Abstract
The definition of ‘head and neck cancer’ (HNC) identifies squamous cell carcinoma arising from the pharynx, the larynx and the oral cavity. Most of them are induced by smoking and alcohol abuse, but tumours arising in the nasopharynx and in the oropharynx may be virus induced, Epstein-Barr virus and human papillomavirus, respectively. Medical oncologists are involved in HNC in locally advanced disease and in relapsed/metastatic disease not suitable for salvage radiotherapy or surgery. A close cooperation with surgeons and in particular with radiation oncologists is required in the first situation. The second situation is almost completely responsibility of medical oncologists while surgeons and radiation oncologists are involved in specific situations requiring palliative treatments. Interventions in locally advanced diseases change according to the goal of treatment. Indeed, the target may be the cure of patients unresectable disease or that have refused surgery, the adjuvant treatment of resected diseases at high risk of relapse, or organ preservation, which means sparing demolitive surgery requiring severe functional impairment, such as definitive laryngectomy. In all these situations, a close cooperation between the medical oncologist and the radiation oncologist is mandatory. Treatment of relapsed/metastatic disease is rapidly changing due to the development of immunotherapy. Although the results of immune checkpoint inhibitors in HNC are less impressive than in other tumours such as melanoma or lung cancer, these drugs are effective and allow for long-term survivors that were not expected with chemotherapy and target therapy. In particular, first-line treatment will change soon. Indeed, due to the result of a large randomised trial, immunotherapy will replace the combination of cisplatin, fluorouracil and cetuximab at least in a large proportion of patients.
Keywords: how i treat cancer
Introduction
Head and neck cancer (HNC) is a complex tumour that develops in an area rich in organs critical for social life and survival: the upper tract of air and digestive ways. It requires an expert multidisciplinary team able to face the multiple problems induced by the tumour and by the treatments. There is evidence that patients followed by such a team have better outcome compared with those treated by a single specialist.1 The clinical situations requiring a multidisciplinary team are described in figure 1.
Figure 1.
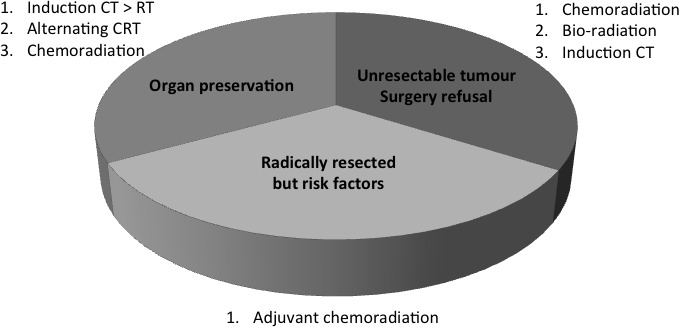
Locally advanced head and neck cancer: clinical situations and treatment.
While surgeons or radiation oncologists can easily manage early stage HNC, namely those without nodal involvement and with limited extension of the ‘T’, locally advanced disease (LA-HNC) and relapsed and metastatic disease (R/M-HNC) represent a major clinical issue.
Lastly, human papillomavirus (HPV)-related oropharyngeal cancers represent a subset of tumours with a better prognosis compared with the smoking-induced and alcohol-induced cancers. The best treatment of these tumours is still to be defined.
Locally advanced unresectable disease
This situation includes tumours with large primary mass and/or nodal involvement, considered unresectable because surgery cannot assure a high cure rate and requires major demolition, or because patient’s refusal.
In these cases concurrent chemoradiation (CCRT) is the first choice of treatment.2
The best drug to combine with concurrent radiotherapy is cisplatin (100 mg/m2 every 21 days for three courses).2 The best radiation regimen to combine with cisplatin is the standard 2 Gy daily, 5 days per week, per seven consecutive weeks.2
There is no evidence that altered fractionation radiotherapy may improve results over standard fractionation.3 Similarly, randomised trials failed to show benefits by weekly cisplatin compared with every 3 weeks.4 However, CCRT is a heavy treatment, hampered by a high mortality rate even in selected patients.5 Patients unfit for cisplatin or considered at high risk of severe toxicity (affecting the result of CCRT) can be treated with induction chemotherapy2 or with radiation and cetuximab. There is no formal comparison between these two approaches in HPV-negative tumours, and they are both evidence based. Therefore, the choice should be driven by the physician expertise, case by case. Patients unsuitable for cisplatin will not be treated with induction chemotherapy since cisplatin is a fundamental component of the induction chemotherapy in HNC. Cetuximab should be administered with caution in patients with a history of heavy smoking, alcohol abuse and allergy because of the elevated risk of infusion reactions in patients with all these risk factors.6
Adjuvant therapy
Historical adjuvant therapy after surgery is radiotherapy. Data from the Surveillance Epidemiology and End Results (SEER) report an overall survival benefit of 11% by the addition of radiotherapy to surgery in LA-HNC.7 However, in case of nodal extracapsular extension or positive/close margins of resection, adjuvant CCRT further increases survival of 12.5%.8
Adjuvant CCRT is similar to the schema used for radical purpose, based on the same cisplatin regimen and radiation fractionation, but with a lower cumulative dose of radiotherapy (66 Gy).
Organ preservation
LA-HNC may require major demolitive surgery. This is the case of large larynx or hypopharynx tumours. The combination of chemotherapy and radiotherapy may spare major surgery. Early studies compared induction chemotherapy followed by radiation versus the classical treatment made up of surgery followed by radiotherapy. These studies confirmed that induction chemotherapy followed by radiotherapy is feasible with similar outcome to the standard treatment.
Up to date three different chemoradiation combinations are available.9 They all originate from the last generation of organ preservation studies: the Radiation Therapy Oncology Group (RTOG) 91–11 and the European Organization for Research and Treatment of Cancer (EORTC) 24954.
The RTOG 91–11 was a three-arm trial comparing CCRT, induction chemotherapy followed by radiotherapy and radiotherapy alone. The study accrued patients with advanced larynx tumours and the primary objective was laryngectomy-free survival. Both CCRT and induction chemotherapy showed significant higher benefit in laryngectomy free survival compared with radiotherapy alone, but long-term analysis revealed a trend towards a poorer survival of CCRT compared with both induction chemotherapy and radiotherapy alone.10
The EORTC 24954 was a two-arm trial comparing induction chemotherapy followed by radiation versus alternating chemoradiation The study accrued patients with advanced larynx or hypopharynx cancers and the primary objective was survival with functional larynx.
The long-term update of the study showed that the two treatments had similar survival with functional larynx, similar overall survival and similar late toxicity, although a trend towards higher larynx preservation and better laryngeal function favours alternating chemoradiotherapy.11 Unfortunately, alternating chemoradiotherapy requires a complex organisation in particular for the radiotherapy departments.
Based on the above-reported studies, induction chemotherapy followed by radiotherapy is the preferred approach when organ preservation is the goal of treatment.
In this case, induction chemotherapy should be the combination of cisplatin, fluorouracil and docetaxel, based on the study by Pointreau et al.12
HPV-related oropharyngeal cancers
These tumours have a better prognosis compared with those smoking or alcohol related. It must be stressed that this favourable outcome is limited to HPV-positive oropharyngeal tumours, and in particular in the basis of the tongue and in the tonsils.
Considering both the good prognosis of these tumours and the high toxicity of the treatment, many studies are investigating de-intensification therapy.
Two of these studies have been presented in October 2018 and published by Mehanna et al and Gillison et al. Both trials compared CCRT versus cetuximab plus radiotherapy and failed to reach their main objective: reduction of toxicity in the cetuximab–radiotherapy arm while preserving similar efficacy of CCRT. In particular, the RTOG 1016 showed that cetuximab plus radiotherapy arm experienced significantly lower overall survival and progression-free survival compared with CCRT.13
Therefore, de-intensification through the substitution of cisplatin with cetuximab is not allowed and CCRT remains the standard of care in HPV-positive oropharyngeal tumours, at the present.
Relapsed/metastatic HNC
Till now, the EXTREME regimen is the standard first-line chemotherapy regimen for RM-HNC.
EXTREME is based on the combination of cisplatin, 5-fluorouracil and cetuximab.14 It is the first regimen achieving a significant survival benefit over the combination of cisplatin and fluorouracil developed by Al-Sarraf in the early 1980s. Immunotherapy with anti-programmed death ligand 1 (PD-L1) - PD-1 axis monoclonal antibodies has emerged as the only evidence-based second-line treatment of R/M-HNC.15 Although progression-free survival is rarely improved, namely only in patients with high-inflamed tumours, there is a clear advantage in overall survival including long-lasting survivors. This advantage is limited to a small cohort of patients, but was never seen before with any of the treatments tested in second line.
However, the KN-048 study presented by Burtness et al during the 2018 ESMO meeting in Munich will change the scenario of the R/M-HNC soon.
The KEYNOTE 048 trial is a complex three-arm study comparing first-line single agent pembrolizumab to the EXTREME regimen and the combination of cisplatin, 5-fluorouracil and pembrolizumab to the EXTREME.
Data reported during the conference mainly pointed out the first comparison
Considering patients with PD-L1 combined positivity score (CPS) ≥1 (85% of the whole population) pembrolizumab shows superior overall survival compared with EXTREME (p<0.009; 24-month survival rate 30.2% vs 18.6%). This advantage become stronger limiting the population to patients showing CPS≥20 (44% of the whole population), representing more inflamed tumours (p=0.0007; 24-month survival rate 38.3% vs 22.1%). Based on these data, immunotherapy will move to first-line treatment in R/M-HNC in the near future at least in a portion of patients.
Conclusions
Early HNC is easily managed with surgery and radiotherapy, but LA-HNC represents a problematic disease requiring an expert multidisciplinary staff. Unresectable tumours should be faced with the combination of chemotherapy and radiotherapy, and the same combination plays a fundamental role in different situations such as definitive treatment, adjuvant treatment and organ preservation treatment.
In recent years, the best improvement has been observed in R/M-HNC due to the introduction of immune therapy. Immune checkpoint inhibitors in HNC offer benefits less impressive than in other tumours such as melanoma or lung cancer; however, these drugs are effective and allow long-term survivors not expected with chemotherapy and target therapy. Recent data will favour the introduction of immune therapy in the first-line treatment.
Footnotes
Contributors: MM defined the article schema and its organisation and wrote the manuscript. ND and DG review the literature and contribute in manuscript writing. OG review and edited the manuscript. All the authors approved the manuscript.
Funding: MM received payment for speaking activities by BMS, Merck-serono; conference travel grant from Merck Serono and BMS; research funding by Merck Serono.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Licitra L, Keilholz U, Tahara M, et al. Evaluation of the benefit and use of multidisciplinary teams in the treatment of head and neck cancer. Oral Oncol 2016;59:73–9. 10.1016/j.oraloncology.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 2. Pignon J-P, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 3. Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the radiation therapy oncology group 0129 trial: long-term report of efficacy and toxicity. JCO 2014;32:3858–67. 10.1200/JCO.2014.55.3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noronha V, Joshi A, Patil VM, et al. Once-a-Week Versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. JCO 2018;36:1064–72. 10.1200/JCO.2017.74.9457 [DOI] [PubMed] [Google Scholar]
- 5. Merlano MC, Monteverde M, Colantonio I, et al. Impact of age on acute toxicity induced by bio- or chemo-radiotherapy in patients with head and neck cancer. Oral Oncology 2012;48:1051–7. 10.1016/j.oraloncology.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 6. Palomar Coloma V, Bravo P, Lezghed N, et al. High incidence of cetuximab-related infusion reactions in head and neck patients. ESMO Open 2018;3:e000346 10.1136/esmoopen-2018-000346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kao J, Lavaf A, Teng MS, et al. Adjuvant radiotherapy and survival for patients with node-positive head and neck cancer: an analysis by primary site and nodal stage. Int J Radiat Oncol Biol Phys 2008;71:362–70. 10.1016/j.ijrobp.2007.09.058 [DOI] [PubMed] [Google Scholar]
- 8. Winquist E, Oliver T, Gilbert R, et al. Postoperative chemoradiotherapy for advanced squamous cell carcinoma of the head and neck: A systematic review with meta-analysis. Head Neck 2007;29:38–46. 10.1002/hed.20465 [DOI] [PubMed] [Google Scholar]
- 9. Denaro N, Russi EG, Lefebvre JL, et al. A systematic review of current and emerging approaches in the field of larynx preservation. Radiother Oncol 2014;110:16–24. 10.1016/j.radonc.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 10. Forastiere AA, Zhang Q, Weber RS, et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients With Locally Advanced Larynx Cancer. JCO 2013;31:845–52. 10.1200/JCO.2012.43.6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henriques De Figueiredo B, Fortpied C, Menis J, et al. Long-term update of the 24954 EORTC phase III trial on larynx preservation. Eur J Cancer 2016;65:109–12. 10.1016/j.ejca.2016.06.024 [DOI] [PubMed] [Google Scholar]
- 12. Pointreau Y, Garaud P, Chapet S, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 2009;101:498–506. 10.1093/jnci/djp007 [DOI] [PubMed] [Google Scholar]
- 13. Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. The Lancet 2019;393:40–50. 10.1016/S0140-6736(18)32779-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–27. 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 15. Denaro N, Merlano MC. Immunotherapy in Head and Neck Squamous Cell Cancer. Clin Exp Otorhinolaryngol 2018;11:217–23. 10.21053/ceo.2018.00150 10.21053/ceo.2018.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]


