Abstract
Background
Increasing antimicrobial resistance among pathogens that cause complicated intraabdominal infections (cIAIs) supports the development of new antimicrobials. Eravacycline, a novel member of the fluorocycline family, is active against multidrug-resistant bacteria including extended-spectrum β-lactamase (ESBL) and carbapenem-resistant Enterobacteriaceae.
Methods
IGNITE4 was a prospective, randomized, double-blind trial. Hospitalized patients with cIAI received either eravacycline 1 mg/kg every 12 hours or meropenem 1 g every 8 hours intravenously for 4–14 days. The primary objective was to demonstrate statistical noninferiority (NI) in clinical cure rates at the test-of-cure visit (25–31 days from start of therapy) in the microbiological intent-to-treat population using a NI margin of 12.5%. Microbiological outcomes and safety were also evaluated.
Results
Eravacycline was noninferior to meropenem in the primary endpoint (177/195 [90.8%] vs 187/205 [91.2%]; difference, –0.5%; 95% confidence interval [CI], –6.3 to 5.3), exceeding the prespecified margin. Secondary endpoints included clinical cure rates in the modified ITT population (231/250 [92.4%] vs 228/249 [91.6%]; difference, 0.8; 95% CI, –4.1, 5.8) and the clinically evaluable population (218/225 [96.9%] vs 222/231 [96.1%]; (difference, 0.8; 95% CI –2.9, 4.5). In patients with ESBL-producing Enterobacteriaceae, clinical cure rates were 87.5% (14/16) and 84.6% (11/13) in the eravacycline and meropenem groups, respectively. Eravacycline had relatively low rates of adverse events for a drug of this class, with less than 5%, 4%, and 3% of patients experiencing nausea, vomiting, and diarrhea, respectively.
Conclusions
Treatment with eravacycline was noninferior to meropenem in adult patients with cIAI, including infections caused by resistant pathogens.
Clinical Trials Registration
Keywords: complicated intraabdominal infection, eravacycline, multidrug resistance, gram-negative bacteria, Enterobacteriaceae
This phase 3 trial compared eravacycline to meropenem for treatment of complicated intraabdominal infections. Eravacycline was noninferior to meropenem. High rates of presumed microbiological eradication were found with both regimens.
Gram-positive and gram-negative organisms with novel antimicrobial resistance mechanisms have been highlighted as a pressing global public health threat by learned societies, the World Health Organization, and government agencies [1–4]. For gram-negative organisms, a common resistance mechanism is the production of extended-spectrum β-lactamases (ESBLs) that deactivate most penicillin and cephalosporin molecules [5]. Estimates of morbidity, mortality, and cost stemming from complications caused by these resistant organisms are striking [6, 7]. The emergence of carbapenem resistance in Enterobacteriaceae is a particularly significant problem, as carbapenems have been the standard first choice for treating infections due to ESBLs, with few currently available alternatives [8–12].
Global surveys have provided estimates for the extent of the problem [13, 14]. For ESBL-producing Escherichia coli and Klebsiella pneumoniae isolates in Europe, rates of approximately 15% and 30%, respectively, have been detected. In North America, rates are approximately 10% for both isolates [15], relatively low when compared to the 40% detected for ESBL-producing E. coli in Southeast and East Asia and 60%–70% in China [16]. Carbapenemase-producing pathogens are responsible for a rapidly increasing number of clinical infections in specific geographic regions, the result of both clonal expansion and transfer of carbapenemase genes through mobile genetic elements [17, 18]. Other important pathogens, such as Enterococcus faecium and Acinetobacter baumannii, are also routinely multidrug resistant [19, 20]. These troubling epidemiological findings define the need for novel agents active against these bacterial pathogens.
Complicated intraabdominal infections (cIAIs) are defined as consequences of perforations of the gastrointestinal tract that result in contamination of the peritoneal space. If not immediately dealt with, this further results in abscess formation, peritonitis, and sepsis syndromes [21]. They are common occurrences in clinical practice and result in considerable consumption of resources for healthcare facilities and morbidity and mortality in patients. The infecting flora are typically polymicrobial, involving synergistic interactions between gram-positive, gram-negative facultative, aerobic, and anaerobic organisms [21]. Early empiric initiation of antimicrobial therapy effective against the range of infecting pathogens, intended to serve as both prophylaxis for surgical site infections and as therapy for established invasive infections, is established practice and is recommended in current guidelines [22, 23]. These infections have been important in the investigation of new antimicrobials because the diseases encompassed by the term cIAI are acute, come to clinical attention rapidly, and require invasive procedures to control, affording a high likelihood of pathogen identification.
Eravacycline is a novel, fully synthetic fluorocycline antibiotic developed for the treatment of serious infections, including cIAI, that inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit [24]. It retains activity in the presence of common tetracycline-specific acquired-resistance mechanisms (ie, efflux, ribosomal protection) [25] and has potent in vitro activity against a broad range of susceptible and multidrug-resistant gram-positive and gram-negative aerobic and anaerobic strains, including Staphylococcus aureus, E. faecium, E. coli, K. pneumoniae, A. baumannii, and Bacteroides spp. [9, 26–28].
We previously performed a phase 2 study to provide an initial estimate of efficacy at the 2 highest dose regimens explored in normal volunteer studies [29, 30]. A total of 139 patients with confirmed cIAI were randomized (2:2:1) to receive eravacycline 1.5 mg/kg every 24 hours, eravacycline 1 mg/kg every 12 hours, or ertapenem 1 g every 24 hours, for a minimum of 4 days and a maximum of 14 days. The clinical success rates were greater than 90% in each arm. The incidence of treatment-emergent adverse events (TEAEs) was 35.8%, 28.6%, and 26.7%, respectively.
We then conducted a phase 3 trial wherein patients received eravacycline 1 mg/kg every 12 hours or ertapenem 1 g every 24 hours for a minimum of four 24-hour dosing cycles. For the microbiological intent-to-treat (micro-ITT) population, the rates of clinical cure at the test-of-cure (TOC) visit were 86.8% in the eravacycline group and 87.6% in the ertapenem group. The difference in clinical cure rates between the groups was −0.80% (95% confidence interval [CI], −7.1, 5.5), meeting the prespecified noninferiority (NI) margin of 10%.
The current phase 3 trial was undertaken to satisfy US Food and Drug Administration (FDA) requirements for a second trial in this indication. It provided the opportunity to examine the efficacy of eravacycline compared to another broad-spectrum carbapenem, meropenem.
METHODS
Study Design
This was a phase 3, randomized, double-blind, double-dummy, multicenter, prospective trial designed to test the safety and efficacy of eravacycline compared to meropenem in acutely hospitalized patients diagnosed with cIAI requiring operative or percutaneous intervention. Participants for this study were recruited from 65 sites in 11 countries.
The study protocol and all relevant supporting information were submitted to the institutional review board/independent ethics committee at each study site for approval prior to study initiation. The trial was conducted in accordance with Good Clinical Practice as described by the International Council for Harmonisation Guideline and consistent with the World Medical Assembly Declaration of Helsinki.
Participants
Patients aged ≥18 years who were hospitalized for suspected cIAI and able to provide informed consent were considered for inclusion. Exclusion criteria included the following: considered unlikely to survive the 6- to 8-week study period; creatinine clearance <50 mL/min; presence or possible signs of significant hepatic disease; immunocompromised condition; history of hypersensitivity to tetracyclines, carbapenems, or beta-lactams; participation in any investigational drug or device study within 30 days of study entry; known or suspected nervous system disorder that suggests a predisposition to seizures; and receipt of effective antibacterial drug therapy for cIAI for more than 24 hours in the 72 hours prior to randomization. A complete listing of inclusion and exclusion criteria can be found in the Supplementary Materials.
Sample Size
Estimations of cure rates and numbers of participants in the micro-ITT population came from the recent phase 3 study with eravacycline in cIAI (IGNITE1), in which ertapenem was used as the comparator. Using a 12.5% NI margin, 1-sided alpha of 0.025, 80% power, and response rates of 84% in the eravacycline treatment group and 85% in the meropenem treatment group, 161 participants per arm in the micro-ITT population were required. A sample size of approximately 466 randomized participants was then estimated to provide sufficient numbers for this study based upon an evaluability rate of 70%.
Blinding and Randomization
Patients who met all of the inclusion criteria and none of the exclusion criteria were enrolled into the study and randomized using a computer-based randomization scheme. Randomization was stratified based on primary site of infection (complicated appendicitis vs all other cIAI diagnoses). The randomization process incorporated an enrollment cap of 50% for patients with complicated appendicitis.
A designated randomization administrator maintained the randomization codes in accordance with standard operating procedures to ensure that the blind was properly maintained. Only personnel who required knowledge of treatment assignments were unblinded.
Intervention
The patients were enrolled and randomized to 1 of the 2 study arms: intravenous (IV) eravacycline (1 mg/kg every 12 hours) or IV meropenem (1 g every 8 hours). Due to the varying infusion volumes and times for the 2 study drugs, each patient received 5 infusions, all prepared by an unblinded pharmacist or designee. A duration of therapy of 4 to 14 complete dose cycles of the assigned drug was provided at the treating physician’s discretion. The expected duration of patient participation for the study was approximately 6–8 weeks. Treatment duration at study entry was expected to be a minimum of four 24-hour dosing cycles.
Source Control Review
A single surgical reviewer (J. S. S.) examined the records of all patients considered clinical failures, or cures with an unplanned second procedure, or deaths. Source control was considered adequate when the physical measures at operation or drainage were consistent with current local standards of practice to drain infected fluid collections, eliminate the source of infection, control ongoing contamination, and restore gastrointestinal function [31]. Patients who were considered to have had inadequate source control were assigned indeterminate outcomes and were excluded from per-protocol analyses.
Clinical Outcome Assessments and Statistical Analyses
The primary endpoint was the clinical response at the TOC visit 25–31 days after initiation of the study drug in the micro-ITT population, as required by the FDA. As eravacycline had demonstrated NI at a 10% NI margin in the IGNITE1 study, an NI margin of 12.5% was used in IGNITE4 as agreed to by the FDA. This is the standard margin for the Europeans Medicines Agency (EMA).
Secondary endpoints were clinical and microbiological responses for the micro-ITT, modified ITT, clinically evaluable, and microbiologically evaluable populations at end-of-treatment (EOT), TOC, and follow-up (FU) visits.
Microbiological Specimen Collection and Outcome Assessments
Appropriate aerobic and anaerobic specimens for culture at the time of the on-study source control procedure were collected from the site of infection and directly inoculated into culture media during the procedure. Specimen collection was either by tissue biopsy or aspirate. These specimens were cultured, and the species were identified at a local or regional laboratory. All purified isolates were sent to the central reference laboratory for confirmation of species identification and antimicrobial susceptibility testing. Isolates were screened for possible ESBL or carbapenemase production based on antimicrobial susceptibility testing, which was confirmed by next-generation sequencing.
RESULTS
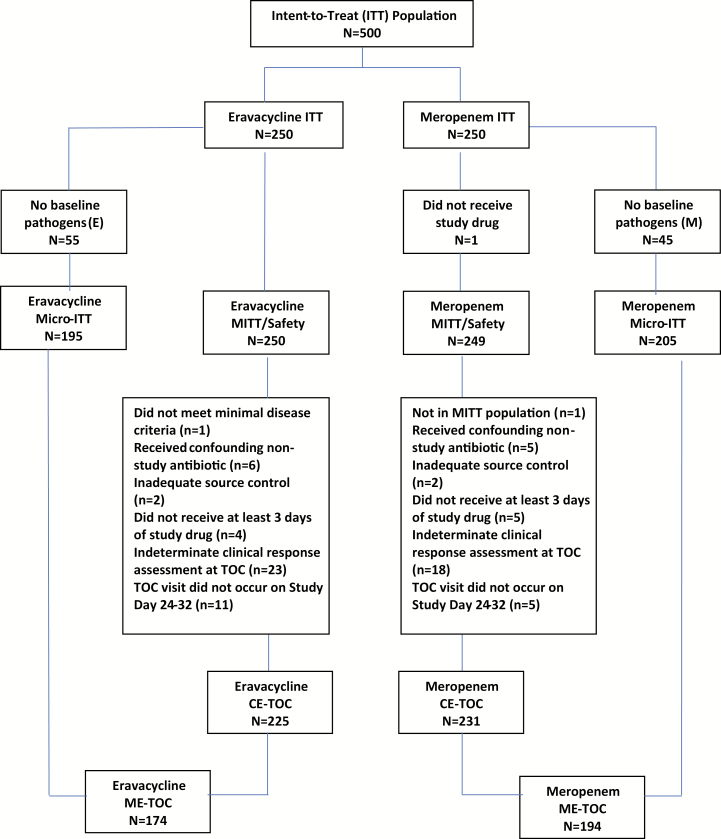
A total of 500 patients were enrolled in the ITT population, 250 in each treatment arm. Figure 1 displays the CONSORT flow diagram. The majority of patients were enrolled in the European Union. Enrollment ran from 13 October 2016 to 1 April 1 2017.
Figure 1.
CONSORT diagram. Abbreviations: CE, clinically evaluable; ME, microbiologically evaluable; micro-ITT, microbiological intent-to-treat; MITT, modified intent-to-treat; TOC, test-of-cure.
Table 1 displays the demographics data for the micro-ITT population. The baseline demographics for patients in both treatment arms were similar. Sixty percent of patients in the eravacycline group and 63.4% in the meropenem group received open surgery; 35.4% and 32.7%, respectively, received laparoscopic surgery; and the remaining received percutaneous or other procedures. As randomized, 48.2% of the eravacycline group were diagnosed with complicated appendicitis vs 43.9% in the meropenem group.
Table 1.
Demographics and Baseline Characteristics: Microbiological Intent-to-Treat Population
| Characteristic | Eravacycline (N = 195) |
Meropenem (N = 205) |
|---|---|---|
| Age, years | ||
| Mean ± standard deviation (min., max.) | 50.3 ± 17.7 (18, 84) | 52.3 ± 18.3 (19, 87) |
| Age group, n (%) | ||
| <65 years | 148 (75.9) | 145 (70.7) |
| 65–75 years | 34 (17.4) | 38 (18.5) |
| >75 years | 13 (6.7) | 22 (10.7) |
| Gender, n (%) | ||
| Female | 86 (44.1) | 100 (48.8) |
| Body mass index, kg/m2 | 27.4 ± 5.3 (17.2, 49.2) | 27.1 ± 5.0 (17.1, 43.8) |
| Acute Physiology and Chronic Health Evaluation II score | 6.6 ± 3.8 (0, 19) | 6.4 ± 4.0 (0, 20) |
| Surgical interventiona | ||
| Open | 117 (60.0) | 130 (63.4) |
| Laparoscopic | 69 (35.4) | 67 (32.7) |
| Percutaneous | 12 (6.2) | 15 (7.3) |
| Other | 0 (0.0) | 1 (0.5) |
aIn some cases, patients initially treated with laparoscopic or percutaneous therapy were converted to other procedures. Therefore, these categories are not mutually exclusive.
Details of the infections encountered are listed in Table 2. The majority of patients in both groups were enrolled post-operatively.
Table 2.
Pathologies: Microbiological Intent-to-Treat Population
| Pathology | Eravacycline (N = 195) |
Meropenem (N = 205) |
|---|---|---|
| Actual primary disease diagnosis | ||
| Complicated appendicitis, n (%) | 94 (48.2) | 90 (43.9) |
| Other complicated intra-abdominal infection | 101 (51.8) | 115 (56.1) |
| Diagnosed and enrolled preoperatively | 7 (3.6) | 11 (5.4) |
| Diagnosed intra-/postoperatively | 188 (96.4) | 194 (94.6) |
| Intra-abdominal abscess(es)a | 119 (63.3) | 110 (56.7) |
| Peritonitis | 94 (50.0) | 95 (49.0) |
| Gastric/duodenal perforation | 11 (5.9) | 12 (6.2) |
| Complicated cholecystitis | 40 (21.3) | 45 (23.2) |
| Perforation of small intestine | 7 (3.7) | 7 (3.6) |
| Complicated appendicitis | 93 (49.5) | 91 (46.9) |
| Perforation of large intestine | 8 (4.3) | 12 (6.2) |
| Diverticulitis with perforation, peritonitis, or abscess | 5 (2.7) | 7 (3.6) |
| Other | 0 | 2 (1.0) |
aThe population included some patients with abscesses and no other diagnosis.
Table 3 shows the clinical response for all populations at the TOC visit. The primary efficacy endpoint, per FDA requirements, was the clinical response at the TOC visit in the micro-ITT population.
Table 3.
Clinical Response at Test-of-cure Visit
| Population | Eravacycline | Meropenem | Difference (95% Confidence Interval) |
|---|---|---|---|
| Modified intent-to-treat | N = 250 | N = 249 | … |
| Clinical cure | 231 (92.4) | 228 (91.6) | 0.8 (–4.1, 5.8) |
| Clinical failure | 7 (2.8) | 9 (3.6) | … |
| Indeterminate/Missing | 12 (4.8) | 12 (4.8) | … |
| Microbiological intent-to-treat | N = 195 | N = 205 | … |
| Clinical cure | 177 (90.8) | 187 (91.2) | –0.5 (–6.3, 5.3) |
| Clinical failure | 7 (3.6) | 7 (3.4) | … |
| Indeterminate/Missing | 11 (5.6) | 11 (5.4) | … |
| Clinically evaluable | N = 225 | N = 231 | … |
| Clinical cure | 218 (96.9) | 222 (96.1) | 0.8 (–2.9, 4.5) |
| Clinical failure | 7 (3.1) | 9 (3.9) | … |
| Indeterminate/Missing | 0 | 0 | … |
| Microbiologically evaluable | N = 174 | N = 194 | … |
| Clinical cure | 167 (96.0) | 187 (96.4) | –0.4 (–4.9, 3.8) |
| Clinical failure | 7 (4.0) | 7 (3.6) | … |
| Indeterminate/Missing | 0 | 0 | … |
For this endpoint, the cure rate was 90.8% for eravacycline and 91.2% for meropenem, a difference of −0.5% with a 95% CI of −6.3% to 5.3%, meeting the predetermined criterion for NI. Clinical cure rates were high across all visits and populations, ranging from 90.8% to 96.9% in the eravacycline arm and from 91.2% to 96.4% in the meropenem arm. The percentages of patients with a response of clinical cure at the FU visit (Table 4) were similar between the treatment groups in all analysis populations and were generally lower than those at the EOT (Table 5) or TOC visits in all populations assessed. The latter observation was due to the higher number of missing responses in both treatment groups. Overall, the results for analysis of clinical cure were supportive of the primary efficacy analysis results.
Table 4.
Clinical Response at Follow-up Visit
| Population | Eravacycline (Clinical Cure/Total) |
Meropenem (Clinical Cure/Total) |
Difference (95% Confidence Interval) |
|---|---|---|---|
| Intent-to-treat | 224/250 (89.6) | 226/250 (90.4) | –0.8 (–6.2, 4.6) |
| Modified intent-to-treat | 224/250 (89.6) | 226/249 (90.8) | –1.2 (–6.5, 4.2) |
| Microbiological intent-to-treat | 170/195 (87.2) | 185/205 (90.2) | –3.1 (–9.5, 3.2) |
| Clinically evaluable | 220/229 (96.1) | 221/231 (95.7) | 0.4 (–3.5, 4.3) |
| Microbiologically evaluable | 168/177 (94.9) | 184/192 (95.8) | –0.9 (–5.7, 3.6) |
Table 5.
Clinical Response at End-of-treatment Visit
| Population | Eravacycline (Clinical Cure/Total) |
Meropenem (Clinical Cure/Total) |
Difference (95% Confidence Interval) |
|---|---|---|---|
| Intent-to-treat | 235/250 (94.0) | 234/250 (93.6) | 0.4 (–4.0, 4.8) |
| Modified intent-to-treat | 235/250 (94.0) | 234/249 (94.0) | 0.0 (–4.3, 4.4) |
| Microbiological intent-to-treat | 181/195 (92.8) | 193/205 (94.1) | –1.3 (–6.5, 3.7) |
| Clinically evaluable | 232/239 (97.1) | 234/237 (98.7) | –1.7 (–4.8, 1.1) |
| Microbiologically evaluable | 180/187 (96.3) | 193/196 (98.5) | –2.2 (–6.2, 1.2) |
The microorganisms identified at the intraabdominal site of infection are detailed in Table 6. All patients in the micro-ITT population for both treatment arms had a baseline intraabdominal specimen, and only 1 patient did not have baseline blood culture samples. Almost all intraabdominal specimens (>99% of patients in both treatment arms) had confirmed bacterial growth in culture. Seven percent of blood cultures from both the eravacycline and the meropenem populations had confirmed growth. The risk of bacteremia regardless of treatment was highest with large or small bowel perforation (15% and 14.3%, respectively). Bacteremia in the micro-ITT population did not have an obvious effect on clinical outcome. Of the 29 such patients with bacteremia, 3 failed, 2 were indeterminant for missing endpoint visit, and 24 were cured. All patients with baseline bacteremia in the eravacycline group and all but 1 in the meropenem group had documented clearance of the baseline organism from the blood.
Table 6.
Clinical Cure at the Test-of-cure Visit by Baseline Pathogen: Microbiological Intent-to-treat Population
| Baseline Pathogena | Eravacycline (N = 195) |
Meropenem (N = 205) |
|---|---|---|
| Gram-negative aerobes | 141/158 (89.2) | 153/166 (92.2) |
| Enterobacteriaceae | 129/146 (88.4) | 142/154 (92.2) |
| Escherichia coli | 111/126 (88.1) | 125/134 (93.3) |
| Klebsiella pneumoniae | 21/21 (100.0) | 23/27 (85.2) |
| Non-enterobacteriaceae | 36/38 (94.7) | 28/30 (93.3) |
| Acinetobacter baumannii complex | 5/5 (100.0) | 2/2 (100.0) |
| Pseudomonas aeruginosa | 18/19 (94.7) | 18/20 (90.0) |
| Gram-positive aerobes | 108/122 (88.5) | 98/107 (91.6) |
| Enterococcus avium | 10/11 (90.9) | 9/10 (90.0) |
| Enterococcus faecalis | 29/31 (93.5) | 26/28 (92.9) |
| Enterococcus faecium | 25/29 (86.2) | 22/23 (95.7) |
| Staphylococcus aureus | 16/16 (100.0) | 7/8 (87.5) |
| Methicillin-susceptible Staphylococcus aureus | 15/15 (100.0) | 7/8 (87.5) |
| Streptococcus species | 52/60 (86.7) | 46/50 (92.0) |
| Streptococcus viridans group | 50/57 (87.7) | 40/44 (90.9) |
| Streptococcus anginosus group | 39/45 (86.7) | 31/33 (93.9) |
| Streptococcus anginosus | 25/29 (86.2) | 21/22 (95.5) |
| Streptococcus constellatus | 13/15 (86.7) | 9/11 (81.8) |
| Streptococcus mitis group | 13/14 (92.9) | 11/12 (91.7) |
| Anaerobes | 99/110 (90.0) | 104/111 (93.7) |
| Bacteroides species | 83/94 (88.3) | 82/88 (93.2) |
| Bacteroides caccae | 5/6 (83.3) | 5/5 (100.0) |
| Bacteroides fragilis | 33/40 (82.5) | 35/38 (92.1) |
| Bacteroides ovatus | 19/24 (79.2) | 28/28 (100.0) |
| Bacteroides thetaiotaomicron | 27/30 (90.0) | 30/33 (90.9) |
| Bacteroides uniformis | 14/16 (87.5) | 14/14 (100.0) |
| Bacteroides vulgatus | 27/28 (96.4) | 23/23 (100.0) |
| Clostridium species | 9/9 (100.0) | 26/26 (100.0) |
| Clostridium perfringens | 7/7 (100.0) | 12/12 (100.0) |
| Fusobacterium species | 5/6 (83.3) | 2/2 (100.0) |
aOrganisms encountered 10 or more times are included, along with those considered of interest. A full listing of baseline pathogens can be found in the Supplementary Materials.
A total of 284 patients (71%) had polymicrobial infection, and 320 patients harbored gram-negatives. Bacteroides species were found in 176 patients, and 158 of these also had at least 1 gram-negative. Bacteroides were cultured from only 1 patient with a monomicrobial infection.
We encountered a variety of ESBLs and carbapenem-resistant Enterobacteriaceae (CRE; see Table 7). The most common ESBL was CTX-M-15. One KPC-2 and 1 OXA-48 were encountered, both patients successfully treated with meropenem.
Table 7.
Extended Spectrum Beta-lactamases
| Eravacycline (Cured/Total) | Meropenem (Cured/Total) | |
|---|---|---|
| Citrobacter freundii | 0 | 1/1 |
| CTX-M-15 | 0 | 1/1 |
| Enterobacter cloacae/asburiae | 3/3 | 1/1 |
| CTX-M-15 | 2/2 | 1/1 |
| Escherichia coli | 8/10 | 5/7 |
| CTX-M-15 | 7/8 | 3/5 |
| CTX-M-3 | 0/1 | 1/1 |
| CTX-M-32 | 1/1 | 0 |
| CTX-M-5 | 0 | 1/1 |
| SHV-12 | 0 | 1/1 |
| Klebsiella pneumoniae | 5/5 | 5/6 |
| CTX-M-15 | 5/5 | 3/4 |
| CTX-M-2 | 0 | 1/1 |
| SHV-12 | 0 | 1/1 |
| Serratia marcescens | 0 | 1/1 |
| CTX-M-15 | 0 | 1/1 |
Organisms encountered often tested positive for more than 1 enzyme.
Clinical failures are detailed in Table 8. The primary reasons for failure in both groups were the need for an unplanned surgical or percutaneous procedure (5 in each group) and initiation of rescue antibiotic therapy for cIAI (6 in each group).
Table 8.
Clinical Failure at Test-of-cure: Microbiological Intent-to-treat Population
| Classification | Eravacycline (N = 195) |
Meropenem (N = 205) |
|---|---|---|
| Clinical failure at test-of-cure | 7a (3.6) | 7 (3.4) |
| Death due to cIAI | 0 | 0 |
| Persistence of clinical symptoms of cIAI | 1 (14.3) | 3 (42.9) |
| Unplanned surgical procedure or percutaneous drainage procedures for complication or recurrence of cIAI | 5 (71.4) | 5 (71.4) |
| Postsurgical wound infection requiring systemic antibiotics | 2 (28.6) | 0 |
| Initiation of rescue antibacterial therapy for cIAI | 6 (85.7) | 6 (85.7) |
| Surgical Adjudication Committee correctionb | 0 | 1 (14.3) |
| Other | 0 | 0 |
Abbreviation: cIAI, complicated intraabdominal infection.
aSome patients fell into multiple categories.
bThis is defined as a patient who was classified as a clinical cure by the investigator but underwent a second procedure and was determined to be a failure by the Surgical Adjudication Committee.
Safety
TEAEs occurred in 37.2% (93/250) of patients in the eravacycline group compared to 30.9% (77/249) in the meropenem group. The incidence of TEAEs reported in this and the 2 other eravacycline trials are well within the range of trials of other antibiotic therapy for cIAI [32, 33]. It is important to note that the reported TEAE rates include all events, regardless of relationship to study drug; less than half of the events reported in either treatment group were considered related to study drug.
The majority of TEAEs seen in patients who received eravacycline were gastrointestinal disorders such as nausea (n = 12), vomiting (n = 9), and diarrhea (n = 6). The full list of TEAEs that occurred in more than 2% of patients in either group can be seen in Table 9. Few events led to discontinuation of study drug in either treatment arm.
Table 9.
Incidence of Adverse Events Occurring in >2% of Patients in Either Group: Safety Population
| Medical Dictionary for Regulatory Activities Term | Eravacycline (N = 250) |
Meropenem (N = 249) |
|---|---|---|
| Nausea | 12 (4.8) | 2 (0.8) |
| Vomiting | 9 (3.6) | 5 (2.0) |
| Infusion site phlebitis | 8 (3.2) | 1 (0.4) |
| Infusion site thrombosis | 6 (2.4) | 1 (0.4) |
| Wound infection (superficial) | 7 (2.8) | 4 (1.6) |
| Diarrhea | 6 (2.4) | 3 (1.2) |
| Anemia | 3 (1.2) | 6 (2.4) |
| Hypertension | 2 (0.8) | 7 (2.8) |
| Hypokalemia | 0 | 6 (2.4) |
| Discontinued because of adverse event | 4 (1.6) | 4 (2.0) |
Localized infusion site reactions, including infusion site phlebitis and infusion site thrombosis, were more common in eravacycline-treated patients compared to meropenem-treated patients in the study. Among these events, 3 were graded moderate in severity, and the remainder was mild. In 2 cases, the study drug was diluted into a larger volume, and in a third case, the infusion rate was decreased to manage the AE. In no case was study drug discontinued as a result of an infusion site reaction. There were 5 deaths, none of which were determined to be treatment related. The causes for these were pulmonary embolism, respiratory failure, chronic obstructive pulmonary disease, pneumonia, and cardiac arrest.
DISCUSSION
The current clinical trial compared eravacycline (1 mg/kg IV every 12 hours) to meropenem (1 g IV every 8 hours) for the management of cIAIs. The key finding was NI of eravacycline to meropenem. These results match the findings in a previously published phase 3 study utilizing ertapenem as the comparator [34]. The particulars of this trial deserving emphasis are the microbiology encountered, response to therapy, and toxicities of eravacycline vs other tetracycline agents.
Microbiology Encountered
The microbiology of infection and resistance encountered in this study is representative of that seen in other recent registration trials [32, 35]. The interpretation of clinical trial data for intraabdominal infection is made complex by the polymicrobial nature of these infections, the varying organ-specific processes, and the central role of source control in determining outcome [36, 37]. In the current study, polymicrobial infections were predominant, and known synergistic pairs, including gram-negative facultative and aerobic isolates and gram-positives along with Bacteroides and Clostridia species, were present in a large percentage of patients. Of note, among the 3 patients in the meropenem group with CRE isolates, 2 had polymicrobial infections.
The high rate of clinical cure among patients with Pseudomonas aeruginosa identified as a baseline pathogen is likely another consequence of this complexity; eravacycline has limited activity against P. aeruginosa, yet patients harboring this organism did equally well when treated with either eravacycline or meropenem in this study. Other trials with similar inclusion criteria have obtained the same result. We note that in the current study, of the 43 patients with P. aeruginosa identified as a baseline pathogen, 38 (20/22 in the eravacycline group and 18/21 in the meropenem group) had polymicrobial infections. In these cases, disruption of bacterial synergy may have contributed to the high rate of clinical cure, particularly in the eravacycline group. For patients in whom P. aeruginosa is suspected to be the predominant pathogen or who are at high risk of poor outcomes from this organism, such as those who are severely immunocompromised, specific therapy against P. aeruginosa should be considered.
As in other recently reported trials, we encountered a variety of ESBL enzyme types and CREs, and most organisms contained multiple ESBL types. Cure rates for ESBLs were uniformly high. Carbapenemase-producing pathogens were found only in patients who received meropenem, so a comparison cannot be made. To specifically address questions of the pathogenicity of ESBL-containing organisms in the presence of adequate source control, we refer to systematic reviews and observational studies that confirm improved outcomes if effective empiric treatment is provided to patients found to be infected with ESBL organisms and/or CRE [38, 39]. In ESBL bacteremia studies, cIAIs are a common source of bacteremic isolates. A recent high-quality, randomized, controlled trial confirmed these findings, with 16% of the bacteremias arising from intraabdominal sources [40]. That study found significant differences in mortality by agent used (comparing piperacillin/tazobactam with meropenem).
Toxicities
Nausea, vomiting, and diarrhea are more common with tetracyclines than other comparable antibiotics. Tigecycline treatment in particular is associated with nausea and vomiting. A pooled analysis of 2 phase 3, double-blind trials designed to evaluate the safety and efficacy of tigecycline (vs imipenem-cilastatin) found that 24% of patients experience treatment-emergent nausea, 19% experienced vomiting, and 14% experienced diarrhea. These were significantly higher than seen in the control [41]. These problems were encountered in other randomized trials with tigecycline [42–45]. Eravacycline in this and our previous trial had comparatively low rates of TEAEs, with less than 5%, 4%, and 3% of patients experiencing nausea, vomiting, and diarrhea, respectively.
Potential Value for Empiric and Definitive Treatment in cIAI
Eravacycline as monotherapy has demonstrated broad antimicrobial activity in both in vitro activity studies and in the 3 trials completed for cIAIs, each using a broad-spectrum carbapenem as a comparator. This agent has more potent in vitro activity than tigecycline and a substantially lessened side-effect profile. Eravacycline thus appears to be an effective agent in the therapy of cIAIs and may be an appropriate empiric choice when coverage of resistant organisms such as ESBL and CRE is desired.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Sergey Izmailyan and Melanie Olesky for their contributions to data retrieval and analysis.
Financial support. This work was supported by Tetraphase Pharmaceuticals Inc
Potential conflicts of interest. J. S. S. has received support from Tetraphase Pharmaceuticals Inc. during the production of this manuscript and has served as a consultant to Merck, Pfizer, GlaxoSmithKline, and Melinta outside of the submitted work. A. S received support from Tetraphase Pharmaceuticals Inc. during the production of this manuscript. J. G. received research funding from Tetraphase Pharmaceuticals Inc. for his role as principal investigator of this study. K. L. and L. T. are employed by Tetraphase Pharmaceuticals Inc. D. E. reports grants from Tetraphase Pharmaceuticals Inc. during the conduct of the study and grants from Merck outside of the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Antimicrobial resistance threats in the United States, 2013 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf. Accessed 14 November 2018.
- 2. Antimicrobial resistance 2018. Available at: https://ecdc.europa.eu/en/antimicrobial-resistance. Accessed 14 November 2018.
- 3. Antimicrobial resistance 2018. Available at: https://www.idsociety.org/public-health/antimicrobial-resistance/antimicrobial-resistance/. Accessed 14 November 2018.
- 4. Antimicrobial resistance 2018. Available at: http://www.who.int/antimicrobial-resistance/en/. Accessed 14 November 2018.
- 5. Chong Y, Shimoda S, Shimono N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Genet Evol 2018; 61:185–8. [DOI] [PubMed] [Google Scholar]
- 6. Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 2007; 60:913–20. [DOI] [PubMed] [Google Scholar]
- 7. Thabit AK, Crandon JL, Nicolau DP. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin Pharmacother 2015; 16:159–77. [DOI] [PubMed] [Google Scholar]
- 8. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 2017; 215:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez-Bano J, Gutierrez-Gutierrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing enterobacteriaceae. Clinical Microbiology Reviews 2018; 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris P TP, David Lye D, Mo Y.. The MERINO trial: piperacillin-tazobactam versus meropenem for the definitive treatment of bloodstream infections caused by third-generation cephalosporin nonsusceptible Escherichia coli or Klebsiella spp.: an international multi-centre open label non-inferiority randomised controlled trial. Madrid: European Congress of Clinical Microbiology and Infectious Diseases, 2018. [Google Scholar]
- 11. Frère JM, Sauvage E, Kerff F. From “An Enzyme Able to Destroy Penicillin” to carbapenemases: 70 years of beta-lactamase misbehaviour. Curr Drug Targets 2016; 17:974–82. [DOI] [PubMed] [Google Scholar]
- 12. Molton JS, Tambyah PA, Ang BS, Ling ML, Fisher DA. The global spread of healthcare-associated multidrug-resistant bacteria: a perspective from Asia. Clin Infect Dis 2013; 56:1310–8. [DOI] [PubMed] [Google Scholar]
- 13. Yang Q, Xu YC, Kiratisin P, Dowzicky MJ. Antimicrobial activity among gram-positive and gram-negative organisms collected from the Asia-Pacific region as part of the tigecycline evaluation and surveillance trial: comparison of 2015 results with previous years. Diagn Microbiol Infect Dis 2017; 89:314–23. [DOI] [PubMed] [Google Scholar]
- 14. Pfaller MA, Huband MD, Streit JM, Flamm RK, Sader HS. Surveillance of tigecycline activity tested against clinical isolates from a global (North America, Europe, Latin America and Asia-Pacific) collection (2016). Int J Antimicrob Agents 2018; 51:848–53. [DOI] [PubMed] [Google Scholar]
- 15. Lob SH, Biedenbach DJ, Badal RE, Kazmierczak KM, Sahm DF. Antimicrobial resistance and resistance mechanisms of Enterobacteriaceae in ICU and non-ICU wards in Europe and North America: SMART 2011-2013. J Glob Antimicrob Resist 2015; 3:190–7. [DOI] [PubMed] [Google Scholar]
- 16. Jean SS, Hsueh PR; SMART Asia-Pacific Group Distribution of ESBLs, AmpC β-lactamases and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal and urinary tract infections in the Asia-Pacific region during 2008-14: results from the study for monitoring antimicrobial resistance trends (SMART). J Antimicrob Chemother 2017; 72:166–71. [DOI] [PubMed] [Google Scholar]
- 17. van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017; 8:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tracking CRE 2016. Available at: https://www.cdc.gov/hai/organisms/cre/TrackingCRE.html. Accessed 24 June 2018.
- 19. Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs 2014; 74:1315–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Harten RM, Willems RJL, Martin NI, Hendrickx APA. Multidrug-resistant enterococcal infections: new compounds, novel antimicrobial therapies? Trends Microbiol 2017; 25:467–79. [DOI] [PubMed] [Google Scholar]
- 21. Mazuski JE, Solomkin JS. Intra-abdominal infections. Surg Clin North Am 2009; 89:421–37, ix. [DOI] [PubMed] [Google Scholar]
- 22. Mazuski JE, Tessier JM, May AK, et al. . The Surgical Infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt) 2017; 18:1–76. [DOI] [PubMed] [Google Scholar]
- 23. Solomkin JS, Mazuski JE, Bradley JS, et al. . Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:133–64. [DOI] [PubMed] [Google Scholar]
- 24. Chukwudi CU. rRNA binding sites and the molecular mechanism of action of the tetracyclines. Antimicrob Agents Chemother 2016; 60:4433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grossman TH, Starosta AL, Fyfe C, et al. . Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother 2012; 56:2559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Lin X, Bush K. In vitro susceptibility of β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE) to eravacycline. J Antibiot (Tokyo) 2016; 69:600–4. [DOI] [PubMed] [Google Scholar]
- 27. Seifert H, Stefanik D, Sutcliffe JA, Higgins PG. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int J Antimicrob Agents 2018; 51:62–4. [DOI] [PubMed] [Google Scholar]
- 28. Zhanel GG, Baxter MR, Adam HJ, Sutcliffe J, Karlowsky JA. In vitro activity of eravacycline against 2213 gram-negative and 2424 gram-positive bacterial pathogens isolated in Canadian hospital laboratories: CANWARD Surveillance Study 2014-2015. Diagn Microbiol Infect Dis 2018; 91:55–62. [DOI] [PubMed] [Google Scholar]
- 29. Newman JV, Zhou J, Izmailyan S, Tsai L. Randomized, double-blind, placebo-controlled studies of the safety and pharmacokinetics of single and multiple ascending doses of eravacycline. Antimicrob Agents Chemother 2018; 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Solomkin JS, Ramesh MK, Cesnauskas G, et al. . Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob Agents Chemother 2014; 58:1847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schein M, Marshall J. Source control for surgical infections. World J Surg 2004; 28:638–45. [DOI] [PubMed] [Google Scholar]
- 32. Solomkin J, Hershberger E, Miller B, et al. . Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 2015; 60:1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazuski JE, Gasink LB, Armstrong J, et al. . Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016; 62:1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solomkin J, Evans D, Slepavicius A, et al. . Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the Investigating Gram-Negative Infections Treated with Eravacycline (IGNITE 1) Trial: a randomized clinical trial. JAMA Surg 2017; 152:224–32. [DOI] [PubMed] [Google Scholar]
- 35. Mazuski JE, Gasink LB, Armstrong J, et al. . Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection—results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016; 62:1380–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berne TV, Yellin AW, Appleman MD, Heseltine PN. Antibiotic management of surgically treated gangrenous or perforated appendicitis. Comparison of gentamicin and clindamycin versus cefamandole versus cefoperazone. Am J Surg 1982; 144:8–13. [DOI] [PubMed] [Google Scholar]
- 37. Heseltine PN, Yellin AE, Appleman MD, et al. . Perforated and gangrenous appendicitis: an analysis of antibiotic failures. J Infect Dis 1983; 148:322–9. [DOI] [PubMed] [Google Scholar]
- 38. Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 2012; 67:2793–803. [DOI] [PubMed] [Google Scholar]
- 39. Girometti N, Lewis RE, Giannella M, et al. . Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore) 2014; 93:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris PNA, Tambyah PA, Lye DC, et al. ; MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Babinchak T, Ellis-Grosse E, Dartois N, Rose GM, Loh E; Tigecycline 301 Study Group; Tigecycline 306 Study Group The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin Infect Dis 2005; 41 (Suppl 5):S354–67. [DOI] [PubMed] [Google Scholar]
- 42. Lauf L, Ozsvár Z, Mitha I, et al. . Phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis. Diagn Microbiol Infect Dis 2014; 78:469–80. [DOI] [PubMed] [Google Scholar]
- 43. Matthews P, Alpert M, Rahav G, et al. ; Tigecycline 900 cSSSI Study Group A randomized trial of tigecycline versus ampicillin-sulbactam or amoxicillin-clavulanate for the treatment of complicated skin and skin structure infections. BMC Infect Dis 2012; 12:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O’Riordan W, Mehra P, Manos P, Kingsley J, Lawrence L, Cammarata S. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis 2015; 30:67–73. [DOI] [PubMed] [Google Scholar]
- 45. Towfigh S, Pasternak J, Poirier A, Leister H, Babinchak T. A multicentre, open-label, randomized comparative study of tigecycline versus ceftriaxone sodium plus metronidazole for the treatment of hospitalized subjects with complicated intra-abdominal infections. Clin Microbiol Infect 2010; 16:1274–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.