Abstract
Angiotensin II (AngII) and the mineralocorticoid receptor (MR) ligand aldosterone both contribute to cardiovascular disorders, including hypertension and adverse vascular remodeling. We previously demonstrated that AngII activates MR-mediated gene transcription in human vascular smooth muscle cells (SMCs), yet the mechanism and the impact on SMC function are unknown. Using an MR-responsive element-driven transcriptional reporter assay, we confirm that AngII induces MR transcriptional activity in vascular SMCs and endothelial cells, but not in Cos1 or human embryonic kidney-293 cells. AngII activation of MR was blocked by the MR antagonist spironolactone or eplerenone and the protein kinase C-δ (PKCδ) inhibitor rottlerin, implicating both in the mechanism. Similarly, small interfering RNA knockdown of PKCδ in SMCs prevented AngII-mediated MR activation, whereas knocking down of MR blocked both aldosterone- and AngII-induced MR function. Coimmunoprecipitation studies reveal that endogenous MR and PKCδ form a complex in SMCs that is enhanced by AngII treatment in association with increased serine phosphorylation of the MR N terminus. AngII increased mRNA expression of the SMC-MR target gene, FKBP51, via an MR-responsive element in intron 5 of the FKBP51 gene. The impact of AngII on FKBP51 reporter activity and gene expression in SMCs was inhibited by spironolactone and rottlerin. Finally, the AngII-induced increase in SMC number was also blocked by the MR antagonist spironolactone and the PKCδ inhibitor rottlerin. These data demonstrate that AngII activates MR transcriptional regulatory activity, target gene regulation, and SMC proliferation in a PKCδ-dependent manner. This new mechanism may contribute to synergy between MR and AngII in driving SMC dysfunction and to the cardiovascular benefits of MR and AngII receptor blockade in humans.
The renin–angiotensin–aldosterone system (RAAS) is a hormonal cascade that is critical for maintenance and control of blood pressure, but it also contributes to the development of cardiovascular disease. Classically, the peptide hormone angiotensin II (AngII) stimulates the adrenal cortex to produce and secrete the steroid hormone aldosterone, which activates the mineralocorticoid receptor (MR) in the kidney to regulate sodium homeostasis [reviewed in (1, 2)]. AngII and aldosterone also act directly on the vasculature through cell surface angiotensin type 1 receptors (AT1Rs) and intracellular MRs, respectively, to contribute to blood pressure control, vascular remodeling, and the development of cardiovascular disease [reviewed in (3–5)]. Many of the pathophysiological effects of RAAS activation are mediated by direct AT1R and MR activation in vascular smooth muscle cells (SMCs), resulting in enhanced vasoconstriction, SMC proliferation, and vascular fibrosis (6–10). Excessive activation of AT1Rs and MRs contributes to cardiovascular aging (10–12) and to the development of hypertension, heart attack, stroke, and heart failure (13–16). Indeed, multiple large clinical trials show that angiotensin receptor blockers and MR antagonists improve outcomes and decrease mortality in cardiovascular patients (17–22). These profound benefits of RAAS blockade are evident even in the absence of elevated aldosterone levels, but the molecular mechanisms are still incompletely understood.
The MR is a steroid hormone receptor that classically regulates cellular functions by acting as a ligand-activated transcription factor to modulate gene expression [slow “genomic” effects, reviewed in (23)] and through modulation of cell signaling in the cytoplasm [rapid “nongenomic” effects, reviewed in (24)]. Both aldosterone and cortisol can act as MR ligands with aldosterone specificity determined by tissue-specific expression of the enzyme 11β-hydroxysteroid dehydrogenase-2 (HSD2) that locally inactivates cortisol (25). Originally identified in the kidney, multiple studies show that HSD2 is expressed and inactivates cortisol in vascular SMCs, including human coronary artery and aortic SMCs (26, 27). In animal models, many pathological effects of AngII on the cardiovascular system can be blocked by MR inhibition, an observation that has long been attributed solely to blockade of AngII effects mediated by adrenal aldosterone production to activate the MR (28, 29). However, clinical data showing cardiovascular benefits of MR blockade, even when aldosterone is not elevated, combined with recent preclinical data supporting a direct role for SMC-MR in mediating components of the vascular impact of AngII (10, 11) suggest the potential for ligand-independent mechanisms of MR activation by AngII. Potential mechanistic explanations for this crosstalk include MR regulation of AT1R expression and synergistic activation of signaling cascades by AT1R and nongenomic actions of MR in the cytoplasm (30–33).
As both AngII signaling and MR expression in vascular SMCs increase in the setting of hypertension and aging, substantial recent research has focused on exploring AngII/MR crosstalk mechanisms in vascular SMCs as a contributor to vascular pathology (10–12, 14, 34). In mouse models of hypertension or aging, AngII-induced vasoconstriction, hypertension, vascular oxidative stress, and cardiac and vascular remodeling are all attenuated by specific deletion of MRs from SMCs with no change in serum aldosterone (4, 10, 12, 35, 36). Analogous in vitro studies in SMCs also show that AngII-induced SMC proliferation, senescence, oxidative stress, and fibrosis gene upregulation are all attenuated by MR inhibition (37–41), suggesting direct crosstalk between AT1R and MR signaling in SMCs by mechanisms that are not completely elucidated.
In human coronary artery SMCs, we previously showed that AngII, acting via the AT1R, activates MR-mediated gene transcription in an aldosterone-independent manner (26). Such hormone-independent activation in response to growth factor or G protein–coupled receptor signaling has been demonstrated for all other members of the steroid hormone receptor family, including the estrogen, progesterone, and androgen receptors, by a mechanism involving phosphorylation of the receptor N terminus (42–46). In this study, we explored the mechanism by which AngII activates MR transcriptional activity in vascular SMCs and identified a specific role for the δ isoform of protein kinase C (PKCδ) that contributes to regulation of SMC gene expression and survival/proliferation.
Materials and Methods
All reagents were purchased from Sigma-Aldrich or Gibco (cell culture medium and serum) unless otherwise stated.
Cell culture
Human embryonic kidney-293 (HEK293) and Cos1 cell lines (American Type Culture Collection) and EAhy926 cells (a human umbilical vein endothelial cell hybrid line, originally from C. J. Edgell, University of North Carolina at Chapel Hill) were maintained in phenol red–containing DMEM with 10% bovine growth serum (BGS) (GE Healthcare Life Sciences). Pac1 cells (rat pulmonary arterial SMC line) and CO396 cells [human coronary artery SMCs transformed by infection with retrovirus expressing E6 or E7 (E6/E7) proteins from human papilloma virus] were maintained in phenol red–free DMEM with 10% BGS. All cells were switched to phenol red–free, serum-free medium 24 hours prior to treatment with the exception of CO396 cells, which were changed to phenol red–free DMEM with 2% charcoal-stripped BGS to remove endogenous steroid ligands.
Transient transfections, viral infection, and luciferase assay
Cells were transiently cotransfected with a luciferase reporter construct along with a β-galactosidase expression plasmid to normalize for transfection efficiency using the FuGene transfection reagent (Promega) according to the manufacturer’s protocol. For cells not expressing endogenous MRs (HEK293 and Cos1), an MR expression plasmid was added containing the full-length human MR cDNA (47) cloned into the CMX expression vector with an N-terminal hemagglutinin (HA) tag, as described (48). The MR reporter plasmid contains the mouse mammary tumor virus (MMTV) long-terminal repeat, which contains multiple glucocorticoid receptor and MR response elements (MREs) cloned into the PGL2 luciferase reporter vector (Promega) (48, 49). Reporter plasmids containing the FK506-binding protein-51 (FKBP51) steroid response elements (SRE1, SRE2, SRE2/SRE1, SRE2 mutant) were a gift from J. G. Scammell (50). As they are not easily transfected, human coronary artery SMCs (CO396) were infected with the MMTV-luciferase reporter adenovirus, as described (26). Cells were treated with aldosterone (10 nM or 100 nM, as indicated) or AngII (10 nM to 1 µM, as indicated) with or without the MR antagonist spironolactone (1 µM) or eplerenone (4 µM), the AT1R blocker losartan (10 µM), or the pharmacologic PKC inhibitors bisindolylmaleimide I (2 μM, a global PKC inhibitor), GO6976 (2 μM, an α/β isoform–specific PKC inhibitor), GO6983 (1 μM, a PKCα/β/γ/δ/ζ isoform inhibitor), and rottlerin (1 μM, a PKCδ-specific inhibitor) for 24 hours. Cells were lysed in reporter lysis buffer (Promega) and luciferase (luciferase assay system, Promega), and β-galactosidase assays (Tropix) were performed according to the manufacturers’ protocols as previously described (26, 48). Each treatment was carried out in triplicate, and each experiment was performed a minimum of three times as indicated in the figure legends.
Confocal fluorescence microscopy
Pac1 cells were cultured in 12-well tissue culture plates on coverslips in phenol red–free DMEM with 10% BGS. The cells were switched to DMEM serum-free medium for 24 hours followed by treatment with vehicle, AngII (1 μM), or aldosterone (10 nM) in the presence or absence of rottlerin (1 μM) for another 24 hours. The cells were fixed in 3.7% paraformaldehyde for 10 minutes and permeabilized with 0.3% Triton X-100 for 10 minutes. After blocking with 10% donkey serum for 1 hour, immunostaining was performed by incubating the cells with mouse anti-MR [1:50; Developmental Studies Hybridoma Bank (DSHB), catalog no. rMR1-18 1D5 (51)] for 1 hour and washing three times with PBS, followed by incubation with Cy3-conjugated donkey anti-mouse secondary antibody [1:500; Jackson ImmunoResearch Laboratories, catalog no. 715-165-150 (52)]. The images were acquired with a Nikon A1 laser scanning confocal microscope with ×10 objective magnification, a 561-nm laser, and 1024 pixels.
Small interfering RNA transfection
Small interfering RNAs (siRNAs) targeting MR or PKCδ were obtained from Integrated DNA Technologies. Human coronary artery SMCs (CO396) were transfected with control siRNA, PKCδ-targeted siRNA, or MR-targeted siRNA using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer’s protocol. PKCδ and MR knockdown were confirmed by immunoblotting.
Immunoprecipitation and immunoblotting
For the coimmunoprecipitation experiments, Pac1 cells were transfected with empty expression vector or one expressing full-length human HA-MR (amino acids 1 to 902 tagged with HA on the N terminus) or the MR ABC domain (amino acids 1 to 670) by FuGene transfection for 24 hours. Pac1 cells were grown until ∼80% confluence and then switched to phenol red–free, serum-free medium for 24 hours, followed by treatment with vehicle, AngII, rottlerin, or AngII plus rottlerin for 20 minutes. Cells were harvested in lysis buffer [20 mM Tris-Cl (pH 7.5), 0.137 M NaCl, 2 mM EDTA (pH 7.4), 1% Triton X-100, 10% glycerol, and 25 mM β-glycerol phosphate]. Phenylmethylsulfonyl fluoride and protease inhibitor mixture were added just prior to cell lysis on ice for 2 hours. After centrifugation, the supernatant was incubated at 4°C overnight with 5 μg of nonimmune mouse IgG or mouse anti-MR antibody [DSHB, catalog no. rMR1-18 1D5 (51)]. Protein G beads (Amersham Biosciences) were then added, followed by further incubation at 4°C for 2 hours. The pellets obtained after centrifugation were washed five times with wash buffer [50 mM Tris (pH 7.5), 7 mM MgCl2, 2 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride]. The washed immunopellets were then used for immunoblotting or in vitro phosphorylation assays. Cell lysates or immunopellets were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore), and probed with the indicated primary antibody. Antibodies used include mouse anti-MR antibody [1:100; DSHB, catalog no. rMR1-18 1D5 (51)], mouse anti–phosphorylated (phospho)-serine [1:500; Sigma-Aldrich, catalog no. P5747 (53)], mouse anti–phospho-threonine [1:1000; Cell Signaling Technology, catalog no. 9386 (54)], rabbit anti-PKCδ [1:500; Cell Signaling Technology, catalog no. 9616 (55)], and rabbit anti-GAPDH [1:2000; Cell Signaling Technology, catalog no. 5174 (56)], followed by incubation with anti-mouse [1:1000; Cell Signaling Technology, catalog no. 7076 (57)] or anti-rabbit [1:1000; Cell Signaling Technology, catalog no. 7074 (58)] horseradish peroxidase secondary antibody and developed with enhanced chemiluminescence reagent (Fisher Scientific).
In vitro phosphorylation assay
MR immunopellets and nonimmune pellets from Pac1 cells overexpressing the MR ABC domain were mixed with recombinant active PKCδ (SignalChem) or kinase buffer as a control, along with ATP mix and PKC lipid activator for 20 minutes at 30°C. After the incubation period, the reaction was terminated by adding an equal volume of 2× protein sample buffer and heating to 95°C for 10 minutes, followed by immunoblotting with anti–phospho-serine (53), anti–phospho-threonine (54), or anti-MR antibody (51). MAPK kinase-7 (MKK7) protein after incubation with or without a kinase known to phosphorylate MKK7 at threonine residues are included as a positive and negative control, respectively, for the phospho-threonine immunoblot (59).
Quantitative RT-PCR
Total RNA was isolated using an RNeasy mini kit (Qiagen) from human coronary artery SMCs after transfection with control siRNA, PKCδ-targeted siRNA, or MR-targeted siRNA, as well as treatment with hormones as indicated for 16 hours. RNA was reverse transcribed using a QuantiTect reverse transcription kit (Qiagen), and quantitative RT-PCR was carried out using SsoFast EvaGreen supermix (Bio-Rad Laboratories). The FKBP51 mRNA level was normalized to GAPDH, and data were presented as fold change vs vehicle-treated cells using the 2−ΔΔCT method. The primer sequences are as follows: FKBP51, forward, 5′- threshold CATCAAGGCATGGGACATTGG-3′ and reverse, 5′-TCGAGGGAATTTTAGGGAGACT-3′; GAPDH, forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′.
Smooth muscle viability assay
Pac1 cells were seeded in a 96-well plate at a density of 2000 cells per well in phenol red–free DMEM with 10% BGS for 6 hours. After the cells adhered to the dish, they were switched to phenol red–free, serum-free medium containing vehicle, aldosterone, or AngII, with or without the MR antagonist spironolactone, the PKCδ inhibitor rottlerin, spironolactone alone, or rottlerin alone. After 48 hours, the number of viable cells was quantified using a CellTiter-Glo luminescent cell viability assay (Promega) according to the manufacturer’s protocol.
Statistical analysis
All values are reported as mean fold change compared with vehicle controls ± SEM. Within-group differences were assessed with one-way ANOVA with the exception of Fig. 5A, for which a Student t test was used, as there are only two groups. Individual comparisons were made using the appropriate post hoc test based on the normality and distribution of the data to adjust for multiple testing. P < 0.05 was considered significant.
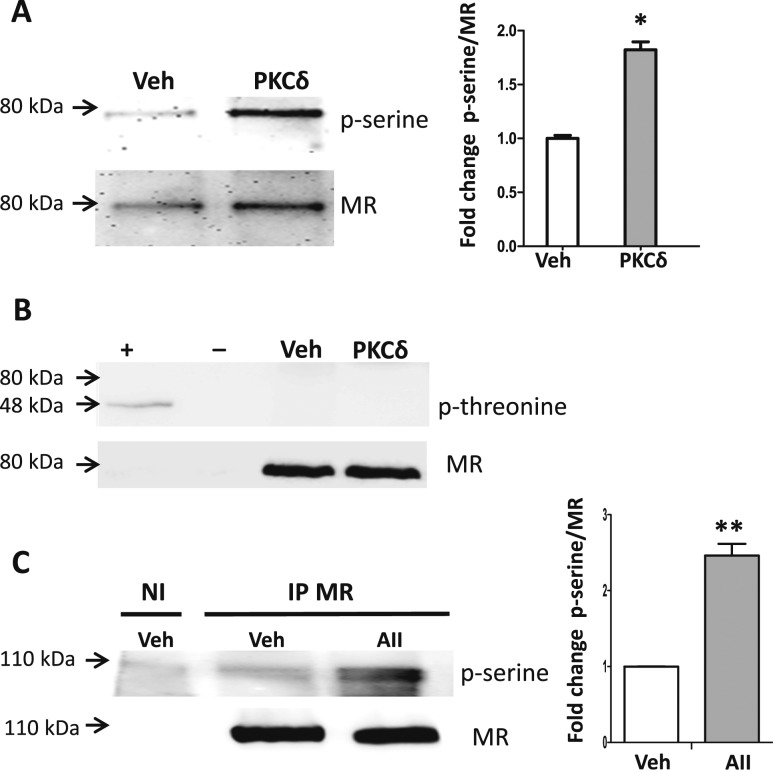
Figure 5.
PKCδ enhances MR N terminus serine phosphorylation in vascular SMCs. (A and B) The MR N-terminal ABC domain was overexpressed in Pac1 SMCs. MR was immunoprecipitated (IP) and the immunopellet was incubated with kinase buffer (Veh) or recombinant PKCδ and immunoblotted for phospho-serine, phospho-threonine, or MR. (A) Representative blot for phospho-serine with quantified phospho-serine normalized to total MR ABC protein. n = 3 experiments. *P < 0.05 vs vehicle (by Student t test). (B) Immunoblotting for phospho-threonine revealed a band only in the positive control despite ample MR ABC expression (80 kDa). Positive and negative controls are the MKK7 protein (48 kDa) after incubation with (+) or without (−) an enzyme known to phosphorylate MKK7 at threonine residues. (C) Pac1 cells overexpressing full-length MR were treated with vehicle or AngII (AII, 1 μM) for 2 h. MR was immunoprecipitated (IP) with nonimmune (NI) IgG as a control. Phospho-serine was quantified normalized to total full-length MR protein. n = 4 experiments. **P < 0.01 vs vehicle (by Student t test).
Results
AngII activates MR transcriptional activity in vascular cell lines
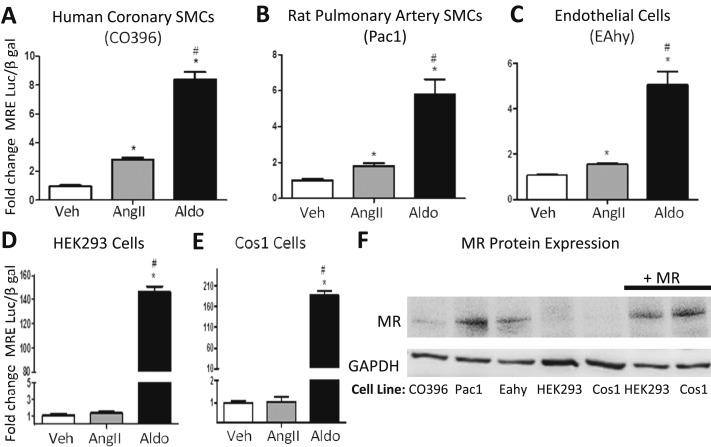
We previously demonstrated that AngII activates MR-mediated gene transcription in human coronary artery SMCs and that this effect was inhibited by both AT1R blockade and MR antagonism (26). We now confirm and extend this finding by quantifying AngII induction of MR-driven gene transcription in other vascular and nonvascular cell lines. The classical MR-responsive MMTV-luciferase reporter was used to quantify the fold increase in promoter activity induced by AngII (1 μM) compared with aldosterone (100 nM) in cell lines derived from human coronary artery SMCs (CO396), rat pulmonary artery SMCs (Pac1), and hybrid human umbilical vein endothelial cells (EAhy926) compared with commonly used lines, including HEK293 cells and Cos1 cells (Fig. 1A–1E). AngII significantly induces endogenous MR-mediated gene transcription in these vascular SMC and endothelial cell lines. AngII did not impact MR promoter activity in HEK293 and Cos1 cell lines, despite exogenous overexpression of MR. The expression level of endogenous MR is confirmed in the vascular cell lines and is lacking in these HEK293 and Cos1 cells and thus was overexpressed in the HEK293 and Cos1 cells to levels similar to those of vascular cells (Fig. 1F).
Figure 1.
AngII activates MR transcriptional activity in vascular cell lines. Cells were transfected with an MRE-luciferase transcriptional reporter. An MR expression plasmid was also added to the MR-negative HEK293 and Cos1 cell lines. Transfected cells were treated with vehicle (Veh), aldosterone (Aldo, 100 nM), or AngII (1 µM) for 24 h. Luciferase reporter activity was quantified relative to vehicle-treated cells. The fold increase in MRE-mediated gene transcription was quantified in response to AngII or Aldo in the following vascular cell lines: (A) human coronary artery SMCs (CO396), (B) rat pulmonary SMCs (Pac1), (C) human endothelial cells (EAhy926), (D) HEK293 cells, or (E) Cos1 cells. n = 3 to 8 experiments. *P < 0.05 vs Veh, #P < 0.05 vs AngII (by one-way ANOVA). (F) The endogenous level of MR protein is demonstrated in CO396, Pac1, EAhy, HEK293, and Cos1 cells and in HEK293 and Cos1 cells overexpressing human MR (+MR).
PKCδ is necessary for AngII-induced activation of MR
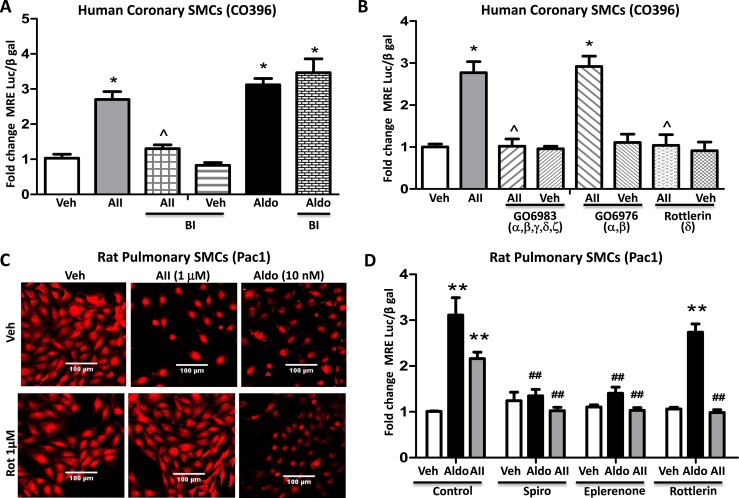
To begin to explore the mechanism of AngII-induced MR activation, the impact of AngII on MRE reporter activity in human coronary artery SMCs (CO396) was compared in the presence of pharmacologic inhibitors of known AngII-activated signaling pathways [reviewed in (60)]. Although the tyrosine kinase inhibitor PP2 (10 nM), MAPK inhibitor PD98059 (50 μM), and the Rho kinase inhibitor Y-27632 (10 μM) did not attenuate the impact of AngII on MRE activity (data not shown), the nonselective PKC inhibitor bisindolylmaleimide (2 μM) significantly reduced AngII-induced MR reporter activity without affecting aldosterone-mediated activation of MR (Fig. 2A). More specific pharmacological inhibitors were next tested to interrogate which of the 11 PKC isoforms might contribute to AngII-induced MR activation. In addition to the nonselective PKC inhibitor bisindolylmaleimide, the PKCα/β/γ/δ/ζ inhibitor GO6983 (1 μM) and the PKCδ inhibitor rottlerin (1 μM), but not the PKCα/β inhibitor GO6976 (2 μM), prevented AngII-mediated MR activation (Fig. 2B). The mechanism was further interrogated in Pac1 cells in serum-free media comparing the AngII effect to that of aldosterone at the more physiologically relevant and MR-specific concentration of 10 nM. Fluorescence confocal microscopy with MR-specific antibody demonstrated that AngII, similar to aldosterone, induces nuclear translocation of the MR, confirming prior studies in human coronary SMCs (26). Only MR localization induced by AngII, but not aldosterone, was attenuated by PKCδ inhibition with rottlerin (Fig. 2C). AngII similarly activated MRE reporter activity in Pac1 cells comparable to the level of activation by 10 nM aldosterone, and this was inhibited by either the MR antagonist spironolactone or the less potent but more specific inhibitor eplerenone (Fig. 2D). Finally, the PKCδ inhibitor rottlerin also blocked MR activation by AngII, but not by aldosterone, in Pac1 cells (Fig. 2D).
Figure 2.
PKCδ contributes to AngII, but not aldosterone, activation of SMC-MR. (A and B) Human coronary artery SMCs were infected with the MR-responsive MMTV-luciferase reporter adenovirus followed by treatment with aldosterone (Aldo, 100 nM) or AngII (AII, 1 µM) with or without PKC inhibitors. (A) The nonselective PKC inhibitor bisindolylmaleimide (BI, 2 μM) attenuated AngII, but not Aldo, activation of MR. (B) AngII activation of MR transcriptional activity is prevented by the pharmacologic inhibitors GO6983 (1 μM, PKCα/β/γ/δ/z inhibitor) and rottlerin (1 μM, PKCδ inhibitor) but not by GO6976 (2 μM, PKCα/β inhibitor). n = 4 experiments. *P < 0.05 vs vehicle (Veh), ^P < 0.05 vs AngII alone (by one-way ANOVA). (C) Pac1 cells were treated with aldosterone (Aldo, 10 nM) or AngII (AII, 1 µM) for 24 h in the presence or absence of PKCδ inhibitor rottlerin (1 μM) and MR localization was determined by fluorescence confocal microscopy. Representative images are shown. (D) Pac1 cells were transfected with the MMTV-luciferase reporter plasmid followed by treatment with aldosterone (Aldo, 10 nM) or AngII (AII, 1 µM) with or without the MR antagonist spironolactone (Spiro, 1 µM) or eplerenone (4 µM) or the PKCδ inhibitor rottlerin (1 μM) for 24 h. n = 5 experiments. **P < 0.01 vs Veh, ##P < 0.01 vs AngII or Aldo alone (by two-way ANOVA).
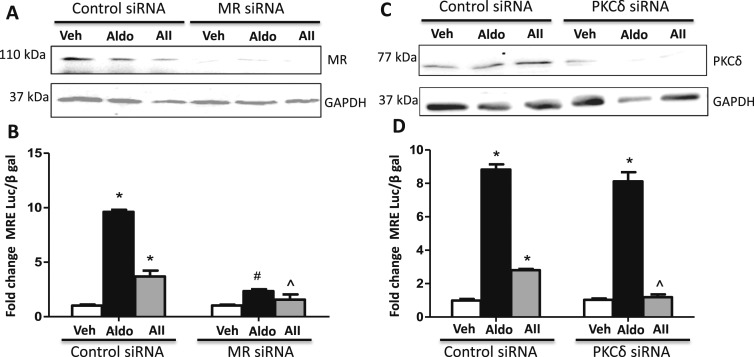
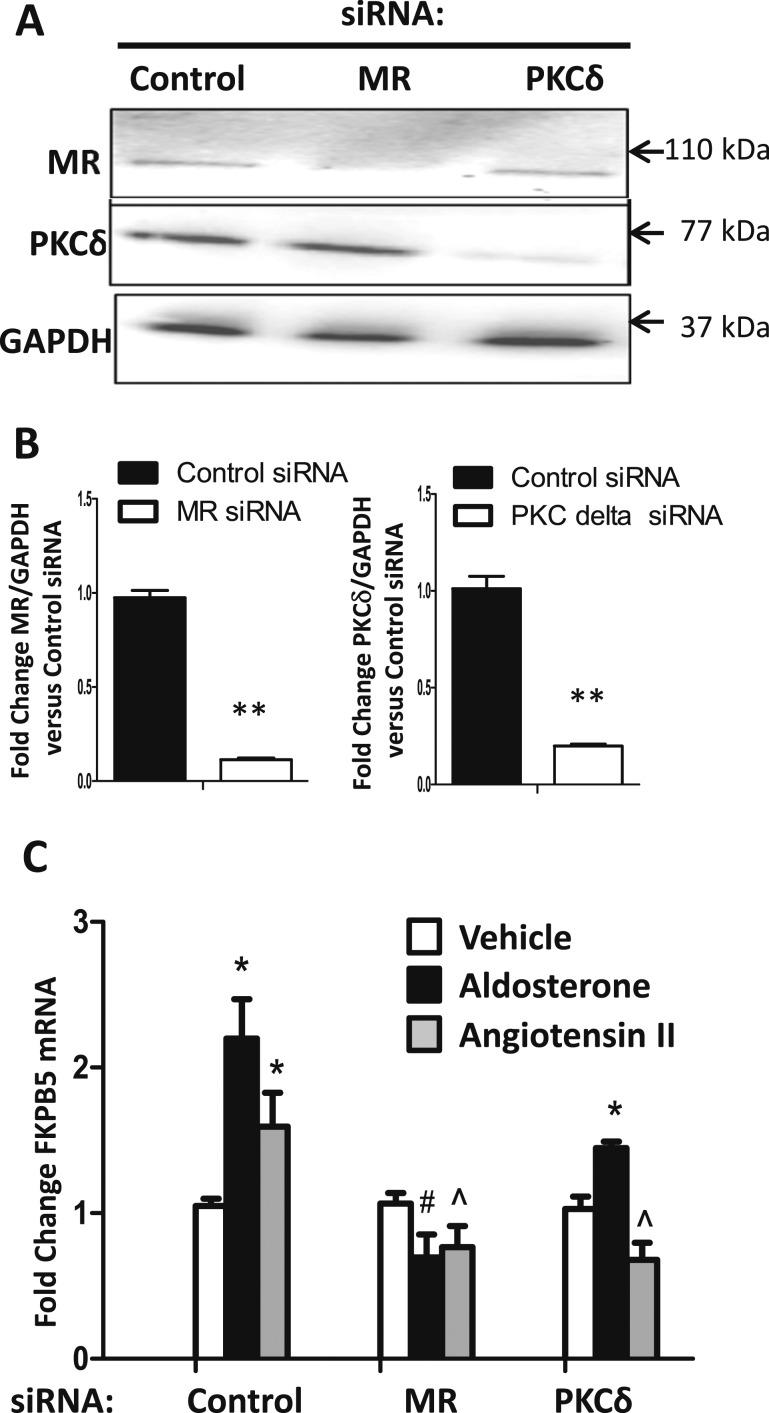
Based on these pharmacologic data, the specific roles of MR and PKCδ were further tested by siRNA knockdown. Human coronary artery SMCs (CO396) were transfected with control siRNA, MR-targeted siRNA, or PKCδ-targeted siRNA followed by infection with an MRE-luciferase reporter adenovirus (Fig. 3). MR and PKCδ knockdown were confirmed by immunoblotting (Fig. 3A and 3C). The MRE-luciferase reporter assay demonstrated that knockdown of MR diminished both AngII- and aldosterone-mediated MR transcriptional activation (Fig. 3B), whereas knockdown of PKCδ diminished only AngII-mediated MR transcriptional activation without altering the impact of the ligand aldosterone (Fig. 3D). Taken together, these data support the concept that PKCδ contributes to AngII, but not to aldosterone, induction of MR nuclear translocation and transcriptional regulatory function in human and rodent SMCs.
Figure 3.
AngII activation of MR transcriptional activity requires PKCδ and MR. Human coronary artery SMCs were transfected with (A and B) control siRNA, MR-targeted siRNA or (C and D) PKCδ-targeted siRNA followed by infection with an MRE-luciferase reporter adenovirus. Representative immunoblots show the degree of (A) MR or (C) PKCδ knockdown. (B) MR knockdown diminished both AngII-mediated (AII, 1 μM) and aldosterone-mediated (Aldo, 100 nM) MR transcriptional activation. (D) PKCδ knockdown diminished only AII-mediated MR transcriptional activation. n = 4 experiments. *P < 0.05 vs vehicle (Veh), ^P < 0.05 vs AngII plus control siRNA, #P < 0.05 vs Aldo plus control siRNA (by one-way ANOVA).
PKCδ forms a complex with MR in SMCs in association with increased MR serine phosphorylation upon AngII stimulation
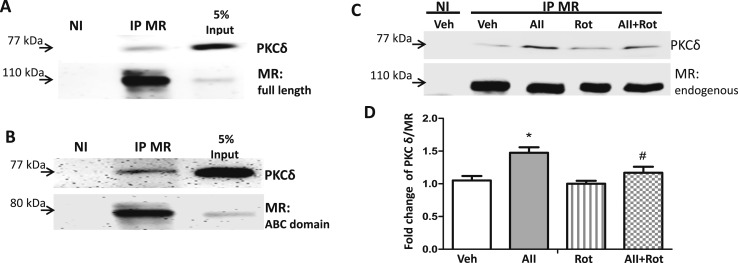
To further explore the mechanism by which PKCδ may contribute to AngII-induced MR activation, we first examined whether PKCδ and MR can form a complex in SMCs. Pac1 SMCs were transfected with the full length or the N terminus only of human MR expression plasmid, and cell lysates were subjected to immunoprecipitation (IP) using anti-MR antibody or nonimmune IgG (negative control) followed by immunoblotting for MR and PKCδ (Fig. 4). PKCδ was detected in the immunopellet from the MR IP but not in the nonimmune IgG control pellet, supporting that the two proteins form a complex in SMCs (Fig. 4A). MR IP of lysate from Pac1 cells overexpressing the MR ABC domain, which includes the N-terminal activation function (A/B domain) and DNA binding domain (C domain) but lacks the C-terminal hinge and ligand-binding domains (no DEF domains) (47), similarly pulled down PKCδ (Fig. 4B). To further investigate whether endogenous MR can form a complex with PKCδ in vascular cells and to determine the impact of AngII on this interaction, IP of MR was performed in nontransfected Pac1 cells. SMCs grown in phenol red–free, serum-free medium for 24 hours were treated for 20 minutes with vehicle or AngII in the presence or absence of rottlerin. Immunoblotting confirmed that PKCδ is part of a complex that immunoprecipitates with endogenous MR in vascular SMCs and that AngII treatment significantly enhances the PKCδ/MR ratio in the complex, an effect that was blocked by the PKCδ inhibitor rottlerin (Fig. 4C and 4D).
Figure 4.
MR and PKCδ form a complex in vascular SMCs and AngII enhances the interaction. IP was performed with anti-MR antibody or nonimmune (NI) IgG using cell lysates of Pac1 cells: (A) overexpressing full-length MR; (B) overexpressing the MR N-terminal ABC domain; or (C) with endogenous MR. (C and D) The cells with endogenous MR alone were treated with vehicle (Veh), AngII (AII, 1 μM), rottlerin (Rot, 1 μM), or AII plus Rot for 20 min prior to IP. (D) Quantification of PKCδ normalized to total endogenous MR. n = 6 experiments. *P < 0.05 vs Veh, #P < 0.05 vs AII alone (by one-way ANOVA).
To test whether PKCδ has the potential to contribute to MR phosphorylation, MR was immunoprecipitated from Pac1 cells overexpressing the MR ABC domain. The MR immunopellet was extensively washed and then incubated with recombinant active PKCδ enzyme or kinase buffer alone as a control. Immunoblotting of the resulting MR pellet was performed to quantify phospho-serine, phospho-threonine, and total MR ABC domain protein (80 kDa). Addition of recombinant PKCδ significantly increased MR serine phosphorylation (Fig. 5A) but did not affect phospho-threonine (Fig. 5B), as there was no band at the predicted MR ABC domain size (80 kDa) despite evidence of ample MR ABC protein in the immunoprecipitate and a positive and negative control for the anti–phospho-threonine antibody (MKK7, a protein known to be threonine phosphorylated after incubation with (positive control) and without (negative control) the appropriate MKK7 threonine kinase). These data support that incubation with PKCδ in vitro increases MR phosphorylation at serine residues in the MR ABC domain. We further examined serine phosphorylation of full-length MR expressed in Pac1 SMCs after treatment with vehicle vs AngII for 2 hours followed by MR IP and immunoblot for MR and phospho-serine. AngII treatment significantly increased serine phosphorylation of full-length MR in vivo in SMCs (Fig. 5C).
AngII regulates FKBP51 mRNA expression and gene transcription via a PKCδ- and MR-dependent mechanism
The data in Figs. 1–3 demonstrate that AngII can activate MR transcriptional regulation of an idealized MR-responsive reporter (MMTV-luciferase). To determine whether AngII activation of SMC-MR can modulate transcription of an endogenous vascular MR target gene, we focused on FKBP51, a gene we previously identified as an aldosterone-regulated MR target gene in SMCs (61). We examined the impact of AngII on FKBP51 mRNA expression in SMCs and explored whether PKCδ is involved (Fig. 6). Human coronary artery SMCs were transfected with control siRNA, MR-targeted siRNA, or PKCδ-targeted siRNA, and reproducible knockdown was confirmed by immunoblotting (Fig. 6A and 6B). Cells were then treated with aldosterone or AngII for 24 hours and FKBP51 mRNA expression was quantified. AngII increased FKBP51 mRNA expression in SMCs in an MR-dependent manner (Fig. 6C). Knockdown of PKCδ significantly diminished AngII induction but not aldosterone induction of FKBP51 mRNA expression (Fig. 6C). These data support that, similar to aldosterone, AngII increases FKBP51 expression via the MR in SMCs and that PKCδ is specifically required for AngII-mediated FKBP51 gene regulation.
Figure 6.
AngII regulates expression of the MR target gene FKPB5 in vascular SMCs via PKCδ and MR. Human coronary artery SMCs (CO396) were transfected with control siRNA, PKCδ-targeted siRNA, or MR-targeted siRNA for 48 h. (A) Representative immunoblot and (B) quantification confirms MR and PKCδ knockdown. n = 4 experiments. **P < 0.01 vs control siRNA. (C) After knockdown, cells were treated with vehicle, aldosterone (100 nM), or AngII (1 μM) and FKBP51 mRNA expression was quantified by qRT-PCR and expressed relative to vehicle and control siRNA-treated cells. N = 4 experiments. *P < 0.05 vs respective vehicle, #P < 0.05 vs control siRNA plus aldosterone, ^P < 0.05 vs control siRNA plus AngII (by one-way ANOVA).
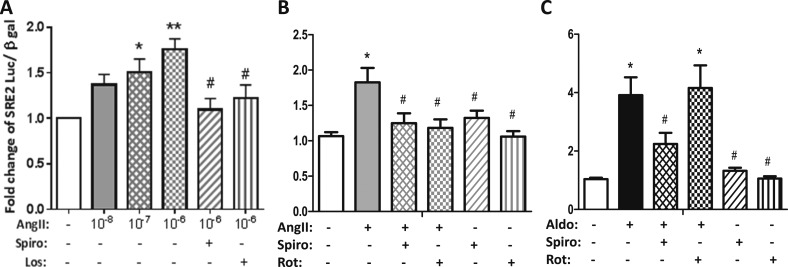
To explore whether AngII regulates FKBP51 through an MR-dependent traditional steroid-responsive genomic mechanism, we first identified the MR-responsive promoter element in the FKBP51 gene. Using a series of previously validated FKBP51 promoter-reporter vectors (50, 62), we compared the transactivation capacity of aldosterone via MR to that of cortisol via the glucocorticoid receptor and determined that MR activates the same glucocorticoid receptor-responsive element previously termed SRE2 that is located in intron 5 of the FKBP51 gene (data not shown) (50, 62). Next, Pac1 cells were transfected with the FKBP51 SRE2-luciferase reporter and treated with AngII (10 nM to 1 μM). AngII activated the FKBP51 SRE2 reporter in a dose-dependent manner that was blocked by the AT1R blocker losartan (Fig. 7A). AngII induction of FKBP51 SRE2 reporter activity was inhibited by the MR antagonist spironolactone and the PKCδ inhibitor rottlerin (Fig. 7A and 7B), whereas aldosterone-mediated FKBP51 reporter transactivation was abolished only by spironolactone with no effect of PKCδ inhibition (Fig. 7C). Thus, Figs. 6 and 7 demonstrate that AngII can regulate an endogenous SMC-MR target gene, FKBP51, by modulation of gene transcription via the steroid-responsive SRE2 binding site and that the mechanism requires AT1R, PKCδ, and MR, whereas aldosterone-mediated FKBP51 transcriptional regulation is dependent only on MR and not on PKCδ.
Figure 7.
AngII regulates the FKBP51 steroid-responsive promoter in SMCs via the AT1R, PKCδ, and the MR. Pac1 SMCs were transfected with the FKBP51 SRE2-luciferase reporter plasmid. (A) Cells were treated with AngII at increasing doses in the presence or absence of the MR antagonist spironolactone (Spiro, 1 μM) or the AT1R antagonist losartan (Los, 10 μM). AngII activated the SRE2 promoter in an MR and AT1R-dependent manner. Cells were treated with (B) AngII (1 μM) or (C) aldosterone (Aldo, 100 nM) in the presence or absence of the MR antagonist spironolactone (Spiro, 1 μM) or the PKCδ inhibitor rottlerin (Rot, 1 μM). n = 3 to 7 experiments. *P < 0.05 vs vehicle, **P < 0.01 vs vehicle, #P < 0.05 vs AngII or Aldo alone (by one-way ANOVA).
PKCδ mediates the AngII-induced increase in viable SMCs
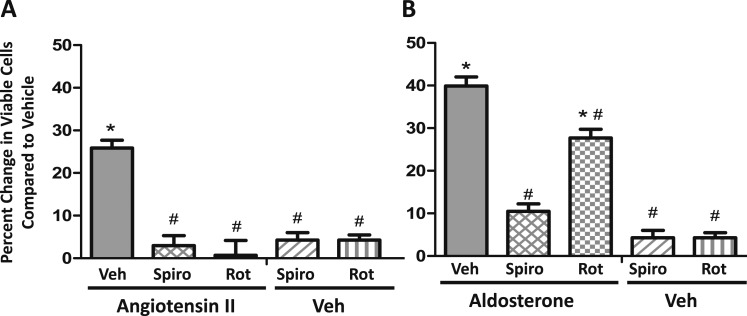
It is well known that vascular SMC proliferation plays an important role in vascular physiology and pathophysiology and that AngII promotes SMC proliferation in vitro and in vivo [reviewed in (63)]. In this study, we confirm that exposure of Pac1 SMCs to AngII for 48 hours in serum-free media significantly increases the number of viable SMCs by 25%. This effect of AngII to increase viable SMCs is blocked by the MR antagonist spironolactone and the PKCδ inhibitor rottlerin (Fig. 8A). In contrast to AngII, the impact of aldosterone on viable SMCs is blocked by spironolactone but not rottlerin (Fig. 8B), whereas spironolactone and rottlerin alone do not significantly impact the number of viable vehicle-treated cells (Fig. 8).
Figure 8.
AngII increases viable SMCs in an MR- and PKCδ-dependent manner. After plating the same number of Pac1 SMCs in serum for 6 h, cells were changed to serum- free media for 24 h and then treated for 48 h in serum-free medium with vehicle (Veh) compared with (A) AngII (1 μM) or (B) aldosterone (100 nM) and viable cells were quantified. Each hormone was tested with or without the MR antagonist spironolactone (Spiro, 1 μM), the PKCδ inhibitor rottlerin (Rot, 1 μM), or with Spiro or Rot alone. Results are expressed as the percentage increase in viable cells after 48 h of treatment relative to vehicle alone. n = 7 experiments. *P < 0.05 vs Veh, #P < 0.05 vs AngII or aldosterone alone (by one-way ANOVA).
Discussion
In summary, this study demonstrates that AngII activates MR transcriptional activity in vascular cell lines and that the mechanism is dependent on PKCδ signaling and contributes to SMC gene expression and function. Based on our results, we propose a model depicted in Fig. 9 that is consistent with ligand-independent MR activation by AngII in SMCs. In response to stimulation of vascular SMCs with AngII, the G protein–coupled AT1R has been found to initiate a signaling cascade that phosphorylates and activates PKCδ (64). In this study, we show that AngII promotes formation of a complex in vascular SMCs that contains PKCδ, the MR, and potentially other proteins. AngII treatment is also associated with increased serine phosphorylation in the N terminus of the MR protein. This AngII-mediated PKCδ-dependent activation of the MR in SMCs results in increased expression of the SMC-MR target gene, FKBP51, via a steroid-responsive intronic promoter element. Furthermore, AngII promotes SMC proliferation (41), and the expected AngII-induced increase in the number of viable vascular SMCs is also dependent on MR and PKCδ. Combined with the traditional ligand-dependent mechanism of MR activation by aldosterone, this novel pathway may contribute to the adverse effects of RAAS activation on SMC function and the observed benefits of RAAS inhibition on cardiovascular disease outcomes, even in the absence of elevated aldosterone (65–67).
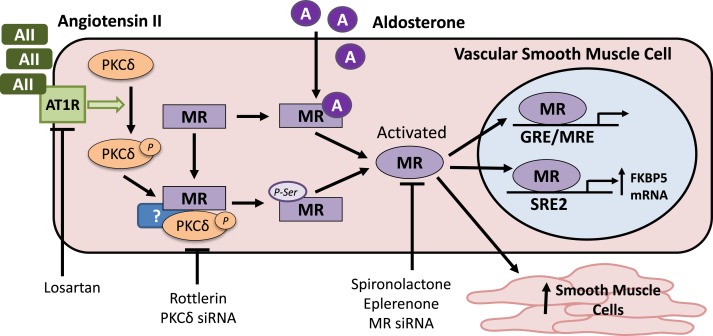
Figure 9.
Proposed model for AngII-mediated MR activation in vascular SMCs. AngII (AII) acts through the AT1R to phosphorylate (p) and activate PKCδ. AII induces formation of a complex in vascular SMCs that includes MR and PKCδ (and potentially other proteins, indicated by “?”). This is associated with enhanced MR phosphorylation on serine residues in the N terminus. Aldosterone (A), the traditional MR ligand, activates the MR by binding to the C-terminal ligand binding domain in a manner independent of PKCδ. Thus, AII activates the MR by a mechanism distinct from its activation by aldosterone. By this PKCδ-dependent mechanism, AII can: induce gene transcription via a traditional glucocorticoid receptor/MR responsive element (GRE/MRE)–driven promoter; increase expression of the SMC-MR target gene FKBP51 via the intronic SRE (SRE2); and enhance SMC number by enhancing survival and/or proliferation.
The MR is a member of the steroid hormone receptor family that includes the sex steroid receptors for estrogen, progesterone, and androgen as well as the glucocorticoid receptor. These receptors are hormone-activated transcription factors that regulate gene transcription in response to hormone binding to their respective C-terminal ligand-binding domains, resulting in conformational change and translocation to the nucleus [reviewed in (68)]. In addition to this traditional mechanism, ample data reveal that the transcriptional regulatory function of all of these other steroid receptors can also be activated in a hormone-independent manner via growth factor or G protein receptor signaling pathways that result in phosphorylation of the N-terminal receptor A/B domain (42–46). This is best characterized for the estrogen receptor α, which is phosphorylated in the AB domain on serine 118 in response to growth factor signaling resulting in nuclear translocation and recruitment to gene-specific promoters to regulate transcription in a fashion that is inhibited by traditional estrogen receptor antagonists (44–46). The current study, combined with our published work (26), supports a similar paradigm for ligand-independent activation of the MR. Specifically, we show that AngII induces MR nuclear localization and activates MR-mediated gene transcription in media lacking traditional ligands and that this is associated with enhanced MR serine phosphorylation, potentially in the AB domain, similar to the impact of growth factor signaling on the estrogen receptor. Although this could be mediated by a direct interaction with, and phosphorylation by, PKCδ of the MR AB domain, our data are also consistent with the possibility that other proteins are required for complex formation and/or phosphorylation, and that other proteins are phosphorylated to mediate MR activation, as well as the potential for an interaction with the MR C terminus via dimerization with endogenous MR in the cells transfected with the N-terminal MR mutant.
The MR has also been found to be activated in a ligand-independent fashion by the oxidative stress–dependent Rho family small GTPase Rac1, which contributes to cardiac and renal disease [reviewed in (69)]. Interestingly, the MR has the largest N-terminal AB domain of all of the steroid receptors, containing >600 amino acids, of which >20 are serine residues that have been previously shown to be dynamically phosphorylated (70). Thus, substantial additional investigation is needed to determine the degree to which specific residues are involved in AngII-mediated MR activation. Previous data, derived predominantly in kidney cells, supports that the phosphorylation state of the MR is dynamically modulated in response to a variety of stimuli to regulate MR protein stability and function [reviewed in (70)]. In rat renal cortical collecting duct cells, aldosterone causes rapid MR phosphorylation, resulting in increased Na+/K+-ATPase α1 transcription (71). In kidney intercalated cells, the phosphorylation status of the MR ligand-binding domain (S843-P) determined aldosterone responsiveness (72). In this study, we show that AngII can activate MR transcriptional activity also in vascular SMC and EC lines in serum-free, phenol red–free media that lacks traditional ligands.
Multiple prior studies have demonstrated crosstalk between aldosterone and AngII signaling in vascular SMCs [reviewed in (30)]. Aldosterone and AngII synergistically enhance vascular SMC proliferation, inflammation, migration, and hypertrophy in vitro (31, 37–41). Previously implicated mechanisms for AngII/aldosterone crosstalk in SMCs include synergistic activation of rapid signaling to serine/threonine kinases, MAPKs, and tyrosine kinases (30). Rapid phosphorylation of MAPKs by aldosterone was found to involve AT1R (31, 38). SMC-MR also enhanced AT1R expression in the aging vasculature via downregulation of the AT1R-targeted miRNA-155 (11). In light of MR increasing SMC AT1R expression (11, 33, 40, 73), AngII-mediated MR activity may reflect a positive feedback mechanism that exacerbates the harmful effects of AngII in vascular cells. These in vitro studies are supported by in vivo data using SMC-specific MR knockout mice demonstrating that SMC-MR is necessary for AngII-induced vascular oxidative stress, vasoconstriction, and blood pressure elevation (10, 11). Some studies have suggested that SMCs can produce aldosterone in response to AngII stimulation (41, 73, 74), although this has not been readily reproduced, and data specifically showing a lack of evidence of vascular aldosterone synthase expression and aldosterone production have also been published (26, 75, 76). The current study adds posttranslational modification involving PKCδ as an additional mechanism by which AngII and MR signaling are integrated in vascular SMCs.
This study further implicates PKCδ as a necessary mediator of AngII-induced MR activation in SMCs. AngII signaling through Gq to induce PKC activity has been previously demonstrated in multiple cell types [reviewed in (60)]. PKC has 11 isoforms, each with distinct roles. In vascular SMCs, AngII stimulation of PKCε signaling contributes to vasoconstriction (77), whereas PKCδ appears to be the major isoform mediating AngII-induced SMC proliferation, hypertrophy, migration, and the resulting adverse vascular remodeling (64, 78–80). In the present study, siRNA and pharmacological tools reveal a specific role for PKCδ in mediating AngII-induced MR transcriptional activity in vascular SMCs. Thus, MR activation may mediate a subset of AngII effects on SMCs that derive from PKCδ signaling, particularly those involved in vascular remodeling. Our data also show that PKCδ does not contribute to aldosterone-induced MR activation, supporting a distinct mechanism by which AngII vs aldosterone activates the MR in vascular cells. PKCδ activation was previously implicated in some of the kidney effects of AngII by stabilizing RNA-binding proteins in the nucleus that regulate expression of PAI-1 and COX-2 genes, although a role for MR was not tested in that study (81). Further studies are needed to determine whether this AngII–PKCδ–MR pathway contributes to the function of other nonepithelial cells, including adipocytes, leukocytes, and cells of the central nervous system.
We further explored the impact of AngII-induced MR activation on SMC gene expression. We previously characterized the aldosterone/MR-regulated transcriptome in blood vessels (12, 61) and identified FKBP51 as an MR-regulated gene in vascular SMCs (61). FKBP51 is a known co-chaperone for steroid hormone receptors, including the glucocorticoid, progesterone, and androgen receptors, thereby modulating hormone responsiveness of cells (82). An SRE in intron 5 of the FKBP51 gene confers glucocorticoid and progesterone responsiveness (50, 62). In this study, we demonstrate that aldosterone acting via the MR regulates FKBP51 gene expression in SMCs via the same SRE. We previously demonstrated a proatherogenic effect of aldosterone that is associated with increased vascular FKBP51 expression in atherosclerotic mice (61, 83). Conversely, MR inhibition decreased FKBP51 expression in vessels from patients with coronary artery disease (61). More recently, a role for FKBP51 in metabolic dysfunction has been identified (84). In the present study, we demonstrate that FKBP51 is also induced by AngII in SMCs through a mechanism that requires PKCδ, MR, and the SRE2 binding site. Thus, AngII activation of MRs can modulate expression of at least one known SMC-MR target gene. Further exploration of the global impact of AngII on the MR-regulated transcriptome in distinct cell types will determine how this might differ from the aldosterone-regulated transcriptome to mediate hormone and cell-specific transcriptional programs.
MR contributes to vascular SMC proliferation and adverse vascular remodeling in vivo in response to vascular injury, hypertension, and aging (8, 9, 12). MR blockade with spironolactone was previously shown to inhibit the proliferative effect of AngII in cultured rat aortic SMCs (41, 73). In this study, we confirm that MR blockade with spironolactone inhibits the proliferative effect of both AngII and aldosterone; however, PKCδ activity was only involved in the AngII affect, supporting divergent signaling pathways. Previous studies suggested that intrinsic SMC aldosterone production may mediate the MR-dependent proliferative effect of AngII (41, 73). However, we previously demonstrated that AngII induction of MR reporter activity in SMCs is not inhibited by an aldosterone synthase inhibitor (26). Thus, the current study further supports a distinct mechanism with AngII activating MR independent of ligand via PKCδ.
Several other observations from this study may be relevant to our understanding of RAAS function in the vasculature. HEK293 and Cos1 cell were insensitive to AngII-mediated MRE activation, even when MR was expressed at levels similar to SMCs and sufficient to mediate strong aldosterone induction of MRE reporter activity. Despite confirmation that those cells express PKCδ, this suggests that additional components, necessary for AngII-mediated MR activation, potentially including the AT1R, are absent in these cells and supports the potential for cell type specificity of this mechanism of MR activation. Also, AngII activation of MR transcriptional activity and the impact of AngII on gene expression and SMC viability were prevented by MR antagonism. Thus, a component of the benefits of MR inhibition observed in the vast preclinical and clinical literature [reviewed in (4)] may be due to inhibition of AngII-induced MR activation in SMCs. Such an interpretation might explain some of the benefits of MR inhibition in improving cardiovascular function and patient outcomes even in the absence of elevated aldosterone or cortisol levels.
We acknowledge several limitations to this study. First, all studies were performed in vitro using cultured cell lines with known limitations due to changes in cell physiology when cultured. Despite these limitations, in vitro studies allow for detailed mechanistic interrogations that would not be easy to interpret in vivo and cannot be performed in humans. To address this limitation, the studies herein were performed in multiple SMC cell lines and using both pharmacologic and genetic approaches. Such use of integrated data limits potential artifacts of a single cell line or approach. It is also reassuring that many of the mechanistic details determined in these in vitro studies are consistent with prior published studies in other cell types and correlate with findings in animal models in vivo. Second, although we find that MR and PKCδ can form a complex in SMCs and that AngII treatment enhances complex formation and serine phosphorylation of the MR ABC domain, we have not confirmed that PKCδ directly phosphorylates the MR, nor have we determined the specific amino acids involved due to the large size and number of serine residues in the MR AB domain. Finally, although these studies strongly implicate PKCδ in the mechanism of AngII-induced MR activation, the data support that there are likely other factors that contribute, which we have not yet identified. Thus, future investigations are needed to address these limitations.
In summary, this study reveals that AngII signaling via PKCδ activates the MR to modulate SMC gene transcription and increases the number of viable SMCs. Upon AngII stimulation, PKCδ forms a complex with MR in association with enhanced MR serine phosphorylation. This provides an additional novel mechanism for the synergistic effects of MR and AngII in vascular SMC function and the impact on cardiovascular disease (Fig. 9). Recent studies have shown that SMC-MR expression increases in the setting of vascular risk factors, particularly with aging, and contributes to adverse vascular remodeling (10, 11, 34). However, with aging, aldosterone levels decline both in mice and humans. This study supports the potential for ligand-independent activation of MR by AngII to enhance MR signaling and contribute to the adverse vascular impact of risk factors even in the absence of elevated aldosterone. This mechanism may contribute to the substantial therapeutic benefits of AT1R and MR antagonists in patients with cardiovascular disease.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants HL095590 and HL119290 (to I.Z.J.), American Heart Association Grants EIA18290005 (to I.Z.J.) and 11POST5390010 (to A.P.M.), and by São Paulo Research Foundation (FAPESP) Grant 2014/26192-6 to (A.P.D.).
Glossary
Abbreviations:
- AngII
angiotensin II
- AT1R
angiotensin type 1 receptor
- BGS
bovine growth serum
- DSHB
Developmental Studies Hybridoma Bank
- FKBP51
FK506-binding protein 51
- HA
hemagglutinin
- HEK293
human embryonic kidney-293
- HSD2
11β-hydroxysteroid dehydrogenase-2
- IP
immunoprecipitation
- MKK7
MAPK kinase-7
- MMTV
mouse mammary tumor virus
- MR
mineralocorticoid receptor
- MRE
mineralocorticoid receptor response element
- phospho-
phosphorylated
- PKCδ
δ isoform of protein kinase C
- RAAS
renin–angiotensin–aldosterone system
- siRNA
small interfering RNA
- SMC
smooth muscle cell
- SRE
steroid response element
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–325. [DOI] [PubMed] [Google Scholar]
- 2. Muñoz-Durango N, Fuentes CA, Castillo AE, González-Gómez LM, Vecchiola A, Fardella CE, Kalergis AM. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int J Mol Sci. 2016;17(7):E797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biwer LA, Wallingford MC, Jaffe IZ. Vascular mineralocorticoid receptor: evolutionary mediator of wound healing turned harmful by our modern lifestyle. Am J Hypertens. 2019;32(2):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DuPont JJ, Jaffe IZ. 30 Years of the mineralocorticoid receptor: the role of the mineralocorticoid receptor in the vasculature. J Endocrinol. 2017;234(1):T67–T82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawai T, Forrester SJ, O'Brien S, Baggett A, Rizzo V, Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res. 2017;125(Pt A):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barrett KV, McCurley AT, Jaffe IZ. Direct contribution of vascular mineralocorticoid receptors to blood pressure regulation. Clin Exp Pharmacol Physiol. 2013;40(12):902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28(8):1511–1518. [DOI] [PubMed] [Google Scholar]
- 8. Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34(2):355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63(3):520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18(9):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DuPont JJ, McCurley A, Davel AP, McCarthy J, Bender SB, Hong K, Yang Y, Yoo JK, Aronovitz M, Baur WE, Christou DD, Hill MA, Jaffe IZ. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight. 2016;1(14):e88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SK, McCurley AT, DuPont JJ, Aronovitz M, Moss ME, Stillman IE, Karumanchi SA, Christou DD, Jaffe IZ. Smooth muscle cell–mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension. 2018;71(4):609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balakumar P, Jagadeesh G. A century old renin–angiotensin system still grows with endless possibilities: AT receptor signaling cascades in cardiovascular physiopathology. Cell Signal. 2014;26(10):2147–2160. [DOI] [PubMed] [Google Scholar]
- 14. Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93(3):583–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol. 2012;350(2):256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGraw AP, McCurley A, Preston IR, Jaffe IZ. Mineralocorticoid receptors in vascular disease: connecting molecular pathways to clinical implications. Curr Atheroscler Rep. 2013;15(7):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. [DOI] [PubMed] [Google Scholar]
- 18. Hirooka Y, Kimura Y, Sagara Y, Ito K, Sunagawa K. Effects of valsartan or amlodipine on endothelial function and oxidative stress after one year follow-up in patients with essential hypertension. Clin Exp Hypertens. 2008;30(3–4):267–276. [DOI] [PubMed] [Google Scholar]
- 19. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J; Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709–717. [DOI] [PubMed] [Google Scholar]
- 20. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–1321. [DOI] [PubMed] [Google Scholar]
- 21. Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108(15):1831–1838. [DOI] [PubMed] [Google Scholar]
- 22. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. [DOI] [PubMed] [Google Scholar]
- 23. Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids. 2000;65(2):61–73. [DOI] [PubMed] [Google Scholar]
- 24. Wehling M. Rapid actions of aldosterone revisited: receptors in the limelight. J Steroid Biochem Mol Biol. 2018;176:94–98. [DOI] [PubMed] [Google Scholar]
- 25. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242(4878):583–585. [DOI] [PubMed] [Google Scholar]
- 26. Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96(6):643–650. [DOI] [PubMed] [Google Scholar]
- 27. Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol. 2007;27(4):799–805. [DOI] [PubMed] [Google Scholar]
- 28. Luther JM, Luo P, Wang Z, Cohen SE, Kim HS, Fogo AB, Brown NJ. Aldosterone deficiency and mineralocorticoid receptor antagonism prevent angiotensin II–induced cardiac, renal, and vascular injury. Kidney Int. 2012;82(6):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002;143(12):4828–4836. [DOI] [PubMed] [Google Scholar]
- 30. Lemarié CA, Paradis P, Schiffrin EL. New insights on signaling cascades induced by cross-talk between angiotensin II and aldosterone. J Mol Med (Berl). 2008;86(6):673–678. [DOI] [PubMed] [Google Scholar]
- 31. Lemarié CA, Simeone SM, Nikonova A, Ebrahimian T, Deschênes ME, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res. 2009;105(9):852–859. [DOI] [PubMed] [Google Scholar]
- 32. Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76(9):834–839. [DOI] [PubMed] [Google Scholar]
- 33. Ullian ME, Fine JJ. Mechanisms of enhanced angiotensin II–stimulated signal transduction in vascular smooth muscle by aldosterone. J Cell Physiol. 1994;161(2):201–208. [DOI] [PubMed] [Google Scholar]
- 34. Krug AW, Allenhöfer L, Monticone R, Spinetti G, Gekle M, Wang M, Lakatta EG. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010;55(6):1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lacolley P, Safar ME, Lucet B, Ledudal K, Labat C, Benetos A. Prevention of aortic and cardiac fibrosis by spironolactone in old normotensive rats. J Am Coll Cardiol. 2001;37(2):662–667. [DOI] [PubMed] [Google Scholar]
- 36. Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res. 2018;114(4):513–528. [DOI] [PubMed] [Google Scholar]
- 37. Mazak I, Fiebeler A, Muller DN, Park JK, Shagdarsuren E, Lindschau C, Dechend R, Viedt C, Pilz B, Haller H, Luft FC. Aldosterone potentiates angiotensin II–induced signaling in vascular smooth muscle cells. Circulation. 2004;109(22):2792–2800. [DOI] [PubMed] [Google Scholar]
- 38. Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res. 2005;97(5):434–442. [DOI] [PubMed] [Google Scholar]
- 39. Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, Tsukuda K, Iwai M, Horiuchi M. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res. 2007;76(3):506–516. [DOI] [PubMed] [Google Scholar]
- 40. Ullian ME, Hutchison FN, Hazen-Martin DJ, Morinelli TA. Angiotensin II-aldosterone interactions on protein synthesis in vascular smooth muscle cells. Am J Physiol. 1993;264(6 Pt 1):C1525–C1531. [DOI] [PubMed] [Google Scholar]
- 41. Xiao F, Puddefoot JR, Vinson GP. Aldosterone mediates angiotensin II-stimulated rat vascular smooth muscle cell proliferation. J Endocrinol. 2000;165(2):533–536. [DOI] [PubMed] [Google Scholar]
- 42. Amin KS, Jagadeesh S, Baishya G, Rao PG, Barua NC, Bhattacharya S, Banerjee PP. A naturally derived small molecule disrupts ligand-dependent and ligand-independent androgen receptor signaling in human prostate cancer cells. Mol Cancer Ther. 2014;13(2):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giulianelli S, Riggio M, Guillardoy T, Pérez Piñero C, Gorostiaga MA, Sequeira G, Pataccini G, Abascal MF, Toledo MF, Jacobsen BM, Guerreiro AC, Barros A, Novaro V, Monteiro FL, Amado F, Gass H, Abba M, Helguero LA, Lanari C. FGF2 induces breast cancer growth through ligand-independent activation and recruitment of ERα and PRBΔ4 isoform to MYC regulatory sequences. Int J Cancer. 2019;145(7):1874–1888. [DOI] [PubMed] [Google Scholar]
- 44. Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270(5241):1491–1494. [DOI] [PubMed] [Google Scholar]
- 45. Duplessis TT, Williams CC, Hill SM, Rowan BG. Phosphorylation of estrogen receptor α at serine 118 directs recruitment of promoter complexes and gene-specific transcription. Endocrinology. 2011;152(6):2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karas RH, Gauer EA, Bieber HE, Baur WE, Mendelsohn ME. Growth factor activation of the estrogen receptor in vascular cells occurs via a mitogen-activated protein kinase-independent pathway. J Clin Invest. 1998;101(12):2851–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268–275. [DOI] [PubMed] [Google Scholar]
- 48. Barrett Mueller K, Lu Q, Mohammad NN, Luu V, McCurley A, Williams GH, Adler GK, Karas RH, Jaffe IZ. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155(11):4461–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lombès M, Binart N, Oblin ME, Joulin V, Baulieu EE. Characterization of the interaction of the human mineralocorticosteroid receptor with hormone response elements. Biochem J. 1993;292(Pt 2):577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones. 2004;9(3):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. RRID:AB_1157909, https://scicrunch.org/resolver/AB_1157909.
- 52. RRID:AB_2340813, https://scicrunch.org/resolver/AB_2340813.
- 53. RRID:AB_477376, https://scicrunch.org/resolver/AB_477376.
- 54. RRID:AB_331239, https://scicrunch.org/resolver/AB_331239.
- 55. RRID:AB_10949973, https://scicrunch.org/resolver/AB_10949973.
- 56. RRID:AB_10622025, https://scicrunch.org/resolver/AB_10622025.
- 57. RRID:AB_330924, https://scicrunch.org/resolver/AB_330924.
- 58. RRID:AB_2099233, https://scicrunch.org/resolver/AB_2099233.
- 59. Calamaras TD, Baumgartner RA, Aronovitz MJ, McLaughlin AL, Tam K, Richards DA, Cooper CW, Li N, Baur WE, Qiao X, Wang GR, Davis RJ, Kapur NK, Karas RH, Blanton RM. Mixed lineage kinase-3 prevents cardiac dysfunction and structural remodeling with pressure overload. Am J Physiol Heart Circ Physiol. 2019;316(1):H145–H159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, Huang PL, Mendelsohn ME, Jaffe IZ. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol. 2011;31(8):1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144(6):2380–2387. [DOI] [PubMed] [Google Scholar]
- 63. Frismantiene A, Philippova M, Erne P, Resink TJ. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018;52:48–64. [DOI] [PubMed] [Google Scholar]
- 64. Nakashima H, Frank GD, Shirai H, Hinoki A, Higuchi S, Ohtsu H, Eguchi K, Sanjay A, Reyland ME, Dempsey PJ, Inagami T, Eguchi S. Novel role of protein kinase C-δ Tyr311 phosphorylation in vascular smooth muscle cell hypertrophy by angiotensin II. Hypertension. 2008;51(2):232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eguchi S, Kawai T, Scalia R, Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71(5):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gaddam KK, Pimenta E, Husain S, Calhoun DA. Aldosterone and cardiovascular disease. Curr Probl Cardiol. 2009;34(2):51–84. [DOI] [PubMed] [Google Scholar]
- 67. Nehme A, Zibara K. Efficiency and specificity of RAAS inhibitors in cardiovascular diseases: how to achieve better end-organ protection? Hypertens Res. 2017;40(11):903–909. [DOI] [PubMed] [Google Scholar]
- 68. Whitfield GK, Jurutka PW, Haussler CA, Haussler MR. Steroid hormone receptors: evolution, ligands, and molecular basis of biologic function. J Cell Biochem. 1999;(Suppl 32–33):110–122. [DOI] [PubMed] [Google Scholar]
- 69. Nagase M, Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol. 2013;9(2):86–98. [DOI] [PubMed] [Google Scholar]
- 70. Faresse N. Post-translational modifications of the mineralocorticoid receptor: how to dress the receptor according to the circumstances? J Steroid Biochem Mol Biol. 2014;143:334–342. [DOI] [PubMed] [Google Scholar]
- 71. Le Moëllic C, Ouvrard-Pascaud A, Capurro C, Cluzeaud F, Fay M, Jaisser F, Farman N, Blot-Chabaud M. Early nongenomic events in aldosterone action in renal collecting duct cells: PKCα activation, mineralocorticoid receptor phosphorylation, and cross-talk with the genomic response. J Am Soc Nephrol. 2004;15(5):1145–1160. [PubMed] [Google Scholar]
- 72. Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18(5):660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xiao F, Puddefoot JR, Barker S, Vinson GP. Mechanism for aldosterone potentiation of angiotensin II–stimulated rat arterial smooth muscle cell proliferation. Hypertension. 2004;44(3):340–345. [DOI] [PubMed] [Google Scholar]
- 74. Matsuzawa Y, Suematsu S, Saito J, Omura M, Nishikawa T. Vascular aldosterone production at the pre-diabetic stage of young Otsuka Long-Evans Tokushima Fatty (OLETF) rats, compared with Long-Evans Tokushima Otsuka (LETO) rats. Molecules. 2013;18(12):15636–15647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ahmad N, Romero DG, Gomez-Sanchez EP, Gomez-Sanchez CE. Do human vascular endothelial cells produce aldosterone? Endocrinology. 2004;145(8):3626–3629. [DOI] [PubMed] [Google Scholar]
- 76. Assersen KB, Jensen PS, Briones AM, Rasmussen LM, Marcussen N, Toft A, Vanhoutte PM, Jensen BL, Hansen PBL. Periarterial fat from two human vascular beds is not a source of aldosterone to promote vasoconstriction. Am J Physiol Renal Physiol. 2018;315(6):F1670–F1682. [DOI] [PubMed] [Google Scholar]
- 77. Sampson LJ, Davies LM, Barrett-Jolley R, Standen NB, Dart C. Angiotensin II-activated protein kinase C targets caveolae to inhibit aortic ATP-sensitive potassium channels. Cardiovasc Res. 2007;76(1):61–70. [DOI] [PubMed] [Google Scholar]
- 78. Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, Nakashima H, Woolfolk EA, Motley ED, Eguchi S. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25(9):1831–1836. [DOI] [PubMed] [Google Scholar]
- 79. Lim S, Lee SY, Seo HH, Ham O, Lee C, Park JH, Lee J, Seung M, Yun I, Han SM, Lee S, Choi E, Hwang KC. Regulation of mitochondrial morphology by positive feedback interaction between PKCδ and Drp1 in vascular smooth muscle cell. J Cell Biochem. 2015;116(4):648–660. [DOI] [PubMed] [Google Scholar]
- 80. Lv P, Miao SB, Shu YN, Dong LH, Liu G, Xie XL, Gao M, Wang YC, Yin YJ, Wang XJ, Han M. Phosphorylation of smooth muscle 22α facilitates angiotensin II–induced ROS production via activation of the PKCδ-P47phox axis through release of PKCδ and actin dynamics and is associated with hypertrophy and hyperplasia of vascular smooth muscle cells in vitro and in vivo. Circ Res. 2012;111(6):697–707. [DOI] [PubMed] [Google Scholar]
- 81. Doller A, Gauer S, Sobkowiak E, Geiger H, Pfeilschifter J, Eberhardt W. Angiotensin II induces renal plasminogen activator inhibitor-1 and cyclooxygenase-2 expression post-transcriptionally via activation of the mRNA-stabilizing factor human-antigen R. Am J Pathol. 2009;174(4):1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jääskeläinen T, Makkonen H, Palvimo JJ. Steroid up-regulation of FKBP51 and its role in hormone signaling. Curr Opin Pharmacol. 2011;11(4):326–331. [DOI] [PubMed] [Google Scholar]
- 83. McGraw AP, Bagley J, Chen WS, Galayda C, Nickerson H, Armani A, Caprio M, Carmeliet P, Jaffe IZ. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J Am Heart Assoc. 2013;2(1):e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Balsevich G, Häusl AS, Meyer CW, Karamihalev S, Feng X, Pöhlmann ML, Dournes C, Uribe-Marino A, Santarelli S, Labermaier C, Hafner K, Mao T, Breitsamer M, Theodoropoulou M, Namendorf C, Uhr M, Paez-Pereda M, Winter G, Hausch F, Chen A, Tschöp MH, Rein T, Gassen NC, Schmidt MV. Stress-responsive FKBP51 regulates AKT2-AS160 signaling and metabolic function. Nat Commun. 2017;8(1):1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.