Abstract
Background
Functional abdominal pain disorders (FAPD) are prevalent in the paediatric population, however, there is currently no consensus regarding best practices for treatment. The use of probiotics is becoming popular to treat FAPD. The goal of this rapid review is to synthesize the best evidence on the use of probiotics in children with FAPD.
Methods
Searches were conducted on five main databases. Randomized controlled trials (RCTs) of probiotic use in children (0 to 18 years) with FAPD were searched. Populations of interest were patients with functional abdominal pain (FAP), irritable bowel syndrome (IBS), and functional dyspepsia (FD), recruited based on Rome criteria. Outcomes of interest were changes in abdominal pain severity, frequency, and duration.
Findings
Eleven RCTs with 829 participants with the diagnosis of FAP (n=400), IBS (n=329), FD (n=45), and mixed population (n=55) were included. Of six studies of children with FAP, two (n=103) used Lactobacillus rhamnosus GG (LGG) and reported no significant effects on pain, and four (n=281) used Lactobacillus (L) reuteri DSM 17938, of which three (n=229) reported significant positive effects on either severity or frequency of pain. Of six trials of children with IBS, four (n=219) used LGG, of which three (n=168) reported a positive effect. One (n=48) used bifidobacteria and one used VSL #3 (n=59), both demonstrating positive effects with probiotics. Two studies of FD reported no benefit. No adverse events were attributed to probiotics.
Conclusions
There is preliminary evidence for use of probiotics, particularly LGG, in reducing abdominal pain in children with IBS. There are inconsistent positive effects of other probiotics, including L. reuteri DSM 17938, in reducing pain in patients with FAP, IBS, or FD. More RCTs with rigorous methodology using single or combination probiotics are warranted.
Keywords: Abdominal pain, Paediatrics, Probiotics, Review
BACKGROUND
Functional gastrointestinal disorders are common in the paediatric population and are associated with lower self-reported quality of life scores, greater school absenteeism, and greater health care utilization (1–3). Functional gastrointestinal disorders are recurring chronic conditions that are not explained by underlying physiological, structural or biomedical etiologies (4,5). The international standard for diagnosis of functional gastrointestinal disorders, Rome IV, classifies childhood functional gastrointestinal disorders into three categories: 1) functional nausea and vomiting disorders, 2) functional abdominal pain disorders (FAPD), and 3) functional defecation disorders (6). FAPD can be further divided into four groups: functional dyspepsia (FD), irritable bowel syndrome (IBS), abdominal migraines, and functional abdominal pain (FAP)—not otherwise specified (6). ‘Functional abdominal pain—not otherwise specified’ is a new category under the Rome IV, previously separated into FAP and functional abdominal pain syndrome (6).
The prevalence of FAPD ranges from 0.3 to 19% (7). IBS is the most common subtype (8.8%), followed by FD (4.5%), FAP (3.5%), abdominal migraine (1.5%), and functional abdominal pain disorder (0.9%) (8). FAPD is 1.5 times more prevalent in females compared with males.
The etiology and pathophysiology of FAPD is likely multifactorial, based on complex interactions between psychosocial factors and gut physiology that lead to an altered brain—gut axis. The symptomology of FAPD is a result of a multitude of factors including visceral hypersensitivity, altered gut motility, microbial dysbiosis, altered mucosal immune function, and central nervous system dysregulation of gut function (6,9). Current pharmacologic therapies for FAPD, such as antispasmodic, antidepressant, antireflux, antihistaminic, and laxative agents, have not been supported by high-quality evidence (10). Nonpharmacological therapies, including dietary restriction, cognitive behavioural therapy, hypnotherapy, and fibre supplementation, have been studied, with some evidence for efficacy for cognitive behavioural therapy and hypnotherapy (11).
There have been an increasing number of studies on the effect of probiotics for functional gastrointestinal disorders. Probiotics, defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (12), have demonstrated beneficial effects in various gastrointestinal conditions (13). Preliminary data also suggest that they may have a role in neurodevelopment and mood in children and adults, representing a ‘microbiome-gut-brain axis’ (14–16). Probiotics are thought to act by promoting protective conditions in the host that affect immune and metabolic activities, bacterial colonization, and gut motility (17,18). Our objective was to synthesize the best evidence on the effects of probiotics in the management of FAPD in the paediatric population.
METHODS
A comprehensive search of MEDLINE, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials, and Google Scholar was conducted for studies published from 1990 through November 2017 (Appendix I). We selected studies if they 1) were English language publications, 2) studied children 0 to 18 years old, 3) were randomized controlled trials (RCTs), 4) recruited patients with FAPD using Rome criteria, 5) used one or more probiotics as intervention, 6) measured frequency, duration, severity of, and/or functional impact of abdominal pain, as rated by the patient or caregiver. The control group could be placebo, usual care, and/or medical interventions. Full text articles were used for final selection of eligible RCTs. A structured data extraction form was used for collecting relevant information from the selected studies. Data were extracted by one (LD) and verified by the second reviewer (MK).
General findings
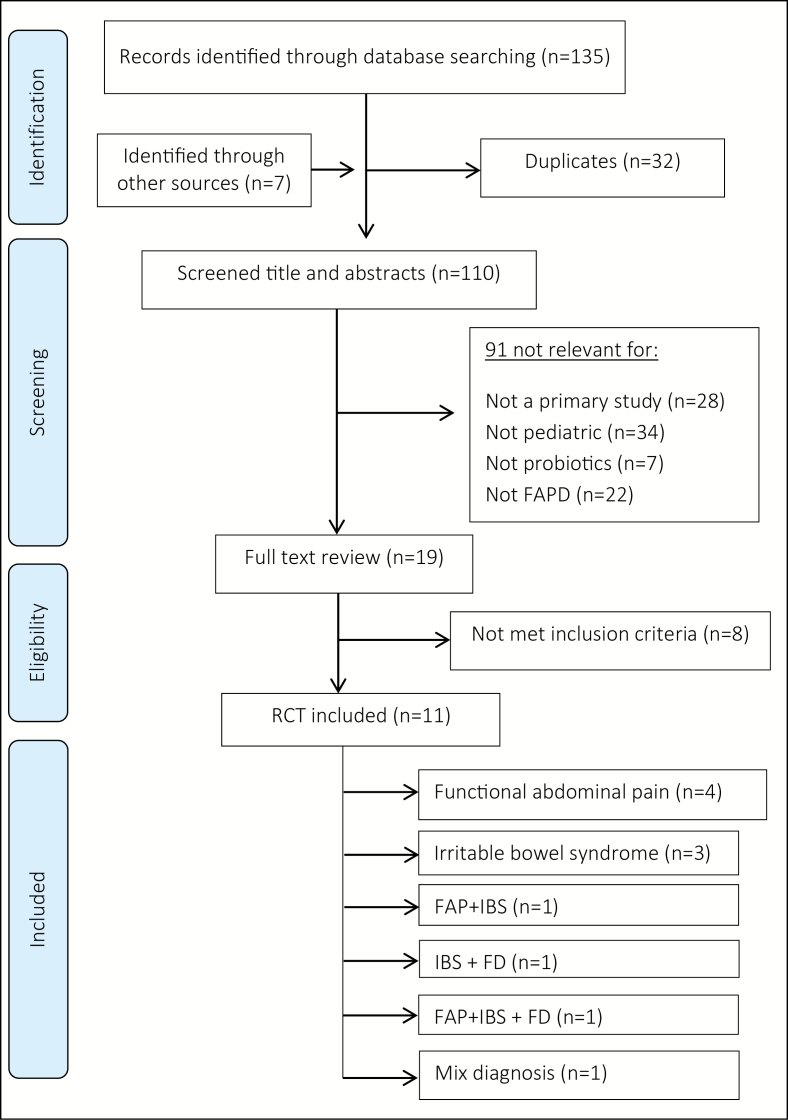
Inclusion criteria were met for 11 RCTs (n=829) including children with FAP (n=400), IBS (n=329), FD (n=45), or mixed FAPD (n=55) (19–29). See PRISMA flow diagram for inclusion and exclusion of studies in Appendix II (30). The RCTs by Gawronska et al., Francavilla et al., and Gianetti et al. presented subgroup analysis for different FAPD populations and subgroup results for FAP, IBS, and/or FD was used for this review. There were no published RCTs on abdominal migraine. Included RCTs had sample sizes of 20 to 141, were published from 2005 to 2017, conducted in eight countries (Croatia, Greece, India, Iran, Israel, Italy, Poland, and USA), and were four to eight weeks in duration. The age range was 4 to 18 years, with no significant age or sex differences between intervention and control groups (Table 1).
Table 1.
General characteristics of RCTs studying probiotics in paediatric FAPD
| First author (year), country | Number of participants (age range) | Recruiting criteria (diagnosis) | Length of trial (weeks) | Intervention | Control | Dose/day | Primary outcome | Authors conclusion |
|---|---|---|---|---|---|---|---|---|
| Giannetti (2017), Italy | 73 (8–18) | Rome III (IBS, FD) | 6 | 3 billion Bifidobacterium (B). longum, 1 billion B. infantis, 1 billion B. breve | Placebo | once | AP resolution | Improvement in AP and QoL in IBS but not confirmed in FD. |
| Jadrešin (2017), Croatia | 55 (5–17) | Rome III (IBS, FAP) | 12 | Lactobacillus (L). reuteri DSM 17938 | Placebo | Once | Days without pain and pain change during the study period | May reduce intensity of pain; more days without pain in FAP and IBS. |
| Maragkoudaki (2017), Greece | 54 (5–16) | Rome III (FAP) | 4 | L. reuteri DSM 17938 108, 2 tablets | Placebo | Once | Pain frequency and intensity | Probiotics not superior to placebo in FAP. |
| Weizman (2016), Israel | 101 (6–15) | Rome III (FAP) | 4 | L. reuteri 17938 1 × 108 CFU | Placebo | Once | Frequency and intensity of AP | Reduced frequency and intensity of FAP. |
| Eftekhari (2015), Iran | 80 (4–16) | Rome III (FAP) | 4 | L. reuteri 17938 1 × 108 CFU, | Placebo | Once | Pain intensity, pain frequency, and response to treatment | Probiotics were not superior to placebo in FAP. |
| Kianifar (2015), Iran | 52 (4–18) | Rome III (IBS) | 4 | Oral LGG (1 × 1010 CFU) with inulin | Inulin | Twice | Severity of AP | LGG resulted in reductions in the severity of AP. |
| Romano (2014), Italy | 60 (6–16) | Rome III (FAP) | 8 | Oral L. reuteri 17938 (1 × 108 CFU), | Placebo | Twice | Reduction in intensity of FAP | Reduced intensity, but not frequency of AP. |
| Francavilla (2010), Italy | 141 (5–14) | Rome II (IBS, FAP) | 8 | Oral LGG (3 × 109 CFU) | Placebo | Twice | Change in AP (frequency/severity) | Reduced frequency and severity of pain in children with IBS. |
| Guandalini (2010), Italy/ India | 59 (4–18) | Rome II (IBS) | 6 | VSL #3 (450 × 109 bacteria/sachet) | Placebo | 1 sachet once (age 4–11); 2 sachets twice (age 12–18) | Sobject’s global assessment of relief (SGARC) | Decreased severity and frequency of AP, abdominal bloating; improved QoL. |
| Gawrońska (2007), Poland | 104 (6–16) | Rome II (IBS, FAP, FD) | 4 | Oral LGG (3 × 109 CFU) | Placebo | Twice | Treatment success (no pain at the end of intervention) | Improved treatment success on IBS, with no effect on IBS or FD. |
| Bauserman (2005), USA | 50 (6–17) | Rome II (IBS) | 6 | Oral LGG (1 × 1010 CFU) with inulin | Placebo with inulin | Twice | Change in AP severity score | Did not improve AP; may help improve symptoms such as perceived abdominal distension. |
Rome II and Rome III definition can be found in Appendix III.AP Abdominal pain; CFU Colony forming units; FAP Functional abdominal pain; FD Functional dyspepsia; FGID Functional gastrointestinal disorder; IBS Irritable bowel syndrome; LGG = Lactobacillus (L) rhamnosus GG; QoL Quality of life; VSL = Bifidobacterium (B) breve, B.longum, B. infantis, L. acidophilus, L. planatarum, L. casei, L. bulgaris, Streptococcus thermophiles; 450 billion CFU per sachet.
Probiotics for functional abdominal pain
Six placebo-controlled RCTs (n=400 children) assessed the effectiveness of probiotics for paediatric FAP(Table 2). The most commonly studied probiotic for FAP was Lactobacillus reuteri DSM 17938(four RCTS, n=295) (21–23,25). Three out of the four studies (n=241) demonstrated a positive effect for probiotic use on reducing pain severity and frequency. Two of the four studies (n=181) found a significant difference in pain frequency (22,23) and two (22,25) indicated a significant difference in pain severity in favour of probiotics (n=161). The fourth trial (21) found no differences between the probiotic and placebo groups. Two trials (n=105) (26,28) used Lactobacillus (L) rhamnosus (LGG) for 4 to 8 weeks and did not report a significant difference in pain severity compared to placebo. Three studies looked at the impact of probiotics on daily function and found no difference in school absenteeism, parental work absenteeism, or use of other medications (21,22,28). Interpretation of the results is complicated by the lack of a sample size calculation (23,25) or being underpowered (21,28). In particular, analysis of functional impact was complicated by small samples and wide confidence intervals. Two studies did not exclude children with probiotics or antibiotics prior to the study period (25,28).
Table 2.
The effectiveness of probiotics for treatment of functional abdominal pain
| First author (Year) | Number of participants | Probiotics | Outcome and measurement unit | Results in intervention(I)/control group (C) | Statistical test results |
|---|---|---|---|---|---|
| Maragkoudaki (2017) | 54 | Lactobacillus (L). reuteri 17938 | Pain severity by WB-FACE | I: 4.3 (SD=8.5) C: 4.0 (SD=5.6) |
(P=0.72) |
| Pain frequency | I: 2.9 (SD=4.5) C: 3.1 (SD=4.1) |
(P=0.68) | |||
| Weizman (2016) | 101 | L. reuteri 17938 | Pain severity by HFPS | I 4.3 (SD=2.7) C: 7.2 (SD=3.1) |
(P<0.01)* |
| Pain frequency | I: 1.9 (SD=0.8) C: 3.6 (SD=1.7) |
(P<0.02)* | |||
| Eftekhari (2015) | 80 | L. reuteri 17938 | Pain resolution (%) by WB-FACEα | I: 50% C: 65% |
NS |
| Pain frequency | I: 2.53 (SD=1.43) C: 2.08 (SD=1.56) |
(P=0001)* | |||
|
Romano
(2014) |
60 | L. reuteri 17938 | Pain severity by WB-FACE | NR | (P<0.05)* |
| Pain frequency (episodes/day) | NR | (NS) | |||
| Francavilla (2010) | 58 | LGG | Pain severity by VAS | I: 2.5 (SD=1.6) C: 3.1 (SD=1.4) |
(P=0.1) |
| Pain severity treatment success (%)† | I: 50% C: 60% |
(P=0.4) | |||
| Pain frequency | I: 1.9 (SD=0.7) C: 1.7 (SD=1.5) |
(P=0.7) | |||
| Pain frequency treatment success (%)† | I: 48% C: 44% |
(P=0.6) | |||
| Gawrońska (2007) | 47 | LGG | Treatment success (n)‡ | I: 25% (n=6) C: 9.1% (n=2) |
RB 2.9 (95% CI: 0.7–11.7, P=0.25) |
| Pain severity by self-report | I: 2.6 (SD=2.0) C: 3.0 (SD=1.5) |
(P=0.57) | |||
| Pain frequency | I: 2.3 (SD=1.8) C:2.4 (SD=1.4) |
(P=0.93) |
All pain frequency measured as number of episodes/week
CI Confidence interval; FOS Fructo-oligosaccharide; HFPS Hicks faces pain scale; LGG = Lactobacillus rhamnosus GG; MD Mean difference; NR Not reported; NS Not significant; RB Relative benefit; VAS Visual analogue scale; WB-FACE Wong-Baker FACES pain rating scale.
α = at least two point reduction in WB-FACE or ‘no pain’ after probiotic.
*Statistically significant.
†At least 50% decrease in number of episodes and intensity of pain
‡No pain (a relaxed face, score of 0, on the Faces Pain Scale)
In summary, three out of six RCTs (n=241) found probiotic significantly reduced abdominal pain treatment response, severity, or frequency. All three studies used L reuteri. There is no difference on functional impacts.
Probiotics for irritable bowel syndrome
Six placebo-controlled RCTs (n=329 children) evaluated the effectiveness of probiotics on IBS specifically. Positive benefits of probiotic were found in five studies. The most commonly studied probiotic was LGG (four RCTs, n=222), with a study period of 4 to 8 weeks (24,26,28,29). Two studies demonstrated improvement in treatment success postintervention, one out of three showed benefit for pain severity, and two out of two for pain frequency. Only the earliest study using LGG found no effect on pain severity or number of responders with probiotic use (29). The fifth study (19) used a combination of three Bifidobacterium species for 6 weeks and found probiotics more effective for pain resolution (defined as no episodes of pain during the treatment period). The sixth trial (31) used VSL #3, a proprietary combination of seven probiotic bacteria, for 6 weeks and demonstrated that the probiotic group had greater improvement in pain severity (Table 3). Four studies evaluated the functional impact of probiotic use, with three studies finding a positive result for probiotic usage. However, a heterogeneous group of probiotics and functional indices were used. Sample size calculation was completed for only one study (26).
Table 3.
The effectiveness of probiotics for treatment of irritable bowel syndrome
| First author (year) | Number of participants | Probiotics | Outcome and measurement unit | Results in intervention (I)/control group (C) | Statistical test results |
|---|---|---|---|---|---|
| Giannetti (2017) | 48 | Bifidobacteria | Pain resolution %† | I: 42% C: 14% |
(P=0.003)* |
| Functional improvement % by FDI | I: 46% C: 16% |
(P=0.002) | |||
| Kianifar (2015) | 52 | LGG | Pain severity by LS5 | I: 0.8 (SD=0.9) C: 1.5 (SD=0.8) |
(P=0.00)* |
| Functional scale by LS3 | I: 2.4 (SD=0.5) C: 1.9 (SD=0.4) |
(P=0.00)* | |||
| Francavilla (2010) | 83 | LGG | Pain severity by VAS | I: 2.5 (SD=1.2) C: 3.6 (SD=2.2) |
(P=0.1) |
| Pain severity treatment success % ‡ | I: 55% C: 30% |
(P=0.01)* | |||
| Pain frequency | I: 1.6 (SD=0.8) C: 3.2 (SD=1.9) |
(P=0.001)* | |||
| Pain frequency treatment success %‡ | I: 79% C: 45% |
(P=0.01)* | |||
| Guandalini (2010) | 59 | VSL#3¥ | Change in pain severity by 5 point scale | I: −1.0 (SD=0.2) C: −0.5 (SD=0.2) |
(P<0.05)* |
| Family life disruptions by caregiver’s report | I: −0.9 (SD=0.2) C: −0.51 (SD=0.3) |
(P<0.01)* | |||
| Gawrońska (2007) | 37 | LGG | Treatment success % (n)$ | I: 33.3% (n=6) C: 5.3% (n=1) |
RB 6.3 (95% CI: 1.2–38, P=0.04)* |
| Pain severity by self-report | I: 2.2 (SD=2.1) C: 3.2 (SD=1.5) |
(P=0.10) | |||
| Pain frequency | I: 1.8 (SD=1.7) C: 3.1 (SD=1.1) |
(P=0.02)* | |||
| Use of medications (n) | I: 4 C: 3 |
RR 1.4 (95% CI 0.4–5.1, P=0.69) | |||
| School absenteeism (n) | I: 1 C: 0 |
(P=0.49) | |||
| Bauserman (2005) | 50 | LGG | Change in pain severity by LS4 | I: −1.7 (SD=0.6) C: −1.3 (SD=0.3) |
(P=0.175) |
| Number of responders** | I: 10 C: 11 |
(P=0.774) |
All pain frequency measured as number of episodes/week.
CI Confidence interval; CFU Colony forming units; FDI Functional disability inventory; FPS Faces pain scale; LGG = Lactobacillus (L) rhamnosus GG LS3 Three-point Likert scale; LS5 Five-point Likert scale; MD Mean difference; RB Relative benefit; VAS Visual analogue scale.
*Statistically significant.
†No episodes of pain during the treatment period.
‡At least 50% reduction in number of episodes and intensity of pain.
$Treatment success as no pain (a relaxed face, score of 0, on the Faces Pain Scale).
**Decrease in abdominal pain severity of one point or more on four-point Likert scale.
Bifidobacteria = 3 billion CFU Bifidobacterium (B). longum BB536, 1 billion CFU B. infantis M-63, 1 billion B. breve M-16V.
¥ - VSL #3:B. breve, B. longum, B. infantis, L. acidophilus, L. planatarum, L. casei, L. bulgaris, Streptococcus thermophiles; 450 billion CFU per sachet.
In summary, five of six RCTS (n=279) demonstrated a beneficial effect of probiotic, however, the specific pain parameter affected was heterogeneous among the studies. Three out of four trials using LGG demonstrated an improvement in pain severity or frequency in the probiotic group. One trial using a bifidobacteria combination product and one using VSL #3, each showed some benefits for pain resolution and pain severity. Three out of four studies using LGG, VSL #3, or bifidobacteria product found a positive effect for functional improvement in the interventional group.
Probiotics for functional dyspepsia
Two placebo-controlled RCTs (n=45) contained subgroup analysis for paediatric functional dyspepsia (Table 4) (19,28). The probiotics used were bifidobacteria and LGG. Neither study found an improvement in abdominal pain resolution rate, severity, frequency, or functional impacts with the use of probiotics. Both studies were limited by very small sample size.
Table 4.
The effectiveness of probiotics for treatment of functional dyspepsia
| First author (Year) | Number of participants | Probiotics | Outcome and measurement unit | Results in intervention(I)/control group (C) | Statistical test results (P-value) |
|---|---|---|---|---|---|
| Giannetti (2017) | 25 | Bifidobacteria | Pain resolution %* | I: 21% C: 32% |
(P=0.5) |
| Functional improvement % by FDI | I: 28% C: 24% |
(P=0.1) | |||
| Gawrońska (2007) | 20 | LGG | Treatment success (n)† | I: 10% (n=1) C: 20% (n=2) |
RB 0.5 (95% CI: 0.07 to 3.3, P = 1) |
| Pain severity by self-report | I: 2.9 (SD=1.5) C: 1.9 (SD=1.3) |
(P=0.14) | |||
| Pain frequency | I: 2.7 (SD=1.3) C:2.0 (SD=1.6) |
(P=0.26) | |||
| Use of medications (n) | I: 3 C: 2 |
RR 1.5 (95% CI 0.4–6.5, P=1) | |||
| School absenteeism (n) | I: 3 C: 0 |
(P=0.21) |
Bifidobacteria = 3 billion CFU Bifidobacterium (B). longum BB536, 1 billion CFU B. infantis M-63, 1 billion B. breve M-16V; FDI Functional disability inventory; LGG Lactobacillus rhamnosus GG; RB Relative benefit; RR Relative risk.
*No episodes of pain during the treatment period.
†Treatment success as no pain (a relaxed face, score of 0, on the Faces Pain Scale).
Probiotics for a mixed diagnosis of FAP, IBS, FD, and abdominal pain syndrome (APS)
One RCTa of children with various FAPD (n=55) was identified. In their study, Jadresin et al. used L. reuteri for 12 weeks (20). Probiotic use significantly increased number of days without pain, however, it did not affect pain severity or frequency (Table 5). The study did not evaluate the functional impact of probiotic use.
Table 5.
The effectiveness of probiotics for treatment of mixed FAPD
| First author (year) | Number of participants | Probiotics | Outcome and measurement unit | Results in intervention(I)/ control group (C) | Statistical test results (P-value) |
|---|---|---|---|---|---|
| Jadresin (2017) | 55 | Lactobacillus reuteri 17938 | Number of days without pain (n) | I: 89.5 (Range=5–108) C: 51 (Range=0–107) |
0.029* |
| Change in pain severity by WBFS | I: 0.42 (Range=−0.31–2.9) C:0.23 (Range=−1.2–2.2) |
0.481 | |||
| Changes in pain duration (minutes) | I: 6.3 (Range=−40–170) C: 23 (Range=−32–395) |
0.143 | |||
| Complete resolution of pain (n) | I: 61.5% (n=16) C: 55.2% (n=16) |
0.633 | |||
| Changes in pain duration (minutes) | I: 19.310 C: 1.800 |
0.012* |
WBFS Wong-Baker faces scale.
*Statistically significant.
Risk of bias assessment
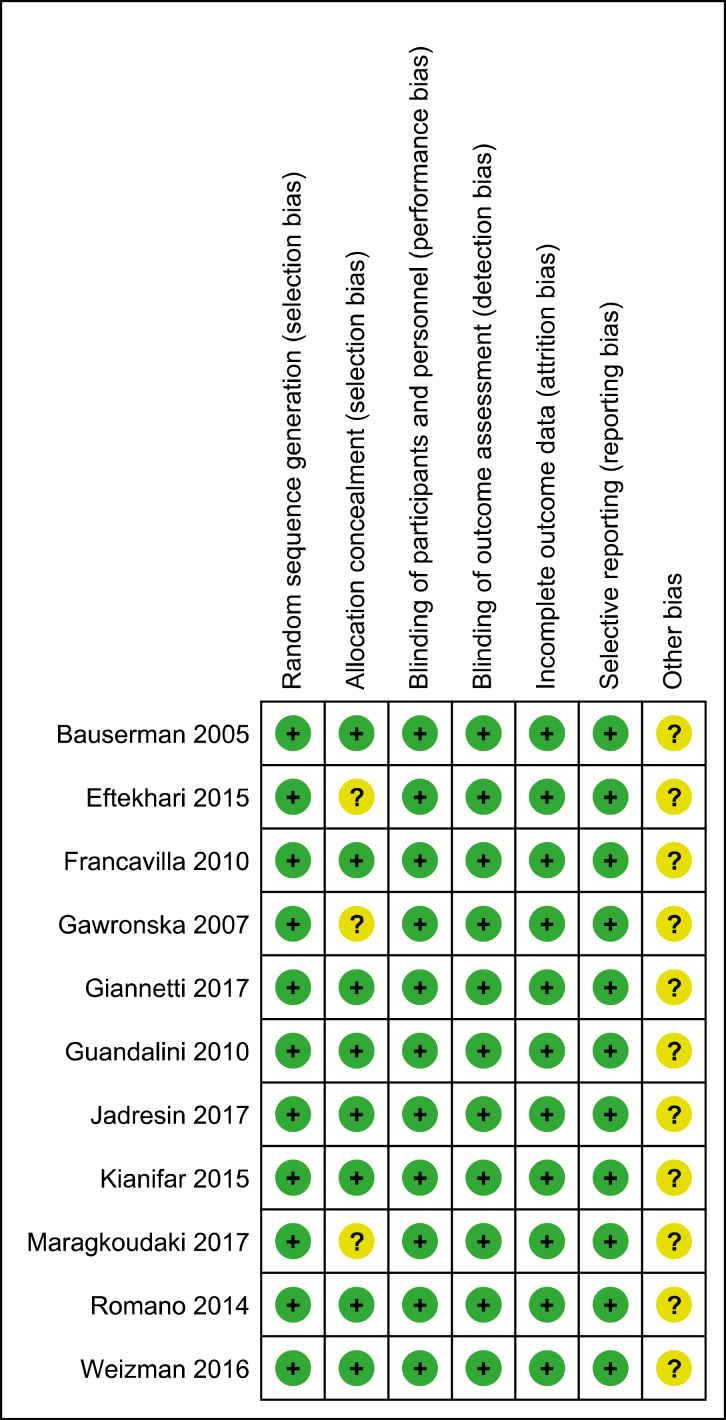
Eight studies were judged to be at low risk of bias in terms of randomization, allocation concealment, blinding, data collection, and selective reporting (19,22,25,27–29,31,32) (Figure 1). Three studies did not report allocation concealment (21,24,30). Nine studies were sponsored by a pharmaceutical company (19–22,25–29). The other two studies (23,24), did not comment on any pharmaceutical company support.
Figure 1.
Assessment of risk of bias in the studies of probiotics in functional abdominal pain disorder*. *Adopted RevMan software by Cochrane Collaboration (http://community.cochrane.org/tools/review-production-tools/revman-5)
Safety
All 11 RCTs in this rapid review reported that probiotics were well tolerated and there were no adverse events attributed to probiotics. A recent systematic review of all available information on the safety of probiotics in humans and animals showed that serious adverse events related to probiotics are exceedingly rare (32). However, selected populations such as critically ill, postoperative/hospitalized, and immunocompromised patients were deemed to be at high risk of invasive infections (32). Consistent with these findings, a population-based evaluation found that increased consumption of Lactobacillus in Finland did not increase serious harms (33).
DISCUSSION
This rapid review looked at the evidence on the use of probiotics for abdominal pain in children with FAP, IBS, and FD. The effect of probiotic use on FAP was mixed, with all positive results coming from studies using L. reuteri. The only negative study with use of L. reuteri had an inadequate sample size. This effect may be strain-specific as both trials using LGG for FAP showed no significant benefit ebo. Inconsistency between the selected populations limits the generalizability of the results as two of the studies did not exclude children with medication use prior to the initiation of the trial. Previous studies have shown that even a short term antibiotic course may shift gut microbiota and affect response to probiotics (34).
Overall, the findings suggest a positive effect on pain reduction with use of probiotics in IBS. LGG is the most studied, with limited evidence for VSL #3 and a product containing bifidobacteria on abdominal pain severity and resolution, respectively.
The number of studies on FD was extremely limited and there is currently no evidence to support use of probiotics in FD.
There have been six systematic reviews (SR) and meta-analyses that included probiotics for paediatric FAP published since 2001 (35–40). The most recent meta-analysis by the Cochrane collaboration assessed dietary interventions for recurrent abdominal pain in children (38). Consistent with this rapid review, the Cochrane meta-analysis demonstrated that probiotic use decreased pain frequency and intensity significantly, especially among IBS patients. However, the evidence was of moderate to low quality due to risk of bias and heterogeneity in probiotic used and measurement (38). In the Cochrane systematic review, meta-analyses of specific probiotics were not performed separately. Our review added two new trials (20,21), both published in 2017, to the ones included in the Cochrane SR. A recent systematic review by Wegh et al. (40) on the effect of probiotic on FAPD and functional constipation also supports the efficacy of LGG in reducing abdominal pain frequency and intensity in children with IBS. Our study includes one additional study (25) as well as specific subgroup analyses for IBS, FAP, and FD, in comparison to the study by Wegh et al.
Strength and limitations
This rapid review used rigorous methods for the accuracy of data extraction and quality assessment whereby two reviewers verified the accuracy of extracted data. Another strength was focusing only on RCTs that used standard criteria to recruit FADP patients. This review also has some limitations. As a rapid review, restrictions regarding time period and language may have omitted some relevant clinical trials.
There are also some limitations pertaining to the included RCTs. The studies were heterogeneous with regards to the probiotic regime, the placebo regime, duration of study, and measurement tool used. This heterogeneity makes it difficult to recommend a single probiotic regime despite some evidence to support its use. Many studies either did not report a sample size calculation or were underpowered, which may have led to some of the negative studies. These limitations would necessitate a cautious interpretation of results.
CONCLUSIONS
Overall, the effect of probiotics on FAP is mixed and there is insufficient evidence to recommend probiotics for alleviation of abdominal pain in FAP. This review shows some evidence for the use of probiotics for reducing abdominal pain in children with IBS, with most studies using LGG. More studies with rigorous methodology are needed to determine the role of probiotics in the management of FAPD in children before they can be recommended.
CLINICAL IMPLICATIONS.
There is insufficient evidence to recommend probiotics for children with FAP. More studies, particularly on L. reuteri are needed to discern effect of the probiotic.
There is some evidence to suggest probiotics can reduce severity of abdominal pain and/or frequency in children with IBS, of which LGG is the most widely studied.
If clinicians and families are considering a therapeutic trial for IBS, LGG 1 × 1010 CFU twice/day for 4 to 8 weeks have been studied with no adverse effects and is available over-the-counter in Canada.
There is no evidence to support use of probiotics in FD
Due to the mixed results of included studies and financial costs, probiotics should be discontinued if there is no clinical improvement in 4 to 6 weeks.
Funding Information: There are no funders to report for this submission.
Financial Disclosure: The authors have no financial relationship relevant to this article to disclose.
Potential Conflicts of Interest: All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX I: Search Strategy for Finding Relevant Rcts of Probiotic use in Paediatric Functional Abdominal Pain Disorder
| 1. Abdominal Pain/ |
| 2. Periumbilical Pain.ti,ab. |
| 3. functional abdominal pain.mp. |
| 4. Dyspepsia/ |
| 5. Irritable Bowel Syndrome/ |
| 6. exp Probiotics/ |
| 7. Lactobacillus/ |
| 8. Bifidobacterium/ |
| 9. Saccharomyces boulardii.ti,ab. |
| 10. Streptococcus thermophilus/ |
| 11. or/6–10 |
| 12. randomized controlled trial.pt. |
| 13. controlled clinical trial.pt. |
| 14. randomized.ab. |
| 15. placebo.ab. |
| 16. drug therapy.fs. |
| 17. randomly.ab. |
| 18. trial.ab. |
| 19. groups.ab. |
| 21. or/12–19 |
| 22. exp animals/ not humans.sh. |
| 23. 20 not 21 |
| 24. or/1–5 |
| 25. 11 and 22 and 23 |
| 26. limit 24 to English language |
| 26. limit 25 to ‘all child (0–18 years)’ |
APPENDIX II: PRISMA Diagram of Probiotics In Pediatric Functional Abdominal Pain Disorder*
APPENDIX III: Rome Ii &Iii Criteria For Diagnosis Of Functional Abdominal Pain And Irritable Bowel Syndrome
| Rome II (1999–2006)* | Rome III (2006–2016) | |
|---|---|---|
| IBS | In children old enough to provide an accurate pain history, at least 12 weeks, which need not be consecutive, in the preceding 12 months of; (1) Abdominal discomfort or pain that has two out of three features: (a) Relieved with defecation; and/or (b) Onset associated with a change in frequency of stool; and/or (c) Onset associated with a change in form (appearance) of stool; and (2) There are no structural or metabolic abnormalities to explain the symptoms. |
Must include all of the following: 1. Abdominal discomfort (an uncomfortable sensation not described as pain) or pain associated with 2 or more of the following at least 25% of the time: a. Improved with defecation b. Onset associated with a change in frequency of stool c. Onset associated with a change in form (appearance) of stool 2. No evidence of an inflammatory, anatomic, metabolic, or neoplastic process that explains the subject’s symptoms *Criteria fulfilled at least once per week for at least 2 months before diagnosis |
| FAP | At least 12 weeks of:(1) Continuous or nearly continuous abdominal pain in a school-aged child or adolescent; and (2) No or only occasional relation of pain with physiological events (e.g., eating, menses, or defecation); and (3) Some loss of daily functioning; and (4) The pain is not feigned (e.g., malingering);and (5) The patient has insufficient criteria for other functional gastrointestinal disorders that would explain the abdominal pain. |
Must include all of the following: 1. Episodic or continuous abdominal pain 2. Insufficient criteria for other FGIDs 3. No evidence of an inflammatory, anatomic, metabolic, or neoplastic process that explains the subject’s symptoms *Criteria fulfilled at least once per week for at least 2 months before diagnosis |
| FD | In children old enough to provide an accurate pain history, at least 12 weeks, which need not be consecutive, in the preceding 12 months of; (1) Persistent or recurrent pain or discomfort centered in the upper abdomen (above the umbilicus) (2) No evidence of organic disease (including at upper endoscopy) that is likely to explain the symptoms; and (3) No evidence that dyspepsia is exclusively relieved by defecation or associated with onset of a change ins tool frequency or stool form (i.e., not irritable bowel) |
Must include all of the following: 1. Persistent or recurrent pain or discomfort centered in the upper abdomen (above the umbilicus) 2. Not relieved by defecation or associated with the onset of a change in stool frequency or stool form (i.e., not irritable bowel syndrome) 3. No evidence of an inflammatory, anatomic, metabolic or neoplastic process that explains the subject’s symptoms *Criteria fulfilled at least once per week for at least 2 months prior to diagnosis |
*Rome IV has been adopted since 2016 (6). No included studies reported Rome IV results.
References
- 1. Assa A, Ish-Tov A, Rinawi F, Shamir R. School attendance in children with functional abdominal pain and inflammatory bowel diseases. J Pediatr Gastroenterol Nutr 2015;61(5):553–7. [DOI] [PubMed] [Google Scholar]
- 2. Youssef NN, Murphy TG, Langseder AL, Rosh JR. Quality of life for children with functional abdominal pain: A comparison study of patients’ and parents’ perceptions. Pediatrics 2006;117(1):54–9. [DOI] [PubMed] [Google Scholar]
- 3. Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: Is it worth it? J Pediatr Gastroenterol Nutr 2010;51(5):579–83. [DOI] [PubMed] [Google Scholar]
- 4. Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2006;130(5):1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collins BS, Thomas DW. Chronic abdominal pain. Pediatr Rev 2007;28(9):323–31. [DOI] [PubMed] [Google Scholar]
- 6. Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016;150(6):1262–79.e2. [DOI] [PubMed] [Google Scholar]
- 7. Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in western countries: A systematic review. Am J Gastroenterol 2005;100(8):1868–75. [DOI] [PubMed] [Google Scholar]
- 8. Korterink JJ, Diederen K, Benninga MA, Tabbers MM. Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PLoS One 2015;10(5):e0126982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saulnier DM, Riehle K, Mistretta TA, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011;141(5):1782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korterink JJ, Rutten JM, Venmans L, Benninga MA, Tabbers MM. Pharmacologic treatment in pediatric functional abdominal pain disorders: A systematic review. J Pediatr 2015;166(2):424–31.e6. [DOI] [PubMed] [Google Scholar]
- 11. Korterink J, Devanarayana NM, Rajindrajith S, Vlieger A, Benninga MA. Childhood functional abdominal pain: Mechanisms and management. Nat Rev Gastroenterol Hepatol 2015;12(3):159–71. [DOI] [PubMed] [Google Scholar]
- 12. National Institutes of Health. Complementary, Alternative, or Integrative Health: Probiotics: In Depth?2016. <https://nccih.nih.gov/health/probiotics/introduction.htm>. Accessed December 2017.
- 13. Barnes D, Yeh AM. Bugs and guts: Practical applications of probiotics for gastrointestinal disorders in children. Nutr Clin Pract 2015;30(6):747–59. [DOI] [PubMed] [Google Scholar]
- 14. Carlson AL, Xia K, Azcarate-Peril MA, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry 2018;83(2):148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slyepchenko A, Carvalho AF, Cha DS, Kasper S, McIntyre RS. Gut emotions - mechanisms of action of probiotics as novel therapeutic targets for depression and anxiety disorders. CNS Neurol Disord Drug Targets 2014;13(10):1770–86. [DOI] [PubMed] [Google Scholar]
- 16. Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes 2014;5(3):404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen CC, Walker WA. Clinical applications of probiotics in gastrointestinal disorders in children. Natl Med J India 2011;24(3):153–60. [PubMed] [Google Scholar]
- 18. Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol 2012;18(30):4012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giannetti E, Maglione M, Alessandrella A, et al. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: A multicenter, randomized, double-blind, placebo-controlled, crossover trial. J Clin Gastroenterol 2017;51(1):e5–e10. [DOI] [PubMed] [Google Scholar]
- 20. Jadrešin O, Hojsak I, Mišak Z, et al. Lactobacillus reuteri DSM 17938 in the treatment of functional abdominal pain in children: RCT study. J Pediatr Gastroenterol Nutr 2017;64(6):925–9. [DOI] [PubMed] [Google Scholar]
- 21. Maragkoudaki M, Chouliaras G, Orel R, Horvath A, Szajewska H, Papadopoulou A. Lactobacillus reuteri DSM 17938 and a placebo both significantly reduced symptoms in children with functional abdominal pain. Acta Paediatr 2017;106(11):1857–62. [DOI] [PubMed] [Google Scholar]
- 22. Weizman Z, Abu-Abed J, Binsztok M. Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: A randomized, double-blind, placebo-controlled trial. J Pediatr 2016;174:160–164.e1. [DOI] [PubMed] [Google Scholar]
- 23. Eftekhari K, Vahedi Z, Kamali Aghdam M, Noemi Diaz D. A randomized double-blind placebo-controlled trial of Lactobacillus reuteri for chronic functional abdominal pain in children. Iran J Pediatr 2015;25(6):e2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kianifar H, Jafari SA, Kiani M, et al. Probiotic for irritable bowel syndrome in pediatric patients: A randomized controlled clinical trial. Electron Physician 2015;7(5):1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romano C, Ferrau’ V, Cavataio F, et al. Lactobacillus reuteri in children with functional abdominal pain (FAP). J Paediatr Child Health 2014;50(10):E68–71. [DOI] [PubMed] [Google Scholar]
- 26. Francavilla R, Miniello V, Magistà AM, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 2010;126(6):e1445–52. [DOI] [PubMed] [Google Scholar]
- 27. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: A multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 2010;51(1):24–30. [DOI] [PubMed] [Google Scholar]
- 28. Gawrońska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther 2007;25(2):177–84. [DOI] [PubMed] [Google Scholar]
- 29. Bauserman M, Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: A double-blind randomized control trial. J Pediatr 2005;147(2):197–201. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151(4):264–9, W64. [DOI] [PubMed] [Google Scholar]
- 31. Guandalini S. The potential for use of probiotics in pediatric irritable bowel syndrome and inflammatory bowel disease. Current Pediatrics Reports 2014;2(3):235. [Google Scholar]
- 32. Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf 2014;13(2):227–39. [DOI] [PubMed] [Google Scholar]
- 33. Salminen MK, Tynkkynen S, Rautelin H, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in finland. Clin Infect Dis 2002;35(10):1155–60. [DOI] [PubMed] [Google Scholar]
- 34. Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis 2016;34(3):260–8. [DOI] [PubMed] [Google Scholar]
- 35. Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther 2011;33(12):1302–10. [DOI] [PubMed] [Google Scholar]
- 36. Korterink JJ, Ockeloen L, Benninga MA, Tabbers MM, Hilbink M, Deckers-Kocken JM. Probiotics for childhood functional gastrointestinal disorders: A systematic review and meta-analysis. Acta Paediatr 2014;103(4):365–72. [DOI] [PubMed] [Google Scholar]
- 37. Rutten JM, Korterink JJ, Venmans LM, Benninga MA, Tabbers MM. Nonpharmacologic treatment of functional abdominal pain disorders: A systematic review. Pediatrics 2015;135(3):522–35. [DOI] [PubMed] [Google Scholar]
- 38. Newlove-Delgado TV, Martin AE, Abbott RA, et al. Dietary interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev 2017;3:CD010972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huertas-Ceballos AA, Logan S, Bennett C, et al. Dietary interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev 2009;1:Cd003019. [DOI] [PubMed] [Google Scholar]
- 40. Wegh CA, Benninga MA, Tabbers MM. Effectiveness of probiotics in children with functional abdominal pain disorders and functional constipation: A systematic review. J Clin Gastroenterol 2018;52(1):S10–26. [DOI] [PubMed] [Google Scholar]