Abstract
Purpose
Mifepristone is a glucocorticoid and progesterone receptor blocker that can be used for patients with hyperglycemia and Cushing syndrome in whom surgery failed to achieve remission or who were ineligible for surgery. We report a case series of patients with Cushing disease (CD) and central hypothyroidism that presented with increased levothyroxine requirements during mifepristone therapy.
Methods
Retrospective longitudinal case series of patients with CD and central hypothyroidism treated with mifepristone in a retrospective database at four pituitary centers in the United States.
Results
Five patients with CD were found, all women, median age 50 (interquartile range 47 to 64.5). They received mifepristone because no adequate response or intolerance to other drugs was observed. Mifepristone initiation was associated with a decrease in free thyroxine levels, mandating a dose increase of a median 1.83 (1.71 to 3.5) times the initial dose of levothyroxine to achieve normal levels. Weight loss was seen in four of five patients, ranging from 3.2 to 42.6 kg in up to 54 months of follow-up.
Conclusions
Although the mechanism behind the decrease in thyroid hormone level is unknown, intestinal malabsorption, decreased residual thyroid function and increased inactivation of T4 via deiodinases are all potential causes. Whereas therapies for hypercortisolism aim to decrease features of hypercortisolemia such as weight gain and depression, hypothyroidism can hamper these goals. This case series raises awareness on the importance of assessment of thyroid status in patients receiving mifepristone to optimize clinical outcomes.
Keywords: hypercortisolism, mifepristone, central hypothyroidism, levothyroxine, Cushing disease
Hypercortisolemia is associated with impaired quality of life and increased morbidity and mortality. Clinical manifestations include weight gain, muscle atrophy, skin thinning, thromboembolic disorders, metabolic disturbances, among others [1, 2]. Medical therapy for the management of surgically persistent or recurrent Cushing disease (CD) includes drugs that target the pituitary, adrenals, or glucocorticoid receptors in peripheral tissues [3].
Central hypothyroidism (CH) is defined by a state of thyroid hormone insufficiency that results from pituitary or hypothalamic dysfunction, causing a decrease in TSH or TRH secretion. Because pituitary thyrotropes are not able to increase thyroid stimulation, CH is diagnosed in patients with low free thyroxine levels (FT4) in association with a low or inappropriately normal TSH. Management includes the administration of levothyroxine, aiming at thyroid hormone values in the normal range, because TSH is no longer an appropriate marker of adequate replacement [4].
Initially used for pregnancy termination [5], mifepristone is a compound that acts as a progesterone receptor blocker and glucocorticoid receptor antagonist. In 2012, mifepristone was Food and Drug Administration-approved for the treatment of patients with hypercortisolemia and diabetes mellitus or glucose intolerance in whom surgery failed to achieve remission or who were not adequate surgical candidates [6]. Weight loss was achieved in most patients, with persistence of this effect after a median of 29 months, shown in an extension study of the same cohort [7].
Because the glucocorticoid and progesterone receptors have a myriad of functions in all systems, some studies were dedicated to evaluating its effects in different organs. In 1983, a study showed an increase in pituitary ACTH, arginine vasopressin, and plasma cortisol levels in patients receiving the progesterone blocker, but no effect on gonadotropins or TSH [8]. Later, it was suggested that the use of mifepristone (or RU486) could impair thyroid function by reducing iodine uptake in normal porcine thyrocytes treated previously with hydrocortisone [9]. Also, in meningiomas, it was shown that long-term use of mifepristone could increase TSH levels within normal range with a slight decrease in FT4 levels [10]. In 2015, an increase in the expression of critical proteins in thyroid function, such as the sodium iodide symporter, thyroglobulin, and thyroperoxidase, was shown after the administration of progesterone, an effect that was blunted by the administration of mifepristone [11]. In other tissues, thyroid hormone receptors were shown to be downregulated in placenta and decidua after mifepristone administration [12] and, as expected with an antiprogesterone agent, gynecological follow-up should be instituted in patients treated with mifepristone because of endometrial thickening [13].
Among patients with hypercortisolemia treated with mifepristone, the Study of the Efficacy and Safety of Mifepristone in the Treatment of Endogenous Cushing Syndrome (SEISMIC) reported primary subclinical hypothyroidism in 8 of 50 patients within six months of administration of mifepristone [1]. However, the mechanisms involved in these findings remain unclear. Furthermore, the effect of mifepristone on FT4 levels in patients with central hypothyroidism receiving thyroid hormone replacement has not been evaluated previously.
Because low thyroid hormone levels may lead to impaired weight loss, altered mood, and decreased energy expenditure, optimization of thyroid hormone therapy is of particular importance in patients with Cushing syndrome.
A retrospective case series study was conducted to report the effects of mifepristone on the increased requirement of levothyroxine doses in patients with CD and CH seen at four pituitary centers across the United States.
1. Methods
A. Subjects
Consecutive patients (N = 5) with a diagnosis of CD and concomitant CH were selected for inclusion in this series from four different pituitary centers if they had an increase in levothyroxine dose requirement demonstrated during therapy with mifepristone.
Patients receiving mifepristone for hyperglycemia associated with ACTH-dependent hypercortisolemia were included if they had documented CH treated with levothyroxine prior to the initiation of mifepristone, laboratory data on thyroid function tests before and during therapy with mifepristone, and increased requirements of levothyroxine during treatment with mifepristone. Increased requirement was defined as a decrease in FT4 to low or borderline low levels despite a stable or even increasing levothyroxine dose. Exclusion criteria included baseline thyroid test results unavailable for analysis and/or primary (rather than central) hypothyroidism documented prior to mifepristone initiation.
B. Design
A longitudinal analysis was performed with FT4 levels measured at consecutive outpatient care visits over a period of up to 54 months. Each patient was evaluated by a neuroendocrinologist at the respective centers and, if criteria were met, clinical records were reviewed and data recorded following Health Insurance Portability and Accountability Act regulations. Informed consent was waived as retrospective database have been approved by institutional review boards.
C. Assays
FT4 assays were assessed by standard techniques at each center, with normal values for each assay stated in the results section: at Massachusetts General Hospital and Medical College of Wisconsin, Roche Diagnostics Chemiluminescent assay [14]; at Oregon Health and Science University, Siemens Vista Chemiluminescent Immunoassay [15]; at St. Joseph’s Hospital and Medical Center, Abbott Laboratories Chemiluminescent Microparticle Immunoassay [16]. rT3 was measured at Mayo Clinic, using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
D. Statistics
Mean, range, and standard deviations are reported for normally distributed data. Median and interquartile range (IQR) are reported for nonnormally distributed data. Values are shown as the median and IQR unless otherwise indicated.
2. Results
Five women were included, age 50 years (IQR 47.0 to 64.5), with a previous history of CD, all of whom had increasing levothyroxine requirement during mifepristone therapy. Patients were followed for a median time of 16 months (IQR 8.25 to 36; range 8 to 54 months). The median increase in levothyroxine requirement was 83.3% (IQR 71 to 350), with an increase ranging from 67% to 400% despite the fact that four of them lost body weight with a median decrease of 4.7% and an IQR of 0.2 to 26.1 (Table 1). One patient gained <4% of body weight during treatment. Levels of rT3 were measured in two patients to determine possible causes of increased levothyroxine requirement. Both exhibited low-normal rT3 levels (10 and 11 ng/dL, with normal values ranging from 10 to 24). Compliance was confirmed and malabsorption was excluded clinically for each patient. Patients denied nausea, vomiting, failure to take the medication, or the addition of any new medications that could interfere with levothyroxine absorption, such as biotin, calcium supplements, antiepileptic drugs, over-the-counter drugs, among others.
Table 1.
Thyroid Dose Increase and Clinical Characteristics
| Patient | Age | Increased Levothyroxine Dosea | Weight Changeb (%) | Follow-Up (mo) |
|---|---|---|---|---|
| 1 | 57 | 1.83 | −4.3 | 16 |
| 2 | 50 | 1.75 | −34.8 | 18 |
| 3 | 72 | 1.67 | −17.4 | 54 |
| 4 | 48 | 2 | +3.9 | 9 |
| 5 | 46 | 5 | −4.7 | 7.5 |
| Median (IQR) | 50 (47 –64.5) | 1.83 (1.71–3.5) | −4.7 (−26.1 to −0.2) | 16 (8.25–36) |
Calculated as maximum dose required/baseline dose.
Percentage of weight change from initial evaluation to last follow up. Negative values represent weight loss and positive values, weight gain.
A. Case 1
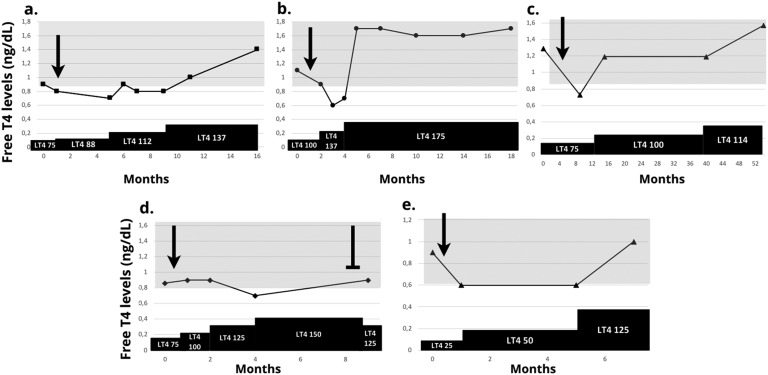
A 57-year-old woman presented with a history of persistent CD after transsphenoidal resection (TSS) and radiation therapy. She had received multiple medical therapies that she had failed to tolerate or for which efficacy was not achieved. Prior to starting mifepristone, she was on a stable replacement of 75 μg/d of levothyroxine. She was started on mifepristone 300 mg/d with a decrease in FT4 levels from 0.9 to 0.8 ng/dL (normal range, 0.9 to 1.8). Levothyroxine dose was increased to 88 μg/d with further decrease in FT4 to 0.7 ng/dL, which led to a continued increase in levothyroxine to 112 μg as FT4 levels remained below normal, and ultimately normalized at a levothyroxine dose of 137 μg/d. Levels of rT3 were low-normal (10 ng/dL). At last follow-up, 16 months since initiation of mifepristone, she achieved substantial weight loss while on mifepristone 600 mg and levothyroxine 137 μg/d with a FT4 of 1.4 ng/dL (Fig. 1a).
Figure 1.
FT4 levels during administration of mifepristone and levothyroxine therapy. (a to e) refer to cases 1 to 5. LT4: levothyroxine (doses in μg/d). ➛: mifepristone initiation. ⊣: mifepristone withdrawal. Shadowed areas depict normal range for respective assays
B. Case 2
A 50-year-old woman with a history of recurrent CD initially underwent TSS with normalization of urinary free cortisol levels and improvement in clinical status. Years after surgery, she developed weight gain and mood disturbances. She was started on monotherapy with ketoconazole, cabergoline, and metyrapone consecutively but she could not tolerate these because of side effects. Mifepristone 300 mg/d was initiated and her FT4 levels decreased from 1.1 to 0.6 ng/dL (normal range, 0.9 to 1.8), requiring a substantial increase in levothyroxine from 100 to 175 μg/d. Levels of rT3 were low-normal (11 ng/dL). At last follow-up, 18 months after initiation of glucocorticoid receptor antagonist, she was receiving mifepristone 600 mg and levothyroxine 175 μg/d, had lost 42.6 kg, and had a substantial improvement in depression, with normal FT4 levels (Fig. 1b).
C. Case 3
A 72-year-old woman with a history of persistent CD developed CH after three TSSs. She developed a decrease in FT4 levels from 1.29 to 0.73 ng/dL (normal levels, 0.82 to 1.77) after initiation of mifepristone that required increasing levothyroxine dose (from 75 to 100 μg/d) to reach normal FT4 levels. Improvement in mood and a 13.6 kg weight loss were noticed after 54 months of follow-up on 300 mg/d of mifepristone (Fig. 1c).
D. Case 4
A 48-year-old woman with a history of persistent CD after one TSS was started on mifepristone with a subsequent decrease in FT4 from 0.9 to 0.7 ng/dL (normal levels, 0.8 to 1.7) requiring an increase of her levothyroxine dose to achieve normal FT4 levels. Her weight remained stable with a mild increase in body weight and her mood improved with mifepristone. She then had to withdraw mifepristone because of the development of ankle and leg edema after nine months of follow-up. Her levothyroxine doses were preemptively reduced although her FT4 levels remained within the normal range (Fig. 1d).
E. Case 5
A 46-year-old woman with CD caused by a 1.2 cm pituitary adenoma had complete initial response after one TSS. She developed recurrent hypercortisolemia one year after surgery and was started on treatment with mifepristone 300 mg for two weeks and then 600 mg daily. After mifepristone initiation, her FT4 decreased from 0.9 (normal levels, 0.6 to 1.2) to 0.6 requiring increasing doses of levothyroxine from 25 to 50 μg/d. After mifepristone increased to 900 mg daily, she noted improvement of diabetes mellitus (HbA1c decreased from 8% to 7.3%) and a 4 kg weight loss, but her FT4 decreased to 0.6 again and required a new levothyroxine increase to 125 μg daily. On this dose, her FT4 was 1.0. She developed vaginal bleeding and stopped mifepristone after 11 months and underwent a second TSS (Fig. 1e).
3. Discussion
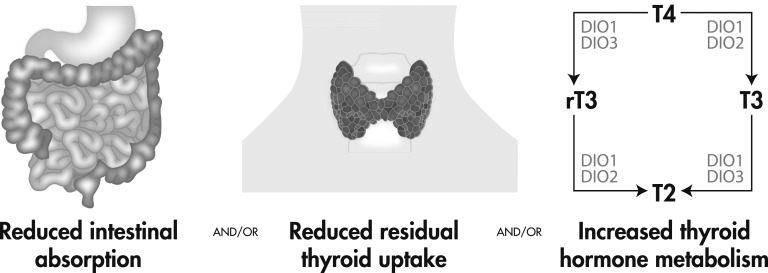
Few reports have discussed longitudinal outcomes in patients with Cushing syndrome receiving mifepristone and only one clinical trial and its extension report have formally assessed the efficacy in this population [1, 17]. Mifepristone is associated with improvement in metabolic profile, including a decrease in plasma fasting glucose and HbA1c, persistent body weight loss in many, and lower diastolic blood pressure in some patients with hypertension [1, 7]. The effects of mifepristone on thyroid function are not well known, but evidence in thyroid cell cultures have shown that mifepristone generates a reduction in iodine uptake as well as a reduced expression of several essential proteins in thyroid hormone synthesis [9, 11]. These effects may explain an increased levothyroxine requirement in hypothyroid patients. This study reviewed levothyroxine requirements in patients with CH who received mifepristone. These cases provide evidence that the initiation of mifepristone can be associated with an increase in thyroid hormone requirement in patients with CH. Several concepts may be considered to explain this phenomenon, including the possibility of decreased intestinal absorption, decreased thyroid residual function, and increased deiodination of thyroid hormones (Fig. 2).
Figure 2.
Proposed mechanisms.
Mifepristone may impair intestinal absorption of levothyroxine, which would lead to decreased FT4 levels and, subsequently an increased dose requirement. To determine that mifepristone causes malabsorption of thyroid hormone, compliance issues and conditions that affect gastrointestinal absorption, such as gastric acidity, bowel resection or celiac sprue, as well as concomitant use of drugs that are known to interfere in levothyroxine absorption should be excluded. Although not done routinely, nor strictly validated, T4 absorption tests could be performed to test this hypothesis.
Regarding residual thyroid function, studies have shown that hydrocortisone stimulates iodine uptake in thyroid cells, but the effect is blunted after mifepristone use [9]. FT4 levels are usually kept at steady state by the negative feedback of the hypothalamic-pituitary-thyroid axis in normal conditions, however, because these patients had central hypothyroidism, the absence of TSH increase as well as the fact that they receive exogenous T4 replacement would make thyroid production negligible. Nevertheless, it could be proposed that even in patients with long-standing central hypothyroidism, the thyroid gland may still produce a small amount of T3 and T4 to keep stable FT4 levels. As it has been shown that mifepristone can lower iodine uptake and lower thyroglobulin production and thyroperoxidase expression [9], it could be inferred that a decrease in these factors could explain a lower hormone secretion and, therefore, a higher levothyroxine requirement to augment the thyroid supply in compensation for this putative decrease in residual endogenous function.
Thyroid hormone metabolism is complex and includes several factors that catalyze deiodination of tyrosine residues, activating or deactivating T4 and T3. Iodothyronine deiodinases type 1, 2, and 3 (DIO1, DIO2, and DIO3, respectively) are the main enzymes involved in the process and are present in different concentrations throughout the body. DIO1 and DIO2 are the primary activators of T4, converting it into T3 so that it can trigger responses via the nuclear thyroid hormone receptor. DIO2 is primarily responsible for T3 production peripherally. DIO3 inactivates T4 and T3, turning them into rT3 and diiodothyronine, which are not usually measured in clinical settings [18, 19]. The increased levothyroxine requirements could be explained by an increased deactivation of T4 by DIO3, which would lead to increased concentrations of rT3 in plasma. As it is not usually performed, only two of the patients had rT3 measured and were both normal, making this theory less likely to explain this effect in mifepristone-treated hypercortisolemic patients, but still viable considering baseline values were not available and both were in the lower normal range.
In terms of clinical manifestations, four of the patients enrolled presented with substantial weight loss that ranged from 4.1 to 42.6 kg in up to 54 months of follow-up, which is concordant with the data published by the SEISMIC extension study [7]. The optimization of thyroid hormone levels could play a role in aiding in this outcome, because it has been shown that hypothyroidism may impair energy expenditure and metabolic rate [20].
This longitudinal case series, although retrospective, reveals that mifepristone decreases FT4 in patients with CH receiving thyroid hormone replacement, even in the presence of substantial weight loss. To determine the exact causes of this increased levothyroxine requirement, more studies are needed to test the different hypotheses and confirm the existence of this entity. Limitations of this study include its retrospective design, which includes selection bias of patients who continued the treatment of a longer duration, the small number of subjects and the observational data taken from real-life clinical practice rather than a standardized protocol. Larger studies would be helpful to confirm this findings, and specific tests to determine the pathophysiology behind this phenomenon, such as thyroxine malabsorption tests and baseline and periodic rT3 levels [21, 22], would be useful.
The experience reported represents all cases with CD and CH treated with mifepristone in three of the four centers and most of such cases in the fourth. Therefore, it appears that this requirement for an increased dose of thyroid hormone commonly occurs with the administration of mifepristone. However, given the retrospective nature of this study and the small number of subjects, the exact frequency of this increased requirement cannot be definitively determined from this series. In addition, regarding published data on this topic, the SEISMIC study reported that 16% of patients developed subclinical hypothyroidism based on elevated TSH levels, but no information on free T4 levels was described on the eight patients with CH [1].
As treatment of persistent hypercortisolemic patients aims to reduce clinical manifestations of Cushing syndrome, including weight gain or mood changes, the presence of untreated hypothyroidism can hamper these changes. We therefore recommend close monitoring of the thyroid status in all patients treated with mifepristone to optimize outcomes. In this case series, the fact that thyroid function was monitored regularly aided in maintaining normal thyroid hormone levels and, probably, may have enhanced the efficacy of mifepristone in maintaining weight loss.
4. Conclusions
Treatment with mifepristone in patients with Cushing syndrome can alter thyroid hormone levels, of which its effects on thyroid hormone metabolism remains poorly understood, especially in patients with concurrent CH. This case series of five patients with CD and CH, in whom increased levothyroxine requirements were observed after mifepristone initiation, suggest that close monitoring and adjustment of thyroid hormone replacement are necessary to optimize the management of these patients. More data are needed to determine its true prevalence and pathophysiology involved. Also, substantial weight loss was achieved in most cases, which is similar to the findings in the literature on mifepristone treatment in the long term.
Acknowledgments
We are grateful for the assistance of Carla Fuenzalida for the design of Figure 2.
Glossary
Abbreviations:
- CD
Cushing disease
- CH
central hypothyroidism
- DIO
deiodinases
- FT4
free thyroxine
- IQR
interquartile range
- SEISMIC
Study of the Efficacy and Safety of Mifepristone in the Treatment of Endogenous Cushing Syndrome
- TSS
transsphenoidal resection
Additional Information
Disclosure Summary: F.J.G. has nothing to disclose. J.F. is and investigator and consultant for Novartis and Corcept. K.C.J.Y received research grants to Barrow Neurologic Institute from Millendo, and Corcept, and consultant fee from Corcept. M.F. has had research support paid to university from Novartis, Millendo, Strongbridge, and Scientific Consultant Fee from Novartis and Strongbridge. L.B.N. has received honoraria as a consultant for Corcept Inc.
Data availability:
All data generated or analyzed during this study are included in this published article.
References and Notes
- 1. Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C; SEISMIC Study Investigators. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97(6):2039–2049. [DOI] [PubMed] [Google Scholar]
- 2. Pivonello R, De Martino MC, De Leo M, Simeoli C, Colao A. Cushing’s disease: the burden of illness. Endocrine. 2017;56(1):10–18. [DOI] [PubMed] [Google Scholar]
- 3. Feelders RA, Newell-Price J, Pivonello R, Nieman LK, Hofland LJ, Lacroix A. Advances in the medical treatment of Cushing’s syndrome. Lancet Diabetes Endocrinol. 2018. [DOI] [PubMed] [Google Scholar]
- 4. Beck-Peccoz P, Rodari G, Giavoli C, Lania A. Central hypothyroidism—a neglected thyroid disorder. Nat Rev Endocrinol. 2017;13(10):588–598. [DOI] [PubMed] [Google Scholar]
- 5. Spitz IM. Mifepristone: where do we come from and where are we going? Clinical development over a quarter of a century. Contraception. 2010;82(5):442–452. [DOI] [PubMed] [Google Scholar]
- 6. Food U, Administration D. Orange Book :Aapproved Drug Products with TherapeuticEequivalence Evaluations. [Mifepristone] 2012. [Google Scholar]
- 7. Fein HG, Vaughan TB III, Kushner H, Cram D, Nguyen D. Sustained weight loss in patients treated with mifepristone for Cushing’s syndrome: a follow-up analysis of the SEISMIC study and long-term extension. BMC Endocr Disord. 2015;15(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Healy DL, Chrousos GP, Schulte HM, Williams RF, Gold PW, Baulieu EE, Hodgen GD. Pituitary and adrenal responses to the anti-progesterone and anti-glucocorticoid steroid RU 486 in primates. J Clin Endocrinol Metab. 1983;57(4):863–865. [DOI] [PubMed] [Google Scholar]
- 9. Takiyama Y, Tanaka H, Takiyama Y, Makino I. The effects of hydrocortisone and RU486 (mifepristone) on iodide uptake in porcine thyroid cells in primary culture. Endocrinology. 1994;135(5):1972–1979. [DOI] [PubMed] [Google Scholar]
- 10. Heikinheimo O, Ranta S, Grunberg S, Lähteenmäki P, Spitz IM. Alterations in the pituitary-thyroid and pituitary-adrenal axes--consequences of long-term mifepristone treatment. Metabolism. 1997;46(3):292–296. [DOI] [PubMed] [Google Scholar]
- 11. Bertoni AP, Brum IS, Hillebrand AC, Furlanetto TW. Progesterone upregulates gene expression in normal human thyroid follicular cells. Int J Endocrinol. 2015;2015:864852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vattai A, Ziegelmüller B, Kost B, Kuhn C, Hofmann S, Bayer B, Anslinger K, Jeschke U, Ditsch N. The expression of thyroid hormone receptors (THR) is regulated by the progesterone receptor system in first trimester placental tissue and in BeWo cells in vitro. Eur J Obstet Gynecol Reprod Biol. 2015;195:31–39. [DOI] [PubMed] [Google Scholar]
- 13. Spitz IM, Grunberg SM, Chabbert-Buffet N, Lindenberg T, Gelber H, Sitruk-Ware R. Management of patients receiving long-term treatment with mifepristone. Fertil Steril. 2005;84(6):1719–1726. [DOI] [PubMed] [Google Scholar]
- 14. RRID:AB_2801661, https://scicrunch.org/resolver/AB_2801661.
- 15. RRID:AB_2801666, https://scicrunch.org/resolver/AB_2801666.
- 16. RRID:AB_2801665, https://scicrunch.org/resolver/AB_2801665.
- 17. Fleseriu M, Findling JW, Koch CA, Schlaffer SM, Buchfelder M, Gross C. Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing’s disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. J Clin Endocrinol Metab. 2014;99(10):3718–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bianco AC, da Conceição RR. The deiodinase trio and thyroid hormone signaling. Methods Mol Biol. 2018;1801:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol. 2015;11(11):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu G, Liang L, Bray GA, Qi L, Hu FB, Rood J, Sacks FM, Sun Q. Thyroid hormones and changes in body weight and metabolic parameters in response to weight loss diets: the POUNDS LOST trial. Int J Obes (Lond). 2017;41(6):878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker JN, Shillo P, Ibbotson V, Vincent A, Karavitaki N, Weetman AP, Wass JA, Allahabadia A. A thyroxine absorption test followed by weekly thyroxine administration: a method to assess non-adherence to treatment. Eur J Endocrinol. 2013;168(6):913–917. [DOI] [PubMed] [Google Scholar]
- 22. de Carvalho GA, Paz-Filho G, Mesa Junior C, Graf H. Management of endocrine disease: pitfalls on the replacement therapy for primary and central hypothyroidism in adults. Eur J Endocrinol. 2018;178(6):R231–R244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.