Abstract
Introduction
In the era of precision medicine, research studies are aiming to design patient-tailored treatment strategies. In this work, we present a clinical case of a patient with non-small cell lung cancer (NSCLC) accompanied by a translational study with the intent to assess the correspondence of drug sensitivity in ex vivo spheroidal tumour cultures and peripheral blood biomarkers with clinical outcome.
Methods
Primary tumour tissue, patient-derived tumour spheroids, peripheral blood mononuclear cells and circulating DNA were analysed to assess drug sensitivity and immunological profiling, and all these data were correlated with clinical and radiological evaluations.
Results
Immunohistochemistry, immunofluorescence, next generation sequencing analysis and T-lymphocyte receptor repertoire assay results showed elevated concordance among primary tumour tissue, ex vivo three-dimensional tumour spheroid specimen and circulating DNA. Cisplatin-based chemotherapy and anti-programmed death 1 drug sensitivity assessed in spheroidal cultures were strictly consistent with patient clinical response to adjuvant chemotherapy and first-line immune therapy.
Conclusion
These results revealed that ex vivo drug sensitivity testing in three-dimensional spheroidal culture can reproduce clinical response to chemotherapy and immunotherapy, with the potential to use those culture models to predict patients‘ outcome from anticancer treatments and, therefore, the feasibility to select individualised therapy.
Keywords: translational research, lung cancer, spheroids culture, immunotherapy
Key questions.
What is already known about this subject?
Individualised research programmes are changing the traditional view about the standard strategy of treatment moving towards a patient-tailored approach.
With the introduction of immunotherapy, it becomes more challenging to establish ex vivo models to study in vitro tumour response.
What does this study add?
To our knowledge this is the first example of an ‘avatar approach’ in non-small cell lung cancer able to demonstrate the correspondence between the treatment response in ex vivo cultures and clinical outcome.
We demonstrated that our model of tumour spheroids maintains original tumour immune profile and genetic profile, further validating them as a good model to test drug sensitivity.
How might this impact on clinical practice?
We are proposing a new tool of immediate impact that could be used prospectively in a larger cohort of patients to validate the ability of ex vivo tumour spheroids as a system to guide personalised therapeutic choices.
Background
Non-small cell lung cancer (NSCLC) comprises about 80% of all lung malignancies and, unfortunately, the survival rate of 5 years remains below 15%.1 The reason for this low survival rate is mostly due to the variegated tumour response to therapy, the evolution of tumour under treatment pressure and the lack of biomarkers driving the choice of the best sequence of treatments for any single patient. In the era of precision medicine, individualised research programmes are changing the traditional view about the standard strategy of treatment moving towards a patient-tailored approach.
Effective therapy should be theoretically designed after careful in vitro and in vivo screening of sensitivity of patient’s cancer cells to a repertoire of drugs in order to choose the best strategy for each patient based on a personal analysis.2 3
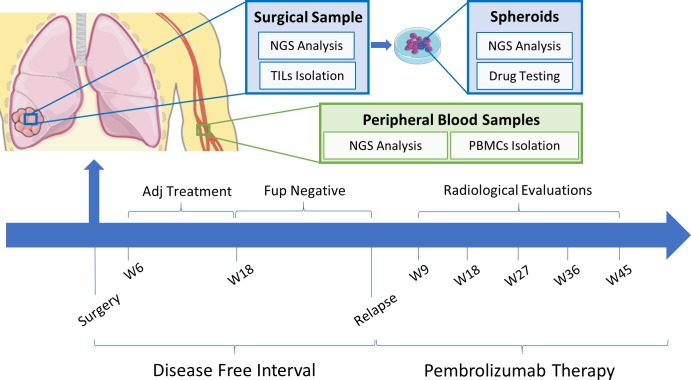
However, with the introduction of immunotherapy, it becomes even more challenging to establish ex vivo models to study in vitro tumour response, considering the need of autologous T cells to estimate the efficacy of such treatments. In this work, we report a clinical case where treatment choices, among the available national health system approved drugs, have been accompanied in parallel by a translational study: we generated patient-derived tumour spheroids, as previously described by our group,4–6 with the intent to assess the correspondence of ex vivo sensitivity with clinical response to specific drugs. To this end, all results from our ex vivo experiments were correlated with the clinical and radiological evaluation of the patient during treatments (figure 1). For material and methods, see online supplementary data-materials and methods.
Figure 1.
Clinical and translational studies timeline. Ex vivo and in vitro experiments and radiological evaluations performed at different time points are shown. Two intervals are described: from the time of surgery to relapse (disease-free interval) and from the start of pembrolizumab therapy thereafter (pembrolizumab therapy). Time from the start of therapy: W6, 6 weeks, W18, 18 weeks, W9, 9 weeks, W18, 18 weeks, W27, 27 weeks, W36, 36 weeks, W45, 45 weeks. Adj, adjuvant; Fup, follow-up; NGS, next generation sequencing; PBMC, peripheral blood mononuclear cells.
esmoopen-2019-000536supp001.pdf (92.7KB, pdf)
The aim of this study was to investigate in a real-life scenario the potentiality of an ex vivo lung cancer patient three-dimensional (3D) model to foresee treatment response to standard treatments including chemotherapy and immunotherapy. The application of this 3D culture model is innovative as a practical tool for individualised therapies, especially in patients with NSCLC undergone to surgical treatment and at high risk of relapse.
Case report
A white female patient aged 51 years underwent basal right lobectomy and lymph-node dissection (including stations 10 and 11, according to the International Association for the Study of Lung Cancer (IASLC) classification)7 for suspected lung neoplasm. At the time of surgery, on patient-specific consent, peripheral blood samples were collected for research purpose and a specimen of primary tumour was used to generate an ex vivo NSCLC spheroid culture. Pathological report defined a pT3N1 lung adenocarcinoma, stage IIIA per TNM classification seventh edition. Standard molecular profile for non-squamous NSCLC was performed and results demonstrated an epidermal growth factor receptor (EGFR) wild type, anaplastic lymphoma kinase (ALK) and ROS-1 negative disease, with a programmed death 1 (PD-1) ligand (PD-L1) expression of 90%. Before surgery, new generation sequencing (NGS) analysis on circulating tumour DNA (ctDNA) was performed without new relevant information for treatment strategy.
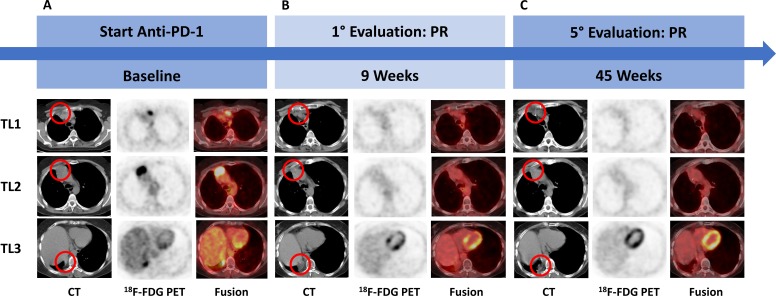
According to international guidelines for this pathological stage of the disease, adjuvant treatment with a platinum-based regimen, consisting of cisplatin 75 mg/m2 day 1 plus vinorelbine 30 mg/m2 day 1 and day 8 every 21 days for four cycles, was started at 6 weeks from surgery. After 11 months from surgery, clinical conditions of the patient worsened, with the onset of chest pain and fatigue and a total body CT scan showed new lymph node and mediastinal tumour lesions. Multidisciplinary committee recommended a first-line therapy with an anti-PD-1 drug. At baseline radiological assessment, three target lesions according to Response Evaluation Criteria in Solid Tumours V.1.1 (RECIST 1.1) were observed: a lymph-node located behind the sternal manubrium of 22 mm in shorter perpendicular axis (target lesion (TL) 1), a wide upper mediastinal lesion of 42 mm in the longest diameter (TL2) and a supradiaphragmatic costovertebral lesion of 28 mm in the longest diameter (TL3) (figure 2A). At a 2-deoxy-2-(fluorine-18)fluoro-D-glucose integrated with CT (18F-FDG positron emission tomography (PET)/CT) scan, a 18F-FDG standardised uptake value of 4.54 for the manubrium lesion, of 11.36 for the upper mediastinal lesion and 6.44 for the supradiaphragmatic one were detected. At first radiological assessment at 9 weeks (W9), imaging showed a partial response (PR) according to RECIST V.1.1 (figure 2B) and no pathological 18F-FDG uptake was found. At next assessments, at 18, 27, 36 and 45 weeks, PR was confirmed and 18F-FDG PET/CT continued to be negative (figure 2C). At the time of writing this article, the patient was still on treatment.
Figure 2.
Radiological evaluation timeline. (A) Start anti-PD-1 treatment, (B) first assessment at 9 weeks and (C) last evaluation visit at 45 weeks. 18F-FDG PET, 2-deoxy-2-(fluorine-18)fluoro-D-glucose positron emission tomography; fusion, 18F-FDG PET/CT fusion image; PD-1, programmed death 1; PR, partial response; TL, target lesion.
Features and drug sensitivity of patient-derived spheroids
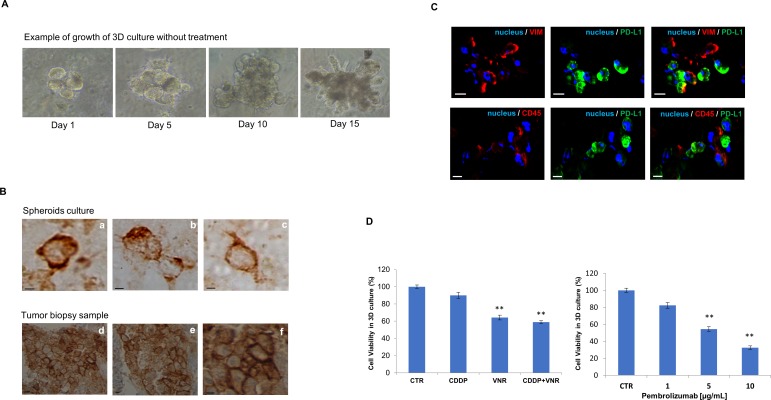
We established 3D ex vivo cultures, as spheroids, from the patient’s surgical sample to estimate the feasibility of an in vitro molecular approach capable of mimicking tumour status ex vivo. The ex vivo growth capacity of patient derived spheroids achieved a minimum diameter of 90 µm 1 week after sowing in matrigel (figure 3A) and continued to grow allowing drug testing for the following 2 weeks, similarly to our previous study in similar models.4
Figure 3.
Example of growth of 3D culture after seeding in Matrigel in the timeline of 14 days without treatment (A) and localisation of PD-L1 protein by immunohistochemistry both in spheroids (B, a–c) and in lung biopsy sample (B, d–f). Scale bar: 10 µm. Localisation by immunofluorescence of PD-L1, vimentin and CD45 markers in patient-derived tumour spheroids (C). Images represent different tumour spheroids deriving from the same patient sample: 4′,6-diamidino-2-phenylindole-fluorescent nuclear staining (blue), vimentin and CD45 staining (VIM and CD45, red), PD-L1 staining (green). Scale bar: 20 µm. Results of 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide assay in 3D ex vivo culture from the patient's surgical sample with indicated treatments. Results are shown as analysis of cell proliferation during treatments (D). Dose–response curve of pembrolizumab. Data are the median of three separate experiments, asterisks indicate significance differences (**p<0.05). 3D, three dimensional; CDDP, cisplatin; CTR, untreated control; PD-L1, programmed death1 ligand; VNR, vinorelbine.
We first verified the presence of PD-L1 by localising the protein by immunohistochemistry both in the spheroids (figure 3B,a–c) and in the lung biopsy sample (figure 3B,d–f). A high expression of PD-L1 of about 90% was found in both samples. As shown in figure 3C, immunofluorescence detected high levels of expression of both PD-L1 and vimentin in the primary tumour (as documented in the pathology report) as well as in tumour spheroids. We next demonstrated that patient-derived tumour spheroids were made of a mixture of cancer cells as well as of immune infiltrating cells, with both CD45+ cells and PD-L1 cells (figure 3C). NGS analysis showed an elevated concordance in both primary tumour tissue, tumour spheroids and ctDNA isolated from patient plasma sample collected at baseline, with a TP53 splice site single nucleotide variant and the KRAS G12V mutation in all three samples (table 1). Collectively, these results confirmed that patient-derived spheroids maintain the primary tumour histology, immune and genetic phenotype, therefore, opening the possibility to use this model to predict response to therapy. To evaluate the ability of such ex vivo model to reflect the sensitivity to specific treatments, we performed an initial drug testing by 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide proliferation assay on patient-derived tumour spheroids with standard chemotherapies which are commonly used in the adjuvant treatment of lung cancer, such as cisplatin and vinorelbine. After 6 days of treatment, we observed a marginal antiproliferative effect by cisplatin monotherapy as compared with vinorelbine. In particular, the vitality of the spheroids was reduced by 10.1% and 35.8%, respectively, while the combination of the two drugs resulted in a more effective spheroid growth inhibition (41.1%), suggesting that the combined treatment could be a better treatment option (figure 3D).
Table 1.
NGS analysis on patient samples
| Sample | Alteration | MF (%) |
| Surgical specimen |
KRAS G12V | 21.12 |
| TP53 Splice Site SNV | 95.7 | |
| Spheroids |
KRAS G12V | 45.6 |
| TP53 Splice Site SNV | 97.1 | |
| Circulating tumour DNA |
KRAS G12V | 39.1 |
| TP53 Splice Site SNV | 17.5 |
MF, molecular fraction; NGS, next generation sequencing; SNV, single nucleotide variant.
Similarly, we also tested pembrolizumab and found that it reduced the viability of tumour spheroids in a dose-dependent manner, reaching the maximum of antiproliferative activity at 10 µg/mL dose (figure 3D), which correspond to the therapeutic dose for clinical use of pembrolizumab.8 9 Of interest, reverse transcription-PCR on messenger RNA extract from treated spheroids confirmed an increased transcript of interferon-γ and CD107a upregulation (a degranulation marker) after pembrolizumab treatment, thus, confirming an engagement of infiltrating T cells in this response.10
Immunological profiling of patient peripheral blood samples
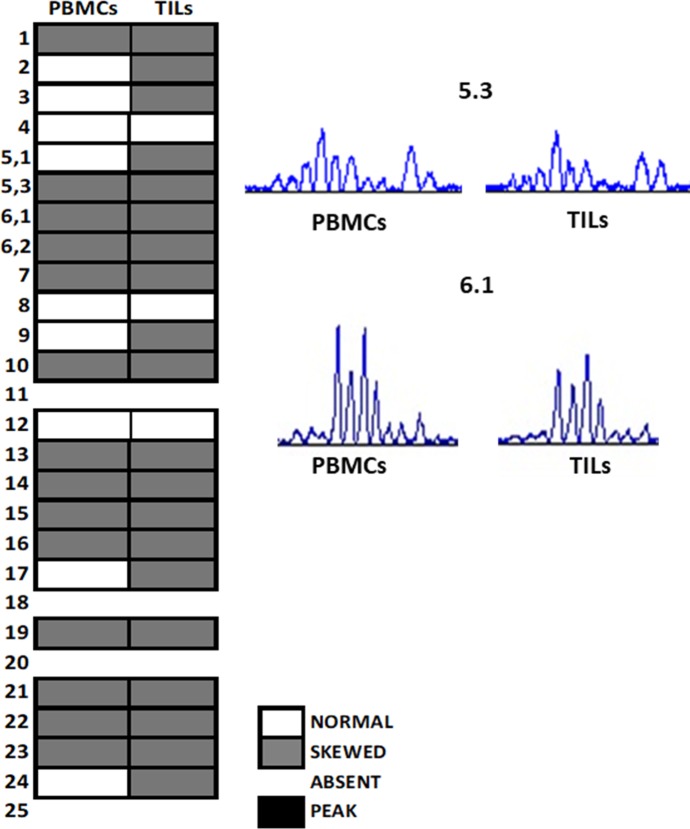
Recently, numerous studies have demonstrated the prognostic value of the presence of inflammatory cells infiltrating tumours, but very few data are available on the potential predictive role of T-lymphocyte receptor (TCR) repertoire for immunotherapy response. To better investigate lymphocytes’ characteristics and activation, both in periphery and in the tumour microenvironment, we analysed the TCR repertoire through a spectratyping assay from both peripheral blood mononuclear cells (PBMCs) and tumour-infiltrating lymphocytes (TILs); this technique allows to discriminate the clonotype length and nucleotide sequences of the TCR repertoire. We studied the CDR3 region of each beta variable chain, which is the most involved in antigen recognition. This analysis shows the PCR products as a series of peaks (chromatograms): a normal condition is displayed with a Gaussian distribution, while alterations of this distribution (skewing) represent anomalies in the TCR family and are synonymous of expansion of T cell clones induced by an antigen. In the samples analysed, we found that many TCR families were skewed and often the same alterations were present in both PBMCs and TILs, as shown in figure 4 for families 5.3 and 6.1. These results suggest a potential role of PBMCs’ TCR alterations as a surrogate for studying the TCR alterations in TILs; moreover, they further confirm the presence of tumour lymphocyte infiltration, which has the same TCR profile of the peripheral lymphocytes. These data suggest that lymphocyte response is specific for any antigen, underlying a pre-primed T cell response. Further analysis should be conducted to analyse the exact function of these family’s sequences alterations.
Figure 4.
Spectratypes showing BV-TCR subfamilies from PBMCs and TILs. Synoptic diagram of TCR repertoire analysed in peripheral blood and TILs of the patient. BV families were considered. The white box represents a Gaussian chromatogram (normal distribution); the grey box indicates a skewed repertoire (polyclonal response); the empty box represents the absence of amplification. Some families of PBMCs and TILs showed very similar alterations, as shown in chromatograms of 5.3 and 6.1 BV families (on the right), where the same clones seem to be expanded. BV, beta variable; PBMC, peripheral blood mononuclear cells; TCR, T lymphocyte receptor; TIL, tumour-infiltrating lymphocytes.
Discussion
Current first-line treatment decisions for advanced NSCLC are guided by the presence of molecular driver as sensitising mutations of EGFR11 or translocations of ALK and by the presence of a high PD-L1 expression which is defined as membranous PD-L1 expression on at least 50% of tumour cells.12 13 This approach to treatment strategy is still based on the results of large phase II–IV clinical trials that provide a great magnitude of clinical evidence on patients’ outcomes and toxicity, but do not account for interpatient variabilities.
Given the large variety of response to all potential therapies for this disease, it is presently challenging to predict whether an individual patient will be sensitive to specific drugs, especially to immunotherapy, where the T cell tumour interactions is patient specific and what alternative treatment could potentially overcome resistance. Here, we provide the proof of concept that tumour spheroids can be used to establish individualised ex vivo model systems for drug sensitivity with a successful establishment rate of culturing method of 64% as previously reported.4 This is of immediate impact, as it may offer a way to predict response to specific chemotherapies and, more importantly, to immunotherapy. First, we have demonstrated the role of tumour spheroids as surrogates of tumour context as immune and genetic profiles completely resemble those of primary tumour, thus, validating them as a model to test drug sensitivity.
This approach represents one of the first attempt of ex vivo evaluation for immunotherapy. One recent work has been a pioneer in testing sensitivity to immunotherapy by co-culturing autologous tumour organoids and peripheral blood lymphocytes in order to enrich tumour-reactive T cells and assess their efficiency in killing matched tumour organoids.14 Additionally, another experience has validated the use of tumour spheroids retaining relevant tumour infiltrating lymphoid and myeloid subpopulations to analyse ex vivo response to immunotherapy.15 However, both approaches did not investigate a comparison between sensitivity to chemotherapy or immunotherapy into the same patient-derived culture models. Despite the statistical disadvantage of a single case report and the costs related to this translational approach, to our knowledge, this is the first example of an ‘avatar approach’ in NSCLC able to demonstrate the correspondence between the ex vivo and clinical scenarios.
Guided by molecular analysis performed at the time of surgery that showed a high PD-L1 expression, we conducted ex vivo experiments to assess the efficacy of both chemotherapy and immunotherapy in patient-derived tumour spheroid culture.
In concordance with clinical response, in patient-derived 3D culture, we did not observe a significant response to standard cisplatin-based chemotherapy commonly used in adjuvant setting in NSCLC, while we saw a high sensitivity to the anti-PD-1 drug pembrolizumab.
Moreover, we verified the TCR expression pattern and observed the same clonality both in the PBMC and TIL samples. Although the specific role of such subfamilies is not still well known, these data leaves us to hypothesise that the TCR alterations that we found may represent a functional and active immune system even before the beginning of the therapy that correlate with immune response.
The case of our patient represents a paradigmatic situation where the poor prognosis of the tumour, at high risk of relapse, may benefit of preliminary drug sensitive testing in order to programme the best treatment choice.
In conclusion, our pilot translational study on a NSCLC clinical case defines a new translational approach for identification of a tailored therapy that can be potentially carried on for every patient that undergo surgical resection or core biopsies. We are proposing a new tool that could be tested prospectively in a larger cohort of patients to validate the ability of ex vivo tumour spheroids as a system to anticipate and monitor response to different treatment and as a new way to guide personalised therapeutic treatment choices.
Footnotes
Contributors: RDL, FC and FM designed the study and wrote the manuscript. VC, GB, CMDC and FP performed in vitro experiments, did data analysis and made the figures, FS, GV, MLI and MF collected clinical data. MV and SM performed immunofluorescence experiments and relative data analysis. FM and FC gave contribution to writing and revising the manuscript and the figures. All authors read and approved the final manuscript.
Competing interests: FC: Advisory Boards: Roche, Amgen, Merck, Pfizer, Sanofi, Bayer, Servier, BMS, Cellgene, Lilly; Institutional Research Grants: Bayer, Roche, Merck, Amgen, AstraZeneca, Ipsen. FM: Advisory Boards MSD, Lilly; Institutional Research Grants: AstraZeneca.
Patient consent for publication: Obtained.
Ethics approval: All human samples were collected after obtaining a written informed consent from patient, in accordance with the Declaration of Helsinki. The use of these samples for research purposes was approved by our local Ethical Committee of Università della Campania ‘Luigi Vanvitelli’.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584–94. 10.1016/S0025-6196(11)60735-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cufer T, Knez L. Update on systemic therapy of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther 2014;14:1189–203. 10.1586/14737140.2014.940327 [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Karachaliou N. Lung cancer in 2014: optimizing lung cancer treatment approaches. Nat Rev Clin Oncol 2015;12:75–6. 10.1038/nrclinonc.2014.225 [DOI] [PubMed] [Google Scholar]
- 4.Della Corte CM, Barra G, Ciaramella V, et al. Antitumor activity of dual blockade of PD-L1 and MEK in NSCLC patients derived three-dimensional spheroid cultures. J Exp Clin Cancer Res. In Press2019;38 10.1186/s13046-019-1257-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orditura M, Della Corte CM, Diana A, et al. Three dimensional primary cultures for selecting human breast cancers that are sensitive to the anti-tumor activity of ipatasertib or taselisib in combination with anti-microtubule cytotoxic drugs. Breast 2018;41:165–71. 10.1016/j.breast.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Ciaramella V, Sasso FC, Di Liello R, et al. Activity and molecular targets of pioglitazone via blockade of proliferation, invasiveness and bioenergetics in human NSCLC. J Exp Clin Cancer Res 2019;38 10.1186/s13046-019-1176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Sherief AH, Lau CT, Wu CC, et al. International association for the study of lung cancer (IASLC) lymph node map: radiologic review with CT illustration. Radiographics 2014;34:1680–91. 10.1148/rg.346130097 [DOI] [PubMed] [Google Scholar]
- 8.Lindauer A, Valiathan CR, Mehta K, et al. Translational pharmacokinetic/pharmacodynamic modeling of tumor growth inhibition supports dose-range selection of the anti-PD-1 antibody pembrolizumab. CPT Pharmacometrics Syst Pharmacol 2017;6:11–20. 10.1002/psp4.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein DA, Gordon N, Davidescu M, et al. A phamacoeconomic analysis of personalized dosing vs fixed dosing of pembrolizumab in firstline PD-L1-positive non-small cell lung cancer. J Natl Cancer Inst 2017;109 10.1093/jnci/djx063 [DOI] [PubMed] [Google Scholar]
- 10.Della Corte CM, Barra G, Ciaramella V, et al. 1851PThe combination of MEK inhibitor and anti PD-L1: effects on organoid models from NSCLC biopsies. Ann Oncol 2018;29(suppl_8):viii649–69. 10.1093/annonc/mdy303.021 [DOI] [Google Scholar]
- 11.Morgillo F, Della Corte CM, Fasano M, et al. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open 2016;1:e000060 10.1136/esmoopen-2016-000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 13.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 14.Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 2018;174:1586–98. 10.1016/j.cell.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins RW, Aref AR, Lizotte PH, et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov 2018;8:196–215. 10.1158/2159-8290.CD-17-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000536supp001.pdf (92.7KB, pdf)