Abstract
Aims
To test whether human immunodeficiency virus (HIV) infection and subclinical cardiovascular disease (sCVD) are associated with expression of CXCR4 and other surface markers on classical, intermediate, and non-classical monocytes in women.
Methods and results
sCVD was defined as presence of atherosclerotic lesions in the carotid artery in 92 participants of the Women’s Interagency HIV Study (WIHS). Participants were stratified into four sets (n = 23 each) by HIV and sCVD status (HIV−/sCVD−, HIV−/sCVD+, HIV+/sCVD−, and HIV+/sCVD+) matched by age, race/ethnicity, and smoking status. Three subsets of monocytes were determined from archived peripheral blood mononuclear cells. Flow cytometry was used to count and phenotype surface markers. We tested for differences by HIV and sCVD status accounting for multiple comparisons. We found no differences in monocyte subset size among the four groups. Expression of seven surface markers differed significantly across the three monocyte subsets. CXCR4 expression [median fluorescence intensity (MFI)] in non-classical monocytes was highest among HIV−/CVD− [628, interquartile range (IQR) (295–1389)], followed by HIV+/CVD− [486, IQR (248–699)], HIV−/CVD+ (398, IQR (89–901)), and lowest in HIV+/CVD+ women [226, IQR (73–519)), P = 0.006 in ANOVA. After accounting for multiple comparison (Tukey) the difference between HIV−/CVD− vs. HIV+/CVD+ remained significant with P = 0.005 (HIV−/CVD− vs. HIV+/CVD− P = 0.04, HIV−/CVD− vs. HIV−/CVD+ P = 0.06, HIV+/CVD+ vs. HIV+/CVD− P = 0.88, HIV+/CVD+ vs. HIV−/CVD+ P = 0.81, HIV+/CVD− vs. HIV−/CVD+, P = 0.99). All pairwise comparisons with HIV−/CVD− were individually significant (P = 0.050 vs. HIV−/CVD+, P = 0.028 vs. HIV+/CVD−, P = 0.009 vs. HIV+/CVD+). CXCR4 expression on non-classical monocytes was significantly higher in CVD− (501.5, IQR (249.5–887.3)) vs. CVD+ (297, IQR (81.75–626.8) individuals (P = 0.028, n = 46 per group). CXCR4 expression on non-classical monocytes significantly correlated with cardiovascular and HIV−related risk factors including systolic blood pressure, platelet and T cell counts along with duration of antiretroviral therapy (P < 0.05). In regression analyses, adjusted for education level, study site, and injection drug use, presence of HIV infection and sCVD remained significantly associated with lower CXCR4 expression on non-classical monocytes (P = 0.003), but did not differ in classical or intermediate monocytes.
Conclusion
CXCR4 expression in non-classical monocytes was significantly lower among women with both HIV infection and sCVD, suggesting a potential atheroprotective role of CXCR4 in non-classical monocytes.
Keywords: Non-classical monocytes, CXCR4, Atherosclerosis, HIV, Cardiovascular risk assessment, Women
Introduction
Infection with the human immunodeficiency virus (HIV) is associated with an increased risk of early-onset and rapidly progressive cardiovascular disease (CVD), even among virally suppressed patients on effective antiretroviral therapy (ART).1–3 Higher incidences of stroke, myocardial infarction, and advanced subclinical cardiovascular disease (sCVD) have been observed in HIV-infected compared to uninfected individuals.4 The mechanism for increased CVD risk in HIV-infected individuals is likely multifactorial, involving both traditional CVD- and HIV-related risk factors. For example, HIV replication and exposure to certain antiretroviral medications like protease inhibitors may have unfavourable influences on the lipid profile.5,6 ART has been associated with progression of subclinical atherosclerosis in some large-scale studies.7 HIV-positive patients are also characterized by a higher frequency of concomitant traditional risk factors like smoking or hyperlipidaemia.8
It is especially important in women with HIV to further investigate risk factors for accelerating CVD to prevent adverse cardiovascular outcome,9,10 as women are underdiagnosed regarding CVD.11–13 There are significant gender differences in screening and diagnosis of CVD.11,12 Even though sex-specific symptoms, traditional and novel risk factors along with expanded understanding of the sex-specific pathophysiology of CVD have been acknowledged in recent years, ischaemic heart disease continues to be the leading cause of morbidity and mortality in women in western countries, especially in women with HIV.11 Traditional CVD risk factors are less frequent in women11–14 resulting in unfavourable impact on diagnosis, prevention, and treatment strategies. Interestingly, there are also significant disparities in CVD burden among subgroups of women, who are socially disadvantaged because of race, ethnicity, income level, and education.11–14
Preventive strategies and early treatment of cardiovascular risk factors, e.g. statins or antiplatelet therapy, have been recommended in high-risk HIV-positive patients. Such treatments may also have pleiotropic effects and reduce circulating levels of pro-inflammatory proteins and cytokines, slowing down inflammatory processes in atherogenesis and inhibiting atheroprogression.15 However, underlying mechanisms of endothelial cell dysregulation and atherosclerotic plaque formation remain elusive.15
Persistent innate immune activation mediated by platelets and monocytes may contribute to atherogenesis and atheroprogression in persons with HIV.16,17 Monocytes are key players in atherogenesis, from the formation of the earliest asymptomatic atherosclerotic lesions to plaque rupture with potentially fatal outcomes.18,19 Three distinct monocyte subpopulations have been defined based on their surface receptor expression of CD14 and CD16: classical (CD14++CD16−), intermediate (CD14+CD16+), and non-classical (CD14dimCD16++) monocytes.20,21 Specific monocyte subsets are believed to be differentially involved in the pathogenesis and outcome of acute coronary syndromes, heart failure, and stroke, amongst other conditions.22–26 Although a subset-specific contribution of monocytes has been proposed in recent years, monocyte heterogeneity has not been analysed thoroughly in the context of HIV-related sCVD.16,17,27 Experimental studies have suggested a causative role of monocytes in atherogenesis,25 but several epidemiologic analyses have shown inconsistent associations between circulating monocyte counts, phenotypes and CVD in HIV infection.7,8,16,28 Baker et al. showed in a prospective cohort of 436 patients with and without HIV infection that higher frequencies of CD16+ monocytes were associated with a greater likelihood of progression of coronary artery calcium (CAC) after adjusting for traditional and HIV-related risk factors.29 Surface marker expression on monocytes was not associated with presence or progression of CAC.29 Another study of 51 patients with HIV and 49 matched controls revealed that surface expression of the chemokine receptor CX3CR1 and the integrin CD11b can serve as independent predictors of carotid intima-media thickness (cIMT) progression in HIV infection.16 In contrast, Longenecker et al.30 found no association between cIMT of individuals with HIV and the proportions of monocyte subsets in peripheral blood. However, increased proportions of CD16+ monocytes have been associated with cardiovascular events and the occurrence of acute coronary syndromes in the general population22,31 and therefore, could be markers of advanced rather than premature sCVD.
Clarifying the immunologic mechanisms and cell types that contribute to premature sCVD among individuals with HIV may help tailor CVD risk assessment in this population. We tested the associations of monocyte characteristics, including subset size, phenotype, and surface marker expression, with HIV serostatus and presence of sCVD in 92 women who participated in the Women’s Interagency HIV Study (WIHS).
Methods
For a detailed description of the study design, patient collective, assessment of clinical parameters, flow cytometry and cell sorting, and statistical analysis, please see Supplementary material online.
Representative fluorescence-activated cell-sorting plots showing gating of monocytes to define subsets by CD14 and CD16 expression are illustrated in Supplementary material online, Figure S1.
Results
Median age of the 92 WIHS participants was 51.5 years [interquartile range (IQR 47–58)]. The majority (96%) was of either black race or Hispanic ethnicity, and 86% reported a history of smoking. Among the HIV-infected participants, 85% were on highly active antiretroviral therapy (HAART) and the median CD4+ T-cell count was 550.5 cells/µL (IQR 284–792). Clinical characteristics of the participants by matched group (HIV−/sCVD−, HIV−/sCVD+, HIV+/sCVD−, and HIV+/sCVD+) are summarized in Table 1. The numbers of classical, non-classical, and intermediate monocytes isolated from PBMCs were not significantly different among the groups (Supplementary material online, Figure S2).
Table 1.
Demographic and clinical characteristics of study participants
| HIV−/sCVD−, N = 23 | HIV+/sCVD−, N = 23 | HIV−/sCVD+, N = 23 | HIV+/sCVD+, N = 23 | P-value | |
|---|---|---|---|---|---|
| Demographic and behaviour-related characteristics | |||||
| Age at baseline vascular study visit (years) (median, IQR) | 45 (40–50) | 43 (40–51) | 45 (43–52) | 48 (43–53) | 0.61 |
| Black race or Hispanic ethnicity | 22 (96) | 22 (96) | 22 (96) | 22 (96) | 1.00 |
| Any history of smoking | 20 (87) | 19 (83) | 20 (87) | 20 (87) | 1.00 |
| Any current substance usea | 10(43) | 11 (48) | 10 (43) | 12 (52) | 0.68 |
| Hepatitis C virus infection status | 6 (26) | 12 (52) | 9 (39) | 13 (57) | 0.15 |
| Study site | <0.01 | ||||
| Bronx, NY | 14 (61) | 19 (83) | 7 (30) | 11 (48) | |
| Brooklyn, NY | 6 (26) | 2 (9) | 2 (9) | 10 (43) | |
| Washington, DC | 1 (4) | 0 (0) | 1 (4) | 0 (0) | |
| Los Angeles, CA | 1 (4) | 0 (0) | 5 (22) | 1 (4) | |
| San Francisco, CA | 1 (4) | 1 (4) | 3 (13) | 0 (0) | |
| Chicago, IL | 0 (0) | 1 (4) | 5 (22) | 1 (4) | |
| Education | 0.94 | ||||
| Completed high school | 15 (65) | 13 (57) | 14 (61) | 13 (57) | |
| Did not finish high school | 8 (35) | 9 (39) | 9 (39) | 10 (43) | |
| HIV-related risk factors | |||||
| CD4+ count, cells/µL (median, IQR) | – | 585 (382–816) | – | 535 (265–792) | 0.72 |
| CD4/CD8 ratio (median, IQR) | – | 0.8 (0.3–1.3) | – | 0.6 (0.3–1.0) | 0.46 |
| Undetectable HIV-1 RNA level | 14 (61) | – | 13 (57) | 0.76 | |
| Current ART use | 1.00 | ||||
| HAART | – | 20 (87) | – | 19 (83) | |
| ART only | – | 1 (4) | – | 2 (9) | |
| No ART | – | 2 (9) | – | 2 (9) | |
| Cardiometabolic risk factors | |||||
| Body mass index (median, IQR) | 30.5 (27–38) | 29 (26–35) | 28 (24–32) | 29 (24–34) | 0.36 |
| Total cholesterol, mg/dL (median, IQR) | 172 (148–183) | 170 (138–200) | 171.5 (145.5–204) | 199.5 (170.5–221) | 0.02 |
| LDL cholesterol, mg/dL (median, IQR) | 96 (72–112) | 93.5 (67–123) | 88.5 (65–116.5) | 116.5 (94.5–131) | 0.04 |
| HDL cholesterol, mg/dL (median, IQR) | 55 (44–60) | 54 (42–59) | 49.5 (43–59.5) | 46 (40–55.5) | 0.51 |
| History of high cholesterol | 16 (69.6) | 17 (73.9) | 21(91.3) | 23 (100) | 0.01 |
| Current use of cholesterol medications | 0 (0) | 0 (0) | 5 (22) | 10 (43) | <0.0001 |
| History of cholesterol medication use | 1 (4) | 0 (0) | 7 (30) | 11 (48) | <0.0001 |
| Systolic blood pressure, mmHg (median, IQR) | 125 (111–138) | 122 (109–134) | 128 (118–151) | 127 (109–139) | 0.38 |
| History of hypertension | 12 (52) | 10 (43) | 17 (74) | 17 (74) | 0.07 |
| Current hypertensive medication use | 10 (43) | 11 (48) | 14 (61) | 15 (65) | 0.39 |
| History of diabetes | 6 (26) | 4 (17) | 8 (35) | 4 (17) | 0.50 |
| Creatinine, mg/dL (median, IQR) | 0.8 (0.7–0.9) | 0.9 (0. 8–1.0) | 0.9 (0.7–1.0) | 0.9 (0.7–1.2) | 0.81 |
| Current aspirin use | 4 (17) | 4 (17) | 6 (26) | 10 (43) | 0.18 |
| Self-reported menopause | 10 (43) | 13 (57) | 9 (39) | 15 (65) | 0.22 |
| Inflammatory biomarker levels | |||||
| sCD163, ng/mL (median, IQR) | 630 (464–1253) | 987 (700–1305) | 772 (465–1118) | 961 (700–1429) | 0.06 |
| sCD14, ng/mL (median, IQR) | 1682 (1401–1943) | 2123 (1842–2275) | 1696 (1584–2219) | 2027 (1779–2670) | <0.01 |
| IL-6, pg/mL (median, IQR) | 1.9 (0.8–2.5) | 1.5 (1.0–2.8) | 1.7 (1.1–2.4) | 1.3 (1.1–2.7) | 0.95 |
| Galectin-3, ng/mL (median, IQR) | 9.4 (8.1–11.1) | 8.7 (5.9–12.8) | 8.6 (7.2–10.4) | 9.9 (7.7–12.8) | 0.52 |
| Galectin-3 binding protein, ng/mL (median, IQR) | 9.2 (4.9–17.6) | 15.4 (5.4–29.7) | 10.5 (4.0–13.1) | 14.4 (7.7–17.7) | 0.15 |
| hsCRP (median, IQR) | 1.9 (0.7–4.8) | 2.0 (0.9–7.7) | 1.8 (0.9–4.3) | 2.5 (1.3–7.4) | 0.74 |
Characteristics of human immunodeficiency virus (HIV)-infected and HIV-uninfected women in the Women's Interagency HIV Study stratified by presence of subclinical cardiovascular disease.
Values are n (%) or median and interquartile range (IQR). Each group contains 23 participants, who were matched by age, race/ethnicity, smoking status, and age of specimen.
ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; HDL, high-density lipoprotein; hsCRP, high sensitive C-reactive protein.
Substance use includes intravenous drug, crack, and cocaine use.
Surface marker expression on monocyte subsets in all WIHS participants
The surface markers CXCR4, CCR5, CCR2, CD11b, CD163, CD36, and CX3CR1 were evaluated in classical, intermediate, and non-classical monocytes (Supplementary material online, Figure S3). Based on ANOVA, there were differences in the expression of many of the analysed surface markers among the three subsets of monocytes (Supplementary material online, Figure S3). The differences in the expression of the chemokine receptors CX3CR1, CCR5 (CD195), CCR2 (CD192) and the scavenger receptor CD36 were expected based on published work.27,32 However, the differences in CD163 (haemoglobin–haptoglobin receptor), CXCR4 and the integrin CD11b are new findings.
Surface marker expression on monocyte subsets stratified by HIV and sCVD status
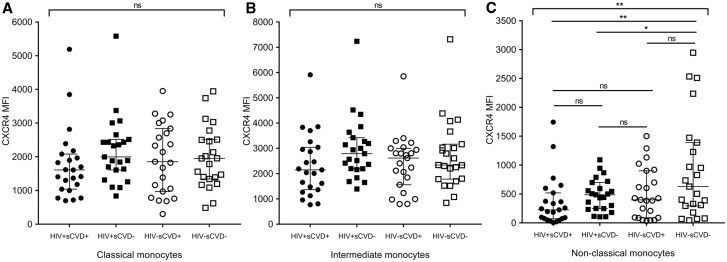
We further assessed the expression of surface markers of interest by HIV and sCVD status within each of the three monocyte subsets. After accounting for multiple comparisons, only CXCR4 expression in non-classical monocytes remained significantly different across the four participant groups. Figure 1A–C shows the association of HIV and sCVD status with CXCR4 expression within classical (1A), intermediate (1B), and non-classical (1C) monocytes. The expression of CXCR4 on non-classical monocytes [given as median fluorescence intensity (MFI)] was highest among HIV−/sCVD− women [median 628, IQR (295–1389)], followed by HIV+/sCVD− [median 486, IQR (248–699)], HIV−/sCVD+ [median 398, IQR (89–901)], and lowest in HIV+/sCVD+ women [median 226, IQR (73–519)], (P = 0.006 for overall comparison in ANOVA analysis). After accounting for multiple comparison (Tukey) the comparison between HIV−/sCVD− vs. HIV+/sCVD+ remained significant with P = 0.005 (HIV−/sCVD− vs. HIV+/sCVD− P = 0.04, HIV−/sCVD− vs. HIV−/sCVD+ P = 0.06, HIV+/sCVD+ vs. HIV+/sCVD− P = 0.88, HIV+/sCVD+ vs. HIV−/sCVD+ P = 0.81, HIV+/sCVD− vs. HIV−/sCVD+, P = 0.99).
Figure 1.
CXCR4 is differentially expressed on monocyte subsets in WIHS participants stratified by HIV and sCVD status. CXCR4 was evaluated by flow cytometry on classical (A), intermediate (B), and non-classical (C) monocytes isolated from frozen PBMCs from WIHS participants stratified by HIV and sCVD status (n = 23 per group). The graphs depict the differential distribution of CXCR4 surface marker expression measured by median fluorescence intensity (MFI) in the different subsets of monocytes and among the different groups of participants stratified by HIV and sCVD status as dot plots [median and interquartile range (IQR) are shown]. ANOVA analysis was performed. For this analysis, a P-value ≤0.050 was considered significant, indicated by *. For this analysis, a P-value ≤0.007 was considered significant after adjustment for multiple testing, indicated by ** (Tukey).
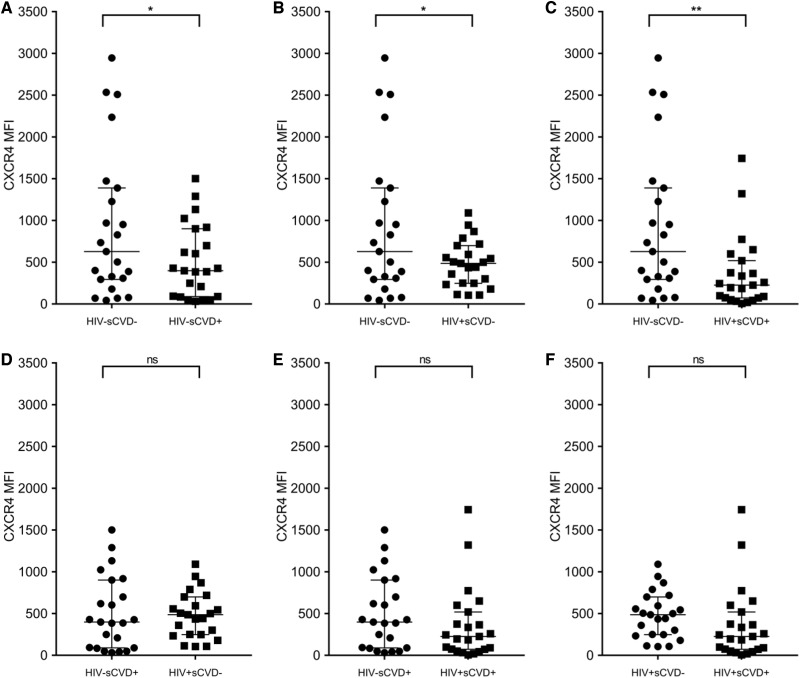
In pairwise comparisons, shown in Figure 2A–F as dot plots, CXCR4 expression on non-classical monocytes was significantly higher in HIV−/sCVD− women vs. (Figure 2A) HIV−/sCVD+ (P = 0.050), (Figure 2B) HIV+/sCVD− (P = 0.028), and (Figure 2C) HIV+/sCVD+ (P = 0.009). There were no differences in the comparison of (Figure 2D) HIV−/sCVD+ vs. HIV+/sCVD− (P = 0.827), (Figure 2E) HIV−/sCVD+ vs. HIV+/sCVD+ (P = 0.266), and (Figure 2F) HIV+/sCVD− vs. HIV+/sCVD+ (P = 0.265).
Figure 2.
Pairwise comparison of CXCR4 expression on non-classical monocytes from WIHS participants stratified by their HIV and sCVD status. CXCR4 was evaluated on non-classical monocytes as in Figure 1 (n = 92; 23 per group) and expressed as MFI. T-test was performed for paired group comparison. The graphs depict the different distribution of CXCR4 expression on non-classical monocytes between the groups as dot plots [median and interquartile range (IQR) are shown]. For this analysis, a P-value ≤0.050 was considered significant, indicated by *, P-value ≤0.010 indicated by **
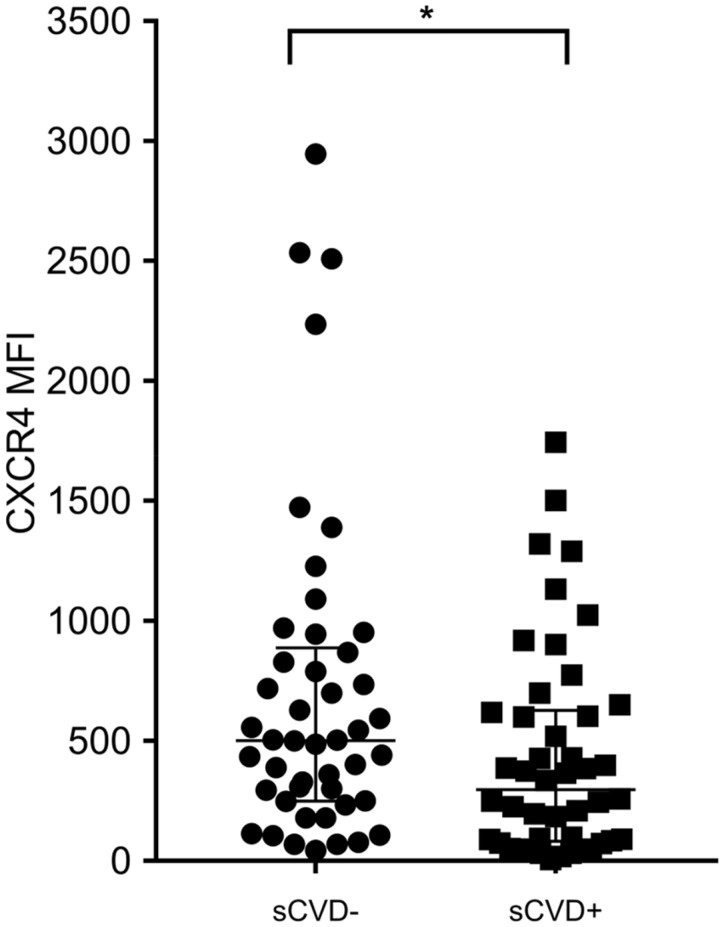
Interestingly, CXCR4 expression on non-classical monocytes was significantly higher in sCVD− [median 501.5, IQR (249.5–887.3)] compared to sCVD+ women [median 297, IQR (81.75–626.8), n = 46 per group, P = 0.028] illustrated in Figure 3.
Figure 3.
CXCR4 expression on non-classical monocytes is significantly different between sCVD+ and sCVD− WIHS participants regardless of HIV staus. CXCR4 was evaluated on non-classical monocytes among WIHS individuals stratified by sCVD status (n = 46 per group). The graphs depict the different distribution of CXCR4 expression on non-classical monocytes between the groups as dot plots [median and interquartile range (IQR) are shown]. T-test was performed for two groups’ comparison. For this analysis, a P-value ≤0.050 was considered significant (*).
CXCR4 expression on non-classical monocytes significantly correlated with several cardiovascular and HIV-related risk factors
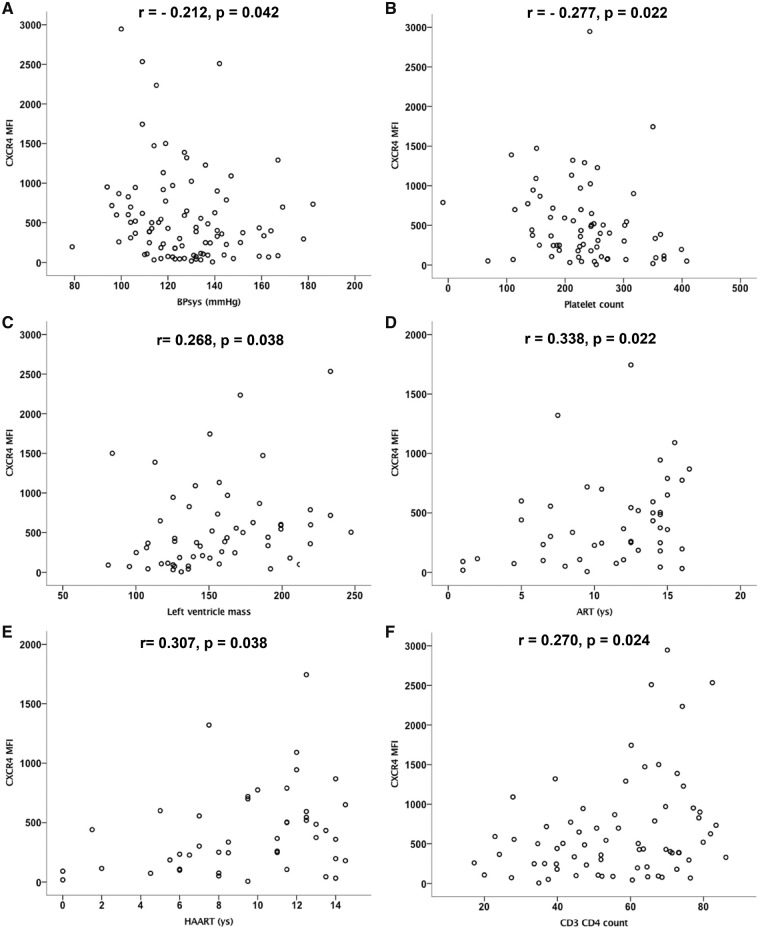
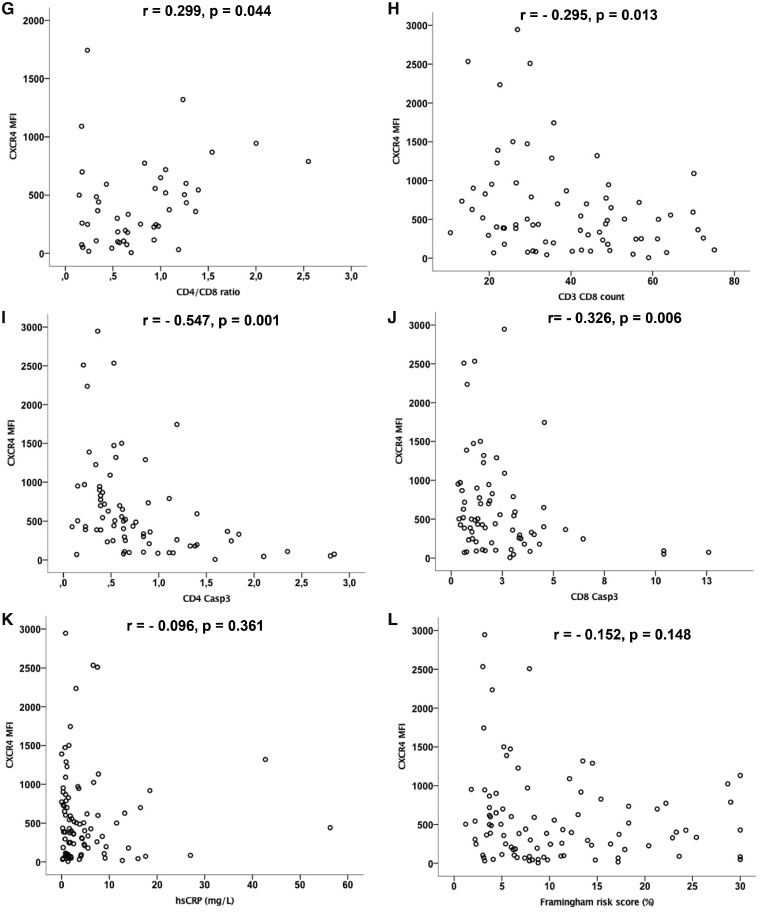
Spearman’s rank correlation analysis to evaluate correlations of CXCR4 expression on non-classical monocytes with cardiovascular and HIV-related risk factors among the whole cohort, given in Figure 4A–P, showed that CXCR4 expression on non-classical monocytes is
Figure 4.
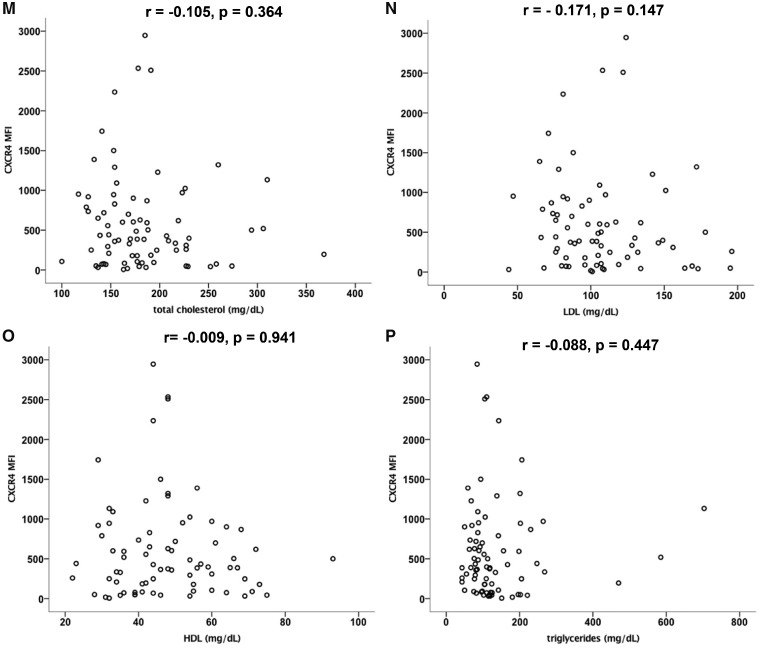
CXCR4 expression on non-classical monocytes correlates with traditional cardiovascular and HIV-related clinical risk factors. Spearman’s rank correlation analysis was performed to evaluate correlations of CXCR4 expression on non-classical monocytes with cardiovascular and HIV-related risk factors among the whole cohort. Values are presented as Spearman’s rank correlation coefficient r. CXCR4 expression on non-classical monocytes is (A) negatively correlated with systolic blood pressure (BPsys, r = −0.212, P = 0.042) and (B) blood platelet count (r = −0.277, P = 0.022) and (C) positively correlated with left ventricular mass (r = 0.268, P = 0.038), (D) the duration of ART (r = 0.338, P = 0.022) and (E) HAART (r = 0.307, P = 0.038 in HIV+ subjects) as well as with (F) blood CD4 T cell counts (CD3CD4 r = 0.270, P = 0.024) and (G) the CD4 to CD8 T cell ratio (r = 0.299, P = 0.044). CXCR4 on non-classical monocytes was negatively correlated with (H) the blood CD8 T cell count (CD3CD8, r = −0.295, P = 0.013) and with (I, J) apoptotic T cells [as detected by activated caspase-3, (I) CD4 Casp3 r = −0.547, P = 0.001, (J) CD8 Casp3 r = −0.326, P = 0.006]. There was no significant correlation of CXCR4 expression on non-classical monocytes with other cardiovascular risk factors as (K) hsCRP (r = −0.096, P = 0.361), (L) Framingham risk score (r = −0.152, P = 0.148), (M) total cholesterol (r = −0.105, P = 0.364), (N) LDL (r = −0.171, P = 0.147), (O) HDL (r = −0.009, P = 0.941),
negatively correlated with systolic blood pressure (r = −0.212, P = 0.042) and platelet count (r = −0.277, P = 0.022), while positively correlated with left ventricular mass (r = 0.268, P = 0.038), all known CVD risk factors (Figure 4A–C),
positively correlated with the duration of ART (r = 0.338, P = 0.022) and HAART (r = 0.307, P = 0.038 in HIV+ subjects, Figure4D,E),
positively correlated with CD4 T cell counts (r = 0.270, P = 0.024) and the CD4 to CD8 T cell ratio (r = 0.299, P = 0.044, Figure4F,G),
negatively correlated with the CD8 T cell count (r = −0.295, P = 0.013) and with apoptotic T cells (as detected by activated caspase-3, CD4 Casp3 r = −0.547, P = 0.001, CD8 Casp3 r = −0.326, P = 0.006, Figure 4H–J),
not correlated with other cardiovascular risk parameters including (Figure 4K) high-sensitive C-reactive protein (hsCRP) (r = −0.096, P = 0.361), (Figure 4L) Framingham risk score (r = −0.152, P = 0.148), (Figure 4M) total cholesterol (r = −0.105, P = 0.364), (Figure 4N) LDL (r = −0.171, P = 0.147), (Figure 4O) HDL (r = −0.009, P = 0.941), and (Figure 4P) triglycerides (r = −0.088, P = 0.447).
Furthermore, we performed subgroup analysis of CXCR4 expression on non-classical monocytes and its correlation with traditional cardiovascular and HIV-related clinical risk factors of the four participant groups stratified by HIV and sCVD status. Data are shown in Supplementary material online, Figures S4, S5, S6, and S7 for each group, respectively. Interestingly, we found a negative correlation of CXCR4 expression on non-classical monocytes with the Framingham risk score in HIV−/sCVD− participants of the WIHS (Supplementary material online, Figure 4I). CXCR4 expression correlated positively with the duration of ART (r = 0.452, P = 0.030) in HIV+/sCVD− participants (Supplementary material online, Figure S6D).
CXCR4 expression on non-classical monocytes was significantly associated with the presence of HIV infection and subclinical cardiovascular disease in women
In unadjusted regression analysis, lower CXCR4 expression on non-classical monocytes was significantly associated with the presence of sCVD (Table 2). Here, β is defined as mean difference in MFI of CXCR4 expression in each group compared with the reference group of HIV−/sCVD− participants. After accounting for education, study site, and history of IDU, lower CXCR4 expression on non-classical monocytes remained significantly associated with the presence of sCVD: HIV+/sCVD+ vs. HIV−/sCVD− [β, −472 U, 95% confidence interval (CI) (−782 to −161), P = 0.003]; HIV−/sCVD+ vs. HIV−/sCVD− [β, −308 U, 95% CI (−645 to 30), P = 0.074]; HIV+/sCVD− vs. HIV−/sCVD− [β, −552 U, 95% CI (−863 to −242), P = 0.001] (overall P = 0.003).
Table 2.
Association between HIV/sCVD status and CXCR4 expression in non-classical monocytes in regression analysis
| Model 1: unadjusted |
Model 2: adjusted for education, study site, history of injection drug use |
|||
|---|---|---|---|---|
| Difference in mean MFI (95% CI) | P-value | Difference in mean MFI (95% CI) | P-value | |
| HIV−/sCVD− | Ref. | – | Ref. | – |
| HIV+/sCVD− | −436 (−761 to −111) | 0.009 | −552 (−863 to −242) | 0.001 |
| HIV−/sCVD+ | −412 (−737 to −87) | 0.014 | −308 (−645 to 30) | 0.074 |
| HIV+/sCVD+ | −557 (−882 to −232) | 0.001 | −472 (−782 to −161) | 0.003 |
The study was designed as a case-control observational study. Four groups of participants of the WIHS cohort were stratified by their HIV and sCVD status (HIV−sCVD− vs. HIV−sCVD+ vs. HIV+sCVD− vs. HIV+sCVD+) and were matched based on participant age, smoking status, race/ethnicity, and age of the specimen collection date of PBMC samples.
Regression analysis of these matched samples adjusted for education, study site, and history of injection drug use. Presence of HIV infection and sCVD remained significantly associated with lower CXCR4 expression on non-classical monocytes.
Test for overall difference by HIV/sCVD status: Model 1, P = 0.006; Model 2: P = 0.003.
Groups in each model were matched by age, race/ethnicity, smoking status, and specimen collection date. Test for overall difference by HIV/sCVD status: Model 1, P = 0.006; Model 2: P = 0.003.
MFI, median fluorescence intensity; sCVD, subclinical cardiovascular disease.
Discussion
In our study, we found differential surface marker expression on three subsets of monocytes in our sample of HIV-infected and uninfected women. Specifically, we showed that both HIV infection status and subclinical cardiovascular disease were associated with reduced CXCR4 expression on non-classical monocytes. Furthermore, Spearman’s rank correlation analysis revealed correlations of CXCR4 expression on non-classical monocytes with traditional cardiovascular and HIV-related risk factors.
Non-classical monocytes play key roles in vascular homeostasis. In particular, non-classical monocytes show patrolling behaviour and actively patrol the vascular endothelium of arteries.33,34 Although patrolling is seen under homeostatic conditions, it is influenced by triggers of the inflammatory response.35,36 Human non-classical monocytes have been shown to patrol in mouse microvessels35 and carotid arteries.33,34 In mice, non-classical monocytes are atheroprotective,36,37 but it is not known whether this translates to humans. Patrolling non-classical monocytes play an important role in several disease settings including atherosclerosis. They probably function to remove damaged cells and debris from the vascular endothelium.33 These cells have also been associated with wound healing and the resolution of inflammation in damaged tissues.35,36
In our study, we described the association of reduced chemokine receptor CXCR4 expression on non-classical monocytes with presence of sCVD. The CXCL12/CXCR4 chemokine ligand/receptor axis plays a key role in cell trafficking during atherogenesis and atheroprogression. Several animal studies have suggested an atheroprotective role for CXCL12/CXCR4 interactions.38–41 CXCR4 is the chemokine receptor for CXCL12 and macrophage migration inhibitory factor (MIF), a chemokine-like molecule with a known pro-atherogenic role.39 CXCR4 is deeply involved in circadian changes in blood monocyte levels42 and thought to be required for disposal of aged leucocytes.43
CXCR4 is also a co-receptor for HIV entry in the infection by T-tropic and M-tropic HIV-1 strains.44,45 CXCR4 expression has been associated with susceptibility of monocytes to HIV infection and atherosclerosis.46,47 Therefore, CXCR4 is a key target in the investigation of chemokine-dependent inflammatory response in HIV infection and sCVD.47 CXCR4 in non-classical monocytes had not been previously investigated in HIV-infected individuals.
Similar to the findings of Longenecker et al.,30 we did not find an association between HIV and the proportions of monocyte subsets in peripheral blood with subclinical atherosclerosis of carotid arteries. In contrast, Baker et al. showed that a higher percentage of CD16+ monocytes were associated with a greater likelihood of progression of CAC.29 Increased proportions of CD16+ monocytes have been associated with cardiovascular events and the occurrence of acute coronary syndromes in non-HIV studies.22,31,48 While expression of certain surface markers on monocytes was not associated with presence or progression of CAC in the Baker study, CXCR4 was not investigated.36 Another study revealed that surface expression of CX3CR1 on CD16+ and CD11b on total monocytes can serve as independent predictors of cIMT progression in HIV infection,16 which we did not find in our cohort. A recent study of treated individuals with HIV showed that cIMT correlated with the count of non-classical monocytes at baseline and with plasma levels of MCP-1 and TNF-α.49
CD16+ monocyte (comprising non-classical and intermediate monocyte) numbers have also been positively correlated with vulnerable plaques in patients with coronary artery disease, and levels of CD16+ monocytes have been found to be significantly decreased in patients receiving statin treatment.26,31 These studies did not distinguish between CD14dimCD16+ (non-classical) and CD14+CD16+ (intermediate) subsets but grouped these two subsets into CD16-positive monocytes. A recent study of >900 patients suggested that it is mainly the CD14+CD16+ intermediate monocytes that are positive predictors for cardiovascular events, whereas the CD14dimCD16+ non-classical monocyte subset showed no correlation.50
Among women with HIV, reduced CXCR4 expression on non-classical monocytes was associated with presence of subclinical carotid artery disease even after adjustment for confounders. Monocyte migration requires signalling via chemokine receptors like CCR2, CX3CR1, and CXCR4 (in response to ligands CCL2, CX3CL1, CXCL12, and MIF, respectively).16,20,38,46 These chemokine and adhesion receptors are differentially expressed on different subsets of monocytes.40,51 Furthermore, chemokines and their receptors play a critical role in HIV infection, acquired immunodeficiency syndrome (AIDS), and atherosclerosis.47 Non-classical monocytes have lower expression of CXCR4 than classical monocytes. That CXCR4 expression on non-classical monocytes from HIV-infected individuals with CVD is even lower may therefore reflect an unfavourable monocyte phenotype or may impair their function. As regression analysis indicated that reduced expression of CXCR4 is associated with sCVD in our cohort and is an independent phenomenon. It would be of interest to extend these findings with a prospective longitudinal study determining whether the identified marker CXCR4 is predictive of clinical CVD. These results may contribute to future diagnostic and therapeutic approaches for the diagnosis and treatment of sCVD in HIV-positive individuals. Understanding the underlying pathogenesis of CVD in HIV patients, including the role of changes in monocyte phenotype, underpins the development of predictive models that will be useful for disease management in chronic HIV infection.
Women, in particular, are underdiagnosed regarding cardiovascular diseases. There are significant gender differences in screening and diagnosis of CVD as it has been defined as a men’s disease for decades.11–14 Even though sex-specific symptoms, traditional and novel risk factors and expanded understanding of the sex-specific pathophysiology of CVD have been acknowledged in recent years, ischaemic heart disease continues to be the leading cause of morbidity and mortality in women in western countries, especially in women with HIV.58–64 Therefore, novel biomarkers to detect high-risk patients at an early stage of the disease are of great clinical interest for an improved risk assessment, especially in women, who less frequently show traditional risk factors for advancing CVD.
Currently prediction of subclinical CVD such as early atherosclerosis in patients with HIV is limited to evaluation of risk factor profiles, which might underestimate the risk of the occurrence of adverse CV events in patients with HIV.52
Interestingly, there are also significant disparities in CVD burden among certain subgroups of women, who are socially disadvantaged, which relates to differences in risk factor prevalence, treatment strategies according to evidence-based guidelines, and other social and environmental factors.58–66 As gender disparities are multifactorial, they reflect under-representation of women at risk of CVD in research, with the resultant unfavourable impact on women’s cardiovascular outcome. For example, women with acute coronary syndrome undergo coronary angiography and revascularization less frequently than men.58–66 Women with CVD are usually older than men when CVD is diagnosed and present more often with unspecific symptoms like dyspnoea, nausea, or vomiting than typical angina pectoris. Traditional risk factors (smoking, diabetes mellitus, and dyslipidaemia) are less frequent in women.58–66 Furthermore, common disorders of pregnancy (gestational hypertension and diabetes) or endocrine disorders (polycystic ovary syndrome and early menopause) are associated with accelerated development of CVD. To further investigate potential, maybe even gender-specific risk factors for adverse cardiovascular outcome is especially important in women with HIV, who are at high risk to develop CVD.9,53,54
This study was based on the WIHS cohort. A similar cohort for HIV-infected men is the Multicenter AIDS Cohort Study (MACS). Our study generates the testable hypothesis that CXCR4 expression on non-classical monocytes may be negatively correlated with CVD also in men which requires further investigations.55
It has been described that CXCR4 expression in CD4+ and CD8+ T cells as well as CD14+ monocytes was significantly reduced in HIV-positive individuals when compared with uninfected controls.46 Down-modulation of CXCR4 has been correlated with HIV disease progression, which further supports the reciprocal role that CXCR4 plays in cellular activation.45,46 This down-regulation of the chemokine receptor expression is associated with elevated levels of the endogenously produced CXCR4 ligand chemokine CXCL12. CXCL12 governs differentiation of haematopoietic progenitors into either endothelial or macrophage-foam cells. CXCL12 ligates CXCR4 and CXCR7 and regulates monocyte/macrophage functions,48–51 in particular cell migration, adhesion, and survival.47,51
Our data suggest that loss of CXCR4 expression on non-classical monocytes may increase the risk of atherogenesis. A potential mechanism for this protective role of CXCR4 might be the maintenance of arterial integrity and preservation of endothelial barrier function, a known function of non-classical monocytes.33,36,56,57 It is possible that enhancing these potentially beneficial functions of CXCR4 by selective modulators could open novel therapeutic options, but more research is needed to corroborate this.
Given the cross-sectional nature and limited sample size of our study, one limitation of our findings is that they are hypothesis-generating, and therefore, the results should be replicated both longitudinally and in other study populations, including in men. Furthermore, our explanations for underlying pathomechanisms are speculative and will require further evidence. Finally, other unmeasured confounding parameters may be present; nonetheless, our careful matching of specimens and use of regression account for major known confounders including age, race, smoking status, and socioeconomic status.
Our findings show that subclinical atherosclerosis in women with HIV-related sCVD is associated with lower expression of CXCR4 on non-classical monocytes, and that surface markers are differentially expressed among monocyte subsets in women with and without HIV and with and without sCVD. Our study suggests that monocyte surface markers, including those on non-classical monocytes, may serve as novel biomarkers and predictors of sCVD in treated individuals with HIV. These findings highlight the important role for monocyte subsets in the progression of HIV-related cardiovascular pathology and need to be investigated in further large-scale studies.
Funding
Women’s Interagency HIV Study (WIHS) (Principal Investigators): Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I–WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute (NCI), the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders (NIDCD), and the National Institutes of Health (NIH) Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). Other funding sources for this study include R01-HL-083760, R01-HL-095140, R01-HL-126543 and R21-HL-120394 to R.C.K., K01-HL-137557 to D.B.H., and P30-AI-051519 to the Einstein/Rockefeller/CUNY Center for AIDS Research. Furthermore, this study was supported, in part, by the TÜFF Frauenförderungsprogramm, University of Tuebingen, Germany, (Karin Mueller, 2241-0-0). This project was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - rojektnummer 374031971 - TRR 240 the Transregio-SFB 240 consortium ‘Platelets - Molecular, cellular and systemic functions in health and disease’.
Supplementary Material
Acknowledgements
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I–WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), and P30-AI-050410 (UNC CFAR). We thank Ms Cornelia Fiessler for excellent statistical supervision.
Conflict of interest: none declared.
Time for primary review: 44 days
References
- 1. Hileman CO, Carman TL, Longenecker CT, Labbato DE, Storer NJ, White CA, McComsey GA.. Rate and predictors of carotid artery intima media thickness progression in antiretroviral-naive HIV-infected and uninfected adults: a 48-week matched prospective cohort study. Antivir Ther 2013;18:921–929. [DOI] [PubMed] [Google Scholar]
- 2. Ross AC, Rizk N, O'Riordan MA, Dogra V, El-Bejjani D, Storer N, Harrill D, Tungsiripat M, Adell J, McComsey GA.. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009;49:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S.. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003;33:506–512. [DOI] [PubMed] [Google Scholar]
- 4. Triant VA, Josephson F, Rochester CG, Althoff KN, Marcus K, Munk R, Cooper C, D'Agostino RB, Costagliola D, Sabin CA, Williams PL, Hughes S, Post WS, Chandra-Strobos N, Guaraldi G, Young SS, Obenchain R, Bedimo R, Miller V, Strobos J.. Adverse outcome analyses of observational data: assessing cardiovascular risk in HIV disease. Clin Infect Dis 2012;54:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saves M, Chene G, Ducimetiere P, Leport C, Le Moal G, Amouyel P, Arveiler D, Ruidavets JB, Reynes J, Bingham A, Raffi F; French WHO MONICA Project and the APROCO (ANRS EP11) Study Group. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003;37:292–298. [DOI] [PubMed] [Google Scholar]
- 6. Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d'Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M, El-Sadr W, De Wit S, Sabin CA, Phillips AN, Lundgren JD; DAD study group. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS 2003;17:1179–1193. [DOI] [PubMed] [Google Scholar]
- 7. Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, Overton ET, Budoff M, Hammer J, Carpenter CC, Hodis HN, Brooks JT; Study To Understand The Natural History Of HIV/AIDS in the Era of Effective Therapy Investigators. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis 2011;53:826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baker JV, Lundgren JD.. Cardiovascular implications from untreated human immunodeficiency virus infection. Eur Heart J 2011;32:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, Cohen MH, Gange SJ, Haberlen SA, Heath SL, Lazar JM, Liu C, Mack WJ, Ofotokun I, Palella FJ, Tien PC, Witt MD, Landay AL, Kingsley LA, Tracy RP, Kaplan RC.. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis 2017;215:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA.. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005;12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA.. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015; 241:211–218. [DOI] [PubMed] [Google Scholar]
- 12. Sorensen NA, Neumann JT, Ojeda F, Schafer S, Magnussen C, Keller T, Lackner KJ, Zeller T, Karakas M, Munzel T, Blankenberg S, Westermann D, Schnabel RB.. Relations of sex to diagnosis and outcomes in acute coronary syndrome. J Am Heart Assoc 2018;7. doi: 10.1161/JAHA.117.007297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, Mendelson MA, Wood MJ, Volgman AS, Mieres JH.. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes 2018;11:e004437. [DOI] [PubMed] [Google Scholar]
- 14. Chester RC, Kling JM, Manson JE.. What the Women's Health Initiative has taught us about menopausal hormone therapy. Clin Cardiol 2018;41:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diamantis E, Kyriakos G, Quiles-Sanchez LV, Farmaki P, Troupis T.. the anti-inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr Cardiol Rev 2017;13:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westhorpe CL, Maisa A, Spelman T, Hoy JF, Dewar EM, Karapanagiotidis S, Hearps AC, Cheng WJ, Trevillyan J, Lewin SR, Sviridov D, Elliott JH, Jaworowski A, Dart AM, Crowe SM.. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol 2014;92:133–138. [DOI] [PubMed] [Google Scholar]
- 17. Haas JG, Riethmuller G, Ziegler-Heitbrock HW.. Monocyte phenotype and function in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related disorders. Scand J Immunol 1987;26:371–379. [DOI] [PubMed] [Google Scholar]
- 18. Weber C, Noels H.. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011;17:1410–1422. [DOI] [PubMed] [Google Scholar]
- 19. Libby P, Ridker PM, Hansson GK.. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 20. Woollard KJ, Geissmann F.. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB.. Nomenclature of monocytes and dendritic cells in blood. Blood 2010;116:e74–e80. [DOI] [PubMed] [Google Scholar]
- 22. Tapp LD, Shantsila E, Wrigley BJ, Pamukcu B, Lip GY.. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J Thromb Haemost 2012;10:1231–1241. [DOI] [PubMed] [Google Scholar]
- 23. Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 2007;81:584–592. [DOI] [PubMed] [Google Scholar]
- 24. Shantsila E, Tapp LD, Wrigley BJ, Pamukcu B, Apostolakis S, Montoro-García S, Lip GYH.. Monocyte subsets in coronary artery disease and their associations with markers of inflammation and fibrinolysis. Atherosclerosis 2014;234:4–10. [DOI] [PubMed] [Google Scholar]
- 25. Shantsila E, Wrigley B, Tapp L, Apostolakis S, Montoro-Garcia S, Drayson MT, Lip GY.. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost 2011;9:1056–1066. [DOI] [PubMed] [Google Scholar]
- 26. Imanishi T, Ikejima H, Tsujioka H, Kuroi A, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Takeshita T, Akasaka T.. Association of monocyte subset counts with coronary fibrous cap thickness in patients with unstable angina pectoris. Atherosclerosis 2010;212:628–635. [DOI] [PubMed] [Google Scholar]
- 27. Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell Immunol 2014;289:135–139. [DOI] [PubMed] [Google Scholar]
- 28. Zungsontiporn N, Tello RR, Zhang G, Mitchell BI, Budoff M, Kallianpur KJ, Nakamoto BK, Keating SM, Norris PJ, Ndhlovu LC, Souza SA, Shikuma CM, Chow DC.. Non-classical monocytes and monocyte chemoattractant protein-1 (MCP-1) correlate with coronary artery calcium progression in chronically HIV-1 infected adults on stable antiretroviral therapy. PLoS One 2016;11:e0149143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, Onen N, Kojic E, Patel P, Brooks JT, Hodis HN, Budoff M, Sereti I; CDC SUN Study Investigators. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 2014;28:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, Lederman MM, McComsey GA.. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med 2013;14:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kashiwagi M, Imanishi T, Tsujioka H, Ikejima H, Kuroi A, Ozaki Y, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Kitabata H, Hirata K, Akasaka T.. Association of monocyte subsets with vulnerability characteristics of coronary plaques as assessed by 64-slice multidetector computed tomography in patients with stable angina pectoris. Atherosclerosis 2010;212:171–176. [DOI] [PubMed] [Google Scholar]
- 32. Frankenberger M, Hofer TP, Marei A, Dayyani F, Schewe S, Strasser C, Aldraihim A, Stanzel F, Lang R, Hoffmann R, Prazeres da Costa O, Buch T, Ziegler-Heitbrock L.. Transcript profiling of CD16-positive monocytes reveals a unique molecular fingerprint. Eur J Immunol 2012;42:957–974. [DOI] [PubMed] [Google Scholar]
- 33. Quintar A, McArdle S, Wolf D, Marki A, Ehinger E, Vassallo M, Miller J, Mikulski Z, Ley K, Buscher K.. Endothelial protective monocyte patrolling in large arteries intensified by western diet and atherosclerosis. Circ Res 2017;120:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buscher K, Marcovecchio P, Hedrick CC, Ley K.. Patrolling mechanics of non-classical monocytes in vascular inflammation. Front Cardiovasc Med 2017;4:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F.. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317:666–670. [DOI] [PubMed] [Google Scholar]
- 36. Thomas G, Tacke R, Hedrick CC, Hanna RN.. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol 2015;35:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC.. Patrolling monocytes control tumor metastasis to the lung. Science 2015;350:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doring Y, Noels H, van der Vorst EPC, Neideck C, Egea V, Drechsler M, Mandl M, Pawig L, Jansen Y, Schroder K, Bidzhekov K, Megens RTA, Theelen W, Klinkhammer BM, Boor P, Schurgers L, van Gorp R, Ries C, Kusters PJH, van der Wal A, Hackeng TM, Gabel G, Brandes RP, Soehnlein O, Lutgens E, Vestweber D, Teupser D, Holdt LM, Rader DJ, Saleheen D, Weber C.. Vascular CXCR4 limits atherosclerosis by maintaining arterial integrity: evidence from mouse and human studies. Circulation 2017;136:388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Vorst EP, Doring Y, Weber C.. MIF and CXCL12 in cardiovascular diseases: functional differences and similarities. Front Immunol 2015;6. doi: 10.3389/fimmu.2015.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW.. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol 2000;67:699–704. [DOI] [PubMed] [Google Scholar]
- 41. Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, Kraemer BF, Siegel-Axel D, May AE, Lindemann S, Gawaz M.. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 2008;117:206–215. [DOI] [PubMed] [Google Scholar]
- 42. Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS.. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011;208:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eash KJ, Means JM, White DW, Link DC.. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 2009;113:4711–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Creery D, Weiss W, Graziani-Bowering G, Kumar R, Aziz Z, Angel JB, Kumar A.. Differential regulation of CXCR4 and CCR5 expression by interleukin (IL)-4 and IL-13 is associated with inhibition of chemotaxis and human immunodeficiency virus (HIV) type 1 replication but not HIV entry into human monocytes. Viral Immunol 2006;19:409–423. [DOI] [PubMed] [Google Scholar]
- 45. Chandrasekaran P, Moore V, Buckley M, Spurrier J, Kehrl JH, Venkatesan S.. HIV-1 Nef down-modulates C-C and C-X-C chemokine receptors via ubiquitin and ubiquitin-independent mechanism. PLoS One 2014;9:e86998.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ostrowski MA, Justement SJ, Catanzaro A, Hallahan CA, Ehler LA, Mizell SB, Kumar PN, Mican JA, Chun TW, Fauci AS.. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol 1998;161:3195–3201. [PubMed] [Google Scholar]
- 47. Kedzierska K, Crowe SM, Turville S, Cunningham AL.. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol 2003;13:39–56. [DOI] [PubMed] [Google Scholar]
- 48. Idzkowska E, Eljaszewicz A, Miklasz P, Musial WJ, Tycinska AM, Moniuszko M.. The role of different monocyte subsets in the pathogenesis of atherosclerosis and acute coronary syndromes. Scand J Immunol 2015;82:163–173. [DOI] [PubMed] [Google Scholar]
- 49. Chow DC, Kagihara JM, Zhang G, Souza SA, Hodis HN, Li Y, Mitchell BI, Nakamoto BK, Kallianpur KJ, Keating SM, Norris PJ, Kohorn LB, Ndhlovu LC, Shikuma CM.. Non-classical monocytes predict progression of carotid artery bifurcation intima-media thickness in HIV-infected individuals on stable antiretroviral therapy. HIV Clin Trials 2016;17:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Große-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Böhm M, Fliser D, Heine GH.. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol 2012;60:1512–1520. [DOI] [PubMed] [Google Scholar]
- 51. Tacke F, Randolph GJ.. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006;211:609–618. [DOI] [PubMed] [Google Scholar]
- 52. D'Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB.. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 53. Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J.. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998;9:117–125. [PubMed] [Google Scholar]
- 54. Shaked I, Hanna DB, Gleißner C, Marsh B, Plants J, Tracy D, Anastos K, Cohen M, Golub ET, Karim R, Lazar J, Prasad V, Tien PC, Young MA, Landay AL, Kaplan RC, Ley K.. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol 2014;34:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stein JH, Hsue PY.. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012;308:405–406. [DOI] [PubMed] [Google Scholar]
- 56. Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC.. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res 2012;110:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F.. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013;153:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kouvari M, Yannakoulia M, Souliotis K, Panagiotakos DB.. Challenges in Sex- and Gender-Centered Prevention and Management of Cardiovascular Disease: Implications of Genetic, Metabolic, and Environmental Paths. Angiology 2018;69:843–853. [DOI] [PubMed] [Google Scholar]
- 59. Peplinski B, McClelland R, Szklo M.. Associations between socioeconomic status markers and depressive symptoms by race and gender: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Ann Epidemiol 2018;28:535–542. [DOI] [PubMed] [Google Scholar]
- 60. Coutinho T, Yam Y, Chow BJW, Dwivedi G, Inácio J.. Sex Differences in Associations of Arterial Compliance With Coronary Artery Plaque and Calcification Burden. J Am Heart Assoc 2017;6:pii:e006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM.. Sex-based differences in early mortality after myocardial infarction. N Engl J Med 1999;341:217–225. [DOI] [PubMed] [Google Scholar]
- 62. Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng Z-J; NRMI Investigators. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012;307:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hillinger P, Twerenbold R, Wildi K, Rubini Gimenez M, Jaeger C, Boeddinghaus J, Nestelberger T, Grimm K, Reichlin T, Stallone F, Puelacher C, Sabti Z, Kozhuharov N, Honegger U, Ballarino P, Miro O, Denhaerynck K, Ekrem T, Kohler C, Bingisser R, Osswald S, Mueller C.. Gender-specific uncertainties in the diagnosis of acute coronary syndrome. Clin Res Cardiol 2017;106:28–37. [DOI] [PubMed] [Google Scholar]
- 64. Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S.. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2015;37:267–315.26320110 [Google Scholar]
- 65. Lee SK, Khambhati J, Varghese T, Stahl EP, Kumar S, Sandesara PB, Wenger NK, Sperling LS.. Comprehensive primary prevention of cardiovascular disease in women. Clin Cardiol 2017;40:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mariani L, Burzotta F, Aurigemma C, Romano A, Niccoli G, Leone AM, Porto I, Trani C, Crea F.. Frequency-domain optical coherence tomography plaque morphology in stable coronary artery disease: sex differences. Coron Artery Dis 2017;28:472–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.