Abstract
Assessment of patients with synchronous primary cancers and metastases is challenging, as it can be difficult to assign the metastases to the correct primary due to low differentiation, high similarity on histology or inaccessibility of tumour tissue. Systemic treatment for metastatic disease, however, needs to be directed at the leading histology or cover multiple tumour types with the same regimen. Considering the additional obstacles in cancer management, including tumour heterogeneity and clonal evolution, blood-based genomic profiling (‘liquid biopsy’) is suggested to be a useful tool to provide accessible tumour-derived biomarkers. We herein report a case of a patient with independent primary tumours of the colon and pancreas, as well as liver metastases. All lesions were resected and genotyped revealing KRAS mutations G12C and G12D in the primary tumours, respectively. The G12D mutation detected in the pancreatic tumour was retrieved in the metastasis, thus confirming the pancreatic cancer to be the origin of the liver lesions. The prevalence of the pancreatic tumour was additionally verified by the detection of the G12D variant in circulating cell-free DNA (cfDNA). This case demonstrates the utility of liquid biopsy to identify the predominant tumour burden in patients with multiple primary cancers, based on the detection of the tumour-associated gene mutation in the plasma. Serial monitoring through liquid biopsies might allow disease surveillance to guide cancer management. The review of the literature highlights the importance of liquid biopsies in personalised oncology, even though only one case report refers to the benefit of cfDNA analysis in a patient affected by synchronous primary tumours.
Keywords: synchronous malignancies, liquid biopsy, cell-free dna, precision medicine
Introduction
Multiple primary cancers have a frequency of 2%–17% and are diagnosed when a patient develops more than one primary tumour at the same time (synchronous) or consecutively more than 6 months apart (metachronous).1 Patients with simultaneous primary tumours represent a major challenge for clinicians since the therapy regimen has to cover multiple cancer types and requires additional caution with respect to pharmacological interactions to avoid increased toxicity levels. Consistent with this, prognosis of these patients is significantly lower compared with those with metachronous tumours.2
The analysis of tissue biopsies is the gold standard for diagnosis and molecular characterisation of the underlying disease. However, the analysis of a single tumour lesion, especially when only a bioptic specimen is acquired, might miss relevant information of tumour genetic diversity and clonal evolution during effective treatment. In contrast, the analysis of blood-based tumour markers from serial liquid biopsies represents a minimally invasive procedure providing potential information about spatial and temporal heterogeneity. Circulating cell-free DNA (cfDNA) is liberated from healthy and diseased cells mainly by rupture, necrosis or apoptosis. Thus, tumour-derived cfDNA can only be identified by the detection of cancer-specific gene variants, which might be present in frequencies as low as 0.01%.3 There is emerging evidence for the clinical utility of cfDNA testing regarding the prediction of therapeutic response. In 2016, the US Food and Drug Administration approved the use of cfDNA as a companion diagnostic test to screen patients with metastatic non-small cell lung cancer for mutations in the epidermal growth factor receptor (EGFR) gene, who might benefit from treatment with EGFR tyrosine kinase inhibitors.4 Moreover, even if not yet used in daily clinical practice, the emergence of RAS mutations in blood from patients with metastatic colorectal cancer (mCRC) treated with anti-EGFR inhibitors has been shown to be a useful tool for early detection of disease progression.5
Here, we present a complex case of a patient with synchronous primary tumours of the colon and the pancreas in the presence of synchronous liver metastases. Immunohistochemistry (IHC) as well as genotyping of both primary tumours and a metastatic lesion verified the pancreatic cancer as the origin of the liver metastasis. Additionally, predominance of the adenocarcinoma of the pancreas was demonstrated by detecting the same KRAS mutation in the corresponding plasma sample.
Case report
In March 2014, a 65-year-old female patient was referred to the Charité Department of General, Visceral and Vascular Surgery due to deteriorating general condition, fever, loss of weight and altered stool passage. Colonoscopy revealed a G2 adenocarcinoma of the hepatic flexure of the colon. A CT scan of the abdomen confirmed the lesion in the right bowel and showed additional lesions of uncertain origin in the tail of the pancreas as well as in the liver segments II, III and VIII. Fine needle aspiration of the pancreas revealed chronic inflammation and fibrosis. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19.9 (CA19-9) levels were 1.31 µg/mL and 17.5 U/mL, respectively. Cardiac evaluation with trans-oesophageal echocardiography showed minimal cardiomyopathy with an ejection fraction of 54% and a grade 1 mitral regurgitation. After admission to the hospital, antibiotic therapy was started, and after providing informed consent the patient was enrolled in the OncoTrack study.6 According to the study procedure, blood samples were collected prior to surgery and plasma was stored until further processing. Under the assumption of a colon carcinoma that metastasised to the liver and potentially to the pancreas, the patient underwent extended right hemicolectomy with further permanent ileostomy, retroperitoneal lymphadenectomy, partial resection of the diaphragm, distal pancreatectomy with splenectomy, and partial left adrenalectomy and liver metastasectomy (segments II, III and VIII). Pathology of the surgical specimen of the colon confirmed R0 resection of a moderately differentiated, ulcerated adenocarcinoma of 50×40×7 mm in size with infiltration of the subserosa and 25 tumour-free lymph nodes. The colon carcinoma was therefore staged as II (pT3pN0(0/25)M0G2R0L0V0). Further molecular characterisation showed a KRAS mutation (exon 2, c.34G>T, G12C). Pathology of the tail of the pancreas revealed a poorly differentiated ductal adenocarcinoma sized 45×35×35 mm with infiltration of the surrounding fat tissue as well as the splenic artery and vein. Both the morphology and IHC analysis (diffuse cytokeratin 7 (CK7) expression and weak expression of cytokeratin 20 (CK20) and SMAD4/B8) indicated a primary carcinoma of the pancreas and not a metastasis of the synchronous adenocarcinoma of the colon. Fourteen peripancreatic lymph nodes did not reveal evidence of tumour. The IHC analysis of the R0-resected liver metastases demonstrated expression of cytokeratin 5/6 (CK5/6) and CK7, moderate positivity of CA19.9, and absence of CK20 and CDX2, thus indicating an origin from the pancreatic tumour, rather than from the colon. The pancreatic carcinoma was therefore staged as IV (pT3pN0(0/14)pM1G3R0L0V1). After surgery, liver and infectious parameters were normalised, and the patient was discharged from the hospital. After 4 weeks, the patient developed fever and fluid retention at the liver resection surface. Therefore, the patient was admitted again to the Charité Department of General, Visceral and Vascular Surgery, where she received antibiotic treatment and underwent drainage of the retained fluid. After 15 days, clinical conditions improved, and infectious parameters and liver enzymes normalised. Therefore, the patient was discharged from the hospital. Eleven days later, before she could start systemic chemotherapeutic treatment, the patient suddenly died at home due to unknown reasons.
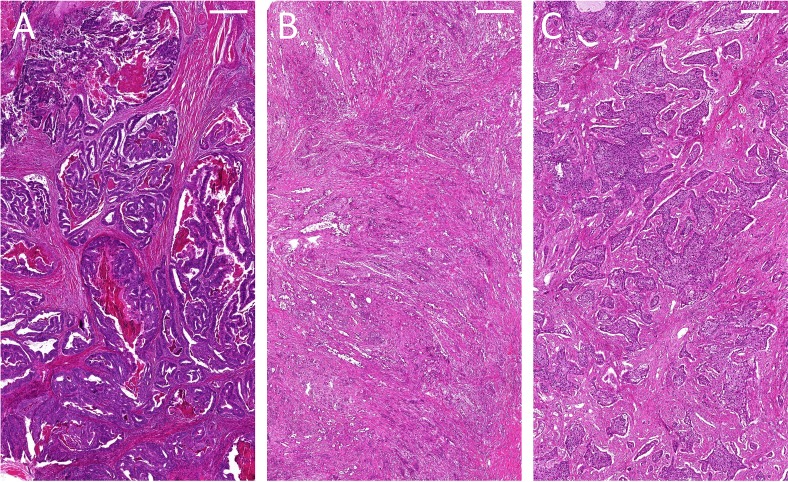
As part of the OncoTrack study, tumour tissue from the colon was freshly collected and sequenced revealing the KRAS G12C mutation, which was further validated by Droplet Digital PCR on the archival formalin-fixed and paraffin-embedded (FFPE) tissue specimen. Moreover, DNA was isolated from archival FFPE tissue of the pancreas and the liver metastases. Tumour purities of the FFPE colon, pancreatic and liver samples were 80%, 30% and 70%, respectively (table 1). The corresponding H&E stainings are shown in figure 1. At the same time, cfDNA was isolated from the stored plasma. By Droplet Digital PCR, and in contrast to the mutation found in the primary colon tumour, we detected KRAS G12D in the cfDNA, liver and pancreatic tissue. Both mutations were somatic and tumour-specific, as they were not present in germline DNA isolated from whole blood at baseline.
Table 1.
KRAS mutations detected via Droplet Digital PCR in tumour tissue and circulating cell-free DNA
| Specimen | Tumour purity %) | KRAS variant | Mutant allele frequency (%) |
| Colon primary tumour | 80 | G12C | 41.2 |
| Pancreas primary tumour | 30 | G12D | 23.2 |
| Liver metastasis | 70 | G12D | 47.4 |
| Cell-free DNA | G12D | 9.7 |
Figure 1.
H&E staining of (A) the adenocarcinoma of the colon, (B) the adenocarcinoma of the pancreas tail and (C) a representative liver metastasis (scale bar: 500 µm).
Discussion
The presented case is a clear example of the utility of cfDNA testing to better understand the predominant cancer component of synchronous multiple primary tumours. The patient died before starting a systemic chemotherapeutic treatment. Therefore, liquid biopsy was, in this case, not used to monitor the molecular dynamics during therapy. Nevertheless, the detection of the pancreas-derived KRAS mutation in peripheral blood could have potentially helped to monitor treatment efficacy. Patients with multiple primary tumours may benefit from liquid biopsy-based cancer monitoring and specifically early recognition of a shift in the leading histology over time, when serial tissue biopsies might be difficult to obtain. Lakis et al 7 reported the case of a patient affected by metastatic lung adenocarcinoma with multiple pulmonary lesions harbouring different driver mutations. The analysis of the liquid biopsy obtained before systemic treatment verified the EGFR exon 19 deletion detected in a lung and bone lesion, and revealed an activating KRAS mutation at a low allele frequency. Since no other malignancy was identified by positron emission tomography-CT, the patient underwent first-line treatment with the tyrosine kinase inhibitor afatinib, resulting in dramatic regression of all lesions except in the right upper lobe. Genotyping of this treatment-resistant lesion revealed the same KRAS mutation detected in the cfDNA, which is why the tumour was diagnosed as a synchronous primary lung cancer. While still under treatment, successive liquid biopsies revealed the enduring absence of the EGFR exon 19 deletion and persistence of the KRAS mutation, which was further identified in new metastases in the soft tissue and in the brain. Despite combination treatment with afatinib and chemotherapy, the patient died on progression due to the KRAS-driven lung tumour. To the best of our knowledge, this is the only reported case of cfDNA analysis in the context of synchronous malignancies that demonstrates the utility of liquid biopsy to identify the predominant cancer component and its evolution during the course of therapy. In addition, the relevance of circulating tumour cells (CTCs) to investigate disease dynamics has previously been reported from our group. Fusi et al 8 provided evidence that the analysis of CTCs from a patient with synchronous metastatic melanoma and colon carcinoma mirrored disease evolution. Two years after diagnosis of a stage IIIC melanoma, the patient presented with pulmonary, pleural and multiple hepatic metastases, of which the latter was biopsied, revealing an adenocarcinoma. Colonoscopy, histological examination, and increased levels of CEA and CA19-9, but not S100 protein, confirmed the diagnosis of mCRC. Therefore, the patient underwent chemotherapy, and a liquid biopsy was collected before and during treatment to detect epithelial CTCs as a surrogate for tumour response. Due to increasing dyspnoea, an additional blood sample was analysed to detect circulating melanoma cells (CMCs). Whereas epithelial CTCs decreased under therapy, CMCs were present at comparably high numbers during treatment, together with clinical progression and a mixed response in the metastatic lesions. Even though biopsies were not obtained from all metastatic lesions and CMCs were not analysed at baseline, CTCs and serum markers were in concordance at re-evaluation. The analysis of liquid biopsies displayed the switch when the colon carcinoma responded to chemotherapy, while the melanoma progressed, demonstrating that serial liquid biopsies can be used to individually assess tumour response in patients with cancer. Consistent with this, Khan et al 9 included patients with RAS wild-type, chemorefractory mCRC receiving single-agent cetuximab into a prospective phase II trial, in which genomic profiling of serial liquid and conventional tissue biopsies was performed for disease surveillance. Including mathematical modelling of clonal evolution, they revealed that primary and acquired resistance were often of polyclonal nature with allele frequencies below the detection threshold of clinically approved methods, explaining why a subcohort of non-responders were initially classified as RAS wild-type. Furthermore, the analysis of frequent serial liquid biopsies captured spatial and temporal heterogeneity, predicting the emergence of resistant clones in a quantitative manner to forecast the time to progression in individual patients. Finally, Almodovar et al.10 clearly showed, in a series of 25 patients affected by small cell lung cancer, that cfDNA analysis was able to detect disease recurrence and occult disease that were not evident with radiographic imaging. Exemplary, 8 weeks after completion of first-line chemoradiotherapy, recurrence of a TP53 mutation was retrieved in cfDNA from a patient at a low allele frequency (1.3%), further increasing to 74% after 256 days from the end of the treatment. Reappearance of a PIK3CA amplification and deletions in TP53 and RB1 were simultaneously detected despite the absence of clear radiological or clinical progression. Only after 33 days progression became clinically apparent. Consistent with their observation, other studies demonstrated that the analysis of liquid biopsies in patients with breast and colorectal cancer provided earlier evidence of cancer recurrence as compared with conventional imaging.11 12
Despite the previously mentioned examples, we are aware that liquid biopsies might have several limitations regarding the identification of the tumour of origin. Prerequisites include verification of distinct disease markers, verified in the primaries, stability of the disease markers over time, and release of CTCs or cfDNA into the bloodstream at a detectable quantity. To our knowledge, the analysis of cfDNA has never been proposed to inform treatment decision for patients with multiple primary tumours of different histology. Our observation supports our conviction that liquid biopsy is a promising tool to depict changes in the mutational landscape of the underlying disease, thereby identifying the predominant cancer component in patients with synchronous or metachronous malignancies to allow dynamic treatment stratification.
Footnotes
Contributors: FK provided FFPE blocks and performed the H&E staining to confirm the diagnoses and estimate the tumour cell content. SL isolated DNA from FFPE sections, whereas AN isolated cfDNA from plasma. AN established the ddPCR assays and performed analysis of all samples. SL designed and edited the text, table and figure as well as their legends under the supervision of LV and UK. All authors provided scientific advice and critically revised the manuscript. We confirm that all coauthors have contributed to the generation of the manuscript and have given final approval of the version to be published.
Funding: The patient was enrolled at the OncoTrack research project, which received funding from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° 115234, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ inkind contribution. Our analysis was financed by the German Cancer Consortium (DKTK), which is funded as one of the National German Health Centers by the Federal German Ministry of Education and Research.
Competing interests: LV is a member of the GI connect group, spouse is employee and shareholder of Bayer AG. SL, AN, FK and UK have no conflicts of interest to declare.
Patient consent for publication: Informed consent was obtained prior to blood and tissue specimen collection.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Vogt A, Schmid S, Heinimann K, et al. . Multiple primary tumours: challenges and approaches, a review. ESMO Open 2017;2:e000172 10.1136/esmoopen-2017-000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agrawal R. Synchronous dual malignancy: successfully treated cases. J Cancer Res Ther 2007;3:153–6. 10.4103/0973-1482.37408 [DOI] [PubMed] [Google Scholar]
- 3. Diehl F, Li M, Dressman D, et al. . Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A 2005;102:16368–73. 10.1073/pnas.0507904102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. U.S. Food & Drug Administration Cobas EGFR mutation test V2. 06/02/2016. Available: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm504540.htm
- 5. Siravegna G, Mussolin B, Buscarino M, et al. . Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med 2015;21:795–801. 10.1038/nm.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schütte M, Risch T, Abdavi-Azar N, et al. . Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat Commun 2017;8:14262 10.1038/ncomms14262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lakis S, Heukamp LC, Griesinger F. Detection of discrepant driver mutations in a patient with two synchronous primary non-small cell lung cancers (NSCLCs) with liquid biopsy. J Thorac Oncol 2017;12:e186–8. 10.1016/j.jtho.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 8. Fusi A, Liu Z, Schmittel A, et al. . Monitoring of circulating tumor cells in a patient with synchronous metastatic melanoma and colon carcinoma. Ann Oncol 2010;21:1734–5. 10.1093/annonc/mdq330 [DOI] [PubMed] [Google Scholar]
- 9. Khan KH, Cunningham D, Werner B, et al. . Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the PROSPECT-C phase II colorectal cancer clinical trial. Cancer Discov 2018;8:1270–85. 10.1158/2159-8290.CD-17-0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Almodovar K, Iams WT, Meador CB, et al. . Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J Thorac Oncol 2018;13:112–23. 10.1016/j.jtho.2017.09.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dawson S-J, Tsui DWY, Murtaza M, et al. . Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199–209. 10.1056/NEJMoa1213261 [DOI] [PubMed] [Google Scholar]
- 12. Diaz Jr LA, Williams RT, Wu J, et al. . The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012;486:537–40. 10.1038/nature11219 [DOI] [PMC free article] [PubMed] [Google Scholar]