Abstract
Hyperinsulinemia is strongly associated with type 2 diabetes. Racial and ethnic minority populations are disproportionately affected by diabetes and obesity-related complications. This mini-review provides an overview of the genetic and environmental factors associated with hyperinsulinemia with a focus on racial and ethnic differences and its metabolic consequences. The data used in this narrative review were collected through research in PubMed and reference review of relevant retrieved articles. Insulin secretion and clearance are regulated processes that influence the development and progression of hyperinsulinemia. Environmental, genetic, and dietary factors are associated with hyperinsulinemia. Certain pharmacotherapies for obesity and bariatric surgery are effective at mitigating hyperinsulinemia and are associated with improved metabolic health. Hyperinsulinemia is associated with many environmental and genetic factors that interact with a wide network of hormones. Recent studies have advanced our understanding of the factors affecting insulin secretion and clearance. Further basic and translational work on hyperinsulinemia may allow for earlier and more personalized treatments for obesity and metabolic diseases.
Keywords: diabetes, hyperinsulinemia, hypersecretion, insulin clearance
Insulin has been known to be an essential hormone since its discovery in 1921. Insulin influences almost every organ in the body, including adipose tissue, liver, muscle, and brain, as well as bone [1], kidneys [2], and vasculature [3, 4]. Insulin, somatostatin, and glucagon fluctuate in a periodic fashion in subjects without type 2 diabetes (T2D), and pulsatile insulin secretion accounts for 75% of total insulin secretion [5–7]. These fluctuations are essential because continuous delivery of IV insulin induces desensitization to insulin, whereas pulsatile insulin delivery preserves sensitivity to insulin [8–10]. Loss of pulsatile insulin secretion is an early feature in the development of T2D [11].
Insulin concentrations are regulated by a variety of mechanisms affecting insulin clearance and secretion, which are carefully coordinated through signals from the hypothalamic–pituitary–adrenal (HPA) axis, as well as the liver–pancreas axis, the entero–osseous axis, and the bone–pancreas axis [12]. Excessive insulin secretion may lead to hypoglycemia in insulinomas and noninsulinoma pancreatogenous hypoglycemia syndrome, but these conditions are uncommon compared with dysregulated hyperinsulinemia (defined as elevated circulating insulin in relationship to its usual level relative to blood glucose), which does not cause hypoglycemia. Dysregulated insulin secretion and/or clearance resulting in chronically elevated insulin without hypoglycemia is common in obesity and metabolic disorders, and it is referred to herein as hyperinsulinemia. Fasting insulin rises from normal glucose tolerance to impaired glucose tolerance (IGT) to T2D [13]. In subjects with obesity but without diabetes or hypertension, hyperinsulinemia and insulin hypersecretion are more prevalent than insulin resistance [14] and hence may precede and contribute to insulin resistance. Furthermore, cohort studies have shown that different subjects with similar degrees of insulin sensitivity may exhibit a range of insulin secretion. For example, in the Relationship between Insulin Sensitivity and Cardiovasular Disease (RISC) study, individuals with insulin hypersecretion tended to be older and have higher percent fat mass, worse lipid profiles, and higher liver insulin resistance indices compared with the rest of the cohort [15]. In the RISC study, preexposure to hyperinsulinemia stimulated a greater insulin-induced secretory response independently of insulin sensitivity [16]. Hence, hyperinsulinemia is self-perpetuating and is more likely to be a primary defect rather than a compensation for insulin resistance in the general population.
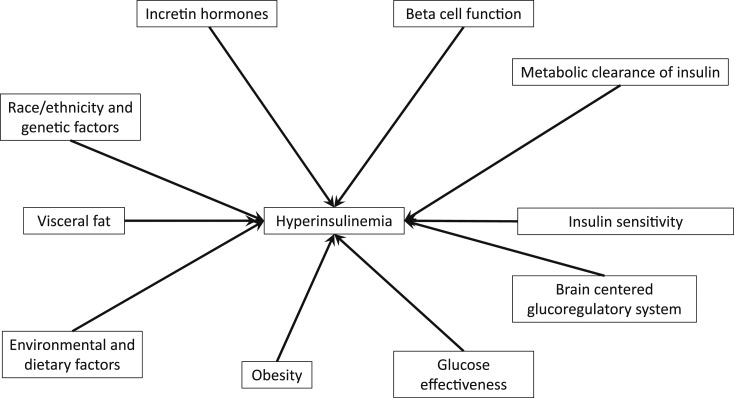
There are racial and ethnic differences in insulin sensitivity and β-cell function [17], and recent research provides insights into their underlying mechanisms. Here, we discuss genetic and environmental factors associated with insulin secretion and clearance and the metabolic consequences of hyperinsulinemia (Fig. 1).
Figure 1.
Diagram of multifactorial etiologies of hyperinsulinemia. Interactions between these various risk factors may also contribute to its development and progression.
1. Methods
We searched PubMed/MEDLINE for English articles with the search terms: hyperinsulinemia, diabetes, race, and obesity. We limited our review primarily to human studies with exceptions when studies have relevance to translational research. We selected mostly recent pertinent publications but did not exclude high-impact older papers. We reviewed the references from key papers to identify additional articles.
2. Methods to Assess Hyperinsulinemia
Insulin has a similar diurnal pattern in subjects with obesity and in lean subjects but is consistently regulated at a higher concentration [18]. The 24-hour urinary c-peptide excretion is a reflection of the area under the curve (AUC) of insulin and has been shown to be negatively correlated with insulin sensitivity assessed by hyperinsulinemic-euglycemic clamp in healthy individuals [19]. Owing to logistic difficulties in obtaining repeated blood or urine samples, most studies assessed only fasting insulin. Fasting insulin has good repeatability within 4 to 8 weeks in the same subjects [20]. Hence, fasting insulin is an important metabolic parameter that is associated with the diurnal insulin exposure and insulin sensitivity and remains fairly stable over time.
Methods to assess insulin sensitivity and β-cell function include the hyperinsulinemic-euglycemic clamp, frequently sampled IV glucose test (FSIVGTT), insulinogenic index, and homeostatic model assessment (HOMA), whicheach have strengths and limitations. Hyperinsulinemic-euglycemic clamps are the gold standard to measure insulin sensitivity, but due to the logistical difficulties in performing these clamps, indices derived from an oral glucose tolerance test (OGTT) or fasting glucose and insulin are widely used. Hyperglycemic clamps provide an accurate assessment of insulin secretion capacity in response to glucose but not insulin sensitivity [21]. HOMA estimates β-cell function and insulin sensitivity based only on fasting glucose and insulin concentrations. The disposition index gives a representation of insulin secretion adjusted for insulin sensitivity.
These methods assess insulin-mediated glucose disposal, but not the ability of glucose to enhance its own disposal (independently of insulin), which is known as glucose effectiveness. Glucose effectiveness accounts for about half of overall glucose disposal, so it is relevant to hyperinsulinemia, and yet its determinants remain poorly understood [22]. Glucose effectiveness can be assessed by FSIVGTT or by pancreatic clamps, which are more difficult to perform [23].
In hyperinsulinemic-euglycemic clamp studies, whole-body insulin clearance and hepatic insulin clearance can be estimated [24]. In contrast, FSIVGTT does not differentiate between hepatic insulin clearance and whole-body insulin clearance [25, 26].
3. Causes of Hyperinsulinemia
A. Factors Associated With Hyperinsulinemia in Epidemiological Studies
A-1. Environmental factors
Diabetogenic dietary and environmental exposures may interact with hormones from the gastrointestinal tract and stimulate insulin hypersecretion under fasting conditions, leading to chronic basal hyperinsulinemia through mechanisms that remain unclear (Table 1) [27–33]. For example, air pollution has been associated with adverse lipid changes and higher fasting glucose and insulin [30] as well as higher childhood body mass index (BMI) trajectories [31]. This association has been hypothesized to be due to chronic ozone exposure and subsequent activation of the HPA axis and hormonal changes [34]. Acute bisphenol A exposure, an endocrine-disrupting chemical, at the maximal daily dose determined to be safe by the U.S. Food and Drug Administration was associated with changes in insulin and c-peptide response to an OGTT and a hyperglycemic clamp [32]. A study of Ghanaians in several countries highlighted the importance of environmental and cultural factors on insulin and glucose metabolism, BMI, insulin sensitivity, and fasting blood glucose [33].
Table 1.
Summary of Environmental Factors Associated With Changes in Glucose and Insulin Metabolism
| Author and Citation | Primary Finding | Study Type/Design | Covariates | Limitations |
|---|---|---|---|---|
| Pories and Dohm 2012 [27] | Fasting insulin rises from normal glucose tolerance lean subjects to normal glucose tolerance subjects with obesity to subjects with T2D Hyperinsulinemia corrects rapidly to normal levels after bariatric surgery | Review | NA | Mechanisms for metabolic improvements following bariatric surgery remain unclear |
| Corkey 2012 [28] | Large numbers of environmental chemicals are detectable in food and human serum, but evidence is lacking on their effects on metabolic health | Review | NA | Further research is necessary to validate and confirm mechanisms |
| Corkey 2012 [29] | In cultured INS-1 cells, monooleoylglycerol, saccharin, aspartame, sucralose, and iron stimulated insulin secretion | Review | NA | Translational work is needed to validate these in vitro findings |
| Chen et al. 2016 [30] | Air pollution exposure is associated with increases in fasting glucose and insulin | Cohort | Socioeconomic status, age, sex, percent body fat | Residual confounding |
| Kim et al. 2018 [31] | Early life near roadway air pollution exposure is associated with greater increases in BMI and higher attained BMI at age 10 y | Cohort | Age, sex, race/ethnicity, parental education, language | Unclear mechanism Residual confounding possible |
| Stahlhut et al. 2019 [32] | Acute bisphenol A exposure is associated with an increase in disposition index | Crossover trial with OGTT and hyperglycemic clamp | NA given crossover design | Acute exposure, small sample size |
| Meeks et al. 2017 [33] | Differences in BMI and waist circumference account for a significant proportion of the geographical variation among sub-Saharan African subjects | Cross-sectional | Age, sex, family history of diabetes, anthropometrics, health-related behaviors, geographical location | Residual confounding |
Abbreviation: NA, not applicable.
A-2. Associations between hyperinsulinemia and race and ethnicity may be partially mediated by differences in body composition
Ethnic differences in insulin sensitivity may be underappreciated owing to the widespread use of OGTT-based surrogate measures and HOMA of insulin resistance (HOMA-IR). HOMA-IR has been validated as a marker of insulin sensitivity in European populations but showed poor correlation with insulin sensitivity assessed by FSIVGTT or clamp in Jamaican adults without diabetes [35]. A similar discrepancy was seen between OGTT-derived surrogate markers and the hyperglycemic clamp parameters in Asian Americans, blacks, whites, and Mexican Americans [36]. Hence, widely used surrogate markers of insulin sensitivity and β-cell function may not be accurate in non-European populations.
The Insulin Resistance and Atherosclerosis Study (IRAS) was a multicenter cross-sectional study of insulin sensitivity assessed by FSIVGTT and cardiovascular risk factors in white, black, and Hispanic patients in the United States [37]. This study provided further insights into the genetic variations underlying the racial and ethnic differences in hyperinsulinemia and diabetes risk. Fasting insulin was higher in subjects with IGT compared with subjects with normal fasting glucose and glucose tolerance [38]. Waist circumference was positively correlated with fasting insulin in white and black patients even after adjusting for glucose tolerance [39]. A 5-year follow-up study showed that the disposition index and glucose effectiveness were independent predictors of incident T2D after adjusting for traditional risk factors [40].
The National Health and Nutrition Examination Survey also demonstrated differences between whites, Hispanics, and blacks in hyperinsulinemia and BMI. There was a significant increase in fasting insulin levels from 1988 to 1994 and 1999 to 2002, which persisted despite adjusting for BMI and waist circumference [41]. Additionally, racial disparities in the prevalence of obesity increased, with black having greater increases in BMI and waist circumference from 1988 to 2004 than did whites or Hispanics [42]. However, National Health and Nutrition Examination Survey data are cross-sectional and lack detailed metabolic assessments of insulin sensitivity and secretion.
Racial differences in hyperinsulinemia are apparent at a young age. The Bogalusa Heart Study of 377 children and adolescents who underwent an OGTT demonstrated that blacks had significantly higher insulin responses than did whites when assessed by the AUC and insulin/glucose ratios at 30 and 60 minutes [43]. These differences were consistent across Tanner stages I to V. Consistent with this, blacks had significantly higher first- and second-phase insulin secretion during a hyperglycemic clamp than did whites [44].
Racial differences in visceral and subcutaneous adipose tissue distributions in women have been reported, with blacks having less visceral fat than whites but paradoxically lower insulin sensitivity [45]. For example, in the Dallas Heart Study, blacks had less visceral fat and hepatic steatosis than did whites and Hispanics, but more insulin resistance by HOMA-IR [46]. In adolescents with obesity, despite similar total percent body fat, Hispanics had greater intramyocellular lipid deposition and blacks had lower hepatic fat accumulation compared with whites [47]. However, these differences in body composition cannot fully account for the differences in β-cell function. In women without diabetes, blacks had greater insulin secretion compared with whites across a wide range of ages, independently of adiposity and insulin sensitivity, and there were no differences in glucose effectiveness [48].
Racial differences have also been reported in the effects of visceral fat mass on serum triglycerides (TGs) and in the upper and lower body subcutaneous fat distribution in women with obesity [49]. Increased visceral adipose tissue (VAT) is associated with higher fasting insulin and insulin AUC during an OGTT, independently of subcutaneous adipose tissue (SAT), skeletal muscle mass [50], insulin resistance, and inflammation [51–55]. In IRAS, VAT and SAT accounted for 27% of the model R2 for insulin sensitivity and 16% of the model R2 for disposition index, adjusting for age, sex, ethnicity, and BMI [56]. VAT contributes to delivery of free fatty acids (FFAs) to the liver, which negatively affects hepatic insulin sensitivity and is associated with reduced insulin clearance [57, 58]. Insulin has an antilipolytic effect on VAT that reduces portal FFAs, and this may be a key mechanism whereby insulin regulates hepatic glucose production in addition to its direct effects on the liver [59, 60].
Central adiposity is associated with lower adiponectin, an adipokine that is normally associated with improved insulin sensitivity [61]. Adiponectin was also negatively associated with VAT, SAT, pericardial fat, and intrathoracic fat in the Framingham Heart Study [62]. There is a strong negative correlation between fasting insulin and adiponectin in whites and Pima Indians [63].
Detailed metabolic studies have shown the important contribution of hyperinsulinemia in Pima Indians, who have a remarkably high prevalence of T2D of 38% [64]. Hormonal and metabolic studies in this population included oral and IV glucose tolerance tests along with body composition. In Pima young adults, high fasting plasma insulin and higher insulin at 30 and 120 minutes were highly heritable and were all predictors of incident T2D [65, 66]. As in IRAS, progression from normal glucose tolerance to IGT to T2D was associated with an increase in fasting insulin levels [67]. Hyperinsulinemia was seen even in Pima prepubertal girls and boys aged 6 to 7. There was no significant difference in the visceral or subcutaneous fat area at L4/L5 in a sample of Pima Indians and whites matched for percent body fat, yet the Pima had significantly higher fasting insulin and lower insulin sensitivity [68]. Hyperinsulinemia was also associated with weight gain and triceps skinfold thickness in the prepubertal years [69].
Racial and ethnic differences in pancreatic fat may account for some of these differences. Fasting and 2-hour insulin during an OGTT were lower in whites than blacks in a study that quantified VAT, pancreatic fat, and hepatic TGs in 100 subjects without T2D. VAT was highest in Hispanics and lowest in blacks [70]. Pancreatic TGs were significantly higher in whites and Hispanics than in blacks [70]. Hepatic TG levels were higher in Hispanics than in whites and blacks [70]. Blacks had the highest disposition index and acute insulin response (AIR) but lowest insulin sensitivity [70]. The effect of a one-unit increase in pancreatic TGs on AIR was largest in blacks compared with whites and Hispanics [70]. However, there was no association between pancreatic fat and β-cell function in another study of young German women [71]. These studies used different methods to estimate β-cell function, which may account for some of these discrepancies.
A systematic review and meta-analysis of studies that measured insulin sensitivity and AIR by the FSIVGTT in Africans, whites, and East Asians confirmed that in subjects with normal glucose tolerance, there is substantially lower insulin sensitivity and higher AIR in African cohorts compared with whites and East Asians, with some subjects exhibiting insulin hypersecretion relative to their degree of insulin sensitivity in each case [72]. Racial and ethnic differences in hyperinsulinemia, as well as glucose and lipid metabolism, are well established [73]. Hence, genetic differences may underlie some of the associations between insulin secretion, insulin resistance, and lipid stores.
A-3. Genetic and epigenetic variants associated with hyperinsulinemia act via several pathways
Genetic differences and epigenetic changes during gestation may underlie the association between in utero exposure to gestational diabetes and increased risk of childhood overweight and obesity [74]. Epigenetic changes in GNAS have also been associated with early-onset obesity [75]. Subjects who had parents with T2D had higher BMI and fasting insulin compared with those who had no family history of diabetes in the RISC study [76].
Studies have implicated several genes involved in obesity and other metabolic outcomes and hyperinsulinemia. The GUARDIAN consortium study of Mexican Americans provided strong evidence for the heritability of insulin sensitivity, AIR, and metabolic clearance of insulin [77]. Distinct clusters of genes have been shown to be associated with β-cell function, body weight, and different diabetes phenotypes [78, 79]. In IRAS, a genome-wide association study identified loci associated with insulin sensitivity and β-cell function in blacks and Hispanics [80], and candidate genes for the disposition index and AIR in blacks were identified [81]. In the IRAS Family Study (IRAS-FS), the heritability of insulin sensitivity assessed by FSIVGTT (0.310) was greater than the heritability of fasting insulin (0.171) and HOMA-IR (0.163) [82].
Genetic variants of FTO also influence the risk of obesity and fasting insulin [83]. Paternal transmission of a polymorphism associated with insulin gene expression conferred an 80% greater risk of early-onset obesity [84]. A genome-wide association study in Indian Asians found that a common variant near MC4R was associated with a higher HOMA-IR, increased waist circumference, and features of metabolic syndrome [85]. Finally, a Hispanic cohort study identified genetic loci that regulate insulin clearance, which has a heritability of 73% [86].
Individuals with ≥17 alleles that raised fasting insulin tended to have higher TG levels, more hepatic steatosis, increased risk of T2D, coronary artery disease, and high blood pressure but a paradoxically lower BMI [87]. In contrast, a Mendelian randomization study of subjects from predominantly European ancestry found a strong association between genes associated with higher insulin concentration at 30 minutes after an OGTT and a higher BMI [88]. These discrepancies may be reconciled by the fact that fasting insulin and insulin secretion may have different genetic determinants. These genetic studies underscore that there are many etiologies for abnormalities in insulin secretion and sensitivity, and they reinforce the paradigm that relative insulin hypersecretion can be pathogenic.
B. Factors Affecting Insulin Clearance With a Focus on Race/Ethnicity
Fasting insulin levels are determined by the dynamic balance between insulin secretion, insulin sensitivity, glucose effectiveness, and insulin clearance, each of which may have different determinants [89]. Estimates of the relative importance of insulin secretion and clearance to hyperinsulinemia have varied depending on the study methodology and population, but both are likely important. One study found that 75% of the hyperinsulinemia is due to a reduction in hepatic metabolic clearance of insulin in subjects with normal fasting glucose and obesity [90]. Using different methods and a different study population, Polonsky et al. [91] found that hyperinsulinemia in subjects with obesity was predominantly driven by increased secretion with a minor contribution of reduced hepatic extraction of insulin. These differences may be due to variations in the measurement of insulin clearance or in the demographic groups. Additionally, reduced clearance may contribute to the early stage of hyperinsulinemia whereas hypersecretion may contribute only to the later stage.
Insulin clearance is associated with physical fitness and metabolic health. Aging is associated with reduced metabolic clearance of insulin and hyperinsulinemia, reduced glucose effectiveness, and an increase in metabolic diseases [92]. Likewise, metabolically healthy subjects with obesity have higher whole-body insulin clearance and hepatic insulin extraction compared with age- and BMI-matched subjects who are metabolically unhealthy [93]. Consistent with this, in nonobese Japanese men without diabetes, low insulin clearance was associated with higher total body fat and lower peak oxygen consumption rate [94].
Blacks had higher insulin levels than did whites and lower fasting c-peptide, consistent with impaired insulin clearance in blacks, which could not be explained by differences in BMI, family history, smoking, or other factors [95]. Lower metabolic clearance of insulin may explain the high prevalence of hyperinsulinemia in blacks. Hepatic first-pass insulin extraction has been estimated to be two-thirds lower in blacks compared with whites, whereas extrahepatic insulin clearance was similar [96]. This low first-pass hepatic extraction was also seen in African immigrants [97]. Consistent with this, in women without diabetes, blacks had a higher insulin response than did whites, as well as lower insulin clearance, but they had similar insulin secretion during OGTT, FSIVGTT, and a mixed meal tolerance test [98].
In IRAS-FS, blacks had lower metabolic clearance of insulin than did Hispanics, which was associated with hyperinsulinemia, greater SAT and VAT, lower high-density lipoprotein, and incident T2D [56, 99], and lower metabolic clearance of insulin was associated with lower insulin sensitivity, higher insulin secretion during FSIVGTT, and higher BMI across race and ethnicities [100].
Ethnic differences in insulin clearance are present in childhood. Black children had 63% higher first-phase insulin secretion and 14% lower clearance along with a 63% higher disposition index compared with whites with similar body composition and insulin sensitivity as assessed by hyperinsulinemic-euglycemic and hyperglycemic clamps [101]. Both greater insulin secretion and reduced clearance make independent contributions to the greater AIR in black children compared with white children [102]. Hispanic children also have a greater second-phase insulin secretion but have similar hepatic insulin extraction compared with whites [103]. In adolescents with obesity, glucose effectiveness was greater in Hispanics than in blacks independent of total fat mass and visceral fat mass [104].
Insulin clearance in whites was lower in subjects with obesity and insulin resistance than in lean subjects, who were similar to subjects with obesity and normal insulin sensitivity [105]. Hyperinsulinemia in whites with obesity but without insulin resistance was mediated by increases in insulin secretion [106]. Additionally, there is evidence that insulin clearance may be associated with carbohydrate intake [107], body composition [108], liver fat [109], insulin sensitivity [110], acute hyperglycemia [111], and glucose intolerance [112]. Hence, both insulin clearance and secretion underlie the racial and ethnic differences in hyperinsulinemia.
C. Diet, Incretins, and Other Hormones Affect Insulin
Dietary differences may also contribute to hyperinsulinemia in black children. Blacks had a higher ratio of dietary fat intake to carbohydrate intake (determined by 24-hour recall), which was associated with higher FFAs, and reduced insulin sensitivity and insulin clearance, as well as upregulated β-cell function [113]. A high-fat diet was associated with reduced insulin sensitivity and insulin clearance in dogs [114, 115]. The short-term effects of lipid infusions on hyperinsulinemia and insulin clearance have shown mixed results [116, 117], but chronically higher FFAs have been associated with a decline in insulin secretion (adjusted for sensitivity) and reduced glucose effectiveness [118, 119].
During puberty, increases in GH, lipolysis, and insulin resistance contribute to hyperinsulinemia [120]. A longitudinal study showed that black girls had higher fasting insulin and AIR, earlier puberty, higher estradiol levels, higher FSH levels throughout puberty, and more rapid fat deposition after menarche compared with whites [121]. In a prospective cohort study of healthy Australian adolescent girls, insulin was negatively associated with ghrelin in boys and a positively associated with PYY [122]. Incretins may play an important role in the insulin response to glucose, as there were marked differences in glucose and insulin indices derived from OGTT and FSIVGTT in black and Hispanic adolescents with obesity [104]. GLP-1 may have paracrine and neural mechanisms to regulate insulin secretion, and hence its serum levels may provide only limited data on its metabolic effects, which makes it more difficult to study [123].
Fasting insulin was positively correlated with cortisol production rate in a study of 24 healthy men [124]. In adolescent girls with hyperinsulinemia and hyperandrogenism, free testosterone was negatively correlated with insulin resistance [125], although in normogonadal men, free testosterone was not associated with insulin sensitivity or β-cell function independent of its effects on adiposity [126]. A hyperinsulinemic-euglycemic clamp was shown to significantly increase ovarian androgen production in women [127]. Hyperinsulinemia contributes to hyperandrogenism in women with polycystic ovarian syndrome [128]. Hence, insulin secretion and sensitivity are associated with many factors, including HPA axis activation and sex hormones.
D. Reactive Oxygen Species, Redox, and Hyperinsulinemia
In vitro studies have suggested that hyperinsulinemia is associated with increases in reactive oxygen species. Exposing β-cells to excess lipids induces excess insulin secretion by increasing the mitochondrial redox state and production of reactive oxygen species, which in turn modulate the thiol redox state [129]. Supplementation with the antioxidant N-acetylcysteine was associated with an increase in HOMA-IR [130]. In healthy blacks without T2D, serum FFAs are positively associated with protein carbonyls, a marker of oxidative stress that were negatively associated with insulin sensitivity. This association was not seen in healthy whites, suggesting that blacks may be more sensitive to oxidative stress-induced insulin resistance than are whites [131]. Further studies of the redox state in vivo and its effects on insulin secretion and oxidative stress are needed.
4. Metabolic Consequences of Hyperinsulinemia
A. Acute Experimental Hyperinsulinemia
In healthy adults, hyperinsulinemia induced by a hyperinsulinemic-euglycemic clamp for 105 minutes increased inflammatory markers and β-amyloid in the cerebrospinal fluid and peripheral circulation [132]. Clamp studies in healthy subjects also demonstrated that chronic euglycemic hyperinsulinemia for 72 to 96 hours is associated with the development of insulin resistance and impaired nonoxidative glucose disposal [133]. The consequences of exposure to hyperinsulinemia may depend on the duration and magnitude of this exposure, as only 24-hour exposure to hyperglycemia and hyperinsulinemia was associated with increased insulin action and glucose effectiveness in healthy males [134].
B. Chronic Hyperinsulinemia
B-1. Hyperinsulinemia and incident diabetes
In youths with obesity, β-cell first-phase insulin secretion showed a stepwise decline from normal glucose tolerance to IGT to T2D [135]. Fasting insulin was an independent predictor of incident T2D in several cohorts [136, 137]. Hence, both postprandial and fasting hyperinsulinemia are associated with incident T2D. The AIR was not associated with subsequent weight gain in a longitudinal study of normoglycemic subjects during a mean time of 26 years [138]. However, the AIR in FSIVGTT does not reflect the incretin effect, and the insulin response to an oral glucose challenge may be a more physiologically relevant outcome. Hyperinsulinemia during an OGTT was associated with an atherogenic lipid profile in a sample of healthy Israelis [139]. Hyperinsulinemia was the most significant predictor of the progression to T2D in a study of 515 normoglycemic men in Israel during a 24-year follow-up period [140, 141]. In whites without diabetes, having a first-degree relative with T2D was associated with a loss of the normal relationship between BMI and insulin response to an OGTT and hyperinsulinemia even with a normal BMI [142]. Similarly, in the offspring of two parents with T2D, hyperinsulinemia was associated with the risk of developing T2D during an average follow-up time of 13 years independent of glucose removal rate [143].
Hyperinsulinemia may lead to incident T2D by affecting insulin resistance, fat storage, and/or direct effects on β-cells or other tissues. Normoglycemic women with a history of gestational diabetes are at increased risk of developing T2D and had significantly higher fasting insulin and fasting glucose, lower disposition index and insulin sensitivity, and reduced suppression of FFAs compared with women with no history of gestational diabetes [144]. The association between hyperinsulinemia and incident T2D and body composition has been seen in several other racial and ethnic groups, including Pacific Islanders [145] and Mexican Americans [146].
B-2. Hyperinsulinemia and NAFLD
In a prospective cohort study of 4954 Koreans without diabetes, baseline fasting hyperinsulinemia and increases in fasting hyperinsulinemia during a 5-year period were associated with incident NAFLD [147]. Fasting insulin was associated with hepatic steatosis in a sample of healthy Italians with normal transaminases [148]. Consistent with this, fasting insulin and insulin exposure during an IV glucose tolerance test were positively correlated with intrahepatocellular lipids, and subjects with NAFLD had higher intrahepatic insulin exposure than did healthy controls [149]. Compared with subjects without NAFLD, subjects with NAFLD had reduced hepatic insulin clearance and there was a negative correlation between hyperinsulinemia and both hepatic and whole-body insulin clearance [150].
Black women with obesity have a lower rate of TG turnover in adipose tissue and lower rates of adipose de novo lipogenesis (DNL) compared with white women with obesity [151]. DNL was originally thought to make only minor contributions to hepatic and adipose tissue lipid contents based on small studies in lean subjects [152]. However, technological improvement and studies in other populations have revealed that DNL is increased 2.4-fold from baseline fasting levels by an oral fructose challenge, and this increase in DNL was positively correlated with fasting insulin levels (r = 0.75) [153]. Blacks also tend to have lower intrahepatic TGs than do age- and BMI-matched whites, yet once NAFLD has developed the prevalence of nonalcoholic steatohepatitis may be similar [154].
B-3. Hyperinsulinemia, hypertension, and endothelial cell function
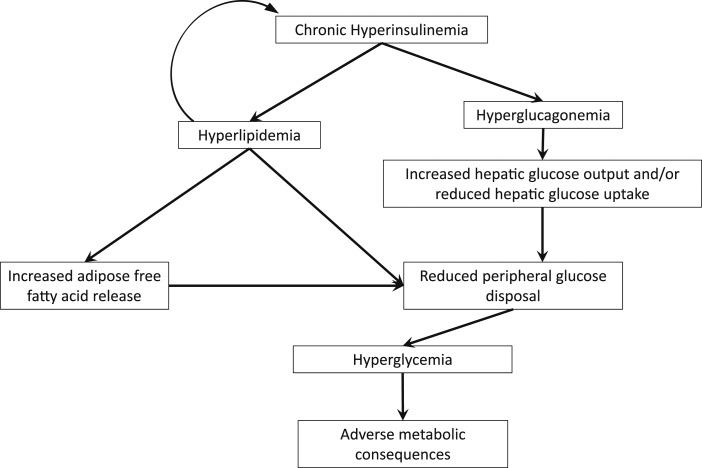
Insulin sensitivity and systolic blood pressure are the dominant determinants of endothelial function in blacks and whites [155]. Subjects with hypertension had higher meal-stimulated c-peptide secretion and lower insulin sensitivity compared with BMI-matched subjects with obesity without hypertension [156]. Similar findings were obtained in a study of Israelis that found that subjects with obesity and hypertension had a higher rise in serum insulin levels during an oral glucose test than did subjects with obesity and without hypertension [157]. Subjects with obesity had diminished endothelium-dependent vasodilation compared with lean controls during a hyperinsulinemic-euglycemic clamp [158]. Hence, hyperinsulinemia is associated with the vascular and lipid abnormalities associated with metabolic syndrome and may underlie its pathogenesis [159]. The exact mechanisms whereby loss of normal insulin pulsatility and hyperinsulinemia can lead to metabolic complications remain under investigation and are summarized in Fig. 2, and the molecular mechanisms for these pathways have been recently reviewed [160].
Figure 2.
Diagram of potential mechanisms for hyperinsulinemia with altered insulin pulsatility to induce metabolic disease. Chronic hyperinsulinemia of any potential etiology is associated with chronic hyperglucagonemia, which may lead to increased hepatic glucose output. Nutrient excess and hyperlipidemia contribute to adipose tissue expansion and dysfunction with eventual ectopic lipid deposition, which is associated with reduced muscle glucose disposal.
5. Therapeutic Implications
A. Differences in Insulin Metabolism May Underlie the Variability in the Response to Dietary Interventions
Racial differences in the response to dietary interventions may be mediated by differences in lipoprotein metabolism [161], which may explain the paradox that blacks have lower insulin sensitivity but often have lower TGs than do whites [162, 163].
Hyperinsulinemia may also modulate the effects of different dietary interventions. Compared with an isoenergetic high-fat low-carbohydrate diet, high simple carbohydrate diets are associated with higher rates of DNL and increases in TGs in lean subjects without hyperinsulinemia [164]. Subjects with hyperinsulinemia and obesity had significantly higher rates of DNL on a high-fat diet than did both subjects with obesity but without hyperinsulinemia and lean subjects without hyperinsulinemia [164]. Subjects with high insulin secretion may lose more weight on a low–glycemic index diet compared with a high–glycemic index diet [165]. Consumption of fructose has been associated with increases in serum insulin and reductions in insulin sensitivity in persons with overweight and obesity [166]. Isocaloric restriction of fructose for 10 days was associated with a significant decrease in liver fat, VAT, DNL, fasting insulin, fasting glucose, insulin secretion, and increased insulin clearance in blacks and Hispanic children with obesity [167]. The role of dietary fat and carbohydrates and insulin in the development of hyperinsulinemia and obesity is an active area of research and debate [168–170].
B. Bariatric Surgery Is Associated With Improvement in Hyperinsulinemia
Because obesity and hyperinsulinemia are often refractory to dietary and lifestyle changes, bariatric surgery is recommended for patients with severe obesity and comorbid conditions. Hyperinsulinemia may underlie the racial differences in bariatric surgical outcomes, such as blacks losing less weight than whites despite adjustment for clinical and behavioral factors [171] and blacks regaining more weight than whites in the years following surgery [172]. Bariatric surgery is associated with a rapid correction of hyperinsulinemia within 1 week of surgery, which may underpin its metabolic and clinical benefits. Unlike the rapid improvement in hyperinsulinemia after bariatric surgery, insulin sensitivity continues to improve between 6 and 24 months postoperatively whereas glucose effectiveness remained constant [173].
C. Exercise Training Is Associated With Improvement in Hyperinsulinemia
Male athletes have lower fasting glucose, lower insulin secretion, increased insulin sensitivity, and increased insulin clearance determined by the insulin/c-peptide ratio following a hyperinsulinemic-euglycemic clamp and arginine stimulation test compared with age- and BMI-matched sedentary males [110]. Consistent with this, exercise training has been shown to acutely lower insulin and gradually increase insulin sensitivity and glucose effectiveness [174, 175]. Compared with untrained subjects, endurance trained subjects had similar nonpulsatile basal insulin secretion, but significantly reduced insulin secreted per secretory burst [176].
D. Pharmacotherapies for Hyperinsulinemia
Hyperinsulinemia is not generally recognized as a primary therapeutic target although this has been debated [27]. Weight loss is associated with improvement in hyperinsulinemia with no change in glucose effectiveness, whereas weight gain is associated with worsening of hyperinsulinemia and reduced glucose effectiveness [177, 178]. Treating obesity with lifestyle modifications, dietary changes, pharmacotherapy, or metabolic surgery improves hyperinsulinemia acutely [179]. Liraglutide at 3.0 mg leads to greater weight loss and decreases in fasting insulin along with a reduction in incident diabetes in subjects with obesity but without diabetes [180].
Several other classes of medications can also affect insulin sensitivity and β-cell function. Fenofibrate, a PPARα agonist, increases fat oxidation and decreases insulin clearance and secretion in mice on a high-fat diet and warrants further trials in humans [181]. Bezafibrate, a pan-PPAR agonist, lowers both lipids and insulin [182]. However, the effectiveness of mediations directly targeting hyperinsulinemia has been mixed [183–186]. Further trials of new classes of medications that can attenuate hyperinsulinemia are warranted [187].
6. Conclusion
Strong evidence implicates hyperinsulinemia as an important precursor to the metabolic diseases associated with obesity. Environmental, genetic, and socioeconomic factors all contribute to the development and progression of hyperinsulinemia. Ethnic and racial differences in hyperinsulinemia are associated with differences in β-cell function and fat distribution. Dietary interventions have differing effects depending on underlying metabolic dysfunction. More research is needed to understand the effects of various genetic and environmental factors associated with hyperinsulinemia to determine which plays a causal role in metabolic disease. Such research in diverse populations will have implications for precision medicine.
Acknowledgments
The authors thank Ashley McCarthy for helpful feedback.
Financial Support: This work was supported in part by the National Institutes of Health Grants P30DK046200, UL1TR001430, and T32DK007201.
Glossary
Abbreviations:
- AIR
acute insulin response
- AUC
area under the curve
- BMI
body mass index
- DNL
de novo lipogenesis
- FFA
free fatty acid
- FSIVGTT
frequently sampled IV glucose test
- HOMA
homeostatic model assessment
- HOMA-IR
HOMA of insulin resistance
- HPA
hypothalamic–pituitary–adrenal
- IGT
impaired glucose tolerance
- IRAS
Insulin Resistance and Atherosclerosis Study
- IRAS-FS
IRAS family study
- OGTT
oral glucose tolerance test
- RISC
Relationship between Insulin Sensitivity and Cardiovasular Disease
- SAT
subcutaneous adipose tissue
- T2D
type 2 diabetes
- TG
triglyceride
- VAT
visceral adipose tissue
Additional Information
Disclosure Summary: C.M.A. reports grants from Aspire Bariatrics, the Vela Foundation, Coherence Laboratory, Energesis, NIH, and PCORI; grants and personal fees from GI Dynamics, Takeda, Novo Nordisk, and Gelesis; and personal fees from Orexigen, EnteroMedics, Nutrisystem, Zafgen, Sanofi-Aventis, Scientific Intake, Xeno Biosciences, Rhythm Pharmaceuticals, Eisai, and Bariatrix Nutrition and other fees from Science-Smart LLC, outside the submitted work. The remaining authors have nothing to disclose.
Data Availability:
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References and Notes
- 1. Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW. Association between insulin resistance and bone structure in nondiabetic postmenopausal women. J Clin Endocrinol Metab. 2016;101(8):3114–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brands MW, Manhiani MM. Sodium-retaining effect of insulin in diabetes. Am J Physiol Regul Integr Comp Physiol. 2012;303(11):R1101–R1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo J, Breen DM, Pereira TJ, Dalvi PS, Zhang H, Mori Y, Ghanim H, Tumiati L, Fantus IG, Bendeck MP, Dandona P, Rao V, Dolinsky VW, Heximer SP, Giacca A. The effect of insulin to decrease neointimal growth after arterial injury is endothelial nitric oxide synthase-dependent. Atherosclerosis. 2015;241(1):111–120. [DOI] [PubMed] [Google Scholar]
- 4. Fornes R, Ormazabal P, Rosas C, Gabler F, Vantman D, Romero C, Vega M. Changes in the expression of insulin signaling pathway molecules in endometria from polycystic ovary syndrome women with or without hyperinsulinemia. Mol Med. 2010;16(3-4):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansen BC, Jen KC, Belbez Pek S, Wolfe RA. Rapid oscillations in plasma insulin, glucagon, and glucose in obese and normal weight humans. J Clin Endocrinol Metab. 1982;54(4):785–792. [DOI] [PubMed] [Google Scholar]
- 6. Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med. 1979;301(19):1023–1027. [DOI] [PubMed] [Google Scholar]
- 7. Pørksen N, Nyholm B, Veldhuis JD, Butler PC, Schmitz O. In humans at least 75% of insulin secretion arises from punctuated insulin secretory bursts. Am J Physiol. 1997;273(5):E908–E914. [DOI] [PubMed] [Google Scholar]
- 8. Ward GM, Walters JM, Aitken PM, Best JD, Alford FP. Effects of prolonged pulsatile hyperinsulinemia in humans. Enhancement of insulin sensitivity. Diabetes. 1990;39(4):501–507. [DOI] [PubMed] [Google Scholar]
- 9. Marangou AG, Weber KM, Boston RC, Aitken PM, Heggie JC, Kirsner RL, Best JD, Alford FP. Metabolic consequences of prolonged hyperinsulinemia in humans. Evidence for induction of insulin insensitivity. Diabetes. 1986;35(12):1383–1389. [DOI] [PubMed] [Google Scholar]
- 10. Shanik MH, Xu Y, Škrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–S268. [DOI] [PubMed] [Google Scholar]
- 11. O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318(19):1225–1230. [DOI] [PubMed] [Google Scholar]
- 12. Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. [DOI] [PubMed] [Google Scholar]
- 14. Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G; European Group for the Study of Insulin Resistance (EGIR). Insulin resistance and hypersecretion in obesity. J Clin Invest. 1997;100(5):1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tricò D, Natali A, Arslanian S, Mari A, Ferrannini E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight. 2018;3(24):124912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mari A, Tura A, Natali A, Anderwald C, Balkau B, Lalic N, Walker M, Ferrannini E; RISC Investigators. Influence of hyperinsulinemia and insulin resistance on in vivo β-cell function: their role in human β-cell dysfunction. Diabetes. 2011;60(12):3141–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasson BR, Apovian C, Istfan N. Racial/ethnic differences in insulin resistance and beta cell function: relationship to racial disparities in type 2 diabetes among African Americans versus Caucasians. Curr Obes Rep. 2015;4(2):241–249. [DOI] [PubMed] [Google Scholar]
- 18. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81(2):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galgani JE, de Jonge L, Rood JC, Smith SR, Young AA, Ravussin E. Urinary C-peptide excretion: a novel alternate measure of insulin sensitivity in physiological conditions. Obesity (Silver Spring). 2010;18(9):1852–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poon AK, Meyer ML, Reaven G, Knowles JW, Selvin E, Pankow JS, Couper D, Loehr L, Heiss G. Short-term repeatability of insulin resistance indexes in older adults: the Atherosclerosis Risk in Communities Study. J Clin Endocrinol Metab. 2018;103(6):2175–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uwaifo GI, Parikh SJ, Keil M, Elberg J, Chin J, Yanovski JA. Comparison of insulin sensitivity, clearance, and secretion estimates using euglycemic and hyperglycemic clamps in children. J Clin Endocrinol Metab. 2002;87(6):2899–2905. [DOI] [PubMed] [Google Scholar]
- 22. Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care. 1996;19(9):1018–1030. [DOI] [PubMed] [Google Scholar]
- 23. Alford FP, Henriksen JE, Rantzau C, Beck-Nielsen H. Glucose effectiveness is a critical pathogenic factor leading to glucose intolerance and type 2 diabetes: an ignored hypothesis. Diabetes Metab Res Rev. 2018;34(4):e2989. [DOI] [PubMed] [Google Scholar]
- 24. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe RM, Volund A, Roy S, Bergman RN. Prehepatic β-cell secretion during the intravenous glucose tolerance test in humans: application of a combined model of insulin and C-peptide kinetics. J Clin Endocrinol Metab. 1989;69(4):790–797. [DOI] [PubMed] [Google Scholar]
- 26. Cobelli C, Pacini G. Insulin secretion and hepatic extraction in humans by minimal modeling of C-peptide and insulin kinetics. Diabetes. 1988;37(2):223–231. [DOI] [PubMed] [Google Scholar]
- 27. Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care. 2012;35(12):2438–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corkey BE. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care. 2012;35(12):2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corkey BE. Banting Lecture 2011: hyperinsulinemia: cause or consequence? Diabetes. 2012;61(1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP, Trigo E, Gilliland FD. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care. 2016;39(4):547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim JS, Alderete TL, Chen Z, Lurmann F, Rappaport E, Habre R, Berhane K, Gilliland FD. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environ Health. 2018;17(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stahlhut RW, Myers JP, Taylor JA, Nadal A, Dyer JA, Vom Saal FS. Experimental BPA exposure and glucose-stimulated insulin response in adult men and women. J Endocr Soc. 2018;2(10):1173–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meeks KA, Stronks K, Adeyemo A, Addo J, Bahendeka S, Beune E, Owusu-Dabo E, Danquah I, Galbete C, Henneman P, Klipstein-Grobusch K, Mockenhaupt FP, Osei K, Schulze MB, Spranger J, Smeeth L, Agyemang C. Peripheral insulin resistance rather than beta cell dysfunction accounts for geographical differences in impaired fasting blood glucose among sub-Saharan African individuals: findings from the RODAM study. Diabetologia. 2017;60(5):854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kodavanti UP. Air pollution and insulin resistance: do all roads lead to Rome? Diabetes. 2015;64(3):712–714. [DOI] [PubMed] [Google Scholar]
- 35. Thompson DS, Boyne MS, Osmond C, Ferguson TS, Tulloch-Reid MK, Wilks RJ, Barnett AT, Forrester TE. Limitations of fasting indices in the measurement of insulin sensitivity in Afro-Caribbean adults. BMC Res Notes. 2014;7(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chiu KC, Chuang LM, Yoon C. Comparison of measured and estimated indices of insulin sensitivity and β cell function: impact of ethnicity on insulin sensitivity and β cell function in glucose-tolerant and normotensive subjects. J Clin Endocrinol Metab. 2001;86(4):1620–1625. [DOI] [PubMed] [Google Scholar]
- 37. Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad MF, Bergman RN, Hamman R. The Insulin Resistance Atherosclerosis Study (IRAS): objectives, design, and recruitment results. Ann Epidemiol. 1995;5(6):464–472. [DOI] [PubMed] [Google Scholar]
- 38. Festa A, Williams K, Hanley AJ, Haffner SM. β-Cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes: comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes. 2008;57(6):1638–1644. [DOI] [PubMed] [Google Scholar]
- 39. Karter AJ, Mayer-Davis EJ, Selby JV, D’Agostino RB Jr, Haffner SM, Sholinsky P, Bergman R, Saad MF, Hamman RF. Insulin sensitivity and abdominal obesity in African-American, Hispanic, and non-Hispanic white men and women. The Insulin Resistance and Atherosclerosis Study. Diabetes. 1996;45(11):1547–1555. [DOI] [PubMed] [Google Scholar]
- 40. Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, Haffner SM. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010;33(9):2098–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li C, Ford ES, McGuire LC, Mokdad AH, Little RR, Reaven GM. Trends in hyperinsulinemia among nondiabetic adults in the U.S. Diabetes Care. 2006;29(11):2396–2402. [DOI] [PubMed] [Google Scholar]
- 42. Beydoun MA, Wang Y. Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity (Silver Spring). 2009;17(1):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Svec F, Nastasi K, Hilton C, Bao W, Srinivasan SR, Berenson GS. Black-white contrasts in insulin levels during pubertal development. The Bogalusa Heart Study. Diabetes. 1992;41(3):313–317. [DOI] [PubMed] [Google Scholar]
- 44. Arslanian S, Suprasongsin C. Differences in the in vivo insulin secretion and sensitivity of healthy black versus white adolescents. J Pediatr. 1996;129(3):440–443. [DOI] [PubMed] [Google Scholar]
- 45. Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45(9):1119–1124. [DOI] [PubMed] [Google Scholar]
- 46. Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49(3):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liska D, Dufour S, Zern TL, Taksali S, Calí AM, Dziura J, Shulman GI, Pierpont BM, Caprio S. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2(6):e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chandler-Laney PC, Phadke RP, Granger WM, Fernández JR, Muñoz JA, Man CD, Cobelli C, Ovalle F, Gower BA. Age-related changes in insulin sensitivity and β-cell function among European-American and African-American women. Obesity (Silver Spring). 2011;19(3):528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marcus MA, Murphy L, Pi-Sunyer FX, Albu JB. Insulin sensitivity and serum triglyceride level in obese white and black women: relationship to visceral and truncal subcutaneous fat. Metabolism. 1999;48(2):194–199. [DOI] [PubMed] [Google Scholar]
- 50. Ross R, Fortier L, Hudson R. Separate associations between visceral and subcutaneous adipose tissue distribution, insulin and glucose levels in obese women. Diabetes Care. 1996;19(12):1404–1411. [DOI] [PubMed] [Google Scholar]
- 51. Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, Kuller LH, Pahor M, Schaap LA, Visser M, Rubin SM, Goodpaster BH, Harris TB; Health ABC study. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring). 2009;17(5):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hyatt TC, Phadke RP, Hunter GR, Bush NC, Muñoz AJ, Gower BA. Insulin sensitivity in African-American and white women: association with inflammation. Obesity (Silver Spring). 2009;17(2):276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740–E747. [DOI] [PubMed] [Google Scholar]
- 54. Sun Q, Li J, Gao F. New insights into insulin: the anti-inflammatory effect and its clinical relevance. World J Diabetes. 2014;5(2):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miyazaki Y, DeFronzo RA. Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc Diabetol. 2009;8(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52(10):2490–2496. [DOI] [PubMed] [Google Scholar]
- 57. Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005;288(2):E454–E461. [DOI] [PubMed] [Google Scholar]
- 58. Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 1997;46(11):1768–1774. [DOI] [PubMed] [Google Scholar]
- 59. Bergman RN, Iyer MS. Indirect regulation of endogenous glucose production by insulin: the single gateway hypothesis revisited. Diabetes. 2017;66(7):1742–1747. [DOI] [PubMed] [Google Scholar]
- 60. Edgerton DS, Lautz M, Scott M, Everett CA, Stettler KM, Neal DW, Chu CA, Cherrington AD. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest. 2006;116(2):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. [DOI] [PubMed] [Google Scholar]
- 62. Jain SH, Massaro JM, Hoffmann U, Rosito GA, Vasan RS, Raji A, O’Donnell CJ, Meigs JB, Fox CS. Cross-sectional associations between abdominal and thoracic adipose tissue compartments and adiponectin and resistin in the Framingham Heart Study. Diabetes Care. 2009;32(5):903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930–1935. [DOI] [PubMed] [Google Scholar]
- 64. Schulz LO, Bennett PH, Ravussin E, Kidd JR, Kidd KK, Esparza J, Valencia ME. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care. 2006;29(8):1866–1871. [DOI] [PubMed] [Google Scholar]
- 65. Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329(27):1988–1992. [DOI] [PubMed] [Google Scholar]
- 66. Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49(12):2094–2101. [DOI] [PubMed] [Google Scholar]
- 67. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104(6):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gautier JF, Milner MR, Elam E, Chen K, Ravussin E, Pratley RE. Visceral adipose tissue is not increased in Pima Indians compared with equally obese Caucasians and is not related to insulin action or secretion. Diabetologia. 1999;42(1):28–34. [DOI] [PubMed] [Google Scholar]
- 69. Odeleye OE, de Courten M, Pettitt DJ, Ravussin E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes. 1997;46(8):1341–1345. [DOI] [PubMed] [Google Scholar]
- 70. Szczepaniak LS, Victor RG, Mathur R, Nelson MD, Szczepaniak EW, Tyer N, Chen I, Unger RH, Bergman RN, Lingvay I. Pancreatic steatosis and its relationship to β-cell dysfunction in humans: racial and ethnic variations. Diabetes Care. 2012;35(11):2377–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Popp D, Aertsen S, Luetke-Daldrup C, Coppenrath E, Hetterich H, Saam T, Rottenkolber M, Seissler J, Lechner A, Sommer NN. No correlation of pancreatic fat and β-cell function in young women with and without a history of gestational diabetes. J Clin Endocrinol Metab. 2018;103(9):3260–3266. [DOI] [PubMed] [Google Scholar]
- 72. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, Sosa JA, Sumner AE, Anton B. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579–E1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pitchika A, Vehik K, Hummel S, Norris JM, Uusitalo UM, Yang J, Virtanen SM, Koletzko S, Andrén Aronsson C, Ziegler AG, Beyerlein A; TEDDY study group. Associations of maternal diabetes during pregnancy with overweight in offspring: results from the prospective TEDDY study. Obesity (Silver Spring). 2018;26(9):1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grüters-Kieslich A, Reyes M, Sharma A, Demirci C, DeClue TJ, Lankes E, Tiosano D, Schnabel D, Jüppner H. Early-onset obesity: unrecognized first evidence for GNAS mutations and methylation changes. J Clin Endocrinol Metab. 2017;102(8):2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Natali A, Muscelli E, Mari A, Balkau B, Walker M, Tura A, Anderwald C, Golay A, Ferrannini E; Relationship Between Insulin Sensitivity and Cardiovascular Disease Investigators. Insulin sensitivity and β-cell function in the offspring of type 2 diabetic patients: impact of line of inheritance. J Clin Endocrinol Metab. 2010;95(10):4703–4711. [DOI] [PubMed] [Google Scholar]
- 77. Goodarzi MO, Langefeld CD, Xiang AH, Chen YDI, Guo X, Hanley AJ, Raffel LJ, Kandeel F, Nadler JL, Buchanan TA, Norris JM, Fingerlin TE, Lorenzo C, Rewers MJ, Haffner SM, Bowden DW, Rich SS, Bergman RN, Rotter JI, Watanabe RM, Wagenknecht LE. Insulin sensitivity and insulin clearance are heritable and have strong genetic correlation in Mexican Americans. Obesity (Silver Spring). 2014;22(4):1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Udler MS, Kim J, von Grotthuss M, Bonàs-Guarch S, Cole JB, Chiou J, Boehnke M, Laakso M, Atzmon G, Glaser B, Mercader JM, Gaulton K, Flannick J, Getz G, Florez JC; Christopher D. Anderson on behalf of METASTROKE and the ISGC. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 2018;15(9):e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, Vikman P, Prasad RB, Aly DM, Almgren P, Wessman Y, Shaat N, Spégel P, Mulder H, Lindholm E, Melander O, Hansson O, Malmqvist U, Lernmark Å, Lahti K, Forsén T, Tuomi T, Rosengren AH, Groop L. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. [DOI] [PubMed] [Google Scholar]
- 80. Rich SS, Bowden DW, Haffner SM, Norris JM, Saad MF, Mitchell BD, Rotter JI, Langefeld CD, Wagenknecht LE, Bergman RN; Insulin Resistance Atherosclerosis Study Family Study. Identification of quantitative trait loci for glucose homeostasis: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2004;53(7):1866–1875. [DOI] [PubMed] [Google Scholar]
- 81. Palmer ND, Langefeld CD, Campbell JK, Williams AH, Saad M, Norris JM, Haffner SM, Rotter JI, Wagenknecht LE, Bergman RN, Rich SS, Bowden DW. Genetic mapping of disposition index and acute insulin response loci on chromosome 11q. The Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2006;55(4):911–918. [DOI] [PubMed] [Google Scholar]
- 82. Bergman RN, Zaccaro DJ, Watanabe RM, Haffner SM, Saad MF, Norris JM, Wagenknecht LE, Hokanson JE, Rotter JI, Rich SS. Minimal model-based insulin sensitivity has greater heritability and a different genetic basis than homeostasis model assessment or fasting insulin. Diabetes. 2003;52(8):2168–2174. [DOI] [PubMed] [Google Scholar]
- 83. Do R, Bailey SD, Desbiens K, Belisle A, Montpetit A, Bouchard C, Pérusse L, Vohl MC, Engert JC. Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes. 2008;57(4):1147–1150. [DOI] [PubMed] [Google Scholar]
- 84. Le Stunff C, Fallin D, Bougnères P. Paternal transmission of the very common class I INS VNTR alleles predisposes to childhood obesity. Nat Genet. 2001;29(1):96–99. [DOI] [PubMed] [Google Scholar]
- 85. Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, Balding D, Scott J, Kooner JS. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40(6):716–718. [DOI] [PubMed] [Google Scholar]
- 86. Guo X, Cui J, Jones MR, Haritunians T, Xiang AH, Chen YD, Taylor KD, Buchanan TA, Davis RC, Hsueh WA, Raffel LJ, Rotter JI, Goodarzi MO. Insulin clearance: confirmation as a highly heritable trait, and genome-wide linkage analysis. Diabetologia. 2012;55(8):2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yaghootkar H, Scott RA, White CC, Zhang W, Speliotes E, Munroe PB, Ehret GB, Bis JC, Fox CS, Walker M. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease and type 2 diabetes. Diabetes. 2014;63(12): 4369–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Astley CM, Todd JN, Salem RM, Vedantam S, Ebbeling CB, Huang PL, Ludwig DS, Hirschhorn JN, Florez JC. Genetic evidence that carbohydrate-stimulated insulin secretion leads to obesity. Clin Chem. 2018;64(1):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab. 2011;301(2):E402–E408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meistas MT, Margolis S, Kowarski AA. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am J Physiol. 1983;245(2):E155–E159. [DOI] [PubMed] [Google Scholar]
- 91. Polonsky KS, Given BD, Hirsch L, Shapiro ET, Tillil H, Beebe C, Galloway JA, Frank BH, Karrison T, Van Cauter E. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988;81(2):435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fink RI, Revers RR, Kolterman OG, Olefsky JM. The metabolic clearance of insulin and the feedback inhibition of insulin secretion are altered with aging. Diabetes. 1985;34(3):275–280. [DOI] [PubMed] [Google Scholar]
- 93. Marini MA, Frontoni S, Succurro E, Arturi F, Fiorentino TV, Sciacqua A, Perticone F, Sesti G. Differences in insulin clearance between metabolically healthy and unhealthy obese subjects. Acta Diabetol. 2014;51(2):257–261. [DOI] [PubMed] [Google Scholar]
- 94. Kaga H, Tamura Y, Takeno K, Kakehi S, Funayama T, Furukawa Y, Nishitani-Yokoyama M, Shimada K, Daida H, Aoki S, Giacca A, Kanazawa A, Kawamori R, Watada H. Correlates of insulin clearance in apparently healthy non-obese Japanese men. Sci Rep. 2017;7(1):1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Harris MI, Cowie CC, Gu K, Francis ME, Flegal K, Eberhardt MS. Higher fasting insulin but lower fasting C-peptide levels in African Americans in the US population. Diabetes Metab Res Rev. 2002;18(2):149–155. [DOI] [PubMed] [Google Scholar]
- 96. Piccinini F, Polidori DC, Gower BA, Bergman RN. Hepatic but not extra-hepatic insulin clearance is lower in African American than in European American women. Diabetes. 2017;66(10):2564–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes. 2016;65(6):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chung ST, Aldana PC, Mabundo LS, DuBose CW, Onuzuruike AU, Walter M, Gharib AM, Courville AB, Sherman AS, Sumner AE. Postprandial insulin response and clearance among black and white women: the Federal Women’s Study. J Clin Endocrinol Metab. J Clin Endocrinol Metab. 2019;104(1):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee CC, Haffner SM, Wagenknecht LE, Lorenzo C, Norris JM, Bergman RN, Stefanovski D, Anderson AM, Rotter JI, Goodarzi MO, Hanley AJ. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care. 2013;36(4):901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lorenzo C, Hanley AJ, Wagenknecht LE, Rewers MJ, Stefanovski D, Goodarzi MO, Haffner SM. Relationship of insulin sensitivity, insulin secretion, and adiposity with insulin clearance in a multiethnic population: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2013;36(1):101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated β-cell function? Diabetes Care. 2008;31(7):1445–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab. 2002;87(5):2218–2224. [DOI] [PubMed] [Google Scholar]
- 103. Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25(12):2184–2190. [DOI] [PubMed] [Google Scholar]
- 104. Hasson RE, Adam TC, Davis JN, Weigensberg MJ, Ventura EE, Lane CJ, Roberts CK, Goran MI. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab. 2010;95(8):4048–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim MK, Reaven GM, Chen YD, Kim E, Kim SH. Hyperinsulinemia in individuals with obesity: role of insulin clearance. Obesity (Silver Spring). 2015;23(12):2430–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim MK, Reaven GM, Kim SH. Dissecting the relationship between obesity and hyperinsulinemia: role of insulin secretion and insulin clearance. Obesity (Silver Spring). 2017;25(2):378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bojsen-Møller KN, Lundsgaard AM, Madsbad S, Kiens B, Holst JJ. Hepatic insulin clearance in regulation of systemic insulin concentrations—role of carbohydrate and energy availability. Diabetes. 2018;67(11):2129–2136. [DOI] [PubMed] [Google Scholar]
- 108. Yki-Järvinen H, Koivisto VA, Karonen SL. Influence of body composition on insulin clearance. Clin Physiol. 1985;5(1):45–52. [DOI] [PubMed] [Google Scholar]
- 109. Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135(1):122–130. [DOI] [PubMed] [Google Scholar]
- 110. Ahrén B, Thorsson O. Increased insulin sensitivity is associated with reduced insulin and glucagon secretion and increased insulin clearance in man. J Clin Endocrinol Metab. 2003;88(3):1264–1270. [DOI] [PubMed] [Google Scholar]
- 111. Tillil H, Shapiro ET, Rubenstein AH, Galloway JA, Polonsky KS. Reduction of insulin clearance during hyperglycemic clamp. Dose-response study in normal humans. Diabetes. 1988;37(10):1351–1357. [DOI] [PubMed] [Google Scholar]
- 112. Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism. 1983;32(5):438–446. [DOI] [PubMed] [Google Scholar]
- 113. Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51(10):3014–3019. [DOI] [PubMed] [Google Scholar]
- 114. Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of β-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–S220. [DOI] [PubMed] [Google Scholar]
- 115. Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292(6):E1590–E1598. [DOI] [PubMed] [Google Scholar]
- 116. Shah P, Vella A, Basu A, Basu R, Adkins A, Schwenk WF, Johnson CM, Nair KS, Jensen MD, Rizza RA. Effects of free fatty acids and glycerol on splanchnic glucose metabolism and insulin extraction in nondiabetic humans. Diabetes. 2002;51(2):301–310. [DOI] [PubMed] [Google Scholar]
- 117. Balent B, Goswami G, Goodloe G, Rogatsky E, Rauta O, Nezami R, Mints L, Angeletti RH, Stein DT. Acute elevation of NEFA causes hyperinsulinemia without effect on insulin secretion rate in healthy human subjects. Ann N Y Acad Sci. 2002;967(1):535–543. [DOI] [PubMed] [Google Scholar]
- 118. Johnston LW, Harris SB, Retnakaran R, Giacca A, Liu Z, Bazinet RP, Hanley AJ. Association of NEFA composition with insulin sensitivity and beta cell function in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort. Diabetologia. 2018;61(4):821–830. [DOI] [PubMed] [Google Scholar]
- 119. Kishore P, Tonelli J, Koppaka S, Fratila C, Bose A, Lee DE, Reddy K, Hawkins M. Time-dependent effects of free fatty acids on glucose effectiveness in type 2 diabetes. Diabetes. 2006;55(6):1761–1768. [DOI] [PubMed] [Google Scholar]
- 120. Arslanian SA, Kalhan SC. Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes. 1994;43(7):908–914. [DOI] [PubMed] [Google Scholar]
- 121. Casazza K, Goran MI, Gower BA. Associations among insulin, estrogen, and fat mass gain over the pubertal transition in African-American and European-American girls. J Clin Endocrinol Metab. 2008;93(7):2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cheng HL, Sainsbury A, Garden F, Sritharan M, Paxton K, Luscombe G, Hawke C, Steinbeck K. Ghrelin and peptide YY change during puberty: relationships with adolescent growth, development, and obesity. J Clin Endocrinol Metab. 2018;103(8):2851–2860. [DOI] [PubMed] [Google Scholar]
- 123. D’Alessio D. Is GLP-1 a hormone: whether and when? J Diabetes Investig. 2016;7(Suppl 1):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11β-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab. 2009;296(2):E351–E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Burt Solorzano CM, Knudsen KL, Anderson AD, Hutchens EG, Collins JS, Patrie JT, Marshall JC, McCartney CR. Insulin resistance, hyperinsulinemia, and LH: relative roles in peripubertal obesity-associated hyperandrogenemia. J Clin Endocrinol Metab. 2018;103(7):2571–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Abate N, Haffner SM, Garg A, Peshock RM, Grundy SM. Sex steroid hormones, upper body obesity, and insulin resistance. J Clin Endocrinol Metab. 2002;87(10):4522–4527. [DOI] [PubMed] [Google Scholar]
- 127. Stuart CA, Nagamani M. Insulin infusion acutely augments ovarian androgen production in normal women. Fertil Steril. 1990;54(5):788–792. [PubMed] [Google Scholar]
- 128. Nestler JE. Role of hyperinsulinemia in the pathogenesis of the polycystic ovary syndrome, and its clinical implications. Semin Reprod Endocrinol. 1997;15(2):111–122. [DOI] [PubMed] [Google Scholar]
- 129. Saadeh M, Ferrante TC, Kane A, Shirihai O, Corkey BE, Deeney JT. Reactive oxygen species stimulate insulin secretion in rat pancreatic islets: studies using mono-oleoyl-glycerol. PLoS One. 2012;7(1):e30200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hildebrandt W, Hamann A, Krakowski-Roosen H, Kinscherf R, Dugi K, Sauer R, Lacher S, Nöbel N, Bodens A, Bellou V, Edler L, Nawroth P, Dröge W. Effect of thiol antioxidant on body fat and insulin reactivity. J Mol Med (Berl). 2004;82(5):336–344. [DOI] [PubMed] [Google Scholar]
- 131. Fisher G, Alvarez JA, Ellis AC, Granger WM, Ovalle F, Man CD, Cobelli C, Gower BA. Race differences in the association of oxidative stress with insulin sensitivity in African- and European-American women. Obesity (Silver Spring). 2012;20(5):972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S. Hyperinsulinemia provokes synchronous increases in central inflammation and β-amyloid in normal adults. Arch Neurol. 2005;62(10):1539–1544. [DOI] [PubMed] [Google Scholar]
- 133. Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo RA. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia. 1994;37(10):1025–1035. [DOI] [PubMed] [Google Scholar]
- 134. Kahn SE, Bergman RN, Schwartz MW, Taborsky GJ Jr, Porte D Jr. Short-term hyperglycemia and hyperinsulinemia improve insulin action but do not alter glucose action in normal humans. Am J Physiol. 1992;262(4 Pt 1):E518–E523. [DOI] [PubMed] [Google Scholar]
- 135. Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. β-Cell function across the spectrum of glucose tolerance in obese youth. Diabetes. 2005;54(6):1735–1743. [DOI] [PubMed] [Google Scholar]
- 136. Ghasemi A, Tohidi M, Derakhshan A, Hasheminia M, Azizi F, Hadaegh F. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol. 2015;52(5):905–915. [DOI] [PubMed] [Google Scholar]
- 137. Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults: the atherosclerosis risk in communities study: 1987–1998. Diabetes Care. 2002;25(8):1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Silver RJ, Mehta S, Soeldner JS, Martin BC, Warram JH, Goldfine AB. Acute insulin secretion as a predictor of weight gain in healthy humans. Obesity (Silver Spring). 2006;14(1):67–72. [DOI] [PubMed] [Google Scholar]
- 139. Modan M, Halkin H, Lusky A, Segal P, Fuchs Z, Chetrit A. Hyperinsulinemia is characterized by jointly disturbed plasma VLDL, LDL, and HDL levels. A population-based study. Arteriosclerosis. 1988;8(3):227–236. [DOI] [PubMed] [Google Scholar]
- 140. Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: a preliminary report. Diabetes Care. 2009;32(8):1464–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal state hyperinsulinemia in healthy normoglycemic adults heralds dysglycemia after more than two decades of follow up. Diabetes Metab Res Rev. 2012;28(7):618–624. [DOI] [PubMed] [Google Scholar]
- 142. Ishikawa M, Pruneda ML, Adams-Huet B, Raskin P. Obesity-independent hyperinsulinemia in nondiabetic first-degree relatives of individuals with type 2 diabetes. Diabetes. 1998;47(5):788–792. [DOI] [PubMed] [Google Scholar]
- 143. Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113(12):909–915. [DOI] [PubMed] [Google Scholar]
- 144. Kousta E, Lawrence NJ, Godsland IF, Penny A, Anyaoku V, Millauer BA, Cela E, Johnston DG, Robinson S, McCarthy MI. Insulin resistance and β-cell dysfunction in normoglycaemic European women with a history of gestational diabetes. Clin Endocrinol (Oxf). 2003;59(3):289–297. [DOI] [PubMed] [Google Scholar]
- 145. Sicree RA, Zimmet PZ, King HO, Coventry JS. Plasma insulin response among Nauruans. Prediction of deterioration in glucose tolerance over 6 yr. Diabetes. 1987;36(2):179–186. [DOI] [PubMed] [Google Scholar]
- 146. Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK. Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body-fat distribution. Diabetes. 1990;39(3):283–288. [DOI] [PubMed] [Google Scholar]
- 147. Rhee EJ, Lee WY, Cho YK, Kim BI, Sung KC. Hyperinsulinemia and the development of nonalcoholic fatty liver disease in nondiabetic adults. Am J Med. 2011;124(1):69–76. [DOI] [PubMed] [Google Scholar]
- 148. Ardigò D, Numeroso F, Valtueña S, Franzini L, Piatti PM, Monti L, Delsignore R, Reaven GM, Zavaroni I. Hyperinsulinemia predicts hepatic fat content in healthy individuals with normal transaminase concentrations. Metabolism. 2005;54(12):1566–1570. [DOI] [PubMed] [Google Scholar]
- 149. Mehta SR, Godsland IF, Thomas EL, Pavitt DV, Morin SX, Bell JD, Taylor-Robinson SD, Johnston DG. Intrahepatic insulin exposure, intrahepatocellular lipid and regional body fat in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2012;97(6):2151–2159. [DOI] [PubMed] [Google Scholar]
- 150. Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F, Hecht J, Cusi K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(6):2178–2187. [DOI] [PubMed] [Google Scholar]
- 151. White UA, Fitch MD, Beyl RA, Hellerstein MK, Ravussin E. Racial differences in in vivo adipose lipid kinetics in humans. J Lipid Res. 2018;59(9):1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Diraison F, Yankah V, Letexier D, Dusserre E, Jones P, Beylot M. Differences in the regulation of adipose tissue and liver lipogenesis by carbohydrates in humans. J Lipid Res. 2003;44(4):846–853. [DOI] [PubMed] [Google Scholar]
- 153. Hudgins LC, Parker TS, Levine DM, Hellerstein MK. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab. 2011;96(3):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bril F, Portillo-Sanchez P, Liu I-C, Kalavalapalli S, Dayton K, Cusi K. Clinical and histologic characterization of nonalcoholic steatohepatitis in African American patients. Diabetes Care. 2018;41(1):187–192. [DOI] [PubMed] [Google Scholar]
- 155. Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112(1):32–38. [DOI] [PubMed] [Google Scholar]
- 156. Istfan NW, Plaisted CS, Bistrian BR, Blackburn GL. Insulin resistance versus insulin secretion in the hypertension of obesity. Hypertension. 1992;19(4):385–392. [DOI] [PubMed] [Google Scholar]
- 157. Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M, Shitrit A, Fuchs Z. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. 1985;75(3):809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34(6):416–422. [DOI] [PubMed] [Google Scholar]
- 160. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Furtado JD, Campos H, Sumner AE, Appel LJ, Carey VJ, Sacks FM. Dietary interventions that lower lipoproteins containing apolipoprotein C-III are more effective in whites than in blacks: results of the OmniHeart trial. Am J Clin Nutr. 2010;92(4):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Goran MI. Ethnic-specific pathways to obesity-related disease: the Hispanic vs. African-American paradox. Obesity (Silver Spring). 2008;16(12):2561–2565. [DOI] [PubMed] [Google Scholar]
- 163. Sumner AE, Vega GL, Genovese DJ, Finley KB, Bergman RN, Boston RC. Normal triglyceride levels despite insulin resistance in African Americans: role of lipoprotein lipase. Metabolism. 2005;54(7):902–909. [DOI] [PubMed] [Google Scholar]
- 164. Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77(1):43–50. [DOI] [PubMed] [Google Scholar]
- 165. Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, Stark PC, Greenberg AS, Roberts SB. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28(12):2939–2941. [DOI] [PubMed] [Google Scholar]
- 166. Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Schwarz JM, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW, Jones GM, Palii SP, Velasco-Alin M, Pan K, Patterson BW, Gugliucci A, Lustig RH, Mulligan K. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology. 2017;153(3):743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hall KD, Guyenet SJ, Leibel RL. The carbohydrate-insulin model of obesity is difficult to reconcile with current evidence. JAMA Intern Med. 2018;178(8):1103–1105. [DOI] [PubMed] [Google Scholar]
- 169. Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out”. JAMA Intern Med. 2018;178(8):1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Branis NM, Etesami M, Walker RW, Berk ES, Albu JB. Effect of a 1-week, eucaloric, moderately high-fat diet on peripheral insulin sensitivity in healthy premenopausal women. BMJ Open Diabetes Res Care. 2015;3(1):e000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wee CC, Jones DB, Apovian C, Hess DT, Chiodi SN, Bourland AC, Davis RB, Schneider B, Blackburn GL, Marcantonio ER, Hamel MB. Weight loss after bariatric surgery: do clinical and behavioral factors explain racial differences? Obes Surg. 2017;27(11):2873–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Thomas DD, Anderson WA, Apovian CM, Hess DT, Yu L, Velazquez A, Carmine B, Istfan NW. Weight recidivism after Roux‐en‐Y gastric bypass surgery: an 11‐year experience in a multiethnic medical center. Obesity (Silver Spring). 2019;27(2):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Purnell JQ, Johnson GS, Wahed AS, Dalla Man C, Piccinini F, Cobelli C, Prigeon RL, Goodpaster BH, Kelley DE, Staten MA, Foster-Schubert KE, Cummings DE, Flum DR, Courcoulas AP, Havel PJ, Wolfe BM. Prospective evaluation of insulin and incretin dynamics in obese adults with and without diabetes for 2 years after Roux-en-Y gastric bypass. Diabetologia. 2018;61(5):1142–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Karstoft K, Clark MA, Jakobsen I, Knudsen SH, van Hall G, Pedersen BK, Solomon TPJ. Glucose effectiveness, but not insulin sensitivity, is improved after short-term interval training in individuals with type 2 diabetes mellitus: a controlled, randomised, crossover trial. Diabetologia. 2017;60(12):2432–2442. [DOI] [PubMed] [Google Scholar]
- 175. Nishida Y, Tokuyama K, Nagasaka S, Higaki Y, Shirai Y, Kiyonaga A, Shindo M, Kusaka I, Nakamura T, Ishibashi S, Tanaka H. Effect of moderate exercise training on peripheral glucose effectiveness, insulin sensitivity, and endogenous glucose production in healthy humans estimated by a two-compartment-labeled minimal model. Diabetes. 2004;53(2):315–320. [DOI] [PubMed] [Google Scholar]
- 176. Engdahl JH, Veldhuis JD, Farrell PA. Altered pulsatile insulin secretion associated with endurance training. J Appl Physiol (1985). 1995;79(6):1977–1985. [DOI] [PubMed] [Google Scholar]
- 177. Weiss R, Magge SN, Santoro N, Giannini C, Boston R, Holder T, Shaw M, Duran E, Hershkop KJ, Caprio S. Glucose effectiveness in obese children: relation to degree of obesity and dysglycemia. Diabetes Care. 2015;38(4):689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Escalante-Pulido M, Escalante-Herrera A, Milke-Najar ME, Alpizar-Salazar M. Effects of weight loss on insulin secretion and in vivo insulin sensitivity in obese diabetic and non-diabetic subjects. Diabetes Nutr Metab. 2003;16(5-6):277–283. [PubMed] [Google Scholar]
- 179. Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018;16(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. [DOI] [PubMed] [Google Scholar]
- 181. Ramakrishnan SK, Russo L, Ghanem SS, Patel PR, Oyarce AM, Heinrich G, Najjar SM. Fenofibrate decreases insulin clearance and insulin secretion to maintain insulin sensitivity. J Biol Chem. 2016;291(46):23915–23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ogawa S, Takeuchi K, Sugimura K, Fukuda M, Lee R, Ito S, Sato T. Bezafibrate reduces blood glucose in type 2 diabetes mellitus. Metabolism. 2000;49(3):331–334. [DOI] [PubMed] [Google Scholar]
- 183. Page MM, Johnson JD. Mild suppression of hyperinsulinemia to treat obesity and insulin resistance. Trends Endocrinol Metab. 2018;29(6):389–399. [DOI] [PubMed] [Google Scholar]
- 184. Zdravkovic M, Kruse M, Rost KL, Møss J, Kecskes A, Dyrberg T. The effects of NN414, a SUR1/Kir6.2 selective potassium channel opener, in healthy male subjects. J Clin Pharmacol. 2005;45(7):763–772. [DOI] [PubMed] [Google Scholar]
- 185. Carr RD, Brand CL, Bodvarsdottir TB, Hansen JB, Sturis J. NN414, a SUR1/Kir6.2-selective potassium channel opener, reduces blood glucose and improves glucose tolerance in the VDF Zucker rat. Diabetes. 2003;52(10):2513–2518. [DOI] [PubMed] [Google Scholar]
- 186. Alemzadeh R, Fledelius C, Bodvarsdottir T, Sturis J. Attenuation of hyperinsulinemia by NN414, a SUR1/Kir6.2 selective K+-adenosine triphosphate channel opener, improves glucose tolerance and lipid profile in obese Zucker rats. Metabolism. 2004;53(4):441–447. [DOI] [PubMed] [Google Scholar]
- 187. Dobbins RL, Greenway FL, Chen L, Liu Y, Breed SL, Andrews SM, Wald JA, Walker A, Smith CD. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol. 2015;308(11):G946–G954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.