–96. 10.1200/JCO.2010.28.0107 [DOI] [PubMed] [Google Scholar]
- 28.Roche N, Reddel H, Martin R, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc 2014;11(Suppl 2):S99–S104. 10.1513/AnnalsATS.201309-300RM [DOI] [PubMed] [Google Scholar]
- 29.National Research Council (U.S.) Panel on Handling Missing Data in Clinical Trials., National Research Council (U.S.). Committee on National Statistics., National Academies Press (U.S.) : The prevention and treatment of missing data in clinical trials. Washington, D.C.: National Academies Press, 2010. [PubMed] [Google Scholar]