Abstract
Context
The aldosterone–to–active renin ratio (AARR) is the recommended screening test for primary aldosteronism (PA), but prospective study data on its sensitivity and specificity are sparse.
Objective
To investigate the diagnostic accuracy of the AARR for detecting PA.
Design
Prospective diagnostic accuracy study.
Setting
This study was conducted from February 2009 to August 2015 at the outpatient clinic of the Department of Endocrinology and Diabetology of the Medical University of Graz, Austria.
Participants
Four hundred patients with arterial hypertension who were referred to a tertiary care center for screening for endocrine hypertension.
Intervention
Participants had a determination of the AARR (index test) and a second AARR determination followed by a saline infusion test (SIT) after 2 to 6 weeks. PA was diagnosed in individuals with any AARR ≥3.7 ng/dL/µU/mL [including a plasma aldosterone concentration (PAC) of ≥9 ng/dL] who had a PAC ≥10 ng/dL after the SIT. We did not substantially alter antihypertensive drug intake.
Main Outcome Measures
Primary outcome was the receiver-operating characteristic (ROC) curve of the AARR in diagnosing PA.
Results
A total of 382 participants were eligible for analyses; PA was diagnosed in 18 (4.7%) patients. The area under the ROC curve of the AARR in detecting PA was 0.973 (95% CI, 0.956 to 0.990). Sensitivity and specificity for a positive AARR in diagnosing PA were 100% (95% CI, 81.5% to 100.0%) and 89.6% (95% CI, 86.0% to 92.5%), respectively.
Conclusions
The AARR has good diagnostic accuracy for detecting PA.
Keywords: aldosterone, diagnostic accuracy, primary aldosteronism, prospective, renin
Primary aldosteronism (PA) affects approximately 5% to 10% of patients with arterial hypertension and is associated with an excess risk of morbidity and mortality when compared with essential hypertension [1–3]. Therefore, the Endocrine Society clinical practice guideline for the management of PA recommends wide screening for PA in ∼50% of patients with arterial hypertension, including patients with resistant hypertension or hypokalemia [1]. Real-life data, however, show that screening for PA is rarely performed, and PA remains an underdiagnosed and undertreated disease [3].
The recommended screening test for PA is determination of the aldosterone-to-renin ratio because it reflects the degree of aldosterone synthesis that is autonomous with regard to renin [1, 4]. Measuring and interpreting the aldosterone-to-renin ratio is, however, a challenge because several factors affect aldosterone and renin concentrations [5, 6]. Moreover, few prospective studies have evaluated the diagnostic accuracy of the aldosterone-to-renin ratio in detecting PA. Several previous studies in this field were limited by, for example, a missing prespecified statistical analysis plan, restricting confirmatory tests for PA to participants with a positive screening test result (and thus risk of verification bias), or incomplete adherence to guidelines for study reporting [7–16]. In many of those studies, common antihypertensive drugs were discontinued for the purpose of PA diagnostics, an approach that may limit the implementation of such procedures into routine clinical care. Hence, diagnostic accuracy studies on the aldosterone-to-renin ratio in detecting PA are still needed.
Herein we present the results of the prospective Graz Endocrine Causes of Hypertension (GECOH) study, a diagnostic accuracy study with the primary aim of evaluating the sensitivity and specificity of the aldosterone–to–active renin ratio (AARR) in detecting PA [17, 18].
1. Methods
A. Design
The GECOH study is a single-center, prospective diagnostic accuracy study of the AARR in detecting PA. Details of the study design and methods, including sample size calculation and statistical analysis plan, have been published previously, and we adhere to the Standards for Reporting of Diagnostic Accuracy Studies (STARD) statement and the Declaration of Helsinki [17–20]. The Ethics Committee of the Medical University of Graz, Austria, approved the study, and all study participants gave written informed consent.
According to our published study protocol, patients were scheduled to have two determinations of the AARR 2 to 6 weeks apart [17]. A saline infusion test (SIT) in the recumbent position with infusion of 2 L 0.9% saline intravenously over 4 hours was performed on the day of the second AARR determination in the first consecutive 200 study participants; afterward, SIT was exclusively done in participants with a positive (pathologic) AARR [17]. The SIT was chosen as the reference standard test in accordance with published guidelines and because of our clinical expertise with this test [1]. PA was diagnosed in individuals with any AARR ≥3.7 ng/dL/µU/mL (including a plasma aldosterone concentration (PAC) ≥9 ng/dL] who had a PAC ≥10 ng/dL after the SIT, whereas PA was excluded if results of only one or none of these two tests was positive [21, 22]. The cutoff for the AARR is derived from the Endocrine Society clinical practice guideline for PA; the PAC cutoffs are also based on previous publications that, in terms of the post-SIT PAC, used the same assay as in our study [1, 21–23].
B. Participants
We enrolled 400 patients with arterial hypertension, age ≥18 years, who were routinely referred to our department for screening for endocrine hypertension. Because certain medications have a substantial effect on aldosterone and renin concentrations, and according to the Endocrine Society guideline, we did not include participants who received spironolactone, canrenoate, eplerenone, amiloride, and/or triamterene within 4 weeks before study inclusion but did not change intake of first-line antihypertensive drugs, such as angiotensin-converting enzyme inhibitors, angiotensin-2–blockers, thiazides, and calcium antagonists [1, 17]. Therefore, patients taking these latter medications had to withdraw them at least 4 weeks before study inclusion, a common practice for patients routinely referred to our outpatient clinic for screening for endocrine hypertension. Other exclusion criteria were a glomerular filtration rate <30 mL/min/1.73 m2, liver failure with Child-Pugh class B or C, severe heart failure with New York Heart Association class 3 or 4, acute coronary syndrome within the last 2 weeks, immunosuppressive therapy, oral glucocorticoid therapy (because glucocorticoids suppress aldosterone), pregnancy, ongoing chemotherapy, and any other disease with an estimated life expectancy <1 year.
Without any specific advertisement, study participants were informed about the study by a conversation and recruited from the outpatient clinic by the principal investigators of the GECOH study (S.P. and A.T.). Therefore, our study population is a convenience sample because participants were enrolled when the principal investigators were on duty in the outpatient clinic and had enough time for this investigation. The entire GECOH study was performed from 3 February 2009 to 10 August 2015 in the outpatient clinic of the Department of Endocrinology and Diabetology at the Medical University of Graz, Austria. Data entry was finished in December 2018.
C. Outcome Measures
The primary outcome measure was the receiver-operating characteristic (ROC) curve for the first AARR (index test) in detecting PA. The secondary outcome measure was the ROC curve for the SIT in detecting PA.
D. Measurements
Details of laboratory measurements and study procedures have been published elsewhere [17, 18]. In brief, all blood samples for this study were obtained after an overnight fast in the morning (8:00 to 11:00 am) after the patients had been seated for 10 minutes. Participants were advised to avoid smoking and taking their antihypertensive drugs in the morning before the blood collection.
Active renin concentration was measured in EDTA plasma by a Renin III Generation radioimmunoassay (Renin IRMA RIA-4541; DRG Instruments GmbH, Marburg, Germany), which has been calibrated against a World Health Organization standard [24]. Intra-assay and interassay coefficients of variation of this assay are 0.6% to 4.5% and 2.7% to 14.5%, respectively. PAC was measured by radioimmunoassay (Active Aldosterone RIA DSL-8600; Diagnostic Systems Laboratories, Inc., Webster, TX; now distributed by Beckman Coulter, Inc, Brea, CA.) with intra-assay and interassay coefficients of variation of 3.3%. to 4.5% and 5.9% to 9.8%, respectively [21].
E. Data Analysis
Continuous data that followed a normal distribution are shown as means with SDs, and parameters with a skewed distribution are shown as medians with interquartile ranges. Categorical data are presented as percentages. Where appropriate, skewed variables were log(e)-transformed before they were used in parametric analyses. Group differences were calculated by Student t test, χ2 test, or Fisher exact test, as appropriate. We calculated ROC curves with area under the curve and respective 95% CIs for the first AARR and for the SIT in detecting PA. We also calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratios for both tests [25]. A P value <0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed by using SPSS software, version 23.0 (IBM Inc., Chicago, IL).
2. Results
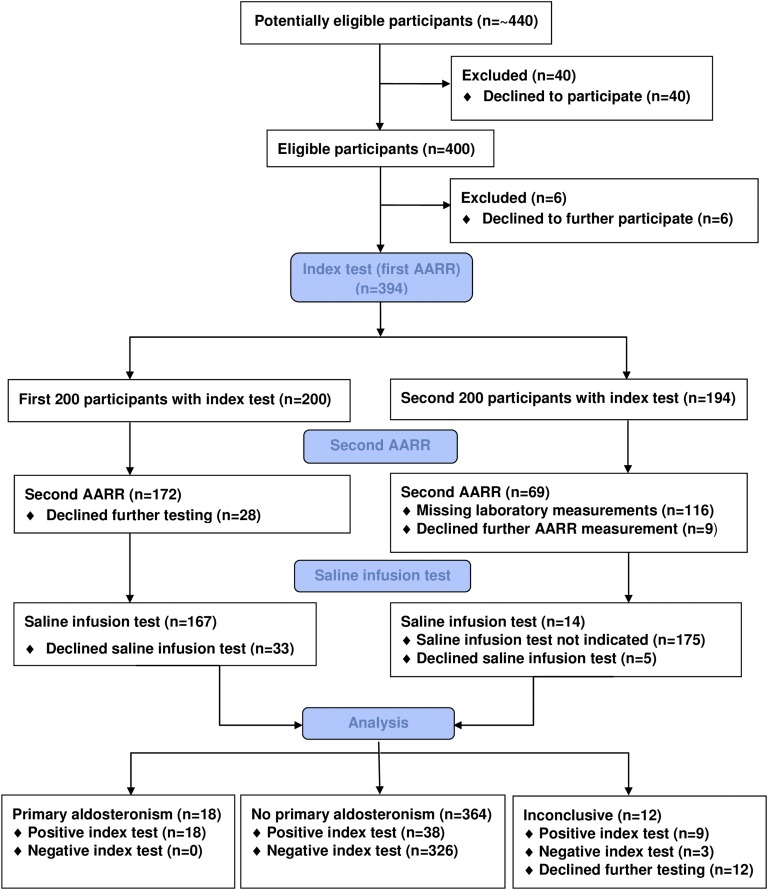
The participant flow chart of the GECOH study is shown in Fig. 1. In brief, of ∼440 patients who were approached for study inclusion, 400 agreed to participate in the GECOH study. The SIT was performed regardless of AARR values in 167 of the first 200 study participants; 33 of the first 200 study participants declined to undergo the SIT. Afterward, the SIT was exclusively performed in participants with a positive AARR (i.e., AARR ≥ 3.7 ng/dL/µU/mL), including a PAC ≥9 ng/dL. Because of insufficient funding for this study, a second AARR determination was routinely performed in the first consecutive 268 participants only (resulting in 231 available values) and was thereafter not measured except in participants who had undergone a SIT. In the entire study, two determinations of the AARR with a median (interquartile range) time interval in between of 30 days (22 to 38 days) were available in 241 participants, and the SIT was performed in 181 participants. We excluded 18 participants who declined to undergo further testing to exclude or confirm PA. Therefore, 382 were finally eligible for analyses because they had sufficient data to exclude or confirm the diagnosis of PA with consistently negative (normal) AARR or any positive AARR, plus the result of the SIT. PA was diagnosed in patients with a positive AARR and a positive result of the SIT, whereas PA was excluded if results of only one or none of these two tests was positive. We did not impute for missing data, and no severe adverse events resulted from the index or reference standard test.
Figure 1.
Participant flow chart for the GECOH study.
Baseline characteristics of all eligible study participants and stratified according to the presence or absence of PA are shown in Table 1. PA was diagnosed in 18 of the 382 participants (4.7%). In addition, two patients were diagnosed with pheochromocytoma on the basis of elevated plasma metanephrines and/or normetanephrines and two were diagnosed with hypercortisolism [26, 27]. Hypercortisolism was diagnosed according to the Endocrine Society guideline (i.e., pathologic result on a 1-mg dexamethasone suppression test plus elevated midnight salivary cortisol concentration); one patient had ACTH-dependent and one had ACTH-independent hypercortisolism [27]. These patients were not excluded from the ROC analyses.
Table 1.
Baseline Characteristics of GECOH Study Population
| Variable | All Study Participants (n = 382) | No PA (n = 364) | PA (n = 18) | P Valuea |
|---|---|---|---|---|
| Age, y | 50.3 ± 14.9 | 50.3 ± 15.1 | 48.9 ± 9.3 | 0.543 |
| Women, % | 56.3 | 56.6 | 50.0 | 0.582 |
| BMI, kg/m2 | 28.7 ± 5.7 | 28.7 ± 5.7 | 28.7 ± 6.1 | 0.984 |
| Systolic blood pressure, mm Hg | 155 ± 22 | 154 ± 22 | 177 ± 22 | <0.001 |
| Diastolic blood pressure, mm Hg | 96 ± 33 | 96 ± 34 | 107 ± 13 | 0.235 |
| Aldosterone, ng/dL | 16.1 (11.9–23.3) | 15.7 (11.6–22.0) | 41.6 (25.9–59.8) | <0.001 |
| Renin, µU/mL | 13.4 (6.6–30.6) | 14.9 (7.4–33.0) | 3.2 (2.5–5.8) | <0.001 |
| Baseline AARR, ng/dL/µU/mL | 1.15 (0.47–2.39) | 1.06 (0.45–2.12) | 9.35 (6.24–15.95) | <0.001 |
| Second AARR, ng/dL/µU/mL | 1.47 (0.64–3.17) | 1.38 (0.57–2.57) | 10.48 (6.97–13.58) | <0.001 |
| Aldosterone after SIT, ng/dL | 5.6 (4.2–7.9) | 5.3 (4.0–7.2) | 20.7 (14.8–43.0) | <0.001 |
| Serum potassium, mmol/L | 3.9 ± 0.4 | 3.9 ± 0.4 | 3.1 ± 0.4 | <0.001 |
| Serum potassium < 3.5 mmol/L, % | 12.3 | 9.1 | 77.8 | <0.001 |
| Serum sodium, mmol/L | 141 ± 2 | 141 ± 2 | 144 ± 2 | <0.001 |
| Creatinine, mg/dL | 0.92 ± 0.51 | 0.92 ± 0.52 | 0.92 ± 0.23 | 0.989 |
| eGFR-MDRD, mL/min/1.73 m2 | 80.4 ± 18.0 | 80.4 ± 18.0 | 80.5 ± 18.4 | 0.984 |
| PTH, pg/mL | 48.6 ± 20.7 | 47.2 ± 19.2 | 75.4 ± 31.6 | 0.002 |
| Fasting glucose, mg/dL | 90 (84–100) | 90 (83–99) | 90 (86–109) | 0.386 |
| HbA1c, mmol/mol | 36 (33–39) | 36 (33–39) | 34 (34–40) | 0.926 |
| HDL cholesterol, mg/dL | 61 ± 19 | 61 ± 19 | 58 ± 23 | 0.492 |
| LDL cholesterol, mg/dL | 119 ± 31 | 119 ± 31 | 113 ± 19 | 0.258 |
| Triglycerides, mg/dL | 103 (74–146) | 103 (73–146) | 125 (78–154) | 0.678 |
| C-reactive protein, mg/dL | 1.8 (1.0–3.9) | 1.8 (1.0–3.9) | 2.8 (1.1–5.3) | 0.299 |
| No. of antihypertensive drugs | 2 (1–3) | 2 (1–3) | 4 (2–4) | 0.002 |
| ACE inhibitors, % | 34.6 | 35.2 | 22.2 | 0.318 |
| Angiotensin-2–receptor blockers, % | 24.9 | 24.2 | 38.9 | 0.159 |
| Calcium-channel blockers, % | 35.9 | 34.1 | 72.2 | 0.001 |
| β-blockers, % | 53.7 | 52.7 | 72.2 | 0.106 |
| Diuretics, % | 27.5 | 27.2 | 33.3 | 0.569 |
| Thiazide diuretics, % | 32.7 | 32.4 | 38.9 | 0.568 |
| Loop diuretics, % | 1.8 | 1.9 | 0 | 1.000 |
| NSAIDs, % | 11.5 | 11.8 | 5.6 | 0.707 |
Continuous data are presented as mean ±SD or as median with interquartile range. Categorical data are presented as percentages.
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; eGFR-MDRD, estimated glomerular filtration rate calculated according to the Modification of Diet in Renal Disease criteria; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NSAID, nonsteroidal anti-inflammatory drug.
P value for paired Student t test for continuous variables and for χ2 or Fisher exact test for categorical variables comparing patients with PA vs no PA.
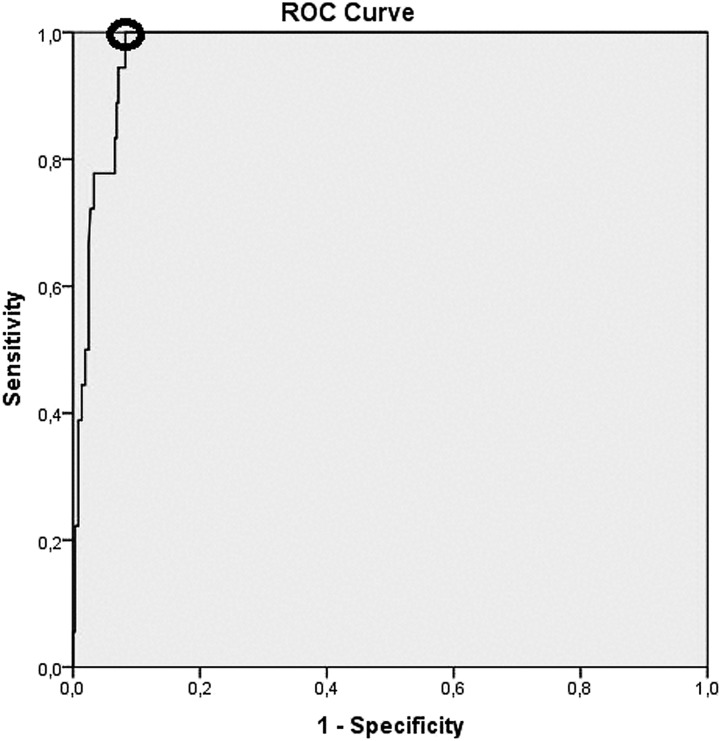
The contingency table for the diagnostic accuracy of a positive AARR at the first study visit (at baseline), and for a positive SIT result (i.e. PAC ≥ 10 ng/dL after the SIT) is shown in Table 2. The area under the ROC curve for the AARR at the first study visit in detecting PA was 0.973 (95% CI, 0.956 to 0.990) (Fig. 2). The point on the ROC curve with the minimum distance from the left-upper corner of the unit square was at an AARR of 4.33 ng/dL/µU/mL with a sensitivity of 100% and a specificity of 91.8% (Fig. 2). The sensitivity, specificity, PPV, NPV, and positive and negative likelihood ratios (with 95% CIs) of a positive AARR in detecting PA were 100% (81.5% to 100.0%), 89.6% (86.0% to 92.5%), 32.1% (26.0% to 39.0%), 100%, 9.58 (7.09 to 12.94), and 0, respectively. The respective area under the ROC curve for the PAC concentration after the SIT was 0.998 (95% CI, 0.994 to 1.000). The sensitivity, specificity, PPV, NPV, and positive and negative likelihood ratios (with 95% CIs) of a positive SIT result for detecting PA were 100% (81.5% to 100.0%), 96.1% (90.6% to 97.9%), 69.2% (53.4% to 81.6%), 100%, 20.4 (10.4 to 40.1), and 0, respectively. Characteristics of patients without PA who had a positive AARR at the first study visit or a positive SIT result are shown in Table 3. Of note, among participants without PA, the PAC after the SIT (median with interquartile ranges) did not significantly different between those with (n = 38) and without (n = 125) a positive baseline AARR [5.7 (4.6 to 7.4) vs 5.2 (3.8 to 7.0) ng/dL; P = 0.071].
Table 2.
Contingency Table for Diagnostic Accuracy of AARR and SIT in Detecting PA
| Test Result | No PA | PA | Total |
|---|---|---|---|
| AARR | |||
| Positive | 38 | 18 | 56 |
| Negative | 326 | 0 | 326 |
| Total | 364 | 18 | 382 |
| SIT | |||
| Positive | 8 | 18 | 26 |
| Negative | 155 | 0 | 155 |
| Total | 163 | 18 | 181 |
Values are numbers of patients. PA was diagnosed in patients with a positive AARR and a positive SIT result, whereas PA was excluded if results of only one or none of these two tests were positive.
Figure 2.
ROC curve for the AARR in detecting PA. The point on the ROC curve with the minimum distance from the left-upper corner of the unit square (marked with a circle) was at an AARR of 4.33 ng/dL/µU/mL, with a sensitivity of 100% and a specificity of 91.8%.
Table 3.
Characteristics of Participants Without PA Who Had Positive AARR at Baseline or Positive SIT Result
| Variable | Positive AARR and No PA (n = 38) | Positive SIT Result and No PA (n = 8) |
| Age, y | 58.6 ± 11.6 | 42.9 ± 13.6 |
| Women, % | 60.5 | 25.0 |
| BMI, kg/m2 | 27.3 ± 4.1 | 31.1 ± 6.4 |
| Systolic blood pressure, mm Hg | 162 ± 25 | 153 ± 27 |
| Diastolic blood pressure, mm Hg | 96 ± 12 | 95 ± 11 |
| Aldosterone, ng/dL | 19.5 (15.3–24.6) | 26.1 (17.4–32.5) |
| Renin, µU/mL | 3.4 (2.3–4.2) | 17.7 (7.2–41.0) |
| Baseline AARR, ng/dL/µU/mL | 5.63 (4.53–7.42) | 1.42 (0.66–2.59) |
| Second AARR, ng/dL/µU/mL | 5.59 (3.06–9.23) | 1.01 (0.31–2.04) |
| Aldosterone after SIT, ng/dL | 5.7 (4.6–7.4) | 11.2 (10.2–11.9) |
| Serum potassium, mmol/L | 3.8 ± 0.3 | 3.8 ± 0.5 |
| Serum potassium < 3.5 mmol/L, % | 10.5 | 25.0 |
| Serum sodium, mmol/L | 142 ± 2 | 142 ± 3 |
| Creatinine, mg/dL | 0.93 ± 0.21 | 0.94 ± 0.16 |
| GFR-MDRD, mL/min/1.73 m2 | 73.8 ± 15.7 | 82.8 ± 14.4 |
| PTH, pg/mL | 48.2 ± 14.8 | 50.2 ± 11.4 |
| Fasting glucose, mg/dL | 90 (82–106) | 91 (84–104) |
| HbA1c, mmol/mol | 37 (35–38) | 34 (31–47) |
| HDL cholesterol, mg/dL | 59 ± 13 | 56 ± 26 |
| LDL cholesterol, mg/dL | 122 ± 30 | 111 ± 30 |
| Triglycerides, mg/dL | 103 (81–159) | 121 (81–165) |
| C-reactive protein, mg/dL | 1.7 (1.0–3.1) | 3.4 (1.3–26.4) |
| No. of antihypertensive drugs | 3 (1–4) | 3 (1–4) |
| ACE inhibitors, % | 23.7 | 37.5 |
| Angiotensin-2–receptor blockers, % | 34.2 | 25.0 |
| Calcium-channel blockers, % | 52.6 | 50.0 |
| β-blockers, % | 68.4 | 37.5 |
| Diuretics, % | 34.2 | 62.5 |
| Thiazide diuretics, % | 39.5 | 50.0 |
| Loop diuretics, % | 0 | 12.5 |
| NSAIDs, % | 18.4 | 0 |
Continuous data are presented as mean ± SD or as median with interquartile range. Categorical data are presented as percentages. PA was diagnosed in patients with a positive AARR and a positive SIT result, whereas PA was excluded if results of only one or none of these two tests were positive.
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; GFR-MDRD, glomerular filtration rate calculated according to the Modification of Diet in Renal Disease criteria; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NSAID, nonsteroidal anti-inflammatory drug.
Some of the prespecified secondary outcomes could not be analyzed because we did not have funding to measure PAC by liquid chromatography mass spectrometry or 24-hour urine aldosterone concentrations with subsequent comparisons of the ROC curves using these parameters with the ROC curve of the AARR in detecting PA. Furthermore, we did not compare the ROC curves before (first AARR determination) and after (second AARR determination) discontinuation of β-blockers because the study participants were largely unwilling to alter their antihypertensive treatment; only 10 patients receiving β-blocker therapy stopped this treatment before the second visit. Of note, in all GECOH study participants with two available AARR measurements (n = 241), values at the first measurement did not significantly differ from those at the second measurement [1.58 (0.67 to 3.51) vs 1.54 (0.66 to 3.38) ng/dL/µU/mL; P = 0.685; Spearman correlation coefficient, 0.841; P < 0.001].
Although this was not prespecified in the study protocol, according to previously defined criteria, the six patients in the GECOH study with an aldosterone-producing adenoma who were subsequently treated by unilateral adrenalectomy showed a complete biochemical success; four of these patients had a complete clinical success and two had a partial clinical success (data not shown) [28]. Follow-up data for the remaining 12 patients with PA who were treated with mineralocorticoid receptor–blocker therapy are not reported because there are no clear criteria for medical treatment success of PA; thus, we did not perform a prespecified follow-up of the patients [29].
3. Discussion
This prospective diagnostic accuracy study in hypertensive patients showed that the area under the ROC curve for the AARR at the first study visit in detecting PA was 0.973 (95% CI, 0.956 to 0.990). The SIT also had good diagnostic accuracy, and we found good intraindividual reproducibility of the AARR.
The results from the GECOH study substantially add to the limited knowledge on the diagnostic accuracy of the AARR in detecting PA. Our findings are roughly in line with those of a meta-analysis of 974 patients from nine studies that reported a sensitivity, specificity, and area under the ROC of the AARR in detecting PA of 0.89 (95% CI, 0.84 to 0.93), 0.96 (95% CI, 0.95 to 0.98), and 0.985, respectively [14]. Methodological differences and limitations are, however, inherent in the existing literature on the diagnostic accuracy of the AARR and account for a relatively high heterogeneity [14]. Of note, our AARR cutoff of ≥3.7 ng/dL/µU/mL is equivalent to a cutoff of ≥30 ng/dL/ng/ml/h and ≥2.5 ng/dL/pmol/L/min, respectively, for measuring plasma renin activity instead of direct renin concentration [1]. Notably, Vorselaars et al. [10] performed a prospective study in 233 patients with difficult-to-control hypertension who were all referred to a SIT. In that study, the PA prevalence was 6.9% and the sensitivity, specificity, PPV, and NPV for the aldosterone-to-renin activity ratio in detecting PA were 100%, 86.7%, 35.6%, and 100%, respectively [10]. Despite the use of different laboratory methods, these findings are similar to those from the GECOH study [10].
One major difference between our study and the existing literature is that we performed PA diagnostic testing under ongoing antihypertensive treatment without substantially altering drug intake, except for spironolactone, canrenoate, eplerenone, amiloride, and/or triamterene, which interfere with aldosterone action. We are well aware that several antihypertensive drugs may alter renin concentrations and PAC, but we have shown that AARR measurements performed under standardized conditions (as in the GECOH study) have excellent reproducibility, with very low intraindividual variability [18]. In this context, we found that the second AARR determination did not lead to the diagnosis of any additional PA case that would have been overlooked by just a single AARR determination. We cannot rule out, however, that we missed PA cases because of ongoing drug intake; however, among individuals without PA, the PAC after the SIT did not significantly differ between those with and without a positive baseline AARR. Furthermore, only eight participants with a positive SIT result had a negative AARR and thus were not classified as having PA. These participants were, however, likely to have secondary aldosteronism with relatively high renin concentrations and only slightly elevated post-SIT PAC.
Apart from this, it must be acknowledged that diagnostic studies on PA are, in general, prone to verification bias and are limited because there is no gold standard for the confirmation or exclusion of PA, although there are excellent approaches for standardization [29]. This diagnostic challenge may be attributed to the fact that the association of AARR with blood pressure and cardiovascular risk exists on a continuum [30]. In this context, a recent trial has shown that the blood pressure–lowering effects of spironolactone is linearly associated with the prevailing AARR that was measured, as in the GECOH study, under ongoing antihypertensive treatment [31]. Although it may therefore appear arbitrary to choose an AARR cutoff for PA diagnostics, it has to be stressed that correct diagnosis of PA is pivotal due to highly effective treatment of this disease with either unilateral adrenalectomy or mineralocorticoid receptor blocker therapy resulting in significantly improved overall health outcome [32]. Of note, the six patients with an aldosterone-producing adenoma undergoing unilateral adrenalectomy showed excellent responses to treatment; four of these patients reached normotensive blood pressure without the aid of antihypertensive drugs. For clinical practice, the ARR carries important quantitative information—previous research found that increasing ARR values identifies patients with an exponentially increasing probability of carrying an aldosterone-producing adenoma [15].
Our data are limited because we studied a cohort of patients referred to a tertiary care center, and therefore we cannot uncritically generalize our results to other populations. In addition, there are no gold standard criteria for the diagnosis of PA; although we strictly adhered to published criteria in terms of assay cutoffs and guideline-recommended case confirmation by the SIT, we cannot rule out some misclassifications. Moreover we could not (mainly because of a lack of funding) analyze some prespecified secondary outcomes. Furthermore, our participants were not willing to stop β-blocker intake; this suggests that at least in our study setting, but probably also in other populations, it may not be possible to perform AARR measurements without potentially interfering drugs, such as e.g. β-blockers or angiotensin-converting enzyme inhibitors. Therefore, we consider it a main strength of our study that we provide data on the diagnostic accuracy of the AARR under ongoing antihypertensive treatment, an approach that is supported by the Endocrine Society clinical practice guideline for the management of PA [1]. Finally, our results confirm some previously published characteristics of patients with PA, such as the high PTH concentrations [33].
In conclusion, we have documented in the GECOH study that even without significantly altering intake of first-line antihypertensive drug treatment, the AARR has good diagnostic accuracy in detecting PA, as does the SIT. These findings may contribute to a broader implementation of PA diagnostic tests.
Acknowledgments
We thank all study participants and the team at the Endocrinology Laboratory Platform and from our outpatient clinic who helped us to conduct the GECOH study.
Glossary
Abbreviations:
- AARR
aldosterone–to–active renin ratio
- GECOH
Graz Endocrine Causes of Hypertension
- NPV
negative predictive value
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PPV
positive predictive value
- ROC
receiver-operating characteristic
- SIT
saline infusion test
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 2. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39(6):1057–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buffolo F, Monticone S, Burrello J, Tetti M, Veglio F, Williams TA, Mulatero P. Is primary aldosteronism still largely unrecognized? Horm Metab Res. 2017;49(12):908–914. [DOI] [PubMed] [Google Scholar]
- 4. Stowasser M, Gordon RD. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev. 2016;96(4):1327–1384. [DOI] [PubMed] [Google Scholar]
- 5. Viola A, Monticone S, Burrello J, Buffolo F, Lucchiari M, Rabbia F, Williams TA, Veglio F, Mengozzi G, Mulatero P. Renin and aldosterone measurements in the management of arterial hypertension. Horm Metab Res. 2015;47(6):418–426. [DOI] [PubMed] [Google Scholar]
- 6. Stowasser M, Ahmed A, Guo Z, Wolley M, Ungerer J, McWhinney B, Poglitsch M, Gordon R. Can screening and confirmatory testing in the management of patients with primary aldosteronism be improved? Horm Metab Res. 2017;49(12):915–921. [DOI] [PubMed] [Google Scholar]
- 7. Rossi GP, Barisa M, Belfiore A, Desideri G, Ferri C, Letizia C, Maccario M, Morganti A, Palumbo G, Patalano A, Roman E, Seccia TM, Pessina AC, Mantero F; PAPY study Investigators. The aldosterone-renin ratio based on the plasma renin activity and the direct renin assay for diagnosing aldosterone-producing adenoma. J Hypertens. 2010;28(9):1892–1899. [DOI] [PubMed] [Google Scholar]
- 8. Rossi GP, Seccia TM, Palumbo G, Belfiore A, Bernini G, Caridi G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Patalano A, Rizzoni D, Rossi E, Pessina AC, Mantero F; Primary Aldosteronism in the Prevalence in hYpertension (PAPY) Study Investigators. Within-patient reproducibility of the aldosterone: renin ratio in primary aldosteronism. Hypertension. 2010;55(1):83–89. [DOI] [PubMed] [Google Scholar]
- 9. Song Y, Yang S, He W, Hu J, Cheng Q, Wang Y, Luo T, Ma L, Zhen Q, Zhang S, Mei M, Wang Z, Qing H, Bruemmer D, Peng B, Li Q; Chongqing Primary Aldosteronism Study (CONPASS) Group†. Confirmatory tests for the diagnosis of primary aldosteronism: a prospective diagnostic accuracy study. Hypertension. 2018;71(1):118–124. [DOI] [PubMed] [Google Scholar]
- 10. Vorselaars WMCM, Valk GD, Vriens MR, Westerink J, Spiering W. Case detection in primary aldosteronism: high-diagnostic value of the aldosterone-to-renin ratio when performed under standardized conditions. J Hypertens. 2018;36(7):1585–1591. [DOI] [PubMed] [Google Scholar]
- 11. Burrello J, Monticone S, Buffolo F, Lucchiari M, Tetti M, Rabbia F, Mengozzi G, Williams TA, Veglio F, Mulatero P. Diagnostic accuracy of aldosterone and renin measurement by chemiluminescent immunoassay and radioimmunoassay in primary aldosteronism. J Hypertens. 2016;34(5):920–927. [DOI] [PubMed] [Google Scholar]
- 12. Morimoto R, Ono Y, Tezuka Y, Kudo M, Yamamoto S, Arai T, Gomez-Sanchez CE, Sasano H, Ito S, Satoh F. Rapid screening of primary aldosteronism by a novel chemiluminescent immunoassay. Hypertension. 2017;70(2):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manolopoulou J, Fischer E, Dietz A, Diederich S, Holmes D, Junnila R, Grimminger P, Reincke M, Morganti A, Bidlingmaier M. Clinical validation for the aldosterone-to-renin ratio and aldosterone suppression testing using simultaneous fully automated chemiluminescence immunoassays. J Hypertens. 2015;33(12):2500–2511. [DOI] [PubMed] [Google Scholar]
- 14. Li X, Goswami R, Yang S, Li Q. Aldosterone/direct renin concentration ratio as a screening test for primary aldosteronism: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2016;17(3):1470320316657450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maiolino G, Rossitto G, Bisogni V, Cesari M, Seccia TM, Plebani M, Rossi GP; PAPY Study Investigators. Quantitative value of aldosterone-renin ratio for detection of aldosterone-producing adenoma: the Aldosterone-Renin Ratio for Primary Aldosteronism (AQUARR) Study. J Am Heart Assoc. 2017;6(5):e005574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045–1050. [DOI] [PubMed] [Google Scholar]
- 17. Pilz S, Tomaschitz A, Stepan V, Obermayer-Pietsch B, Fahrleitner-Pammer A, Schweighofer N, Portugaller HR, Sourij H, Dobnig H, Meinitzer A, Pieber TR. Graz Endocrine Causes of Hypertension (GECOH) study: a diagnostic accuracy study of aldosterone to active renin ratio in screening for primary aldosteronism. BMC Endocr Disord. 2009;9(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pilz S, Kienreich K, Gaksch M, Grübler M, Verheyen N, Bersuch LA, Schmid J, Drechsler C, Ritz E, Moosbrugger A, Stepan V, Pieber TR, Meinitzer A, März W, Tomaschitz A. Aldosterone to active renin ratio as screening test for primary aldosteronism: reproducibility and influence of orthostasis and salt loading. Horm Metab Res. 2014;46(6):427–432. [DOI] [PubMed] [Google Scholar]
- 19. Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, Irwig L, Levine D, Reitsma JB, de Vet HC, Bossuyt PM. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen JF, Korevaar DA, Gatsonis CA, Glasziou PP, Hooft L, Moher D, Reitsma JB, de Vet HC, Bossuyt PM; STARD Group. STARD for Abstracts: essential items for reporting diagnostic accuracy studies in journal or conference abstracts. BMJ. 2017;358:j3751. [DOI] [PubMed] [Google Scholar]
- 21. Schirpenbach C, Seiler L, Maser-Gluth C, Beuschlein F, Reincke M, Bidlingmaier M. Automated chemiluminescence-immunoassay for aldosterone during dynamic testing: comparison to radioimmunoassays with and without extraction steps. Clin Chem. 2006;52(9):1749–1755. [DOI] [PubMed] [Google Scholar]
- 22. Diederich S, Bidlingmaier M, Quinkler M, Reincke M. [Diagnosis of primary hyperaldosteronism]. Med Klin (Munich). 2007;102(1):16–21. [DOI] [PubMed] [Google Scholar]
- 23. Mosso L, Carvajal C, González A, Barraza A, Avila F, Montero J, Huete A, Gederlini A, Fardella CE. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161–165. [DOI] [PubMed] [Google Scholar]
- 24. Roth HJ. Validation of immunoradiometric assay “RENIN III GENERATION” for the determination of active renin concentrations. Clin Lab (Zaragoza). 1994;40:1007–1015. [Google Scholar]
- 25. Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med. 1981;94(4 Pt 2):557–592. [PubMed] [Google Scholar]
- 26. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 27. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr, Gomez-Sanchez CE, Funder JW, Reincke M; Primary Aldosteronism Surgery Outcome (PASO) investigators. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calhoun DA. Use of aldosterone antagonists in resistant hypertension. Prog Cardiovasc Dis. 2006;48(6):387–396. [DOI] [PubMed] [Google Scholar]
- 30. Tomaschitz A, Maerz W, Pilz S, Ritz E, Scharnagl H, Renner W, Boehm BO, Fahrleitner-Pammer A, Weihrauch G, Dobnig H. Aldosterone/renin ratio determines peripheral and central blood pressure values over a broad range. J Am Coll Cardiol. 2010;55(19):2171–2180. [DOI] [PubMed] [Google Scholar]
- 31. Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, Ford I, Cruickshank JK, Caulfield MJ, Padmanabhan S, Mackenzie IS, Salsbury J, Brown MJ; British Hypertension Society programme of Prevention And Treatment of Hypertension With Algorithm based Therapy (PATHWAY) Study Group. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pilz S, Kienreich K, Drechsler C, Ritz E, Fahrleitner-Pammer A, Gaksch M, Meinitzer A, März W, Pieber TR, Tomaschitz A. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J lin Endocrinol Metab. 2012;97(1):E75–E79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.