Abstract
Context
Von Hippel-Lindau (VHL) disease is an autosomal dominant syndrome caused by germline mutations in the VHL gene. Guidelines recommend pheochromocytoma (PHEO) biochemical screening should start at age 5 years.
Objective
Genotype–phenotype correlations in VHL, focusing on PHEO penetrance in children, were studied.
Design
We retrospectively evaluated 31 individuals (median age at diagnosis was 26 years) with diagnosed VHL disease.
Results
PHEO was diagnosed in six children with VHL. A large PHEO (5 cm) was detected in a 4-year-old boy with p.Gly114Ser mutation. PHEO penetrance was 55% starting at age 4 years. VHL missense mutations were identified in 11 of 22 families (50%), frameshift mutations in four (18.2%), stop codon in three (13.6%), splicing site in two (9.1%), and large gene deletion in two (9.1%). The codon 167 (n = 10) was a hotspot for VHL mutations and was significantly associated with PHEO (90% vs. 38%; P = 0.007). PHEOs and pancreatic neuroendocrine tumors (PNETs) were strongly associated with VHL missense mutations compared with other mutations (89.5% vs. 0% and 73.7% vs. 16.7%; P = 0.0001 and 0.002, respectively). In contrast, pancreatic cysts (91.7% vs. 26.3%; P = 0.0001), renal cysts (66.7% vs. 26.3%; P = 0.027), and central nervous system hemangioblastomas (91.7% vs. 47.3%; P = 0.012) were more frequent in VHL with nonmissense mutations.
Conclusion
VHL missense mutations were highly associated with PHEO and PNETs. Our data support that in children with VHL harboring missense mutations, biochemical screening for PHEO should be initiated at diagnosis.
Keywords: von Hippel-Lindau, pheochromocytoma, pancreatic neuroendocrine tumors, surveillance
Von Hippel-Lindau disease (VHL; Online Mendelian Inheritance in Man no. 193300) is an autosomal dominant, inherited tumor predisposition syndrome caused by germline mutations in the VHL tumor suppressor gene [1, 2]. Most cases (80%) are inherited from an affected parent and approximately 20% are de novo. More than 500 germline mutations have been identified since the VHL gene was cloned in 1993 [1, 3]. The incidence of VHL is approximately one in 36,000 live births and lifetime penetrance approaches 100% by age 65 years [4]. The VHL tumor spectrum includes central nervous system (CNS) and retinal hemangioblastomas (HBs), renal cell carcinomas (RCCs), renal cysts, pancreatic cysts (PCs) or pancreatic neuroendocrine tumors (PNETs), pheochromocytomas (PHEOs), endolymphatic-sac tumors, and papillary cystadenomas of the epididymis or broad ligament [2, 4].
VHL is primarily caused by inactivation of the VHL tumor-suppressor protein, which plays a key role in cellular oxygen sensing by targeting hypoxia-inducible factors (HIFs) for ubiquitination and proteasomal degradation [5]. Approximately 95% to 100% of individuals with a clinical diagnosis of VHL have a disease-causing mutation [1, 2]. Elevated levels of HIFs subsequently result in overactivation of the vascular endothelial growth factor, platelet-derived growth factor, and transforming growth factor-α downstream pathways [5, 6]. In addition, VHL tumors differ with respect to the level of HIFα activation required for tumorigenesis and HIF-independent tumor suppressor functions of the VHL tumor-suppressor protein, which can explain the heterogeneity of VHL clinical presentation [7, 8].
VHL diagnosis is established in an individual with a single characteristic VHL-related tumor (i.e., CNS, HB, RCC, or PHEO tumor) and a family history of VHL. In the absence of a VHL family history, a diagnosis requires two or more typical VHL tumors (excluding epididymal and renal cysts) [2]. VHL diagnosis can also be confirmed if a VHL germline mutation has been identified. VHL genetic diagnosis is also essential for family counseling before disease onset in individuals harboring VHL mutations.
Because VHL has marked phenotypic variability, genetic guided surveillance would be essential to decrease morbidity related to VHL-associated tumors [2]. To date, genotype–phenotype correlations in patients with VHL disease mainly rely on predisposition for PHEO development [1, 9, 10]. Type 1 VHL is characterized by truncating or large deletion mutations that confer a low risk for PHEOs, whereas type 2 VHL is characterized by missense mutations and an increased risk for PHEOs. In addition, PNETs are more often diagnosed in patients with VHL disease with exon 3 intragenic mutations, compared with patients with VHL disease with large deletions [10–12]. In this study, we investigated genotype–phenotype correlations in patients with VHL disease from a single tertiary referral hospital. In addition, we analyzed a subgroup of young patients with PHEOs to investigate penetrance and clinical presentation and revisit the optimal age at which to initiate screening.
1. Patient and Methods
The study was approved by the ethics committees of the Hospital das Clínicas, University of São Paulo, and informed written consent was obtained from all patients. Thirty-one patients with molecular diagnosis of VHL from 22 unrelated families were retrospectively evaluated until February 2019. After clinical and/or molecular diagnosis, clinical and imaging follow-up was performed according the surveillance guideline proposed by Nielsen et al. [2]. In summary, patients underwent eye/retinal examination with an ophthalmoscope (since diagnosis) and measurement of levels of plasma-free metanephrines or 24-hour urinary metanephrines annually (after 5 years). MRI of the brain and whole spine was performed every 1 or 2 years after 16 years of age. But differently from what suggested by surveillance guidelines [2, 9], we started to perform abdominal MRI or CT scans after 10 years from diagnosis, every 1 or 2 years, even in asymptomatic individuals. Before 10 years, abdominal imaging was performed only if there were biochemical abnormalities.
Pancreatic lesions were classified as cystic or neuroendocrine tumor according to radiological findings. Biochemical and imaging diagnosis of PHEO or paraganglioma followed the Endocrine Society guideline recommendations [13]. Malignant PNET or PHEO was defined only in the presence of local invasion or lymph nodal or distant metastases.
A. Molecular Analysis
Genomic DNA was extracted using standard procedures. All VHL coding regions were analyzed by Sanger sequencing. PCR products were sequenced in an automated ABI Prism 3700 sequencer (Thermo Fisher Scientific, Waltham, MA). The following oligonucleotides were used: exons 1, forward 5′-CTAGCCTCGCCTCCGTTAC-3′ and reverse 5′-GTCACCCTGGATGTGTCCTG-3′; exon 2, forward 5′-TTAGCCAGGACGGTCTTGAT-3′ and reverse 5′- CGTACAAATACATCACTTCCATT -3′; and exon 3, forward 5′-TACTACAGAGGCATGAACACC-3′ and reverse 5′- CCCCTAAACATCACAATGC -3′.
In the patients with clinical VHL diagnosis but without pathogenic coding variants detected by Sanger, multiplex ligation-dependent probe amplification (MLPA) was performed to investigate large deletions. MLPA was performed as previously described using the SALSA® MLPA® P016 VHL probe mix (MRC-Holland, Amsterdam, Netherlands) [14]. This probe mix contains 29 probes with amplification products between 166 and 427 nucleotides: nine probes for the VHL gene (two or more probes for each exon); six probes for genes located close to VHL (FANCD2, BRK1/C3orf10/HSPC300, IRAK2, and GHRL); and 12 reference probes detecting sequences on other chromosomes. PCR product underwent capillary electrophoresis on an ABI Prism 310 Genetic Analyzer (Thermo Fisher Scientific). Data analysis was performed with Genescan 3.7 (Thermo Fisher Scientific). Dosage quotient areas outside the range of 0.70 to 1.3 were considered abnormal.
B. Statistical Analysis
Statistical analysis was performed using SPSS version (25.0 (IBM, Armonk, NY). Continuous data are expressed as median (range) values. The χ2 test was used to investigate dichotomous variables. P < 0.05 was considered significant.
2. Results
Among the 31 individuals with molecular diagnosis of VHL, median age at diagnosis was 26 (range, 5 to 56) years. The median follow-up was 80.6 (2 to 286) months. In our cohort, CNS HB was the most common tumor, followed by PHEO and pancreatic lesions (Table 1). Two patients died as a result of postoperative complications of neurosurgery for HBs (patients 6 and 15; Table 2).
Table 1.
Tumor Spectrum of 31 Patients With VHL Disease
| Tumor | Frequency, No. (%) |
|---|---|
| CNS HB | 20 (64.5) |
| PHEO | 17 (54.8) |
| PNET | 16 (51.6) |
| PC | 16 (51.6) |
| Renal cyst | 13 (41.9) |
| Retinal HB | 12 (38.7) |
| RCC | 8 (25.8) |
Table 2.
Molecular and Clinical Data of Individuals With VHL Disease.
| Family | Age (y) | PPGL | Age at PPGL Diagnosis | PNET | Age at PNET Diagnosis | RCC | RC | PC | CNS HB | Retinal HB | Mutation (cDNA) | Region | Mutation (Protein) | Type of Mutation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 38 | Y | 38 | Y | 38 | N | N | N | N | N | c.340G>A | Exon 1 | p.Gly114Ser | Missense |
| 2 | 1 | 4 | Y | 4 | Y | 20 | N | N | N | Y | N | C.340G.A | Exon 1 | p.Gly114Ser | Missense |
| 3 | 1 | 26 | Y | 26 | Y | 26 | N | Y | N | Y | Y | C.340G.A | Exon 1 | p.Gly114Ser | Missense |
| 4 | 2 | 8 | Y | 8 | Y | 14 | N | N | N | N | N | c.340G>A | Exon 1 | p.Gly114Ser | Missense |
| 5 | 3 | 30 | Y | 33 | Y | 49 | Y | N | Y | Y | Y | c.499C>T | Exon 3 | p.Arg167Trp | Missense |
| 6 | 4 | 18 | Y | 18 | Y | 44 | N | N | N | Y | N | c.500G>A | Exon 3 | p.Arg167Gln | Missense |
| 7 | 4 | 20 | N | — | N | — | Y | N | N | N | N | c.500G>A | Exon 3 | p.Arg167Gln | Missense |
| 8 | 4 | 27 | Y | 27 | N | — | N | N | N | Y | N | c.500G>A | Exon 3 | p.Arg167Gln | Missense |
| 9 | 4 | 49 | Y | 49 | N | — | N | Y | Y | Y | N | c.500G>A | Exon 3 | p.Arg167Gln | Missense |
| 10 | 4 | 12 | Y | 12 | Y | 13 | N | N | N | N | N | c.500G>A | Exon 3 | p.Arg167Gln | Missense |
| 11 | 5 | 26 | Y | 26 | Y | 26 | N | N | N | N | N | c.499C>T | Exon 3 | p.Arg167Trp | Missense |
| 12 | 6 | 36 | Y | 36 | Y | 36 | N | Y | Y | N | Y | c.499C>T | Exon 3 | p.Arg167Trp | Missense |
| 13 | 6 | 16 | Y | 16 | N | — | N | N | N | N | N | c.499C>T | Exon 3 | p.Arg167Trp | Missense |
| 14 | 7 | 29 | Y | 29 | Y | 29 | N | N | N | N | N | c.374A>C | Exon 2 | p.His125Pro | Missense |
| 15 | 8 | 32 | Y | 33 | Y | 33 | Y | Y | Y | Y | N | c.233A>G | Exon 1 | p.Asn78Ser | Missense |
| 16 | 9 | 14 | N | — | N | — | N | N | N | Y | Y | c.540delC | Exon 3 | p.180fs*22 | Frameshift |
| 17 | 10 | 44 | N | — | N | — | Y | N | Y | Y | Y | c.227_229delTCT | Exon 1 | p.Phe76del | Frameshift |
| 18 | 11 | 15 | N | — | N | — | N | Y | Y | Y | Y | c.309_322del14a | Exon 1 | p.G104fs*231 | Frameshift |
| 19 | 12 | 53 | N | — | N | — | Y | Y | Y | Y | N | — | Exon 1 | — | Large deletion |
| 20 | 13 | 24 | N | — | Y | 24 | N | Y | Y | Y | Y | c.541delG | Exon 3 | p.V181S fs*21 | Frameshift |
| 21 | 14 | 36 | N | — | N | — | Y | N | Y | Y | N | c.486C>A | Exon 3 | p.Cys162* | Stop codon |
| 22 | 15 | 19 | N | — | N | — | N | N | Y | Y | N | c.481C>T | Exon 3 | p.Arg161* | Stop Codon |
| 23 | 16 | 16 | N | — | Y | 17 | N | N | Y | Y | Y | c.74C>Tb; 256C>A | Exon 1 | p.Pro25Leu /p.Pro86Thr | Missense |
| 24 | 17 | 31 | N | — | N | — | N | Y | Y | N | Y | c.463+2T>G | Intron 2 | — | Splicing site |
| 25 | 18 | 26 | Y | 26 | Y | 35 | N | N | N | Y | N | c.499C>T | Exon 3 | p.Arg167Trp | Missense |
| 26 | 19 | 13 | Y | 13 | Y | 47 | N | Y | N | N | Y | c.371C>T | Exon 2 | p.Thr124Ile | Missense |
| 27 | 19 | 10 | Y | 10 | N | — | N | N | N | N | N | c.371C>T | Exon 2 | p.Thr124Ile | Missense |
| 28 | 20 | 45 | N | — | N | — | N | Y | Y | Y | N | c.463+1G>A | Intron 2 | — | Splicing site |
| 29 | 21 | 14 | N | — | Y | 25 | N | Y | Y | Y | Y | — | All exons | — | Large deletion |
| 30 | 22 | 56 | N | — | N | — | Y | Y | Y | Y | Y | c.481 C>T | Exon 3 | p.Arg161* | Stop Codon |
| 31 | 22 | 32 | N | — | N | — | Y | Y | Y | Y | N | c.481 C>T | Exon 3 | p.Arg161* | Stop Codon |
Abbreviations: —, case did not develop the tumor; PPGL, pheochromocytomas and paragangliomas.
Mutation previously described only as a somatic event in RCC (15).
The mutation c.74C>T has been reported as likely being in ClinVar (ClinVar accession number VCV000093330.1).
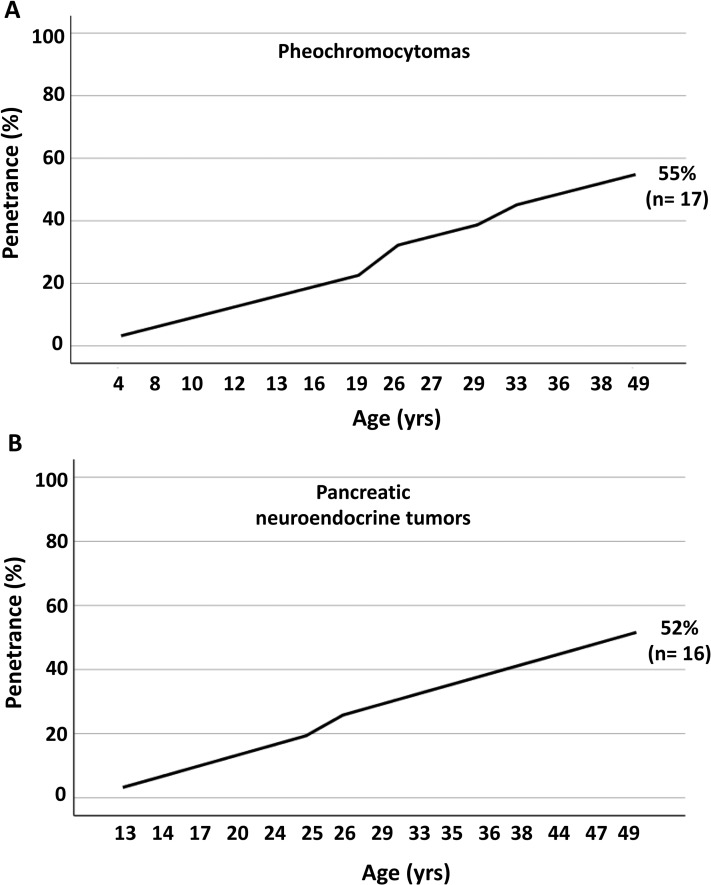
Median age at diagnosis of PHEO was 26 (5 to 49) years. All PHEOs were noradrenergic. The median size was 3.5 (0.8 to 6.8) cm. Ten of 17 PHEOs (59%) were bilateral. Six of the 10 bilateral PHEOs were synchronous. A para-aortic abdominal paraganglioma (2.2 cm) was diagnosed in a young patient with bilateral PHEO (patient 10;, Table 2). PHEO penetrance was 55%, starting at 4 years of age (Fig. 1).
Figure 1.
Cumulative frequency of (A) PHEOs and (B) PNETS in patients with VHL disease (n = 31).
Six of 16 patients (37.5%) with VHL disease with PHEO were younger than 19 (4 to 16) years, and tumor size (largest PHEO) varied from 1.3 to 6.0 cm (Table 3). Five of the six patients with VHL disease (83.3%) had bilateral PHEOs, but only one case was synchronous. We report here the interesting case of a 4-year-old boy with VHL disease with a history of sudoresis, headache, and abdominal pain (case 2; Table 2). During evaluation, he was diagnosed with hypertension. An abdominal CT scan revealed a 5-cm heterogenous mass in the right adrenal and normal left adrenal glands. This patient underwent a right nodulectomy to preserve normal adrenal tissue. Anatomopathological diagnosis confirmed PHEO. After 3 years, a bilateral recurrence was evidenced (4-cm right and 1.5-cm left nodules). The patient underwent a right-side adrenalectomy and a left-side nodulectomy. He remained without hormone replacement. After 13 years from the second surgery, the boy presented with a left-side recurrence (1.3-cm nodule) and elevated plasmatic normetanephrine levels; he underwent left-side adrenalectomy. He is currently receiving hydrocortisone and fludrocortisone therapy.
Table 3.
Characteristics of PHEO and Paraganglioma in Patients Younger Than Age 19 Years With VHL Disease
| Family/Case No. | Age at Diagnosis (y) | Sex (F/M) | PHEOa (cm) (Right or Left Side) | Bilateral (Y/N) | Synchronous (Y/N) | Contralateral PHEOa (cm) (Right or Left Side) | Paragangliomaa (cm) | Time Until Recurrence (mo) |
|---|---|---|---|---|---|---|---|---|
| 1/2 | 4 | M | 5.0 (R) | Y | N | 4.0 (R), 1.5 (L) | — | 36 |
| 1.3 (L) | 156 | |||||||
| 2/4 | 8 | F | 6.0 (L) | Y | N | 5.5 (R) | — | 24 |
| 4/10 | 12 | M | 5.5 (R) | Y | Y | 1.5 (L) | 2.2 | — |
| 6/13 | 16 | M | 1.7 (R) | N | N | — | — | No recurrence (follow-up, 83 mo) |
| 19/26 | 13 | M | NA | Y | N | NA | — | 48 |
| 19/27 | 10 | M | NA | Y | N | NA | — | 48 |
Abbreviations: —, case did not develop the tumor; NA, not available.
Largest diameter.
PNETs and PCs were both identified in 16 of 31 (51.6%) patients. The median age at diagnosis was 29 (13 to 49) years. Median size of PNETs was 1.75 (0.7 to 9.6) cm. All PCs were multiple, whereas seven of 16 PNETs (43.8%) were multiple. PNET penetrance was 52%, starting at 13 years of age (Fig. 1). Two PNETs were malignant: one in a patient with lymph node and hepatic metastases (patient 11; Table 2) and another in a patient with local duodenal invasion (patient 12; Table 2). The sizes of the malignant PNETs were 4.0 and 9.6 cm, respectively.
Among the 16 patients with VHL disease who had PNETs, three (18.8%) were younger than 19 (13 to 17) years; tumor size in this group varied from 0.7 to 2.2 cm (Table 4). Two of them had multiple PNETs and only one had a PC. Two of the three young patients with PNETs had missense VHL mutations. Only the patient with a concomitant PC had a frameshift VHL mutation in exon 3 (case 16; Table 2).
Table 4.
Characteristics of Nonfunctioning Neuroendocrine Pancreatic Lesions in Patients Younger than Age 19 Years With VHL Disease
| Family/Case No. | Age at Diagnosis (y) | Sex (F/M) | PNET Sizea (cm) | Multiple (Y/N) | Pancreatic Cysts (Y/N) |
|---|---|---|---|---|---|
| 2/4 | 14 | F | 1.5 | Y | N |
| 4/9 | 13 | M | 0.7 | N | N |
| 23/16 | 17 | F | 2.2 | N | Y |
Largest diameter of largest nodule.
VHL missense mutations were identified in 11 of 22 families (50%), frameshift mutations in four (18.2%), stop codon in three (13.6%), splicing site in two (9.1%), and large gene deletion in two (9.1%; Table 2). Codon 167 was a hotspot for VHL mutations, identified in 10 cases from 5 of 22 kindreds (22.7%). Codon 167 mutations were significantly associated with a higher risk of developing PHEO (90% vs. 38%; P = 0.007; χ2= 7.37). Among those 10 patients with codon 167 mutations, PNETs developed in six, CNS HBs in five, PCs in three, and RCCs in two.
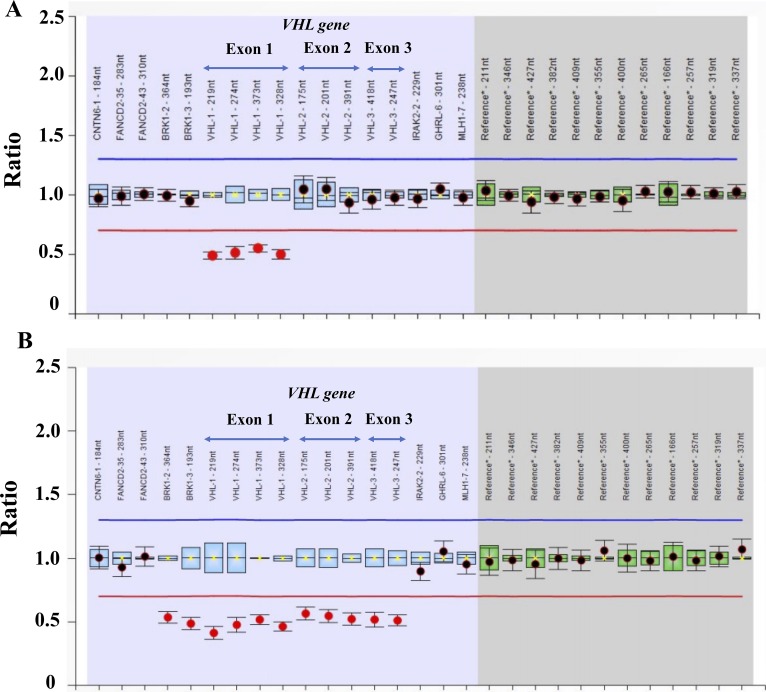
Among the 22 families, intragenic VHL mutations (81.8%) were located in exon 1 (six families; 27.3%), exon 2 (two families; 22, 9.1%), and exon 3 (10 families; 45.5%). Large VHL deletions (two families; 9.1%) and splicing sites (two families; 9,1%) were less frequent among the 22 families. All mutations but one have been previously reported in VHL disease. The germline VHL frameshift mutation c.309_322del14 (p.G104fs*23), leading to a premature stop codon, was previously described as a somatic event in RCCs in the Catalogue of Somatic Mutations in Cancer. Two large VHL deletions were identified in two families of patients with VHL disease: an exon 1 and a complete VHL deletion (Fig. 2).
Figure 2.
MLPA showing heterozygous VHL large deletions in two patients with VHL type 1 disease: (A) exon 1 deletion and (B) complete gene deletion.
VHL missense mutations were highly associated with PHEO when compared with other mutations (89.5% vs. 0%; P = 0.0001; χ2= 23.54; Table 5). In addition, PNETs were significantly more frequent in patients with VHL disease with missense mutations when compared with nonmissense defects (73.7% vs. 16.7%; P = 0.002; χ2= 9.57). In contrast, PCs (91.7% vs. 26.3%; P = 0.0001; χ2= 12,58), renal cysts (66.7% vs. 26.3%; P = 0.027; χ2= 4.92), and CNS HBs (91.7% vs. 47.3%; P = 0.027; χ2= 6.30) were more frequent in patients with nonmissense variants (truncating or large deletion) when compared with missense mutations (Table 5).
Table 5.
Genotype–Phenotype Correlations in Individuals With VHL Disease
| Missense (n = 19), No. (%) | Nonmissense (n = 12), No. (%) | P (χ 2 test) | |
|---|---|---|---|
| PHEO | 17 (89.5) | 0 (0) | 0.0001 (23.74) |
| RCC | 3 (15.7) | 5 (41.7) | 0.11 (2.57) |
| Renal cysts | 5 (26.3) | 8 (66.7) | 0.027 (4.92) |
| PNET | 14 (73.7) | 2 (16.7) | 0.002 (9.57) |
| PC | 5 (26.3) | 11 (91.7) | 0.0001 (12.58) |
| CNS HB | 9 (47.3) | 11 (91.7) | 0.012 (6.3) |
| Retinal HB | 5 (26.3) | 7 (58.3) | 0.075 (3.18) |
3. Discussion
In this study, we analyzed a large cohort of Brazilian patients with VHL disease and investigated genotype–phenotype correlations. We found that missense mutations conferred an increased risk of PHEO and PNET development but a decreased risk of CNS HBs, PCs, and renal cysts. In our cohort, the majority of VHL mutations detected were missense, as previously described [16, 17]. Our study confirmed previous associations between VHL tumors and genotype but also expanded the genotype–phenotype correlation in this disease. These findings underscore the clinical utility in tailoring a personalized approach in the follow-up of patients with VHL disease according mutational status.
We also reported here a 5-cm PHEO in a 4-year-old boy with VHL harboring the p.Gly114Ser mutation. Two cases of PHEO before 5 years of age in VHL were previously reported [18, 19]. In both cases, patients carried VHL missense mutations (Val84Leu and Gln164Arg) [18, 19]. In 2015, Aufforth et al. [20] reported 21 pediatric patients with VHL disease (age <19 years) with PHEO and the earliest age at diagnosis was 5.5 years, suggesting that biochemical screening (i.e., annual measurement of plasma metanephrine levels) for PHEO should start at age 5 years in patients with VHL disease. Indeed, VHL surveillance guidelines recommend starting PHEO screening only after 5 years of age [2, 9]. Data from the Dutch VHL surveillance suggest surveillance for PHEO should be initiated at birth [21]. Similarly, we think PHEO surveillance should initiate before 5 years of age, but only in children with VHL missense mutations and by measuring annually plasma metanephrine levels, without imaging. Although PHEOs in VHL disease are rarely malignant, starting annual screening of plasma metanephrine levels before 5 years of age only in a subset of children with VHL disease (those harboring VHL missense mutations) is cost-effective and will allow an earlier diagnosis of a tumor associated with hypertension and increased cardiovascular morbidity. Although PHEO size has not been reported in the two previously reported patients with VHL disease who were younger than 5 years, both children had night sweats, headache, severe hypertension, and weight loss at diagnosis [18, 19].
Codon 167 was a hotspot for VHL mutations in our cohort. Mutations in this codon represent approximately 43% of mutations in American and Canadian families with type 2 VHL disease [15, 22, 23]. Codon 167 mutations have been associated with an increased risk of PHEO, PNET and RCC development [10, 23]. In our study, codon 167 mutations were associated only with PHEO diagnosis. Recently, Peng et al. [17] demonstrated a strong association between codon 167 mutations and PHEO development in VHL disease, but also a lower risk of CNS HBs, RCCs, and pancreatic lesions (PNETs and cysts were analyzed together).
The frequency of pheochromocytomas and paragangliomas (mostly PHEOs) in patients with VHL disease has been estimated at approximately 10% to 25% [2]. The higher frequency of PHEOs in our cohort was probably due to a referral bias to our Endocrinology Division. In our cohort, patients with VHL disease with PHEO had a higher frequency of bilateral adrenal involvement, low risk of paraganglioma and malignant disease, and early age at diagnosis. In addition, PHEOs were highly associated with missense mutations, as previously reported [1, 9, 10]. Although relevant and from a single center, our study has limitations because of its retrospectively design and rarity of VHL disease.
Genotype–phenotype correlation of PNET in patients with VHL disease is not well established as PHEOs. Here, we demonstrated a higher frequency of PNETs in patients with VHL disease with missense mutations (VHL type 2). In addition, we report three PNETs (one was 2.2 cm) in VHL disease before 19 years of age. Similarly, Igarashi et al. [24] demonstrated that VHL type 2 disease was significantly more related to PNET than VHL type 1 disease. More recently, Krauss et al. [10] showed that PNETs occurred significantly more frequently in patients with VHL disease with intragenic mutations compared with large deletions. In addition, PNETs in VHL disease were significantly associated with mutations affecting exon 3 with hotspots in codons 161 and 167 [10]. In contrast to PNETs, PCs were detected more frequently in patients with VHL disease harboring nonmissense mutations in our cohort. Recently, Vikkath et al. [25] reported a preferential involvement of PCs in exon 1 mutations in 15 Indian families. In our study, frequency of PCs was not associated with exon mutations.
PNET prognosis in VHL disease has been demonstrated to be better than in sporadic cases [11, 26]. PNETs in VHL disease are diagnosed earlier and more often are benign and multiple [26]. PNETs <15 mm usually do not progress and a size >3 cm is a risk factor for malignancy [11, 26]. Recently, a 2.8-cm tumor was demonstrated to represent a better cutoff to predict malignant PNET [10]. Besides size, exon 3 mutations (mostly those in codon 161 and 167) have been also associated with an increased risk of malignancy in PNETs of patients with VHL disease [10, 12, 27]. In our cohort, the two cases of malignant PNETs in VHL disease carried the missense mutation p.Arg167Trp at codon 167 in exon 3.
Early age at onset, truncating VHL mutations, and CNS HB as the first presenting tumor were related to a decreased survival in a Chinese cohort of patients with VHL disease [17]. CNS HBs were associated with nonmissense VHL mutations in our study when compared with missense mutations, as previously reported [2, 17]. Two patients in our cohort died as a result of surgical complications of CNS HBs.
In conclusion, VHL missense mutations were highly associated with PHEOs, whereas CNS HBs were more often diagnosed in patients with VHL truncating mutations, as previously described. Interestingly, PNETs were significantly more associated with VHL missense mutations in our cohort. Therefore, patients with VHL missense mutations should be closely monitored for PNET development, particularly those with exon 3 missense mutations who carry a higher risk of malignancy. In addition, our data support that biochemical screening for PHEO should be initiated at birth for patients with VHL disease harboring missense mutations.
Acknowledgments
Financial Support: This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (Grant 2015/17049-8 to M.Q.A.); and Conselho Nacional de Desenvolvimento Científico e Tecnológico Grants 403256/2016-0 (to MQA), 302849/2015 (to A.C.L.), and 303002/2016-6 (to B.B.M.).
Glossary
Abbreviations:
- CNS
central nervous system
- HB
retinal hemangioblastoma
- HIF
hypoxia-inducible factor
- MLPA
multiplex ligation-dependent probe amplification
- PC
pancreatic cyst
- PHEO
pheochromocytoma
- PNET
pancreatic neuroendocrine tumor
- RCC
renal cell carcinoma
- VHL
Von Hippel-Lindau
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
Data sharing is not applicable to this article because no data sets were generated or analyzed during the current study.
References and Notes
- 1. Chen F, Kishida T, Yao M, Hustad T, Glavac D, Dean M, Gnarra JR, Orcutt ML, Duh FM, Glenn G, Green J, Hsia YE, Lamiell J, Li H, Wei MH, Schmidt L, Tory K, Kuzmin I, Stackhouse T, Latif F, Linehan WM, Lerman M, Zbar B. Germline mutations in the von Hippel-Lindau disease tumor suppressor gene: correlations with phenotype. Hum Mutat. 1995;5(1):66–75. [DOI] [PubMed] [Google Scholar]
- 2. Nielsen SM, Rhodes L, Blanco I, Chung WK, Eng C, Maher ER, Richard S, Giles RH. Von Hippel-Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34(18):2172–2181. [DOI] [PubMed] [Google Scholar]
- 3. Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. [DOI] [PubMed] [Google Scholar]
- 4. Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15(1):55–64. [DOI] [PubMed] [Google Scholar]
- 5. Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8(11):865–873. [DOI] [PubMed] [Google Scholar]
- 6. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108–119. [DOI] [PubMed] [Google Scholar]
- 7. Li M, Kim WY. Two sides to every story: the HIF-dependent and HIF-independent functions of pVHL. J Cell Mol Med. 2011;15(2):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER. Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Genet. 2001;10(10):1029–1038. [DOI] [PubMed] [Google Scholar]
- 9. Rednam SP, Erez A, Druker H, Janeway KA, Kamihara J, Kohlmann WK, Nathanson KL, States LJ, Tomlinson GE, Villani A, Voss SD, Schiffman JD, Wasserman JD. Von Hippel-Lindau and hereditary pheochromocytoma/paraganglioma syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23(12):e68–e75. [DOI] [PubMed] [Google Scholar]
- 10. Krauss T, Ferrara AM, Links TP, Wellner U, Bancos I, Kvachenyuk A, Villar Gómez de Las Heras K, Yukina MY, Petrov R, Bullivant G, von Duecker L, Jadhav S, Ploeckinger U, Welin S, Schalin-Jäntti C, Gimm O, Pfeifer M, Ngeow J, Hasse-Lazar K, Sansó G, Qi X, Ugurlu MU, Diaz RE, Wohllk N, Peczkowska M, Aberle J, Lourenço DM Jr, Pereira MAA, Fragoso MCBV, Hoff AO, Almeida MQ, Violante AHD, Quidute ARP, Zhang Z, Recasens M, Díaz LR, Kunavisarut T, Wannachalee T, Sirinvaravong S, Jonasch E, Grozinsky-Glasberg S, Fraenkel M, Beltsevich D, Egorov VI, Bausch D, Schott M, Tiling N, Pennelli G, Zschiedrich S, Därr R, Ruf J, Denecke T, Link KH, Zovato S, von Dobschuetz E, Yaremchuk S, Amthauer H, Makay Ö, Patocs A, Walz MK, Huber TB, Seufert J, Hellman P, Kim RH, Kuchinskaya E, Schiavi F, Malinoc A, Reisch N, Jarzab B, Barontini M, Januszewicz A, Shah N, Young WF Jr, Opocher G, Eng C, Neumann HPH, Bausch B. Preventive medicine of von Hippel-Lindau disease-associated pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2018;25(9):783–793. [DOI] [PubMed] [Google Scholar]
- 11. de Mestier L, Gaujoux S, Cros J, Hentic O, Vullierme MP, Couvelard A, Cadiot G, Sauvanet A, Ruszniewski P, Richard S, Hammel P. Long-term prognosis of resected pancreatic neuroendocrine tumors in von Hippel-Lindau disease is favorable and not influenced by small tumors left in place. Ann Surg. 2015;262(2):384–388. [DOI] [PubMed] [Google Scholar]
- 12. Tirosh A, Sadowski SM, Linehan WM, Libutti SK, Patel D, Nilubol N, Kebebew E. Association of VHL genotype with pancreatic neuroendocrine tumor phenotype in patients with von Hippel-Lindau disease. JAMA Oncol. 2018;4(1):124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 14. De Luca A, Bottillo I, Dasdia MC, Morella A, Lanari V, Bernardini L, Divona L, Giustini S, Sinibaldi L, Novelli A, Torrente I, Schirinzi A, Dallapiccola B. Deletions of NF1 gene and exons detected by multiplex ligation-dependent probe amplification. J Med Genet. 2007;44(12):800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C, Teague J, Andrews J, Barthorpe S, Beare D, Buck G, Campbell PJ, Forbes S, Jia M, Jones D, Knott H, Kok CY, Lau KW, Leroy C, Lin ML, McBride DJ, Maddison M, Maguire S, McLay K, Menzies A, Mironenko T, Mulderrig L, Mudie L, O’Meara S, Pleasance E, Rajasingham A, Shepherd R, Smith R, Stebbings L, Stephens P, Tang G, Tarpey PS, Turrell K, Dykema KJ, Khoo SK, Petillo D, Wondergem B, Anema J, Kahnoski RJ, Teh BT, Stratton MR, Futreal PA. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463(7279):360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordstrom-O’Brien M, van der Luijt RB, van Rooijen E, van den Ouweland AM, Majoor-Krakauer DF, Lolkema MP, van Brussel A, Voest EE, Giles RH. Genetic analysis of von Hippel-Lindau disease. Hum Mutat. 2010;31(5):521–537. [DOI] [PubMed] [Google Scholar]
- 17. Peng S, Shepard MJ, Wang J, Li T, Ning X, Cai L, Zhuang Z, Gong K. Genotype-phenotype correlations in Chinese von Hippel-Lindau disease patients. Oncotarget. 2017;8(24):38456–38465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sovinz P, Urban C, Uhrig S, Stepan V, Lackner H, Schwinger W, Benesch M, Moser A, Spuller E, Speicher MR. Pheochromocytoma in a 2.75-year-old-girl with a germline von Hippel-Lindau mutation Q164R. Am J Med Genet A. 2010;152A(7):1752–1755. [DOI] [PubMed] [Google Scholar]
- 19. Abbott MA, Nathanson KL, Nightingale S, Maher ER, Greenstein RM. The von Hippel-Lindau (VHL) germline mutation V84L manifests as early-onset bilateral pheochromocytoma. Am J Med Genet A. 2006;140(7):685–690. [DOI] [PubMed] [Google Scholar]
- 20. Aufforth RD, Ramakant P, Sadowski SM, Mehta A, Trebska-McGowan K, Nilubol N, Pacak K, Kebebew E. Pheochromocytoma screening initiation and frequency in von Hippel-Lindau syndrome. J Clin Endocrinol Metab. 2015;100(12):4498–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kruizinga RC, Sluiter WJ, de Vries EG, Zonnenberg BA, Lips CJ, van der Horst-Schrivers AN, Walenkamp AM, Links TP. Calculating optimal surveillance for detection of von Hippel-Lindau-related manifestations. Endocr Relat Cancer. 2013;21(1):63–71. [DOI] [PubMed] [Google Scholar]
- 22. Stolle C, Glenn G, Zbar B, Humphrey JS, Choyke P, Walther M, Pack S, Hurley K, Andrey C, Klausner R, Linehan WM. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12(6):417–423. [DOI] [PubMed] [Google Scholar]
- 23. Crossey PA, Richards FM, Foster K, Green JS, Prowse A, Latif F, Lerman MI, Zbar B, Affara NA, Ferguson-Smith MA, Maher ER. Identification of intragenic mutations in the von Hippel-Lindau disease tumour suppressor gene and correlation with disease phenotype. Hum Mol Genet. 1994;3(8):1303–1308. [DOI] [PubMed] [Google Scholar]
- 24. Igarashi H, Ito T, Nishimori I, Tamura K, Yamasaki I, Tanaka M, Shuin T. Pancreatic involvement in Japanese patients with von Hippel-Lindau disease: results of a nationwide survey. J Gastroenterol. 2014;49(3):511–516. [DOI] [PubMed] [Google Scholar]
- 25. Vikkath N, Valiyaveedan S, Nampoothiri S, Radhakrishnan N, Pillai GS, Nair V, Pooleri GK, Mathew G, Menon KN, Ariyannur PS, Pillai AB. Genotype-phenotype analysis of von Hippel-Lindau syndrome in fifteen Indian families. Fam Cancer. 2015;14(4):585–594. [DOI] [PubMed] [Google Scholar]
- 26. Erlic Z, Ploeckinger U, Cascon A, Hoffmann MM, von Duecker L, Winter A, Kammel G, Bacher J, Sullivan M, Isermann B, Fischer L, Raffel A, Knoefel WT, Schott M, Baumann T, Schaefer O, Keck T, Baum RP, Milos I, Muresan M, Peczkowska M, Januszewicz A, Cupisti K, Tönjes A, Fasshauer M, Langrehr J, von Wussow P, Agaimy A, Schlimok G, Lamberts R, Wiech T, Schmid KW, Weber A, Nunez M, Robledo M, Eng C, Neumann HP; VHL-ICT Consortium; German NET Registry. Systematic comparison of sporadic and syndromic pancreatic islet cell tumors. Endocr Relat Cancer. 2010;17(4):875–883. [DOI] [PubMed] [Google Scholar]
- 27. Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, Alexander HR, Seidel G, Shutack Y, Yuldasheva N, Eugeni M, Bartlett DL, Glenn GM, Middelton L, Linehan WM, Libutti SK. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery. 2007;142(6):814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the current study.