Abstract
Background
Anti-PD-(L)1 agents are standard of care treatments in various cancers but predictive factors for therapy selection are limited. We hypothesised that markers of systemic inflammation would predict adverse outcomes in multiple cancers treated with anti-PD-(L)1 agents.
Material and methods
Discovery cohort consisted of patients who were treated with anti-programmed cell death protein-1 (PD-1) agents for advanced melanoma (MEL), non-small cell lung cancer (NSCLC) or renal and bladder cancers (GU) at Oulu University Hospital and had pretreatment C reactive protein (CRP), or neutrophil/lymphocyte values available. As a validation cohort, we collected patients treated with anti-PD-1 agents from three other hospitals in Finland.
Results
In the discovery cohort (n=56, MEL n=23, GU n=17, NSCLC n=16), elevated CRP over the upper limit of normal (ULN) (>10 mg/mL) indicated poor progression-free (PFS; p=0.005) and overall survival (OS; p=0.000004) in the whole population and in MEL subgroup. Elevated neutrophil-to-lymphocyte ratio (>2.65) also indicated inferior PFS (p=0.02) and OS (p=0.009). In the validation cohort (n=107, MEL n=44, NSCLC n=42, GU n=17, other n=4), CRP over ULN also was a strong indicator for poor PFS (p=0.0000008), and OS (p=0.000006) in the whole population, and in MEL and NSCLC also.
Conclusions
Systemic inflammation suggested by elevated CRP is a very strong indicator for adverse prognosis on patients treated with anti-PD-(L)1 agents and has a potential negative predictive value for treatment with anti-PD-(L)1 agents. Prospective trials should investigate whether patients with elevated CRP gain any significant benefit from anti-PD-1 therapy.
Keywords: PD-1, CRP, prognostic, predictive, survival
Key questions.
What is already known about this subject?
Programmed cell death protein-1 (PD-1) inhibitors are at the centre of clinical practice for number of malignancies yet most patient fail to respond, thus, identifying biomarkers for patient selection is of essence.
Although the negative impact of systemic inflammation on prognosis of cancer patients is widely studied, the predictive meaning of systemic inflammatory status in patients receiving immune checkpoint inhibitors (ICIs) is inadequately defined.
Blood-based markers of systemic inflammation are shown to predict treatment benefit on cancer patients treated with ICIs but the knowledge about the independent prognostic role of C reactive protein (CRP) is scarce.
What does this study add?
The current study investigated markers for systemic inflammation as a poor prognostic marker in cancer patients treated with PD-1 inhibitors using independent, real-world cohorts.
Results imply that elevated pretherapy CRP is strong indicator for adverse prognosis in multiple tumour types.
How might this impact on clinical practice?
CRP value could prove to be a cheap and non-invasive predictive marker and it should be investigated in prospective clinical trials.
Introduction
Progress in the field of immuno-oncology has changed the treatment landscape of multiple cancer types. Immune checkpoint inhibitor (ICI) therapies such as anti-PD-(L)1 and CTLA-4 antibodies, are now at the centre of clinical practice for a number of malignancies and even durable responses are seen in advanced disease.1–10 In the programmed death ligand-1 (PD-L1)/programmed cell death protein-1 (PD-1) pathway, binding of PD-L1 on tumour cells and antigen presenting cells to the PD-1 receptor on T cells halts or limits T cell response by downregulating T cell proliferation, effector function and cytokine production.11 Typically, with PD-(L)1 inhibitors, responses are seen in 20%–40% of the patients yet most fail to respond. Therefore, predictive biomarkers are needed to guide the patient selection.
Despite the pervasive research on biomarker field, only a few biomarkers have proven to be clinically relevant such as tumour PD-L1 expression,12 13 tumour mutation burden (TMB),14 15 and in rare cases microsatellite instability, and mismatch-repair deficiency.16 However, positive and negative predictive values of PD-L1 and TMB are low, and they are valid for patient selection only in particular cancers such as non-small cell lung cancer (NSCLC) and urothelial cancers. Nevertheless, these biomarkers are assessed from tumour biopsies, which are time consuming and not readily available. Blood-based biomarker assays as non-invasive analysis, are thus more compelling in various cancers.
The inflammation process has been proposed as a mechanism of immunoresistance in patients with cancer, promoting cancer growth17 and cancer-related inflammation has been reported to be a marker of poor prognosis.18 19 C reactive protein (CRP), a marker of systemic inflammation, has been used to make prognostic and predictive determinations of clinical outcome in cancer patients treated with ICIs.20–22 Other widely acknowledged markers for systemic inflammation with prognostic value include neutrophil-to-lymphocyte ratio (NLR) and lactate-dehydrogenase (LDH). NLR is a marker for the general immune response to various stress stimuli, and it is shown to predict outcome among NSCLC and melanoma (MEL) patients treated with ICI therapies.23–26 LDH level is a classic inflammatory marker in patients with cancer. High baseline levels of LDH are linked to poor survival and to inferior response to ICIs on MEL and NSCLC patients.27 28
The current retrospective study evaluates the correlation of pretreatment CRP-values and NLR ratio to survival outcomes among cancer patients receiving anti-PD-1 agents. We hypothesised that elevated CRP levels and high NLR ratio would predict poor outcome in multiple tumour types.
Material and methods
Patients
All patients who had received at least one dose of intravenous anti-PD-(L)1 agent at Oulu University Hospital (Finland) 8/2014–9/2018 were retrospectively identified from the pharmacy records (Discovery cohort). Clinical variables included age, cancer type, TNM staging, Eastern Cooperative Oncology Group (ECOG) and blood sample results were manually collected from the electronic patient record. Laboratory values 4 weeks prior and 2 weeks post from the first anti-PD-(L)1 infusion were included, thus, all patients included in the analysis had received only one cycle of anti-PD-(L)1 therapy. If multiple laboratory values existed, the closest value to the first anti-PD-(L)1 infusion was selected. Furthermore, if blood samples were taken during clinically confirmed acute infection, another preinfection or postinfection value was chosen which timely lined the closest to the first anti-PD-(L)1 infusion. All the laboratories used the Finnish Accreditation Service (FINAS) (SFS-EN ISO 15189) immunoturbidometric test for CRP. Progression-free (PFS) and overall survival (OS) were calculated from the date of the first anti-PD-(L)1 infusion to documented tumour progression, death or end of follow-up (PFS) or to death or end of follow-up (OS). Tumour progression and/or death were counted as an event.
A validation cohort constituted of cancer patients treated with anti-PD-(L)1 therapy in three Finnish University Hospitals: Tampere, Helsinki and Kuopio. Patients, who had received at least one dose of anti-PD-(L)1 treatment during 3/2015–9/2018 and had CRP values available as for discovery cohort, were included.
Data collection was carried out according to national legislation and under permit from the medical director of each University Hospital (Oulu University Hospital study no. 299/2016), Helsinki University Hospital (HUS/395/2018), Kuopio University Hospital (112/2018, 192/2018) and Tampere University Hospital (R18612). Anonymisation was performed before data analysis. Individual informed consents were not sought due to the register nature of the study.
Statistical analysis
IBM SPSS Statistics V.24.0.0.0 for Windows was applied for statistical analysis. Survival was analysed by using the Kaplan-Meier method with the log-rank test. Receiver operating characteristic (ROC) curves were calculated for CRP and NLR to define the optimal cut-off-point. Multivariate analysis was performed using Cox regression analysis. Probability values below 0.05 were considered significant.
Results
Discovery cohort
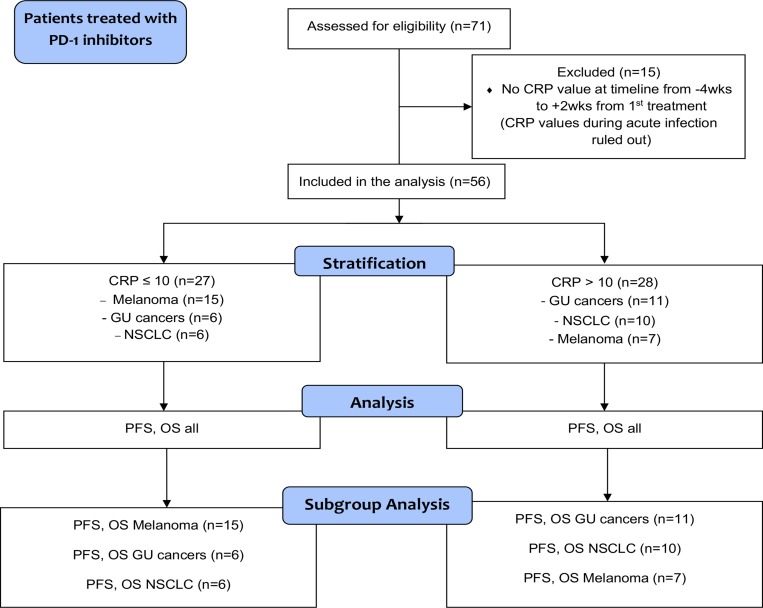
A total of 71 patients treated with single agent anti-PD-1 therapy for advanced cancers in 2014–2018 at Oulu University Hospital Oncology Department were assessed for eligibility, and 56 of them were included in the final analysis (figure 1). Median age of the patients was 66.0 years and majority of the patients were male (73.2%). The cohort included patients with MEL (n=23, 41.1%), renal and bladder cancers (GU) (n=17, 30.4%), and NSCLC (n=16, 28.6%). Detailed patient demographics are presented in table 1.
Figure 1.
Flowchart demonstrating study selection for the discovery cohort. CRP, C reactive protein; GU, renal and bladder cancers; NSCLC, non-small cell lung cancer; OS, overall survival; PD-1, programmed cell death protein-1; PFS, progression-free.
Table 1.
Patientdemographics
| n (%) | |
| Age (median) | 66 |
| Gender | |
| Male | 41 (73.2) |
| Female | 15 (26.8) |
| Tumour type | |
| Melanoma | 23 (41.0) |
| GU cancers | 17 (30.4) |
| NSCLC | 16 (28.6) |
| Stage at diagnosis | |
| Stage IV | 54 (96.4) |
| Stage III | 2 (3.6) |
| ECOG | |
| 0 | 50 (89.3) |
| 1 | 6 (10.7) |
| Therapy line for metastatic cancer | |
| Line therapy | 19 (33.9) |
| Or later line | 37 (66.1) |
GU, renal and bladder cancers; NSCLC, non-small cell lung cancer.
Survival according to CRP in the discovery cohort
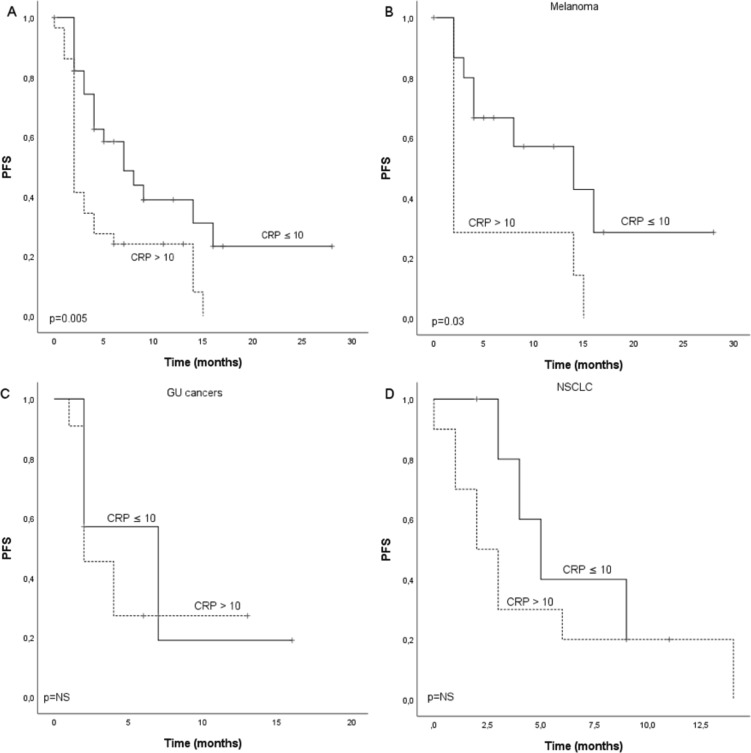
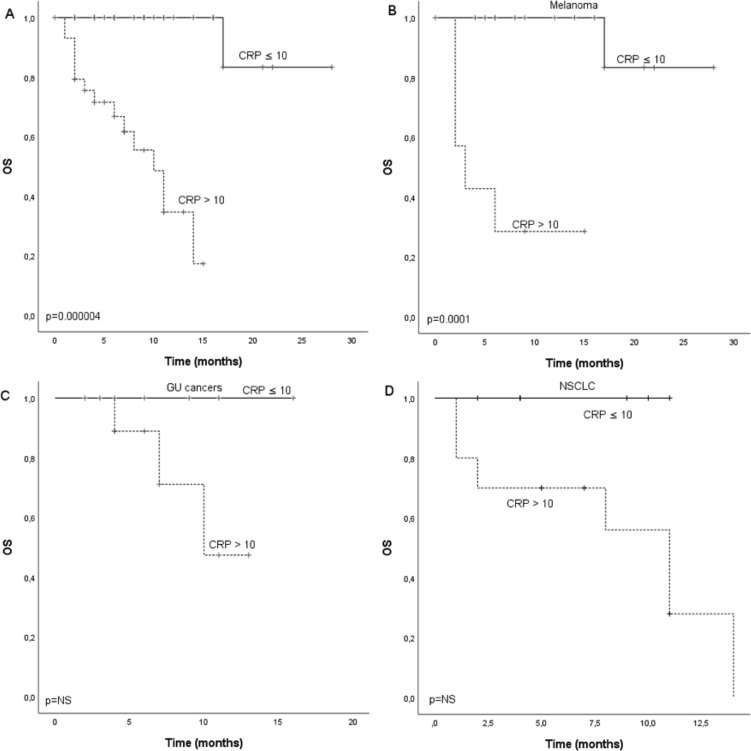
A ROC curve was calculated to define the optimal cut-off point of CRP in the cohort, and value 9.50 mg/mL was selected (online supplementary figure S1). We further rounded the value up to 10.0 which is a validated ULN rank for CRP. Median PFS for the whole cohort was 4.0 months (CI 2.4 to 5.6) and there was statistical difference (p=0.005) between patients with CRP ≤10 (7.0 months, CI 2.9 to 11.1) and CRP >10 (2.0 months, CI 1.6 to 2.4) (figure 2A). Median OS for the whole cohort was 17.0 months and there was statistical difference (p=0.000004) between patients with CRP ≤10 (not reached) and CRP >10 (10.0 months, CI 6.8 to 13.2) (figure 3A). In subgroup analysis for different cancer types (MEL, GU and NSCLC), there was statistically significant difference in PFS (p=0.03) and OS (p=0.0001) according to CRP in MEL but not GU or NSCLC. However, similar tendency for improved survival was seen also in NSCLC and GU cancers (figures 2B–D and 3B–D).
Figure 2.
Kaplan-Meier analysis for progression-free survival according to CRP in (A) whole study population, (B) melanoma patients, (C) GU cancer patients and (D) NSCLC patients of the discovery cohort. Crosses indicate censored events. CRP, C reactive protein; GU, renal and bladder cancers; NSCLC, non-small cell lung cancer; PFS, progression-free.
Figure 3.
Kaplan-Meier analysis for overall survival according to CRP in (A) whole study population, (B) melanoma patients, (C) GU cancer patients and (D) NSCLC patients of the discovery cohort. Crosses indicate censored events. CRP, C reactive protein; GU, renal and bladder cancers; NSCLC, non-small cell lung cancer; OS, overall survival.
esmoopen-2019-000531supp001.pdf (157.1KB, pdf)
Survival according to NLR in the discovery cohort
NLR was calculated by dividing the number of neutrophils by number of lymphocytes. ROC curve was calculated to define the optimal cut-off-point of the NLR ratio in our cohort, and the value 2.65 was chosen (online supplementary figure S2). Median PFS for the whole cohort was 4.0 months (CI 1.8 to 6.3) and there was statistical difference (p=0.02) between patients with NLR ≤2.65 (7.0 months, CI 4.4 to 9.6) and NLR >2.65 (2.0 months, CI 1.5 to 2.5) (online supplementary figure S3A). Median OS for the whole cohort was 19.0 months (CI 9.3 to 28.7) and there was statistical difference (p=0.009) between patients with NLR ≤2.65 (19.0 months, CI 15.0 to 23.0) and NLR >2.65 (7.0 months, CI 0.0 to 15.1) (online supplementary figure S3B). We also calculated PFS and OS with NLR ratio five defined as a cut-off-point in previous studies and the results showed statistically non-significant PFS and OS (table 2). Cox regression model was used to evaluate the dependence between CRP and NLR ratio. In multivariate analysis, these inflammatory biomarkers were non-independent.
Table 2.
Other blood-based markers of systemic inflammation and their correlation to survival
| Laboratory value | P value |
| NLR, cut-off point >5 | |
| n=31 | |
| PFS | 0.21 |
| OS | 0.056 |
| Total leucocytes | |
| n=58 | |
| PFS | 0.57 |
| OS | 0.37 |
| Total lymphocytes | |
| n=31 | |
| PFS | 0.59 |
| OS | 0.37 |
| LDH | |
| n=59 | |
| PFS | 0.54 |
| OS | 0.32 |
LDH, lactate-dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PFS, progression-free.
esmoopen-2019-000531supp002.pdf (156.2KB, pdf)
esmoopen-2019-000531supp003.pdf (130.4KB, pdf)
Survival according other studied blood-based markers in the discovery cohort
We also investigated additional blood-based markers suggestive of systemic inflammation and previously linked to poor survival and benefit from anti-PD-(L)1 agents. We investigated LDH, total leucocytes, total lymphocytes, and their correlation to PFS and OS. However, none of these markers predicted survival difference in the cohort (table 2).
Survival according to CRP in validation cohort
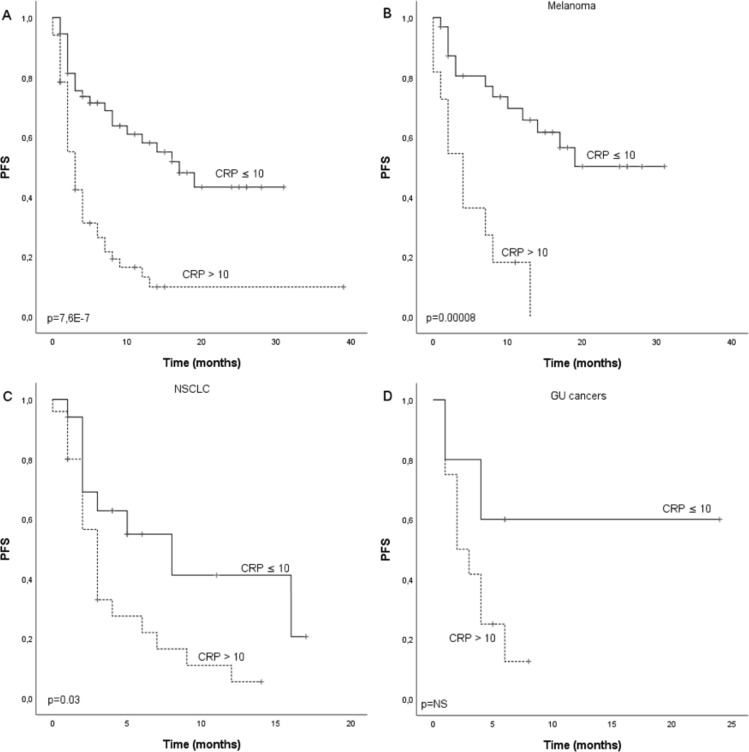
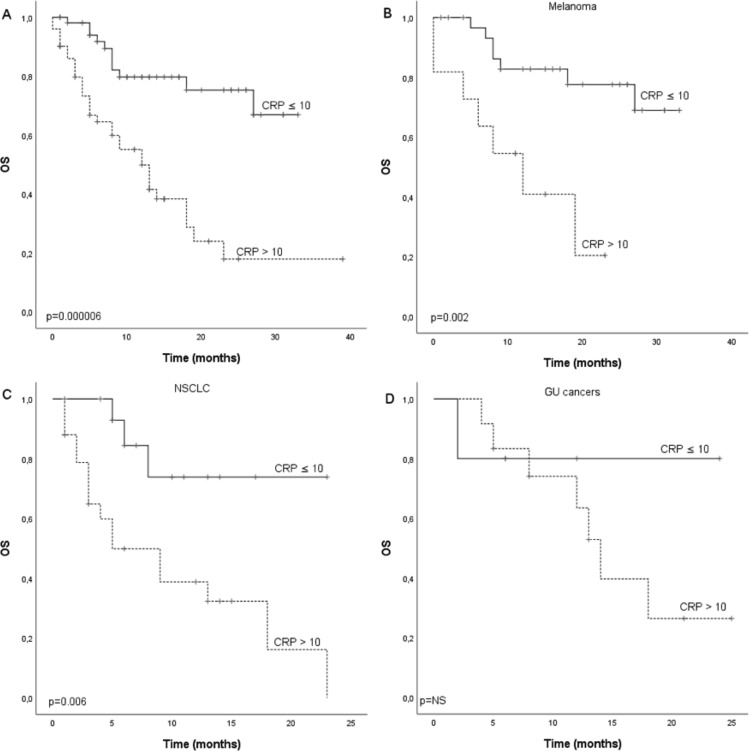
A total of 107 cancer patients treated with single anti-PD-1 therapy in three other Finnish University Hospitals between March 2015 and November 2018 were included in the cohort. Validation cohort consisted of patients with MEL (n=44, 41.1%), NSCLC (n=42, 39.3%), renal cell carcinoma (RCC) (n=13, 12.1%), bladder cancer (n=4, 3.7%) and other not specified (n=4, 3.7%). Median PFS for the whole cohort was 7.0 months (CI 4.1 to 9.9) and there was statistical difference (p=0.0000008) between patients with CRP ≤10 (17.0 months, CI 10.2 to 23.8) and CRP >10 (3.0 months, CI 1.9 to 4.1) (figure 4A). Median OS for the whole cohort was 19.0 months and there was statistical difference (p=0.000006) between patients with CRP ≤10 (not reached) and CRP >10 (12.0 months, CI 7.6 to 16.4) (figure 5A). In subgroup analysis for MEL, GU (RCC and bladder cancer) and NSCLCs, there was a statistically significant difference in PFS (p=0.00008) and OS (p=0.002) according to CRP in MEL and NSCLC PFS (p=0.03) and OS (p=0.006) but not in GU cancer. Though, similar tendency for improved survival was seen in GU cancer (figures 4B–D and 5B–D).
Figure 4.
Kaplan-Meier analysis for progression-free survival according to CRP in (A) whole study population, (B) melanoma patients, (C) NSCLC patients and (D) GU cancer patients of the validation cohort. Crosses indicate censored events. CRP, C reactive protein; GU, renal and bladder cancers; NSCLC, non-small cell lung cancer; PFS, progression-free.
Figure 5.
Kaplan-Meier analysis for overall survival according to CRP in (A) whole study population, (B) melanoma patients, (C) NSCLC patients and (D) GU cancer patients of the validation cohort. Crosses indicate censored events. CRP, C reactive protein; GU, renal and bladder cancers; NSCLC, non-small cell lung cancer; OS, overall survival.
Discussion
The negative impact of systemic inflammation on prognosis of cancer patients receiving chemotherapy and targeted therapies is widely studied,29 30 but the role of systemic inflammatory status to help predict benefit of immunotherapy is inadequately characterised. The role of tumour microenvironment (TME), the area immediately surrounding the tumour which is typically composed of non-malignant lymphoid and/or myeloid cells as well as fibroblasts, vascular cells and lymphatic vessels, in predicting treatment response for ICIs is under fierce investigation. Analysis of genomic and transcriptomic data has led to the discovery of so called metagene signatures and distinctive mutational landscapes with prognostic and predictive nature in cancer patients.31–34 However, implementing a DNA or RNA-based gene signature is challenging from a clinical perspective, and more simplified surrogate biomarkers are needed.
Peripheral blood-based markers for systemic inflammation such as NLR and LDH have been shown to have prognostic,24 35 36 and predictive20 impact on cancer patients receiving ICIs. CRP is an acute phase protein which reflects tissue injury, and its synthesis is influenced by many factors including interleukin 1 (IL-1) and tumour necrosis factor (TNF).37 CRP is a known marker for systemic inflammation but its correlation to treatment benefit from anti-PD-(L)1 agents is scarcely studied,28 38–40 and little is known about the independent prognostic role of CRP level.
The current study investigated the role of CRP and other markers for systemic inflammation in multiple advanced cancers treated with anti-PD-1 therapy. Interestingly, the optimal cut-off value for CRP as prognostic marker in anti-PD-1 treated patients was 10 mg/mL which is also the ULN value for CRP in most laboratories. In the discovery cohort, median PFS and OS were 4.0 and 17.0 months which were substantially lower in patients with elevated CRP (2.0 and 10.0 months, respectively). We also verified our results with validation cohort of patients treated with anti-PD-(L)1 agents in three other hospitals. Median PFS and OS (7.0 and 19.0 months) were similar in the validation cohort and there was statistical difference between survivals according to CRP. These results highlight the role of elevated CRP and systemic inflammation as markers for poor prognosis in patients treated with anti-PD-(L)1 agents. NLR was also investigated but it proved inferior to CRP in the discovery cohort and was not further investigated in the validation cohort. Multivariate analysis, however, revealed that CRP and NLR were not independent prognostic markers suggesting that both reflect systemic inflammation with poor prognosis on anti-PD-(L)1 therapy treated patients.
The strengths of the current study include investigating the role of systemic inflammation using two independent, multicentre cohorts consisting of real-world patients treated with anti-PD-1 agents. Furthermore, our study investigated multiple different tumour types (MEL, GU cancers and NSCLC) which represent the most common cancers treated with anti-PD-(L)1 therapy. To our knowledge, current study is the first investigating systemic inflammation markers in multiple tumour types using independent cohorts. The results enable us to conclude generalisation of elevated CRP as a poor prognostic marker in cancer patients treated with anti-PD-1 therapy.
The current study has some limitations. Retrospective collection of patients and inclusion of only those who were treated with anti-PD-(L)1 agents could bias the results and generate prognostic but not true predictive information of the studied markers. The relatively small sample size is another challenge. Possible confounding factors as concurrent use of medications that could have affected the blood biomarkers were not analysed. However, low PFS figures (median 2–3 months) on patients with elevated CRP levels are highly suggestive of low or no benefit of anti-PD-(L)1 therapy. Survival outcomes of our cohorts were significant in the whole population and in MEL (both cohorts) and NSCLC (validation cohort) but not in the others. In all, survival differences were similar in all the studied cancers even though not reaching statistical significance which is most likely due to small number of patients in some of the cohorts.
Even though results of the current study are convincing, they are hypothesis generating. Prospective trials should investigate whether patients with elevated markers for systemic inflammation gain any significant benefit from single agent anti-PD-(L)1 therapy. We cannot conclude whether our results with systemic inflammation and poor prognosis on anti-PD-1 agents can be generalised to combination or adjuvant treatment with these agents. Future studies should aim to investigate whether systemic inflammation is marker of immunosuppression or functional component in immunosuppression. If latter is true, this might open new therapeutic opportunities for better cancer care. According to preclinical studies, IL-1 beta (IL-1β) is a master cytokine in tumour progression, and besides hindering the tumour growth, blocking IL-1β facilitates checkpoint inhibition by anti-PD-1s. In IL-1β-deficient mouse, low levels of the chemokine CCL2 hamper monocyte recruitment, and together with low levels of colony-stimulating factor-1 (CSF-1) inhibit their differentiation to macrophages in TME. The low levels of macrophages in IL-1β-deficient mouse result in relatively high percentage of specific subtype of dendritic cells in the tumours, which secrete IL-12 fostering the antitumor immunity through activated CD8+ lymphocytes expressing interferon gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α) and granzyme B, infiltrating tumours and inducing regression 41. There are ongoing clinical studies investigating lowering CRP with medications such as IL-1β antibodies in combination to PD-1 agents (ClinicalTrials.gov Identifier: NCT03631199).
In conclusion, the current study investigated markers for systemic inflammation as a poor prognostic marker in cancer patients treated with anti-PD-1 agents using independent, real-world cohorts. The results suggest a strong negative prognostic role of elevated pretherapy CRP in anti-PD-1 therapy treated patients. CRP value could prove to be a cheap and non-invasive predictive marker and it should be investigated in prospective clinical trials.
Footnotes
Contributors: SI and JPK contributed to the conception and design of the study; SI, JPK, JA, AK and ST acquired the data; SI and JPK analysed the data and SI and JPK interpreted the data. SI and JPK contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Funding: This work was supported by Oulu University and Finnish Cancer Institute.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell Non–Small-Cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robert C, Long GV, Brady B, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 3. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med Overseas Ed 2017;377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced Nonsquamous Non–Small-Cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med Overseas Ed 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 8. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 9. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): a phase 3, open-label, multicentre randomised controlled trial. The Lancet 2017;389:255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–Positive Non–Small-Cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 11. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of Anti–PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daud AI, Wolchok JD, Robert C, et al. Programmed Death-Ligand 1 expression and response to the Anti–Programmed death 1 antibody pembrolizumab in melanoma. JCO 2016;34:4102–9. 10.1200/JCO.2016.67.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189–99. 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DT L, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 18. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demaria S, Pikarsky E, Karin M, et al. Cancer and inflammation: promise for biologic therapy. J Immunother 2010;33:335–51. 10.1097/CJI.0b013e3181d32e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hopkins AM, Rowland A, Kichenadasse G, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer 2017;117:913–20. 10.1038/bjc.2017.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naqash AR, Stroud CRG, Butt MU, et al. Co-relation of overall survival with peripheral blood-based inflammatory biomarkers in advanced stage non-small cell lung cancer treated with anti-programmed cell death-1 therapy: results from a single institutional database. Acta Oncol 2018;57:867–72. 10.1080/0284186X.2017.1415460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simeone E, Gentilcore G, Giannarelli D, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 2014;63:675–83. 10.1007/s00262-014-1545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soyano AE, Dholaria B, Marin-Acevedo JA, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer 2018;6 10.1186/s40425-018-0447-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1–7. 10.1016/j.lungcan.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 25. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e551 10.1016/S1470-2045(16)30406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zaragoza J, Caille A, Beneton N, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol 2016;174:146–51. 10.1111/bjd.14155 [DOI] [PubMed] [Google Scholar]
- 27. Martens A, Wistuba-Hamprecht K, Geukes Foppen M, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 2016;22:2908–18. 10.1158/1078-0432.CCR-15-2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced Non–Small cell lung cancer. JAMA Oncol 2018;4:351–7. 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diakos CI, Charles KA, McMillan DC, et al. Cancer-Related inflammation and treatment effectiveness. Lancet Oncol 2014;15:493–503. 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 30. Dolan RD, Laird BJA, Horgan PG, et al. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: a systematic review. Crit Rev Oncol Hematol 2018;132:130–7. 10.1016/j.critrevonc.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 31. Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39:782–95. 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 32. Kirilovsky A, Marliot F, El Sissy C, et al. Rational bases for the use of the immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol 2016;28:373–82. 10.1093/intimm/dxw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333–9. 10.1038/nature12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 2017;168:542 10.1016/j.cell.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 35. Diem S, Kasenda B, Martin-Liberal J, et al. Prognostic score for patients with advanced melanoma treated with ipilimumab. Eur J Cancer 2015;51:2785–91. 10.1016/j.ejca.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 36. Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer 2016;114:256–61. 10.1038/bjc.2015.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Black S, Kushner I, Samols D. C-Reactive protein. J Biol Chem 2004;279:48487–90. 10.1074/jbc.R400025200 [DOI] [PubMed] [Google Scholar]
- 38. Oya Y, Yoshida T, Kuroda H, et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget 2017;8:103117–28. 10.18632/oncotarget.21602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shibata Y, Shimokawaji T, Kondo T, et al. C-Reactive protein (CRP) as a predictive marker for survival in patients with advanced non-small cell lung cancer (NSCLC) treated with first line pembrolizumab monotherapy. JCO 2018;36(15_suppl):e21106 10.1200/JCO.2018.36.15_suppl.e21106 [DOI] [Google Scholar]
- 40. Brustugun OT, Sprauten M, Helland A. C-Reactive protein (CRP) as a predictive marker for immunotherapy in lung cancer. JCO 2016;34(15_suppl):e20623 10.1200/JCO.2016.34.15_suppl.e20623 [DOI] [Google Scholar]
- 41. Kaplanov I, Carmi Y, Kornetsky R, et al. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci USA 2019;116(4):1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000531supp001.pdf (157.1KB, pdf)
esmoopen-2019-000531supp002.pdf (156.2KB, pdf)
esmoopen-2019-000531supp003.pdf (130.4KB, pdf)