Abstract
Context
Premenopausal women with anorexia nervosa (AN) and obesity (OB) have elevated fracture risk. More plate-like and axially aligned trabecular bone, assessed by individual trabeculae segmentation (ITS), is associated with higher estimated bone strength. Trabecular plate and rod structure has not been reported across the weight spectrum.
Objective
To investigate trabecular plate and rod structure in premenopausal women.
Design
Cross-sectional study.
Setting
Clinical research center.
Participants
A total of 105 women age 21 to 46 years: (i) women with AN (n = 46), (ii) eumenorrheic lean healthy controls (HCs) (n = 29), and (iii) eumenorrheic women with OB (n = 30).
Measures
Trabecular microarchitecture by ITS.
Results
Mean age (±SD) was similar (28.9 ± 6.3 years) and body mass index differed (16.7 ± 1.8 vs 22.6 ± 1.4 vs 35.1 ± 3.3 kg/m2; P < 0.0001) across groups. Bone was less plate-like and axially aligned in AN (P ≤ 0.01) and did not differ between OB and HC. After controlling for weight, plate and axial bone volume fraction and plate number density were lower in OB vs HC; some were lower in OB than AN (P < 0.05). The relationship between weight and plate variables was quadratic (R = 0.39 to 0.70; P ≤ 0.0006) (i.e., positive associations were attenuated at high weight). Appendicular lean mass and IGF-1 levels were positively associated with plate variables (R = 0.27 to 0.67; P < 0.05). Amenorrhea was associated with lower radial plate variables than eumenorrhea in AN (P < 0.05).
Conclusions
In women with AN, trabecular bone is less plate-like. In women with OB, trabecular plates do not adapt to high weight. This is relevant because trabecular plates are associated with greater estimated bone strength. Higher muscle mass and IGF-1 levels may mitigate some of the adverse effects of low weight or excess adiposity on bone.
Among the premenopausal women in this study, trabecular bone was less plate-like in those with AN trabecular plates did not adapt to high weight in those with OB.
Trabecular bone is important in skeletal loading because it carries most of the load in a vertebral body and transfers the load from the joint surface to cortical bone in the extremities (1). Although most of the variation in trabecular bone’s estimated strength is explained by its bone volume fraction (BV/TV) (i.e., the ratio of the volume of bone tissue to the overall bulk volume) (2), trabecular microarchitecture is an independent determinant of estimated bone strength (3–6). In recent years, individual trabeculae segmentation (ITS) analysis of high-resolution peripheral quantitative CT (HRpQCT) data has been developed to segment trabecular bone into its component plate and rod number, volume, orientation, and connectivity. This has addressed limitations in standard HRpQCT analysis, in which trabecular bone is not separated into its component plates and rods (7).
The ITS analysis technique has advanced our understanding of trabecular microarchitecture by demonstrating that more plate-like and axially (i.e., longitudinally) aligned trabecular bone is associated with higher estimated bone strength (3, 8, 9) independent of bone volume fraction (10, 11). In contrast, although a certain number of trabecular rods is necessary for trabecular bone to resist failure initiation (12), more rod-like trabecular bone is associated with lower estimated bone strength independent of bone volume fraction (10, 11). Clinically, studies have demonstrated that postmenopausal women with fractures (11, 13, 14), as well as premenopausal women with idiopathic osteoporosis (10) or distal radius fractures (15), have preferential loss of trabecular plates over rods (i.e., a lower plate-to-rod ratio), less axially aligned trabecular bone, and less trabecular connectivity compared with controls.
Although it is known that trabecular microarchitecture and estimated strength by HRpQCT are impaired in premenopausal women with anorexia nervosa (AN) (16–18) and are superior in women with obesity (OB) (19) compared with healthy lean controls, differences in trabecular plate and rod number, volume, orientation, and connectivity by ITS are unknown. These are important groups to study because incident fracture risk is higher in premenopausal women with AN (20), as well as with OB at certain skeletal sites (21), compared with healthy lean controls. We hypothesized the following regarding tibial and radial trabecular microarchitecture across the weight spectrum: (i) women with AN will have fewer trabecular plates, less axially aligned trabecular bone, and less trabecular connectivity compared with healthy lean controls, (ii) women with OB will have more trabecular plates, greater axially aligned trabecular bone, and greater trabecular connectivity compared with healthy lean controls, and (iii) higher muscle mass, as well as higher serum IGF-1 and 25-hydroxyvitamin D [25(OH)D] levels, will be associated with superior, whereas amenorrhea in women with AN will be associated with less favorable, trabecular plate variables.
Materials and Methods
The Partners Healthcare Research Committee approved the study, and all participants provided written informed consent before study participation. We studied 105 women, age 21 to 46 years, who were consecutively screened for two different National Institutes Health trials between 2011 and 2015. Dual-energy x-ray absorptiometry and HRpQCT data from a subset of participants have been published previously (18, 22–25), but none of the ITS data from any participants have been published.
Participants met the following diagnostic criteria:
1. Women with AN (n = 46): According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, including low weight [operationalized as body mass index (BMI) < 18.5 kg/m2].
2. Lean healthy controls (HCs) (n = 29): BMI of 18.5 to 25 kg/m2 and eumenorrheic with no history of AN.
3. Women with OB (n = 30): BMI ≥ 30 kg/m2 and eumenorrheic with ho history of AN.
Exclusion criteria for all participants included conditions other than AN or OB that may affect bone or use of medications known to affect bone. We included women with AN taking oral contraceptive pills (n = 7); these women were later excluded in secondary analyses. Participants self-reported their age and history of fracture; fractures not typically associated with low BMD (i.e., those of the face, skull, finger, toe, or heel) were excluded. Height and weight were measured, and percentage ideal body weight was calculated (26). Physical activity was quantified by using the Paffenbarger scale (27), and hours of vigorous physical activity per week were reported.
DXA
Areal BMD at the postero-anterior spine, total hip, femoral neck, and total radius, as well as body composition, were assessed in all participants by DXA (Hologic 4500, Hologic, Inc., Waltham, MA) [precision of 0.01 g/cm2 at the spine and 3% for fat mass (28)]. Sex- and race-specific BMD Z-scores were calculated from the 2012 National Health and Nutrition Examination Survey (NHANES) reference data.
HRpQCT
HRpQCT was performed at the distal tibia and radius (Xtreme CT; Scanco Medical AG, Brüttisellen, Switzerland) with an isotropic voxel size of 82 μm3 (29). The nondominant arm or leg was scanned unless there was a prior fracture at that region, in which case the contralateral side was scanned. Two-dimensional scout views were obtained and used to locate the distal CT slice at 9.5 mm and 22.5 mm from the radius and tibia endplate, respectively.
Microfinite element analysis
Microfinite element analysis estimates the biomechanical properties of the bone in the setting of simulated axial compression per previously published methods (30). Failure load (in Newtons) was estimated by scaling the resultant load from a 1% apparent compressive strain until 2% of all elements reached an effective strain > 7000 microstrain, per previously published methods (30). Microfinite element analysis–derived estimates of failure load are strongly correlated (r2 = 0.75) with experimentally measured failure loads that produce Colles-type fractures in human cadaveric radii (31).
ITS
All HRpQCT images of the trabecular bone compartment underwent ITS-based morphological analysis, as previously described, to define plate, rod, and plate-rod junction characteristics (32). Plate-to-rod ratio was calculated as the ratio of plate bone volume fraction divided by rod bone volume fraction. More plate-like and axially (i.e., longitudinally) aligned trabecular bone is independently associated with higher estimated bone strength (10, 11). In contrast, although a certain number of trabecular rods is necessary for trabecular bone to resist failure initiation (12), more rod-like trabecular bone is independently associated with lower estimated bone strength (10, 11).
Biochemical analysis
Serum IGF-1 levels were measured by ISYS Analyzer (Luminescence, Immunodiagnostics Systems, Tyne & Wear, UK) with a sensitivity of 4.4 ng/mL, range of 10 to 1200 ng/mL, interassay variability of 5.1%, and intra-assay variability of 2.2%. Serum 25(OH)D levels were measured by using assays certified by the National Institutes of Health Vitamin D Standardization Certification Program.
Data analysis
JMP Statistical Discovery Software, version 12 Professional (SAS Institute, Inc., Cary, NC) was used. Variables were assessed for normality by using the Shapiro-Wilk test and, if nonnormal, were log-transformed. Continuous variables were compared among the three groups by using the Fisher least significant difference test. Additional correction for multiple comparisons is not indicated when this method is used for three-group comparisons (33). Categorical variables were compared among the three groups by using the Fisher exact test; pairwise comparisons were performed only when the overall P value was ≤ 0.05. Data modeling was performed to determine the best fit between trabecular bone structure or estimated strength variables and clinical, body composition, and biochemical variables; Pearson correlation coefficients are reported. Multivariate least-square analyses were performed to control for potential confounders; least-square means ± SEM or partial correlation coefficients are reported. Because adults with OB have greater fall impact forces as a result of greater weight, a secondary analysis was performed in which bone variables were controlled for weight. To differentiate the contributions of ITS measurements to the mechanical properties of trabecular bone from that of bone volume fraction, partial correlation analyses between ITS measurements and estimated bone strength after controlling for bone volume fraction are reported, as previously described by Liu et al. (10, 11). Significance was defined as a two-tailed P value ≤ 0.05. Data are reported as mean ± SD, or n (%), unless otherwise noted.
Results
Clinical characteristics
Clinical characteristics are shown in Table 1. Mean age and physical activity were similar across the groups. Mean BMI and appendicular lean mass were lower in AN and higher in OB than in HC (P < 0.0001). Mean serum IGF-1 levels were lower in AN and OB than in HC (P < 0.05), whereas mean serum 25(OH)D levels were higher in AN and did not differ in OB compared with HC (P < 0.0005). The prevalence of vitamin D deficiency [defined as serum 25(OH)D < 20 ng/mL] was lower in AN (14%) and did not differ in OB (61%) compared with HC (38%) (P < 0.03). Sixty-one percent of the AN group was amenorrheic; mean duration of amenorrhea was 69 ± 112 months.
Table 1.
Clinical Characteristics in Premenopausal Women
| Characteristic | Women With AN (n = 46) | Lean HC (n = 29) | Women With OB (n = 30) | Overall P Value |
|---|---|---|---|---|
| Age, y | 28.9 ± 5.6 | 28.5 ± 5.7 | 31.0 ± 7.8 | 0.31 |
| Body mass index, kg/m2 | 16.7 ± 1.8a | 22.6 ± 1.4b | 35.1 ± 3.3c | <0.0001 |
| Physical activity, h/wk | 4.1 ± 5.3 | 6.2 ± 4.9 | 4.3 ± 4.6 | 0.21 |
| Appendicular lean mass, kg | 14.3 ± 2.1a | 18.6 ± 2.2b | 22.3 ± 3.9c | <0.0001 |
| Serum IGF-1, ng/mL | 177.4 ± 59.0a | 221.5 ± 60.4b | 184.2 ± 50.8a | 0.006 |
| Serum 25(OH)D, ng/mL | 31.8 ± 12.7a | 22.6 ± 8.4b | 19.3 ± 6.7b | <0.0001 |
| Serum 25(OH)D < 20 ng/mL, % | 14a | 38b | 61b | 0.0003 |
| Areal BMD | ||||
| Postero-anterior spine, g/cm2 | 0.83 ± 0.13a | 0.99 ± 0.10b | 1.10 ± 0.07c | <0.0001 |
| Postero-anterior spine Z-score | −1.89 ± 1.17a | −0.45 ± 0.87b | 0.44 ± 0.71c | <0.0001 |
| Total hip, g/cm2 | 0.77 ± 0.11a | 0.98 ± 0.09b | 1.08 ± 0.11c | <0.0001 |
| Total hip Z-score | −1.41 ± 0.88a | 0.30 ± 0.75b | 0.98 ± 0.86c | <0.0001 |
| Femoral neck, g/cm2 | 0.65 ± 0.10a | 0.85 ± 0.10b | 0.94 ± 0.10c | <0.0001 |
| Femoral neck Z-score | −1.68 ± 0.92a | 0.02 ± 0.93b | 0.74 ± 0.88c | <0.0001 |
| Total radius, g/cm2 | 0.53 ± 0.06a | 0.58 ± 0.04b | 0.61 ± 0.05c | <0.0001 |
| Total radius Z-score | −0.71 ± 1.00a | 0.19 ± 0.76b | 0.71 ± 0.90c | <0.0001 |
Data are presented as mean ± SD.
Groups with different superscripts differ significantly at P ≤ 0.05.
Groups with different superscripts differ significantly at P ≤ 0.05.
Groups with different superscripts differ significantly at P ≤ 0.05.
Areal BMD
Mean areal BMD Z-scores at the postero-anterior spine, total hip, femoral neck, and total radius were lower in AN and higher in OB than in HC (P < 0.01) (Table 1). Excluding women with AN who were taking oral contraceptive pills did not change significant differences between groups.
Failure load
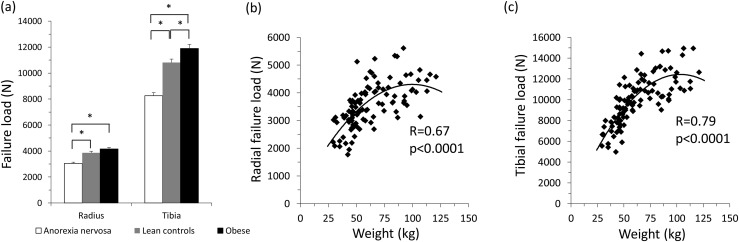
Mean radial failure load was lower in AN and did not differ in OB compared with HC, whereas mean tibial failure load was lower in AN and higher in OB compared with HC (P < 0.05) [Fig. 1(a)]. After controlling for body weight, mean radial and tibial failure load were lower in OB and remained lower in AN compared with HC (P < 0.05). There was a quadratic relationship between total body weight and failure load at the radius and tibia (R = 0.67 to 0.79; P < 0.0001), such that the positive association between weight and failure load was attenuated at high weights [Fig. 1(b) and 1(c)]. Excluding women with AN who were taking oral contraceptive pills did not change significant differences between groups.
Figure 1.
(a) Mean radial failure load was lower in women with AN and did not differ in women with OB compared with lean HC, whereas mean tibial failure load was lower in women with AN and higher in women with OB compared with lean HC (P < 0.05). Values are mean ± SEM. Across the weight spectrum, at the radius (b) and tibia (c), there was a quadratic relationship between total body weight and failure load, such that the positive association between body weight and failure load was attenuated at high weights. *P < 0.05. N, Newtons.
Individual trabeculae segmentation
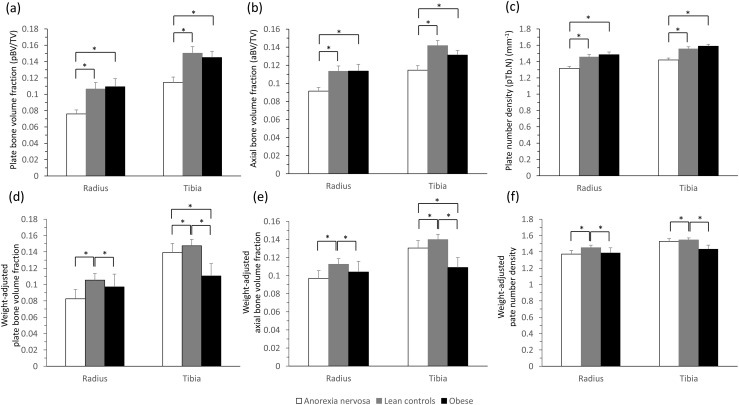
Mean radial and tibial plate variables and axial bone volume fraction were lower in AN (P < 0.05) and did not differ in OB compared with HC [Table 2, Fig. 2(a)-2(c)]. In contrast, mean radial and tibial rod variables were generally lower in AN and higher in OB than in HC (P < 0.05) (Table 2). Consequently, the mean plate-to-rod ratio was lower in AN at the radius and lower in OB at the tibia compared with HC (P < 0.05) (Table 2). Excluding women with AN who were taking oral contraceptive pills did not change significant differences between groups, except that the mean plate-to-rod ratio was no longer significantly different between groups at the radius (P = 0.10) or tibia (P = 0.06).
Table 2.
Individual Trabeculae Segmentation Measurements in Premenopausal Women
| Variable | Women With AN (n = 46) | Lean HC (n = 29) | Women With OB (n = 30) | Overall P Value |
|---|---|---|---|---|
| Radius | ||||
| Bone volume fraction | 0.23 ± 0.05a | 0.27 ± 0.04b | 0.29 ± 0.05b | <0.0001 |
| Plate bone volume fraction | 0.08 ± 0.03 a | 0.11 ± 0.04b | 0.11 ± 0.05 b | 0.0004 |
| Rod bone volume fraction | 0.15 ± 0.03a | 0.17 ± 0.02b | 0.18 ± 0.03c | 0.0001 |
| Axial bone volume fraction | 0.09 ± 0.03 a | 0.11 ± 0.03b | 0.11 ± 0.04 b | 0.002 |
| Plate number density, mm−1 | 1.31 ± 0.17 a | 1.46 ± 0.15b | 1.48 ± 0.18 b | <0.0001 |
| Rod number density, mm−1 | 1.80 ± 0.16a | 1.88 ± 0.12b | 1.96 ± 0.13c | 0.0001 |
| Plate-plate junction density, mm−3 | 1.56 ± 0.55a | 2.08 ± 0.60b | 2.25 ± 0.77b | <0.0001 |
| Plate-rod junction density, mm−3 | 3.24 ± 0.93a | 4.10 ± 0.91b | 4.53 ± 1.11b | <0.0001 |
| Rod-rod junction density, mm−3 | 2.74 ± 0.71a | 2.97 ± 0.71a | 3.43 ± 0.76b | 0.0005 |
| Plate-to-rod ratio | 0.50 ± 0.24a | 0.67 ± 0.35b | 0.62 ± 0.40a,b | 0.05 |
| Tibia | ||||
| Bone volume fraction | 0.24 ± 0.05a | 0.29 ± 0.04b | 0.33 ± 0.05c | <0.0001 |
| Plate bone volume fraction | 0.11 ± 0.04 a | 0.15 ± 0.04b | 0.14 ± 0.04 b | 0.0003 |
| Rod bone volume fraction | 0.13 ± 0.03a | 0.14 ± 0.03b | 0.18 ± 0.04c | <0.0001 |
| Axial bone volume fraction | 0.11 ± 0.03 a | 0.14 ± 0.03b | 0.13 ± 0.03 b | 0.001 |
| Plate number density, mm−1 | 1.42 ± 0.16 a | 1.56 ± 0.12b | 1.59 ± 0.13 b | <0.0001 |
| Rod number density, mm−1 | 1.68 ± 0.16a | 1.76 ± 0.17b | 1.95 ± 0.15c | <0.0001 |
| Plate-plate junction density, mm−3 | 1.87 ± 0.54 a | 2.42 ± 0.54b | 2.69 ± 0.57 b | <0.0001 |
| Plate-rod junction density, mm−3 | 3.27 ± 0.85a | 4.13 ± 1.06b | 5.13 ± 1.05c | <0.0001 |
| Rod-rod junction density, mm−3 | 2.08 ± 0.68a | 2.31 ± 0.82a | 3.26 ± 1.0b | <0.0001 |
| Plate-to-rod ratio | 0.95 ± 0.44ab | 1.15 ± 0.57a | 0.85 ± 0.31 b | 0.05 |
Data are presented as mean ± SD. Values in bold are significantly lower than lean control group after controlling for body weight (P ≤ 0.05).
Groups with different superscripts differ significantly at P ≤ 0.05.
Groups with different superscripts differ significantly at P ≤ 0.05.
Groups with different superscripts differ significantly at P ≤ 0.05.
Figure 2.
Mean radial and tibial plate bone volume fraction (a), axial bone volume fraction (b), and plate number density (c) were lower in women with AN and similar in women with OB compared with lean HC (P ≤ 0.01). Values are mean ± SEM. After adjustment for total body weight, mean radial and tibial plate bone volume fraction (d), axial bone volume fraction (e), and plate number density (f) were lower in both women with AN and OB than in lean HC (P ≤ 0.05). Values are least-square mean ± SEM. *P ≤ 0.05.
After controlling for body weight, we found that mean radial and tibial plate bone volume fraction, axial bone volume fraction, and plate number density, as well as tibial plate-plate junction density, were lower in OB and remained lower in AN compared with HC (P ≤ 0.05) [Table 2, Fig. 2(d)–2(f)]. Moreover, weight-adjusted mean tibial plate and axial bone volume fraction were lower in OB than AN (P ≤ 0.05) [Fig. 2(d)–2(f)]. Excluding women with AN who were taking oral contraceptive pills did not change significant differences between groups, except that mean radial plate number density and tibial plate–plate junction density did not differ between AN and HC after controlling for body weight.
Determinants of trabecular plate and rod structure
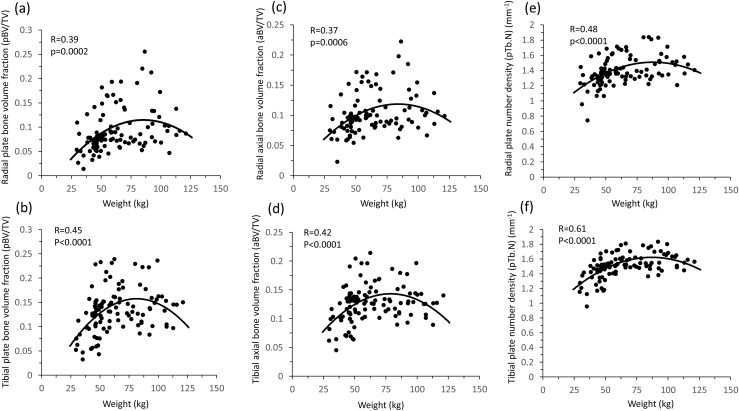
Across the weight spectrum, there was a quadratic relationship between total body weight; plate number, volume, and connectivity; axial alignment; and plate-to-rod ratio at the radius and tibia (R = 0.39 to 0.70; P ≤ 0.0006), such that the positive association between body weight and these variables was attenuated at high weights (Fig. 3). In contrast, the relationship between total body weight and rod variables was linear (R = 0.39 to 0.61; P ≤ 0.0006).
Figure 3.
Across the weight spectrum at the radius and tibia, there was a quadratic relationship between total body weight and plate bone volume fraction (a and b), axial bone volume fraction (c and d), and plate number density (e and f), such that the positive association between body weight and plate variables was attenuated at high weights.
At both the radius and tibia, there was a positive linear relationship between plate number, volume, and connectivity, as well as axial alignment, with (i) appendicular lean mass (R = 0.27 to 0.67; P ≤ 0.006), and (ii) serum IGF-1 levels (R = 0.27 to 0.35; P ≤ 0.007). After controlling for body weight, serum IGF-1 levels remained significantly associated with these variables (β = 0.23 to 0.32; P < 0.05). The association between trabecular plate and rod variables with physical activity was not significant.
Participants with serum 25(OH)D level < 20 ng/mL (n = 34) had similar mean plate variables but higher mean radial rod bone volume fraction (0.17 ± 0.02 vs 0.16 ± 0.03), rod number density (1.92 ± 0.11 vs 1.84 ± 0.17 mm−1), and rod-rod junction density (3.25 ± 0.63 vs 2.88 ± 0.79 mm−3) than participants with serum 25(OH)D level > 20 ng/mL (P < 0.05). However, participants with 25(OH)D level < 20 ng/dL also had greater mean body weight (73.8 ± 20.1 kg vs 58.4 ± 21.5 kg; P = 0.0001). After controlling for body weight, the relationship between 25(OH)D deficiency and trabecular plate and rod variables was not significant.
At the radius and tibia, mean failure load was lower in both amenorrheic and eumenorrheic women with AN compared with HC (P < 0.05) (Table 3). Plate and rod variables were impaired in amenorrheic women with AN (P < 0.05) and did not differ in eumenorrheic women with AN compared with HC (Table 3). Amenorrheic women with AN had lower mean radial, but not tibial, plate and rod variables compared with eumenorrheic women with AN (P < 0.05).
Table 3.
Skeletal Variables in Premenopausal Women
| Variable | Amenorrheic Women With AN (n = 27) | Eumenorrheic Women With AN (n = 10) | Lean HC (n = 29) | Overall P Value |
|---|---|---|---|---|
| Estimated strength by HRpQCT | ||||
| Radial failure load, N | 2998.48 ± 667.14a | 3344 ± 588.76a | 3863.29 ± 636.52b | <0.0001 |
| Tibial failure load, N | 8255.33 ± 1774.88a | 9000 ± 1176.72a | 10804.28 ± 1483.18b | <0.0001 |
| Individual trabeculae segmentation measurements | ||||
| Radius | ||||
| Bone volume fraction | 0.22 ± 0.05a | 0.26 ± 0.04b | 0.27 ± 0.04b | 0.0001 |
| Plate bone volume fraction | 0.07 ± 0.03a | 0.09 ± 0.04a,b | 0.11 ± 0.04b | 0.004 |
| Rod bone volume fraction | 0.15 ± 0.03a | 0.17 ± 0.02b | 0.17 ± 0.02b | 0.03 |
| Axial bone volume fraction | 0.09 ± 0.03a | 0.10 ± 0.03a,b | 0.11 ± 0.03b | 0.01 |
| Plate number density, mm−1 | 1.29 ± 0.16a | 1.41 ± 0.17a | 1.46 ± 0.15b | 0.002 |
| Rod number density, mm−1 | 1.78 ± 0.18a | 1.88 ± 0.10a,b | 1.88 ± 0.12b | 0.03 |
| Plate-plate junction density, mm−3 | 1.48 ± 0.47a | 1.92 ± 0.63b | 2.08 ± 0.60b | 0.001 |
| Plate-rod junction density, mm−3 | 3.07 ± 0.75a | 3.91 ± 0.98b | 4.10 ± 0.91b | 0.0005 |
| Rod-rod junction density, mm−3 | 2.65 ± 0.78 | 3.01 ± 0.62 | 2.97 ± 0.71 | 0.15 |
| Plate-to-rod ratio | 0.51 ± 0.27 | 0.56 ± 0.23 | 0.67 ± 0.35 | 0.09 |
| Tibia | ||||
| Bone volume fraction | 0.24 ± 0.06a | 0.26 ± 0.03a,b | 0.29 ± 0.04b | 0.0007 |
| Plate bone volume fraction | 0.11 ± 0.05a | 0.13 ± 0.02a,b | 0.15 ± 0.04b | 0.01 |
| Rod bone volume fraction | 0.12 ± 0.03 | 0.13 ± 0.02 | 0.14 ± 0.03 | 0.06 |
| Axial bone volume fraction | 0.12 ± 0.04a | 0.13 ± 0.02a,b | 0.14 ± 0.03b | 0.01 |
| Plate number density, mm−1 | 1.42 ± 0.18a | 1.49 ± 0.09a,b | 1.56 ± 0.12b | 0.002 |
| Rod number density, mm−1 | 1.67 ± 0.18 | 1.69 ± 0.12 | 1.76 ± 0.17 | 0.13 |
| Plate-plate junction density, mm−3 | 1.86 ± 0.59a | 2.10 ± 0.37a,b | 2.42 ± 0.54b | 0.001 |
| Plate-rod junction density, mm−3 | 3.22 ± 0.88a | 3.61 ± 0.74a,b | 4.13 ± 1.06b | 0.002 |
| Rod-rod junction density, mm−3 | 2.04 ± 0.79 | 2.03 ± 0.47 | 2.31 ± 0.82 | 0.36 |
| Plate-to-rod ratio | 1.02 ± 0.52 | 1.02 ± 0.23 | 1.15 ± 0.57 | 0.47 |
Data are presented as mean ± SD.
Abbreviation: N, Newtons.
Groups with different superscripts differ significantly at P ≤ 0.05.
Groups with different superscripts differ significantly at P ≤ 0.05.
Association between trabecular plate and rod variables and failure load
Across the weight spectrum at the radius and tibia, plate bone volume fraction (β = 0.21 to 0.39; P < 0.05), axial bone volume fraction (β = 0.20 to 0.30; P ≤ 0.05), and plate-to-rod ratio (β = 0.02 to 0.40; P < 0.05) were positively associated with failure load independent of bone volume fraction and appendicular lean mass, whereas rod bone volume fraction (β = −0.09 to −0.40; P < 0.05), rod number density (β = −0.26 to −0.47; P ≤ 0.01), and rod-rod junction density (β = −0.25 to −0.43; P ≤ 0.01) were negatively associated.
Association between trabecular plate and rod variables and self-reported fracture
After controlling for body weight, women with a self-reported history of fracture, compared with those without fracture, had lower mean radial and tibial plate number density (radius, 1.37 ± 0.20 vs 1.42 ± 0.17 mm−1; tibia, 1.48 ± 0.18 vs 1.52 ± 0.14 mm−1) and plate-rod junction density (radius, 3.64 ± 1.22 vs 3.97 ± 1.04 mm−3; tibia, 3.90 ± 1.30 vs 4.14 ± 1.98 mm−3), as well as lower mean radial bone volume fraction (0.25 ± 0.06 vs 0.27 ± 0.05), rod bone volume fraction (0.16 ± 0.03 vs 0.17 ± 0.03), rod number density (1.83 ± 0.16 vs 1.89 ± 0.14 mm−1), plate-plate junction density (1.78 ± 0.72 vs 1.97 ± 0.68 mm−3), and rod-rod junction density (2.83 ± 0.67 vs 3.11 ± 0.82 mm−3) (P ≤ 0.05).
Discussion
We demonstrate that premenopausal women with AN have preferential loss of trabecular plates over rods, resulting in a lower plate-to-rod ratio, and less axially aligned trabeculae compared with lean HC. In premenopausal women with OB, although trabecular rod variables are proportionally greater at higher weight, trabecular plate structure does not adapt proportionally to a higher weight load, thus resulting in a lower plate-to-rod ratio compared with lean HC. This is clinically relevant because more plate-like and axially aligned trabecular bone is associated with higher estimated bone strength. This study shows differences in trabecular bone structure by ITS in premenopausal women across the weight spectrum, which may contribute to the higher risk for fracture at certain skeletal sites in both women with AN and women with OB.
Our findings that premenopausal women with AN have preferential loss of trabecular plates over rods, less axially aligned trabeculae bone, and less trabecular connectivity compared with lean HC are consistent with prior studies in premenopausal women with idiopathic osteoporosis (10) or fractures (15), as well as in female adolescents with AN (34). We have added to the literature by evaluating an additional female population at increased fracture risk: women with AN who have reached their peak bone mass, which, according to longitudinal studies, occurs by ∼19 to 21 years of age (35, 36). We found that trabecular bone structure at the non–weight-bearing radius may be especially impaired in amenorrheic vs eumenorrheic women with AN, which is consistent with prior data in amenorrheic athletes (32). This suggests that hypogonadism may be more detrimental to trabecular bone structure at non–weight-bearing sites, or that weight bearing may attenuate some of the adverse effects of hypogonadism on trabecular bone.
In premenopausal women with OB, although trabecular rod variables are proportionally greater at higher weight, neither trabecular plate structure nor axial alignment adapts proportionally to a higher weight load. In fact, in women with obesity, some weight-adjusted trabecular plate variables are more impaired than in lean HC and women with AN. In addition, we demonstrate that failure load is lower in women with OB relative to body weight compared with lean HC, which is similar to studies in postmenopausal women (37), nonosteoporotic adults with morbid obesity (38), and female adolescents (39). The inability of the skeleton of women with OB to adapt to the greater fall impact forces due to high weight may explain the higher risk of fractures at certain skeletal sites in these women (40).
Although we found that both low body weight and excess adiposity may be detrimental to trabecular bone structure, the positive linear relationships between trabecular plate structure and muscle mass and serum IGF-1 levels suggest that muscle and IGF-1 may be beneficial to trabecular bone. Both muscle and IGF-1 have anabolic effects on bone (41, 42), and we contribute to the literature by adding trabecular microarchitecture by ITS. Because both women with AN and those with OB may be at risk for relatively low muscle mass [because of undernutrition (24) or sarcopenic obesity (43), respectively] and low serum IGF-1 levels [due to growth hormone resistance in AN (44) and growth hormone deficiency in OB (45)], therapeutic interventions that increase muscle mass or serum IGF-1 levels may improve trabecular bone. Our data showing significant differences in ITS variables between participants with and without a self-reported history of fracture are consistent with prior studies (11, 13–15) and further suggest that trabecular plate and rod structure may be a therapeutic target to reduce fracture risk.
Limitations of this study include its cross-sectional design, such that causality between anthropometric and biochemical variables with trabecular bone structure cannot be determined. Future longitudinal studies that include incident fracture data, including morphometric vertebral fractures, would be beneficial. Further investigation using fracture risk modeling that incorporates the applied load during a fall may also be beneficial because the applied load during a fall is much higher in women with OB; such an analysis may support our data that trabecular bone structure in obesity is not commensurate with greater body weight. Small subgroup analyses, such as with the amenorrheic and eumenorrheic women with AN, may have been underpowered to detect a difference.
In conclusion, we demonstrate that premenopausal women with AN have preferential loss of trabecular plates over rods, less axially aligned trabecular bone, and less trabecular connectivity compared with lean HC. In premenopausal women with OB, although trabecular rod variables are proportionally greater at higher weight, trabecular plate structure, orientation, and connectivity do not adapt proportionally to a higher weight load. We report differences in trabecular bone structure by ITS in premenopausal women across the weight spectrum, which may contribute to the higher fracture risk in women with AN and OB. Therefore, interventions targeting trabecular bone structure may have therapeutic potential. Interventions that increase muscle mass or IGF-1 levels may also be able to mitigate some of the adverse effects of low weight or excess adiposity on bone.
Acknowledgments
Financial Support: This study was funded in part by the National Institutes of Health (Bethesda, MD) R01 DK052625 (A.K.), R24 DK084970 (A.K.), K24 HL092902 (K.K.M.), K23 DK115903 (M.S.), T32 DK007028, and 1 UL1 TR001102; 8 UL1 TR000170, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science; 1 UL1 RR025758, Harvard Clinical and Translational Science Center, from the National Center for Research Resources; and The Hilda and Preston Davis Foundation (K.K.M.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- AN
anorexia nervosa
- BMI
body mass index
- BV/TV
bone volume fraction
- DXA
dual-energy x-ray absorptiometry
- HC
healthy control
- HRpQCT
high-resolution peripheral quantitative CT
- ITS
individual trabeculae segmentation
- OB
obesity
Data Availability:
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Oftadeh R, Perez-Viloria M, Villa-Camacho JC, Vaziri A, Nazarian A. Biomechanics and mechanobiology of trabecular bone: a review. J Biomech Eng. 2015;137(1):010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ulrich D, van Rietbergen B, Laib A, Rüegsegger P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone. 1999;25(1):55–60. [DOI] [PubMed] [Google Scholar]
- 3. Liu XS, Sajda P, Saha PK, Wehrli FW, Guo XE. Quantification of the roles of trabecular microarchitecture and trabecular type in determining the elastic modulus of human trabecular bone. J Bone Miner Res. 2006;21(10):1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu XS, Zhang XH, Guo XE. Contributions of trabecular rods of various orientations in determining the elastic properties of human vertebral trabecular bone. Bone. 2009;45(2):158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fields AJ, Lee GL, Liu XS, Jekir MG, Guo XE, Keaveny TM. Influence of vertical trabeculae on the compressive strength of the human vertebra. J Bone Miner Res. 2011;26(2):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi X, Liu XS, Wang X, Guo XE, Niebur GL. Effects of trabecular type and orientation on microdamage susceptibility in trabecular bone. Bone. 2010;46(5):1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laib A, Häuselmann HJ, Rüegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6(5-6):329–337. [PubMed] [Google Scholar]
- 8. Liu XS, Sajda P, Saha PK, Wehrli FW, Bevill G, Keaveny TM, Guo XE. Complete volumetric decomposition of individual trabecular plates and rods and its morphological correlations with anisotropic elastic moduli in human trabecular bone. J Bone Miner Res. 2008;23(2):223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou B, Liu XS, Wang J, Lu XL, Fields AJ, Guo XE. Dependence of mechanical properties of trabecular bone on plate-rod microstructure determined by individual trabecula segmentation (ITS). J Biomech. 2014;47(3):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu XS, Cohen A, Shane E, Stein E, Rogers H, Kokolus SL, Yin PT, McMahon DJ, Lappe JM, Recker RR, Guo XE. Individual trabeculae segmentation (ITS)-based morphological analysis of high-resolution peripheral quantitative computed tomography images detects abnormal trabecular plate and rod microarchitecture in premenopausal women with idiopathic osteoporosis. J Bone Miner Res 2010;25(7):1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu XS, Stein EM, Zhou B, Zhang CA, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Cosman F, Nieves J, Shane E, Guo XE. Individual trabecula segmentation (ITS)-based morphological analyses and microfinite element analysis of HR-pQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J Bone Miner Res. 2012;27(2):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu XS, Bevill G, Keaveny TM, Sajda P, Guo XE. Micromechanical analyses of vertebral trabecular bone based on individual trabeculae segmentation of plates and rods. J Biomech. 2009;42(3):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stein EM, Kepley A, Walker M, Nickolas TL, Nishiyama K, Zhou B, Liu XS, McMahon DJ, Zhang C, Boutroy S, Cosman F, Nieves J, Guo XE, Shane E. Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res 2014;29(5):1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang J, Stein EM, Zhou B, Nishiyama KK, Yu YE, Shane E, Guo XE. Deterioration of trabecular plate-rod and cortical microarchitecture and reduced bone stiffness at distal radius and tibia in postmenopausal women with vertebral fractures. Bone. 2016;88:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rozental TD, Deschamps LN, Taylor A, Earp B, Zurakowski D, Day CS, Bouxsein ML. Premenopausal women with a distal radial fracture have deteriorated trabecular bone density and morphology compared with controls without a fracture. J Bone Joint Surg Am. 2013;95(7):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milos G, Häuselmann HJ, Krieg MA, Rüegsegger P, Gallo LM. Are patterns of bone loss in anorexic and postmenopausal women similar? Preliminary results using high resolution peripheral computed tomography. Bone. 2014;58:146–150. [DOI] [PubMed] [Google Scholar]
- 17. Frølich J, Hansen S, Winkler LA, Andresen AK, Hermann AP, Støving RK. The role of body weight on bone in anorexia nervosa: a HR-pQCT study. Calcif Tissue Int. 2017;101(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fazeli PK, Faje AT, Cross EJ, Lee H, Rosen CJ, Bouxsein ML, Klibanski A. Serum FGF-21 levels are associated with worsened radial trabecular bone microarchitecture and decreased radial bone strength in women with anorexia nervosa. Bone. 2015;77:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Evans AL, Paggiosi MA, Eastell R, Walsh JS. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res. 2015;30(5):920–928. [DOI] [PubMed] [Google Scholar]
- 20. Solmi M, Veronese N, Correll CU, Favaro A, Santonastaso P, Caregaro L, Vancampfort D, Luchini C, De Hert M, Stubbs B. Bone mineral density, osteoporosis, and fractures among people with eating disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2016;133(5):341–351. [DOI] [PubMed] [Google Scholar]
- 21. Ishii S, Cauley JA, Greendale GA, Nielsen C, Karvonen-Gutierrez C, Ruppert K, Karlamangla AS. Pleiotropic effects of obesity on fracture risk: the Study of Women's Health Across the Nation. J Bone Miner Res. 2014;29(12):2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bachmann KN, Fazeli PK, Lawson EA, Russell BM, Riccio AD, Meenaghan E, Gerweck AV, Eddy K, Holmes T, Goldstein M, Weigel T, Ebrahimi S, Mickley D, Gleysteen S, Bredella MA, Klibanski A, Miller KK. Comparison of hip geometry, strength, and estimated fracture risk in women with anorexia nervosa and overweight/obese women. J Clin Endocrinol Metab. 2014;99(12):4664–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bachmann KN, Bruno AG, Bredella MA, Schorr M, Lawson EA, Gill CM, Singhal V, Meenaghan E, Gerweck AV, Eddy KT, Ebrahimi S, Koman SL, Greenblatt JM, Keane RJ, Weigel T, Dechant E, Misra M, Klibanski A, Bouxsein ML, Miller KK. Vertebral strength and estimated fracture risk across the BMI spectrum in women. J Bone Miner Res. 2016;31(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schorr M, Thomas JJ, Eddy KT, Dichtel LE, Lawson EA, Meenaghan E, Lederfine Paskal M, Fazeli PK, Faje AT, Misra M, Klibanski A, Miller KK. Bone density, body composition, and psychopathology of anorexia nervosa spectrum disorders in DSM-IV vs DSM-5. Int J Eat Disord. 2017;50(4):343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fazeli PK, Faje A, Bredella MA, Polineni S, Russell S, Resulaj M, Rosen CJ, Klibanski A. Changes in marrow adipose tissue with short-term changes in weight in premenopausal women with anorexia nervosa. Eur J Endocrinol. 2018;EJE-18-0824.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Metropolitan Life Foundation New weight standards for men and women. Stat Bull Metrop Life Insur Co. 1959;40:1–4. [Google Scholar]
- 27. Paffenbarger RS Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25(1):60–70. [DOI] [PubMed] [Google Scholar]
- 28. Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51(6):1106–1112. [DOI] [PubMed] [Google Scholar]
- 29. Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, Bouxsein ML, Misra M. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011;96(10):3123–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pistoia W, van Rietbergen B, Laib A, Rüegsegger P. High-resolution three-dimensional-pQCT images can be an adequate basis for in-vivo microFE analysis of bone. J Biomech Eng. 2001;123(2):176–183. [DOI] [PubMed] [Google Scholar]
- 31. Pistoia W, van Rietbergen B, Lochmüller EM, Lill CA, Eckstein F, Rüegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842–848. [DOI] [PubMed] [Google Scholar]
- 32. Mitchell DM, Tuck P, Ackerman KE, Cano Sokoloff N, Woolley R, Slattery M, Lee H, Bouxsein ML, Misra M. Altered trabecular bone morphology in adolescent and young adult athletes with menstrual dysfunction. Bone. 2015;81:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meier U. A note on the power of Fisher’s least significant difference procedure. Pharm Stat. 2006;5(4):253–263. [DOI] [PubMed] [Google Scholar]
- 34. Singhal V, Tulsiani S, Campoverde KJ, Mitchell DM, Slattery M, Schorr M, Miller KK, Bredella MA, Misra M, Klibanski A. Impaired bone strength estimates at the distal tibia and its determinants in adolescents with anorexia nervosa. Bone. 2018;106:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen TV, Maynard LM, Towne B, Roche AF, Wisemandle W, Li J, Guo SS, Chumlea WC, Siervogel RM. Sex differences in bone mass acquisition during growth: the Fels Longitudinal Study. J Clin Densitom. 2001;4(2):147–157. [DOI] [PubMed] [Google Scholar]
- 36. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729–1739. [DOI] [PubMed] [Google Scholar]
- 37. Sornay-Rendu E, Boutroy S, Vilayphiou N, Claustrat B, Chapurlat RD In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: the Os des Femmes de Lyon (OFELY) study. J Bone Miner Res. 2013;28(7):1679–1687. [DOI] [PubMed] [Google Scholar]
- 38. Andersen S, Frederiksen KD, Hansen S, Brixen K, Gram J, Støving RK. Bone structure and estimated bone strength in obese patients evaluated by high-resolution peripheral quantitative computed tomography. Calcif Tissue Int. 2014;95(1):19–28. [DOI] [PubMed] [Google Scholar]
- 39. Singhal V, Sanchita S, Malhotra S, Bose A, Flores LPT, Valera R, Stanford FC, Slattery M, Rosenblum J, Goldstein MA, Schorr M, Ackerman KE, Miller KK, Klibanski A, Bredella MA, Misra M. Suboptimal bone microarchitecure in adolescent girls with obesity compared to normal-weight controls and girls with anorexia nervosa. Bone. 2019;122:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johansson H, Kanis JA, Oden A, McCloskey E, Chapurlat RD, Christiansen C, Cummings SR, Diez-Perez A, Eisman JA, Fujiwara S, Gluer CC, Goltzman D, Hans D, Khaw KT, Krieg MA, Kroger H, LaCroix AZ, Lau E, Leslie WD, Mellstrom D, Melton LJ 3rd, O'Neill TW, Pasco JA, Prior JC, Reid DM, Rivadeneira F, van Staa T, Yoshimura N, Zillikens MC. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–233. [DOI] [PubMed] [Google Scholar]
- 41. Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, Rosenblum L, Donoho D, Gupta R, Klibanski A. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone. 2010;46(2):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gibbs JC, Giangregorio LM, Wong AKO, Josse RG, Cheung AM. Appendicular and whole body lean mass outcomes are associated with finite element analysis-derived bone strength at the distal radius and tibia in adults aged 40years and older. Bone. 2017;103:47–54. [DOI] [PubMed] [Google Scholar]
- 43. McKee A, Morley JE, Matsumoto AM, Vinik A. Sarcopenia: An Endocrine Disorder? Endocr Pract. 2017;23(9):1140–1149. [DOI] [PubMed] [Google Scholar]
- 44. Støving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, Kristiansen J, Hagen C. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab. 1999;84(6):2056–2063. [DOI] [PubMed] [Google Scholar]
- 45. Pijl H, Langendonk JG, Burggraaf J, Frölich M, Cohen AF, Veldhuis JD, Meinders AE. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab. 2001;86(11):5509–5515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.