Abstract
It was shown previously that high dietary fiber (DF) and immune system stimulation (ISS) with systemic Escherichia coli lipopolysaccharide independently increased the threonine (Thr) requirement to maximize growth performance and protein deposition (PD). However, no additive effects on the Thr requirement were observed when both DF and ISS were present. The objective of the present study was to investigate whether supplementing Thr to meet previously estimated requirements for high DF and systemic immune challenge would maintain performance of pigs exposed to an enteric immune challenge when fed high DF. A total of 128 pigs (22.6 ± SD = 1.6 kg initial BW) were assigned to 1 of 4 dietary treatments in a 2 × 2 factorial arrangement in a randomized complete block design (n = 8 pens/treatment and 4 pigs/pen) for 28 d. Treatments were a low-fiber (LF; 13% total DF) or high-fiber (HF; 20% total DF) diet with either a standard (STD; 0.65% SID) or supplemental (SUP; 0.78% SID) Thr level. After a 7-d adaptation, pigs were orally inoculated with 2 mL (2.3 × 109 CFU/mL) of Salmonella typhimurium (ST). Blood samples and rectal swabs were obtained and rectal temperature recorded to determine clinical responses and ST shedding. On day 7 postinoculation, 1 pig/pen was euthanized and mesenteric lymph nodes, spleen, and digesta (ileum, cecum, and colon) were sampled to assess ST colonization and translocation. Body weight and feed intake were recorded on day 0, 7, and 21 postinoculation to calculate ADG, ADFI, and G:F. Rectal temperature increased (P < 0.05) 24 h postinoculation and remained elevated at day 6. Serum albumin concentration decreased (P < 0.05), whereas haptoglobin concentration increased (P < 0.05) postinoculation. There was no fiber or Thr effect (P > 0.05) on ST counts in the ileum and cecum, but a fiber × Thr interaction (P < 0.05) was observed in the colon. Supplemental Thr improved (P < 0.05) growth performance in LF- and HF-fed challenged pigs. However, performance of supplemented HF challenged pigs was less than (P < 0.05) supplemented LF challenged pigs. These results suggest that Thr supplemented to meet requirements for high DF and systemic immune challenge was not sufficient to maintain growth performance of pigs fed HF diets and challenged with an enteric pathogen.
Keywords: average daily gain, dietary fiber, pigs, Salmonella typhimurium, threonine
INTRODUCTION
Optimal growth performance can be affected by a wide range of factors in the production system of growing-finishing pigs. For instance, factors such as subclinical disease challenges, inappropriate levels of essential nutrients such as amino acids (AA), and the negative effects of high-fiber ingredients, which are poorly digestible (Stein et al., 1999) can adversely affect pig performance. Dietary fiber (DF) has been reported to independently increase the threonine (Thr) requirement for maximum growth and protein deposition (PD) in growing pigs (Mathai et al., 2016; Wellington et al., 2018). Similarly, Blank et al. (2012) and Libao-Mercado et al. (2006, 2007) have reported fiber-associated Thr losses in growing pigs and concluded that those effects were due to high endogenous loss of mucins. Commercial production systems are likely to predispose pigs to subclinical disease, which may affect immune system function. Immune system stimulation (ISS) has been reported to partition nutrients away from growth to support the immune response (Reeds et al., 1994; Reeds and Jahoor, 2001) and has been shown to increase maintenance energy (Huntley et al., 2018) and AA requirements (Litvak et al., 2013; Rakhshandeh et al., 2014; Wellington et al., 2018) in pigs. In contrast, Rudar et al. (2016) reported no effect of feeding supplemental leucine on nitrogen retention in starter pigs challenged with Escherichia coli lipopolysaccharide. In a previous study, it was demonstrated that high DF and ISS independently increased the threonine (Thr) requirement to maximize PD (Wellington et al., 2018). Interestingly, when both high DF and a systemic immune challenge were present, no further increase in the Thr requirement for PD was observed. Thus, ISS and DF effects on Thr requirement were not additive. Since systemic LPS challenge may have limited direct effects on the gastrointestinal tract, the objective of the present study was to investigate whether supplementing Thr to meet previously estimated requirements for high DF and systemic immune challenge would maintain performance of pigs exposed to an enteric immune challenge when fed high DF.
MATERIALS AND METHODS
The experimental protocol was approved by the University of Saskatchewan’s Animal Research Ethics Board (AUP #20170123) and followed the Canadian Council on Animal Care guidelines (CCAC, 2009).
Animals, Housing, and Diets
The experiment duration was 28 d and consisted of a 7-d adaptation period (no challenge) and 21 d postinoculation period. A total of 128 pigs (Camborough Plus × C3378; PIC Canada Ltd.) of 22.6 ± 1.6 kg initial BW were obtained from the Prairie Swine Centre, Inc. (Saskatoon, SK) and transported to the Animal Care Unit of the Western College of Veterinary Medicine (Saskatoon, SK). The pigs were placed on trial in 2 blocks using 2 experimental rooms for each block. In each experimental room (22 °C ambient temperature), pigs were housed in groups of 4 pigs/pen on solid floors lined with rubber mats. Pens were randomly assigned to 1 of 4 dietary treatments in a 2 × 2 factorial arrangement in a randomized complete block design (n = 8 pens/treatment). Treatments consisted of a low (LF; 13% total DF) or high (HF; 20% total DF) fiber diet with either a standard (STD; 0.65% SID) or supplemental (SUP; 0.78% SID) Thr level. The SUP Thr level was based on previous studies examining the effects of systemic ISS and DF on Thr requirements in growing pigs (Wellington et al., 2018, 2019). Diets were wheat- soybean-based and were formulated using the analyzed content of AA and published SID coefficients of AA for ingredients to meet or exceed nutrient requirements for 25 to 50 kg pigs according to NRC (2012) and Evonik (AMINODat 5.0). All other nutrient content was estimated using published nutrient content of ingredients (NRC, 2012). The HF diets were formulated by partly replacing corn in the LF diet with 5% wheat bran and 10% sugar beet pulp (Table 1). Pigs were fed ad libitum and had unrestricted access to water. Samples of experimental diets were collected during the trial and ground in a centrifugal mill (ZM 100, RETSCH GmbH & Co., Rheinische Straße, Germany) through a 1 mm sieve. The diets were analyzed (Table 2) for AA contents via ion-exchange chromatography with postcolumn derivatization with ninhydrin (Evonik Nutrition & Care GmbH, Hanau, Germany; Llames and Fontaine, 1994). The DM content of the diets was determined according to AOAC (2007) method 930.15. Nitrogen (N) content of the experimental diets was determined using an automatic analyzer (LECO FP 528; MI, USA; Method 990.03; AOAC, 2007) and CP content of the diets calculated as N× 6.25. Total dietary fiber (TDF), soluble dietary fiber (SDF), and insoluble dietary fiber (IDF) of the experimental diets was analyzed according to the AOAC (2007) method 991.43 using an ANKOMTDF analyzer (ANKOM Technology, Macedon, NY). All analyses were conducted in duplicate. Individual pig BW and feed disappearance were measured on day 0, 7, and 21 postinoculation for calculation of ADG, ADFI, and feed efficiency (G:F).
Table 1.
Composition of experimental diets (as-fed basis)
| Low fiber | High fiber | |||
|---|---|---|---|---|
| Ingredients, % | STD Thr1 |
SUP Thr2 |
STD Thr |
SUP Thr |
| Corn | 21.84 | 21.72 | 3.96 | 3.83 |
| Wheat | 47.0 | 47.0 | 47.0 | 47.0 |
| Barley | 10.0 | 10.0 | 10.0 | 10.0 |
| Wheat bran | - | - | 5.0 | 5.0 |
| Sugar beet pulp | - | - | 10.0 | 10.0 |
| Canola oil | 0.90 | 0.90 | 3.80 | 3.80 |
| Soybean meal | 17.0 | 17.0 | 17.0 | 17.0 |
| L-Lys.HCl3 | 0.53 | 0.52 | 0.50 | 0.50 |
| DL-Met3 | 0.20 | 0.20 | 0.20 | 0.20 |
| L-Thr3 | 0.15 | 0.28 | 0.16 | 0.29 |
| L-Trp3 | 0.03 | 0.03 | 0.03 | 0.03 |
| L-Val3 | 0.05 | 0.05 | 0.05 | 0.05 |
| Limestone | 1.25 | 1.25 | 1.25 | 1.25 |
| Monocalcium phosphate | 0.75 | 0.75 | 0.75 | 0.75 |
| Salt | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin-mineral premix4 | 0.10 | 0.10 | 0.10 | 0.10 |
| Calculated nutrient content 5 | ||||
| DM, % | 87.4 | 87.4 | 84.7 | 84.7 |
| CP, % | 17.3 | 17.4 | 17.5 | 17.6 |
| ME, kcal/kg | 3285 | 3287 | 3318 | 3317 |
| NE, kcal/kg | 2488 | 2489 | 2486 | 2484 |
| Amino Acids, % SID 6 | ||||
| Arg | 0.92 | 0.92 | 0.92 | 0.92 |
| His | 0.40 | 0.40 | 0.39 | 0.39 |
| Ile | 0.60 | 0.60 | 0.59 | 0.59 |
| Leu | 1.15 | 1.15 | 1.07 | 1.07 |
| Lys | 1.08 | 1.08 | 1.08 | 1.08 |
| Met | 0.44 | 0.44 | 0.42 | 0.42 |
| Cys | 0.29 | 0.29 | 0.30 | 0.30 |
| Phe | 0.74 | 0.74 | 0.72 | 0.72 |
| Tyr | 0.45 | 0.45 | 0.46 | 0.46 |
| Thr | 0.65 | 0.78 | 0.65 | 0.78 |
| Trp | 0.22 | 0.22 | 0.23 | 0.23 |
| Val | 0.73 | 0.73 | 0.73 | 0.73 |
1STD Thr, Standard Thr (0.65% standardized ileal digestible).
2SUP Thr, Supplemental Thr (0.78% standardized ileal digestible).
3Amino acids provided by Evonik Nutrition & Care GmbH (Hanau-Wolfgang, Germany).
4Supplied per kg of complete diet: vitamin A, 8000 IU; vitamin D, 1500 IU; vitamin E, 30 IU; menadione, 2.5 mg; vitamin B12, 0.025 mg; thiamine, 1.00 mg; biotin, 0.10 mg; niacin, 20 mg; riboflavin, 4 mg; pantothenate, 12 mg; folic acid, 0.50 mg; pyridoxine, 2.0 mg; Fe,100 mg; Zn, 100 mg; Mg, 40 mg; Cu, 15 mg; Se, 0.30 mg and I, 1 mg.
5Nutrient content of diets based on estimated nutrient contents of ingredients according to NRC (2012).
6SID, Standardized ileal digestible.
Table 2.
Analyzed nutrient content of experimental diets (as-fed basis)
| Low fiber | High fiber | |||
|---|---|---|---|---|
| Item, % | STD Thr1 | SUP Thr2 | STD Thr | SUP Thr |
| Dry matter | 89.20 | 90.10 | 90.60 | 90.10 |
| Crude protein | 18.57 | 18.54 | 18.13 | 18.60 |
| Soluble dietary fiber | 1.80 | 1.90 | 3.60 | 3.70 |
| Insoluble dietary fiber | 11.20 | 11.80 | 17.30 | 16.10 |
| Total dietary fiber | 13.00 | 13.70 | 20.90 | 19.80 |
| Total amino acids 3 | ||||
| Arg | 1.07 (0.99) | 1.04 (0.99) | 1.07 (0.99) | 1.08 (0.99) |
| His | 0.43 (0.40) | 0.43 (0.40) | 0.44 (0.41) | 0.45 (0.41) |
| Ile | 0.70 (0.64) | 0.68 (0.64) | 0.70 (0.64) | 0.70 (0.64) |
| Leu | 1.31 (1.23) | 1.29 (1.23) | 1.24 (1.14) | 1.25 (1.14) |
| Lys | 1.22 (1.15) | 1.22 (1.15) | 1.26 (1.16) | 1.23 (1.16) |
| Met | 0.46 (0.45) | 0.47 (0.45) | 0.47 (0.44) | 0.45 (0.44) |
| Cys | 0.34 (0.29) | 0.34 (0.29) | 0.33 (0.31) | 0.33 (0.31) |
| Phe | 0.86 (0.77) | 0.84 (0.77) | 0.84 (0.76) | 0.85 (0.76) |
| Thr | 0.78 (0.72) | 0.87 (0.85) | 0.78 (0.72) | 0.87 (0.85) |
| Trp | 0.26 (0.23) | 0.25 (0.23) | 0.26 (0.24) | 0.26 (0.24) |
| Val | 0.85 (0.77) | 0.84 (0.77) | 0.86 (0.78 | 0.86 (0.78) |
1STD Thr, Standard Thr (0.65% standardized ileal digestible).
2SUP Thr, Supplemental Thr (0.78% standardized ileal digestible).
3Analyzed values of total amino acids with calculated values in brackets.
Inoculation and Rectal Swab Protocol
On day 0 of the challenge period (day 8 of the trial), pigs were orally inoculated twice within 4 h, each time with 1 mL solution containing 2.3 × 109 CFU/mL of Salmonella typhimurium (ST) var Copenhagen selected for antibiotic resistance to Novobiocin and Nalidixic acid (Pieper et al., 2009). Rectal temperatures were obtained from all pigs on day 2 preinoculation and every 24 h postinoculation for 6 d using a digital thermometer (Life Brand, ON, Canada).
On day 2 preinoculation and day 1, 2, 4, 6, 14, and 20 postinoculation, rectal swabs were obtained from individual pigs, diluted 1:10 in buffered peptone water (BPW) and cultured on brilliant green agar (BG agar) plates containing 30 µg/mL Nalidixic acid and 50 µg/mL Novobiocin (Nal+/Nov+). Further, 1 mL of the dilution was enriched in 4 mL of selenite-cysteine broth containing 30 µg/mL Nalidixic acid and 50 µg/mL Novobiocin and incubated overnight at 37 °C and later cultured on BG agar plates (Nal+/Nov+). Colony counts were recorded on all plates after incubation for 24 h at 37 °C. A scoring system was used to assign fecal shedding scores for each swab (Burkey et al., 2004). Plates prepared from swabs with colony counts > 30 were given a shedding score of 3. Plates positive for antibiotic-resistant ST but with colony counts < 30 were given shedding score 2. A shedding score of 1 was assigned to swabs that were negative for ST following direct plating but positive after enrichment. Finally, swabs negative for antibiotic resistant ST following direct plating and on enrichment were given a score of zero.
Blood Sampling and Analyses
Blood samples were obtained from 2 pigs/pen 1 d before the ST inoculation and on day 4 and 7 postinoculation. Blood samples were collected via jugular vein puncture into 10 mL vacutainer tubes containing either EDTA or no additive (BD, Vacutainers Mississauga, ON, Canada). Whole blood samples collected in EDTA tubes were immediately submitted and analyzed for complete blood cell count at Prairie Diagnostic Services (Saskatoon, SK) using an ADVIA 2120i hematology analyzer (Siemens Health Care Diagnostics, Deerfield, IL). Blood samples collected into additive-free tubes were allowed to clot and then centrifuged at 2,500 × g at 4 °C for 15 min. Serum samples were obtained and stored at −20 °C for subsequent analysis for albumin, haptoglobin, and immunoglobulin G (IgG). Briefly, serum albumin was analyzed by bromocresol green method using a Cobas C 311 (Roche Diagnostics, Laval, QC, Canada) according to Doumas et al. (1971). Serum IgG levels were determined by radial immunodiffusion assay as previously described by Chelack et al. (1993) with antiserum incorporated into the gel:goat anti-swine IgG (H+L; Jackson Immuno Research Laboratories, Inc., West Grove, PA). A porcine reference serum was used for a standard curve supplied by Bethyl laboratories, Inc., (Montgomery, TX). A secondary reference serum was used as a control assay (Immuno-Reagents, Raleigh, NC). Serum haptoglobin was analyzed in the Animal Health Laboratory (University of Guelph, Guelph, ON) according to a method described by Makimura and Suzuki (1982) on a Roche Cobas 6000 c501 analyzer.
Digesta and Tissue Collection and Analyses
On day 7 postinoculation, 1 pig/pen representing the average pen BW was humanely euthanized by penetrating captive bolt followed by exsanguination. Subsequently, mesenteric lymph nodes (MLN) and spleen were sampled under aseptic conditions into sterile tubes containing 20 mL BPW and 200 µL of the dilution was plated on Nal+/Nov+ BG agar plates. Further, 1 mL was diluted in 4 mL selenite-cysteine broth (Nal+/Nov+) for enrichment overnight. The enriched samples were incubated overnight at 37 °C with shaking, after which 200 µL was plated and incubated. A shedding score was assigned as previously described. Intestinal digesta samples (~1 g) were obtained from the ileum, cecum, and colon and each diluted in 4 mL BPW and kept at 4 °C. The digesta samples were serially diluted to 10–7 and 200 µL of each dilution was plated on BG agar (Nal+/Nov+) and cultured at 37 °C for 24 h after which colonies were counted on each plate and recorded. Colony counts of 30 to 300 were used in the calculations of colony forming units per gram digesta (CFU/g).
Statistical Analyses
Data were tested for normality and outliers using the PROC UNIVARIATE model in SAS (version 9.4, SAS Institute Inc., Cary, NC) and the Shapiro-Wilk test. Outliers were determined as a value ± 2 standard deviations away from the treatment mean. All data were analyzed as a 2 × 2 factorial in a randomized complete block design (PROC MIXED) with main effects of 1) fiber level, 2) threonine level and their interactions. A block was included in the model as a random effect. For blood data, period was also included as a fixed effect in the statistical model. Thus, the main effects were fiber level, threonine level, period (preinoculation, postinoculation day 4, and postinoculation day 7) and their interaction and block included as random effect. The Tukey-Kramer mean separation test was used to determine significant differences, LSMEANS considered significant at P < 0.05 and a trend toward significance considered at P > 0.05 and P ≤ 0.10.
RESULTS
Response to Salmonella Inoculation
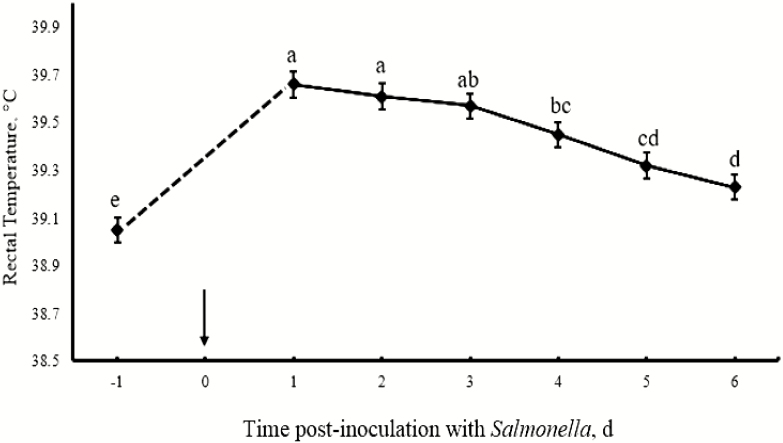
The response of pigs to ST inoculation was determined by measuring rectal temperature over a period of 6 d postinoculation after an initial measurement prior to the oral inoculation. There was an increase (P < 0.01) in rectal temperature on day 1 to 6 compared to the preinoculation levels (Fig. 1). There was no significant (P > 0.05) period effect on white blood cell count (WBC); however, HF reduced WBC count (P < 0.05), whereas SUP Thr tended (P = 0.08) to reduce the WBC count (Table 3). Period had a significant (P < 0.01) effect on serum albumin. Specifically, there was a reduction (P < 0.01) in serum albumin concentration on day 4 postinoculation compared to preinoculation, which returned to preinoculation levels (P > 0.05) on day 7 postinoculation (Table 3). Fiber had no effect (P > 0.05) on serum albumin concentration but SUP Thr tended (P = 0.06) to reduce serum albumin concentration. Period (preinoculation, day 4 postinoculation, and day 7 postinoculation) had a significant effect (P < 0.01) on serum haptoglobin concentration. Specifically, haptoglobin levels increased on day 4 postinoculation and remained elevated on day 7 compared to the preinoculation levels. There were no significant effects (P > 0.05) of fiber or Thr levels on serum haptoglobin and serum IgG concentrations (Table 3).
Figure 1.
Rectal temperature (°C) of pigs prior to Salmonella typhimurium inoculation and monitored for 6 d postinoculation. The arrow indicates time point of S. typhimurium inoculation.
Table 3.
Plasma parameters of immune status in pigs inoculated with Salmonella typhimurium
| Low fiber | High fiber | P-value3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | STD Thr1 | SUP Thr2 | STD Thr | SUP Thr | SEM | Fiber | Thr | Period |
| White blood cell count, ×10 9 g/L | ||||||||
| Preinoculation | 20.32 | 18.98 | 18.86 | 16.36 | 1.03 | <0.05 | 0.08 | NS4 |
| Postinoculation (day 4) | 19.93 | 20.41 | 19.46 | 17.51 | ||||
| Postinoculation (day 7) | 19.41 | 20.37 | 20.17 | 18.23 | ||||
| Albumin, g/L | ||||||||
| Preinoculation | 35.92 | 36.50 | 37.63 | 36.00 | 1.59 | NS | 0.07 | <0.01 |
| Postinoculation (day 4) | 30.08 | 29.47 | 31.00 | 27.60 | ||||
| Postinoculation (day 7) | 36.31 | 34.89 | 37.63 | 34.24 | ||||
| Haptoglobin, g/L | ||||||||
| Preinoculation | 0.94 | 1.26 | 1.22 | 0.96 | 0.27 | NS | NS | <0.01 |
| Postinoculation (day 4) | 1.71 | 1.73 | 1.56 | 1.56 | ||||
| Postinoculation (day 7) | 1.66 | 1.89 | 1.87 | 1.75 | ||||
| Immunoglobulin G, mg/L | ||||||||
| Preinoculation | 10.27 | 11.09 | 10.42 | 10.94 | 1.27 | |||
| Postinoculation (day 4) | 11.24 | 11.79 | 11.5 | 11.52 | NS | NS | NS | |
| Postinoculation (day 7) | 10.44 | 12.39 | 12.38 | 11.85 | ||||
1STD Thr, Standard Thr (0.65% standardized ileal digestible).
2SUP Thr, Supplemental Thr (0.78% standardized ileal digestible).
3No significant two- or three-way interactions were observed.
4NS, not significant (P > 0.05).
Salmonella Shedding
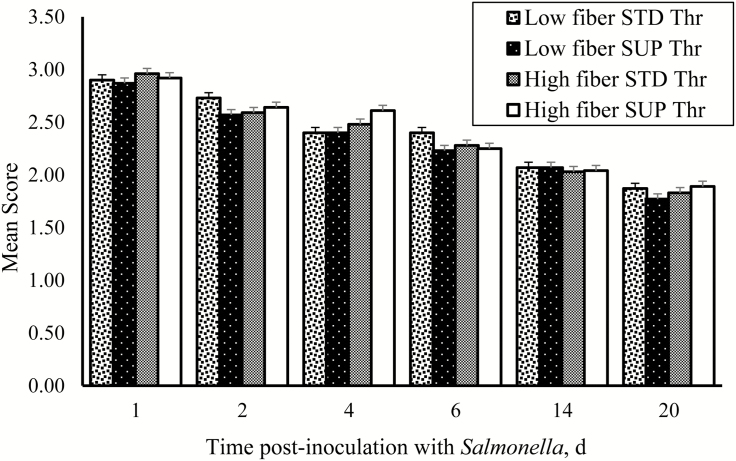
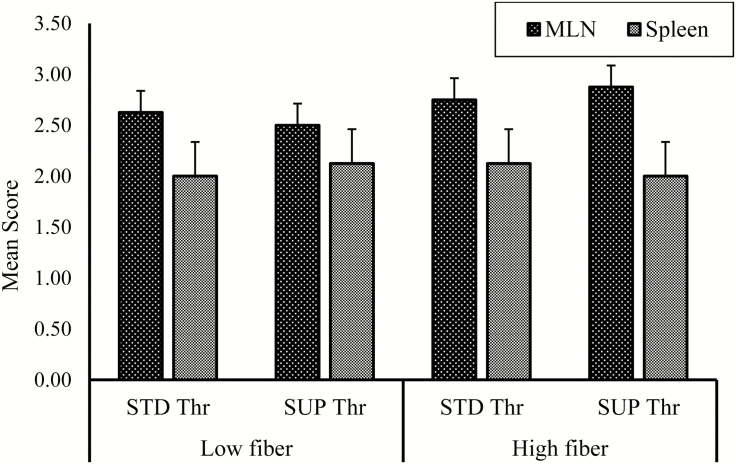
Dietary treatment had no effect (P > 0.05) on ST count in the caecum (Table 4). In the ileum, there were tendencies for fiber (P = 0.06) and SUP Thr (P = 0.06) to increase ST count. In the colon, a significant fiber × Thr interaction (P< 0.01) was observed where SUP Thr decreased ST counts in pigs fed HF diets but not in LF-fed pigs (Table 4). Although ST was present in all pigs (feces, digesta, and tissue samples), there were no significant effects (P > 0.05) of fiber, Thr, or fiber × Thr interaction on ST shedding in feces (Fig. 2). Although we did not analyze the effect of day on fecal shedding, there was a decline in the mean scores from day 1 to 20 postinoculation. There were no significant effects (P > 0.05) of fiber, Thr, or fiber × Thr interaction on ST in the MLN and spleen (Fig. 3) of pigs.
Table 4.
Salmonella typhimurium quantification in intestinal contents (Log10 CFU/g; day 7 postinoculation) of inoculated pigs
| Low fiber | High fiber | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | STD Thr1 | SUP Thr2 | STD Thr | SUP Thr | SEM | Fiber | Thr | Fiber × Thr |
| Ileum | 4.55 | 4.94 | 4.96 | 6.43 | 0.49 | 0.06 | 0.06 | NS3 |
| Cecum | 4.39 | 4.96 | 4.94 | 4.22 | 0.48 | NS | NS | NS |
| Colon | 5.21 | 6.84 | 7.03 | 4.81 | 0.73 | NS | NS | 0.008 |
1STD Thr, Standard Thr (0.65% standardized ileal digestible).
2SUP Thr, Supplemental Thr (0.78% standardized ileal digestible).
3NS, not significant (P > 0.05).
Figure 2.
Postinoculation fecal shedding of Salmonella typhimurium on day 1, 2, 4, 6, 14, and 20 in pigs fed high-fiber diets (HF; 20% total dietary fiber) or low-fiber diets (LF; 13% total dietary fiber) with standard threonine (STD Thr; 0.65% SID) or supplemental threonine (SUP Thr; 0.78% SID). Data presented as mean scores. A shedding score of 3 was assigned to plates positive for the inoculated ST with counts > 30 and plates positive but with counts < 30 were given shedding score of 2. A shedding score of 1 was assigned to plates that were only positive after enrichment and plates negative after enrichment were scored zero. No significant (P > 0.05) fiber, threonine, or interactive effects on S. typhimurium shedding on day 1, 2, 4, 6, 14, and 20 was observed.
Figure 3.
Salmonella typhimurium translocation to the mesenteric lymph nodes (MLN) and spleen in pigs fed high-fiber diets (HF; 20% total dietary fiber) or low-fiber diets (LF; 13% total dietary fiber) with standard threonine (STD Thr; 0.65% SID) or supplemental threonine (SUP Thr; 0.78% SID). Data presented as mean scores. A shedding score of 3 was assigned to plates positive for the inoculated S. typhimurium with counts > 30 and plates positive but with counts < 30 were given shedding score of 2. A shedding score of 1 was assigned to plates that were only positive after enrichment and plates negative after enrichment were scored zero. No significant (P > 0.05) fiber, threonine, or interactive effects on S. typhimurium shedding in either MLN or spleen.
Growth Performance Post-Salmonella Inoculation
All pigs remained healthy and had similar growth performance across dietary treatments in the preinoculation period (Table 5). In the first week (day 0 to 7) of the ST challenge period, there was a significant effect of fiber level (P < 0.01; Table 5) on ADG. Feeding HF diets reduced ADG compared to LF (0.877 vs. 1.023 kg/d). There was also a significant effect of Thr (P < 0.01) level on ADG, where SUP Thr increased ADG compared to STD Thr level (0.99 vs. 0.90 kg/d). Similarly, there were both significant fiber (P < 0.01) and Thr (P < 0.01) effects on ADFI, such that ADFI was higher in the LF-fed pigs compared to the HF-fed pigs (1.71 vs. 1.60 kg/d). Supplemental Thr reduced the ADFI (1.59 kg/d) compared to the STD Thr-fed pigs (1.72 kg/d). For both ADG and ADFI, no significant interactions (P > 0.05) between fiber and Thr were observed. In the present study, we observed an effect of Thr level on pig performance during week 1 (day 0 to 7) and week 2 and 3 (day 8 to 21) postinoculation independent of DF level. During day 0 to 7 postinoculation, pigs fed SUP Thr had lower ADFI (1.593 kg/d) but higher ADG (0.996 kg/d) and G:F (0.63 kg/kg) compared to pigs fed the STD Thr diets which had a higher ADFI (1.720 kg/d). These levels of feed intake resulted in daily Thr intake of 13.5 and 12.4 g/d in SUP Thr and STD Thr-fed pigs, respectively. Based on the observed significant effects of both DF and Thr on ADG and ADFI, G:F also showed a significant effect for fiber (P = 0.02) and Thr (P < 0.01) with no significant interaction. A lower G:F was observed in HF-fed pigs compared to the LF-fed pigs (0.55 vs. 0.60 kg/kg) and SUP Thr improved G:F compared to STD Thr level (0.63 vs. 0.53 kg/kg).
Table 5.
Growth performance of pigs pre- and postinoculation with Salmonella typhimurium
| Low fiber | High fiber | P-value3 | |||||
|---|---|---|---|---|---|---|---|
| Parameters | STD Thr1 | SUP Thr2 | STD Thr | SUP Thr | SEM | Fiber | Thr |
| Initial BW, kg | 22.76 | 22.32 | 22.69 | 22.45 | 0.167 | NS4 | NS |
| Final BW, kg | 49.51 | 51.45 | 46.34 | 48.57 | 1.055 | <0.01 | <0.01 |
| Preinoculation period (day −7–0) | |||||||
| ADG, kg | 0.826 | 0.804 | 0.818 | 0.772 | 0.043 | NS | NS |
| ADFI, kg | 1.339 | 1.359 | 1.218 | 1.185 | 0.089 | <0.01 | NS |
| G:F, kg/kg | 0.620 | 0.595 | 0.636 | 0.655 | 0.020 | <0.01 | NS |
| Postinoculation (day 0–7) | |||||||
| ADG, kg | 0.965 | 1.082 | 0.843 | 0.911 | 0.025 | <0.01 | <0.01 |
| ADFI, kg | 1.788 | 1.635 | 1.653 | 1.551 | 0.047 | <0.01 | <0.01 |
| G:F, kg/kg | 0.543 | 0.665 | 0.511 | 0.588 | 0.022 | <0.05 | <0.01 |
| Postinoculation (day 8–21) | |||||||
| ADG, kg | 0.940 | 1.093 | 0.783 | 0.938 | 0.031 | <0.01 | <0.01 |
| ADFI, kg | 1.983 | 1.944 | 1.934 | 1.831 | 0.075 | NS | NS |
| G:F, kg/kg | 0.483 | 0.566 | 0.409 | 0.514 | 0.022 | <0.01 | <0.01 |
| Overall (day 0–21) | |||||||
| ADG, kg | 0.953 | 1.088 | 0.813 | 0.924 | 0.020 | <0.01 | <0.01 |
| ADFI, kg | 1.885 | 1.789 | 1.793 | 1.691 | 0.042 | <0.05 | <0.05 |
| G:F, kg/kg | 0.514 | 0.615 | 0.459 | 0.553 | 0.017 | <0.01 | <0.01 |
1STD Thr, Standard Thr (0.65% standardized ileal digestible).
2SUP Thr, Supplemental Thr (0.78% standardized ileal digestible).
3No significant Fiber × Thr interaction were observed.
4NS, not significant (P > 0.05).
From day 8 to 21 postinoculation, no significant effect (P > 0.05) of fiber, Thr, or an interaction on ADFI was observed. There were significant effects of fiber (P < 0.01) and Thr (P < 0.01) on ADG, with no significant interaction. High fiber reduced ADG (0.87 vs. 1.02 kg/d) compared to the LF-fed pigs while SUP Thr improved ADG compared with the STD Thr-fed pigs, regardless of fiber level. Feed efficiency during this period showed significant fiber (P < 0.01) and Thr (P < 0.01) effects. The HF-fed pigs had a reduced G:F (0.51 vs. 0.56) compared to the LF-fed pigs. Also, feeding SUP Thr improved the G:F as compared with the STD Thr-fed pigs. Overall (day 0–21 postinoculation), pigs fed SUP Thr had higher ADG (1.01 vs. 0.88 kg/d), lower ADFI (1.74 vs. 1.84 kg/d), and higher G:F (0.58 vs. 0.49) than STD Thr group (P < 0.05). Similarly, pigs fed LF diets had higher ADG (1.02 vs. 0.87 kg/d), ADFI (1.83 vs. 1.74 kg/d), and G:F (0.56 vs. 0.51 kg/kg) than HF-fed pigs (P < 0.05).
DISCUSSION
The objective of the present study was to determine if growth performance of pigs fed high DF and challenged with an enteric pathogen would be maintained when Thr supply was adjusted to meet previously estimated requirements for both high DF and systemic immune challenge (Wellington et al., 2018, 2019). We reported previously that high DF and systemic immune challenge with E. coli LPS independently increased the Thr requirement for maximum PD (Wellington et al., 2018). In the present study, we fed the same level of DF with a standard level (0.65% SID; NRC, 2012) or supplemental level (0.78% SID) of Thr. The SUP Thr (0.78% SID) was previously determined to be sufficient to maximize growth performance (Wellington et al., 2019) and PD (Wellington et al., 2018) when feeding the same high DF as used in the present study. However, when a systemic immune challenge was applied and high DF was fed, we estimated 0.76% SID Thr required to maximize PD. Therefore, the supplemental Thr level (0.78% SID) used in the present study was expected to be sufficient to mount an immune response and support growth (Wellington et al., 2018).
Response to Salmonella Inoculation
The enteric pathogen model (S. typhimurium) used in the current study has been reported to successfully and uniformly stimulate the immune system of pigs (Pieper et al., 2012; Barba-Vidal et al., 2017; Burdick-Sanchez et al., 2017). In the present study, the increase in haptoglobin and decrease in serum albumin postinoculation is in agreement with response observed in previous studies in pigs under immune stimulation (Lipperheide et al., 1998; Petersen et al., 2002; de Ridder et al., 2012; Litvak et al., 2013). Turner et al. (2002) and Gebru et al. (2010), who measured rectal temperature in pigs for 7 d post-Salmonella inoculation and reported the highest rectal temperatures on day 2 and 3, which remained elevated until day 7, similar to the response seen in the current study. Previous studies in which LPS was used to initiate an immune stimulation observed alterations in WBC counts (Litvak et al., 2013; Wellington et al., 2018). This was not observed in and may be due to differences in the infection model used (i.e., enteric pathogen vs. systemic LPS) or time of blood sampling. The lack of response of serum IgG to ST inoculation agrees with Turner et al. (2002), who reported no significant effect of ST challenge model on serum IgG levels in weanling pigs. However, others have reported that increasing dietary Thr levels increased serum IgG concentration in growing pigs (Li et al., 1999). Altogether, the data suggest an effective and uniform ST challenge across all the dietary treatments.
Rectal swabs collected prior to ST inoculation confirmed that pigs used in the current study were negative for the inoculated strain of ST. Salmonella typhimurium shedding in feces was detected in all pigs until day 20 following ST inoculation, consistent with prolonged inflammatory indicators. Furthermore, ST was detected in digesta and tissue samples on day 7 postinoculation indicating intestinal colonization and translocation of the ST. Dietary treatment did not affect fecal shedding which agrees with Thomson et al. (2012) who fed diets containing different fiber sources (sugar beet pulp, distiller’s dried grains with solubles) to pigs inoculated with ST and observed no significant changes in fecal ST shedding. In contrast, others have reported changes in Salmonella colonization, which has been associated with variations in barley fiber (Pieper et al., 2012). No significant treatment effects were observed on ST count in digesta (ileum and cecum), MLN, and spleen tissues, consistent with the observations made on fecal shedding scores. In the colon digesta, however, an interaction of fiber and Thr on shedding was observed, suggesting that supplemental Thr may have supported immune responses contributing to reduced ST colonization when HF diets were fed, however, given this observation limited to the colon.
Growth Performance of Salmonella-Inoculated Pigs
We observed no significant interactions between DF and Thr level on growth performance and therefore only main effects are discussed here. In the present study, the observed effect of HF diets (54% increase in TDF content) on growth performance was consistent during the first week (day 0 to 7) postinoculation, week 2 and 3 (day 8 to 21) postinoculation, and overall (day 0 to 21). In the first week postinoculation, the reduced ADG observed in HF-fed pigs is likely due to lower ADFI as well as reduced feed efficiency. In the postinoculation period day 8 to 21, although all pigs had the same ADFI, ADG, and G:F were reduced in HF-fed pigs. The fibrous ingredients (sugar beet pulp and wheat bran) that were used to produce the HF diet in the present study have been reported by others (Anguita et al., 2007) to reduce feed intake in pigs thereby reducing growth rate and efficiency in the absence of a pathogenic challenge. However, in the current study, dietary nutrient content (NE and AA) was formulated to be consistent across dietary treatments and, therefore, should not have resulted in reduced performance. Under poor sanitary conditions the addition of fiber (16.9% total DF) in pig diets has been reported to negatively affect growth performance by decreasing voluntary feed intake (Montagne et al., 2012). Similarly, when Gebru et al. (2010) inoculated pigs with Salmonella, voluntary feed intake decreased compared to preinoculation intake levels, suggesting that the presence of a disease challenge also affects voluntary feed intake. This is likely why HF diets, despite formulation to similar nutrient levels as LF diets, reduced feed intake and resulted in decreased growth performance in the current study. It has been suggested that during enteric pathogen challenge, DF may act as a substrate for increased bacterial proliferation and hence increased disease prevalence and poor performance of pigs (Montagne et al., 2012). Nevertheless, there was no evidence of increased ST colonization with HF diets in the current study. Factors contributing to lower intake and performance during enteric disease challenge are unclear but appear to be unrelated to differences in ST colonization or supply of Thr.
Under immune-stimulating conditions, such as disease challenge or poor sanitary conditions, nutrient requirements increase to support an effective immune response (Le Floc’h et al., 2009; Van der Meer et al., 2016). Threonine has been reported to support optimal growth, immune function, and intestinal barrier in pigs when supplied at the required levels (Wang et al., 2010; Mao et al., 2014; Trevisi et al., 2015). The addition of supplemental Thr above the requirement for growth has been reported to improve weight gain, feed efficiency, and immune status in growing pigs, with a greater level of supplementation needed to maximize immune response than growth (Li et al., 1999). Other reports have suggested that higher Thr levels are required to optimize growth performance under unsanitary conditions (Jayaraman et al., 2015) and during systemic ISS with LPS (Wellington et al., 2018; McGilvray et al., 2019). In the current study, supplemental dietary Thr improved ADG regardless of DF and resulted in similar effects as high DF on ADFI and G:F during all postinoculation periods. It is unclear why SUP Thr decreased feed intake postinoculation. It is possible the SUP Thr increased susceptibility to ST challenge there is little evidence in support of this on recorded clinical responses or salmonella colonization and translocation in tissues. Moreover, Trevisi et al. (2015) reported that feed intake was increased in pigs (8 to 10 kg BW) that were challenged with E. coli and fed relatively high Thr diets (0.90%) compared to pigs fed lower Thr diets (0.85%) during day 0 to 7 postinoculation. Regardless of the effects on feed intake, in the current study supplementing Thr above NRC (2012) requirements for growth resulted in improved ADG and G:F. The improved growth performance observed in the present study agrees with Trevisi et al. (2015) who reported an improved ADG and G:F in pigs fed high Thr diets and challenged with E. coli. Similarly, Ren et al. (2014) reported improved growth performance (ADG and G:F) and intestinal immune status when higher Thr was fed to weaned pigs challenged with E. coli. Therefore, under conditions of disease challenge where pigs are unable to maintain voluntary feed intake at required levels to meet energy and nutrient requirement for an effective immune response and growth, increased dietary Thr could play an important role in alleviating the effects of the disease challenge by improving growth performance and immune status.
It is noteworthy that while feeding high DF in the present study reduced pig growth performance during an enteric disease challenge and supplemental Thr improved pig performance regardless of the DF content, there was no interaction between Thr supplementation and DF. So, while SUP Thr improved growth performance in challenged pigs fed high DF diets, this growth response was less than the response in LF-fed pigs. This suggests that the level of Thr supplementation used in this study was insufficient to meet requirements in pigs fed high DF and challenged with an enteric pathogen or another factor is limiting for growth under the conditions of the current study. This contrasts with our previous work (Wellington et al., 2018) in which we demonstrated that both high DF and ISS independently increased Thr requirement for PD but did not result in a further increase when both were present (i.e., no additive effect). It has been shown that challenge models (e.g., enteric pathogen, LPS challenge, sanitary conditions) can result in different effects on immune response (Pastorelli et al., 2012) and caution should therefore be used when comparing and interpreting results of studies in which different models have been used.
CONCLUSION
Overall, supplementing dietary Thr above the estimated requirement (NRC, 2012) improved growth performance in LF- and HF-fed challenged pigs. However, performance of supplemented HF challenged pigs was less than supplemented LF challenged pigs. These results suggest that Thr supplementation to meet previously estimated requirements for high DF and systemic immune challenge was not sufficient to maintain growth performance of pigs fed HF diets and challenged with an enteric pathogen.
Footnotes
Acknowledgements: Funding for this project was provided by the Alberta Agriculture and Forestry Strategic Research and Development Section (research Grant no. 2016R046R), Evonik Nutrition & Care GmbH, and Mitacs Accelerate (research Grant no. IT08520). The authors would like to thank the staff at the Animal Care Unit of the Western College of Veterinary Medicine, staff of the Canadian Feed Research Centre, and the staff and students of Prairie Swine Center, Inc., (Raelene Petracek, Jana Rajendram, Rochelle Thiessen, Miriam ter Borgh, and Jack Krone) for their assistance.
LITERATURE CITED
- Anguita M., Gasa J., Nofrarias M., Martín-Orúe S. M., and Pérez J. F.. 2007. Effect of coarse ground corn, sugar beet pulp and wheat bran on the voluntary intake and physicochemical characteristics of digesta of growing pigs. Livest. Sci. 107:182–191. doi: 10.1016/j.livsci.2006.09.016 [DOI] [Google Scholar]
- AOAC International 2007. Official methods of analysis of AOAC International. 18th ed. Assoc. Off. Anal. Chem., Gaithersburg, MD. [Google Scholar]
- Barba-Vidal E., Roll V. F. B., Castillejos L., Guerra-Ordaz A. A., Manteca X., Mello J. J., and Martin-Orue S. M.. 2017. Response to a Salmonella typhimurium challenge in piglets supplemented with protected sodium butyrate or Bacillus licheniformis: Effects on performance, intestinal health and behavior. Transl. Anim. Sci. 1:186–200. doi: 10.2527/tas2017.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank B., Schlecht E., and Susenbeth A.. 2012. Effect of dietary fibre on nitrogen retention and fibre associated threonine losses in growing pigs. Arch. Anim. Nutr. 66:86–101. doi:10.1080/1745039x.2012.663669 [DOI] [PubMed] [Google Scholar]
- Burdick-Sanchez N. C., Broadway P. R., Carroll J. A., Gart E. V., Bryan L. K., and Lawhon S. D.. 2017. Weaned pigs experimentally infected with Salmonella display sexually dimorphic innate immune response without affecting pathogen colonization patterns. Transl. Anim. Sci. 1:69–76. doi: 10.2527/tas2016.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey T. E., Dritz S. S., Nietfeld J. C., Johnson B. J., and Minton J. E.. 2004. Effect of dietary mannanoligosaccharide and sodium chlorate on the growth performance, acute-phase response, and bacterial shedding of weaned pigs challenged with Salmonella enterica serotype typhimurium. J. Anim. Sci. 82:397–404. doi: 10.2527/2004.822397x. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care (CCAC) 2009. Guidelines on the care and use of farm animals in research, teaching and testing. CCAC, Ottawa, ON. [Google Scholar]
- Chelack B. J., Morley P. S., and Haines D. M.. 1993. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can. Vet. J. 34:407–412. [PMC free article] [PubMed] [Google Scholar]
- Doumas B. T., Watson W. A., and Biggs H. G.. 1971. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 31:87–96. doi: 10.1016/0009-8981(71)90365-2 [DOI] [PubMed] [Google Scholar]
- Gebru E., Lee J. S., Son J. C., Yang S. Y., Shin S. A., Kim B., Kim M. K., and Park S. C.. 2010. Effect of probiotic-, bacteriophage-, or organic acid-supplemented feeds or fermented soybean meal on the growth performance, acute-phase response, and bacterial shedding of grower pigs challenged with Salmonella enterica serotype typhimurium. J. Anim. Sci. 88:3880–3886. doi: 10.2527/jas.2010-2939. [DOI] [PubMed] [Google Scholar]
- Huntley N. F., Nyachoti C. M., and Patience J. F.. 2018. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 9:47. doi: 10.1186/s40104-018-0264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman B., Htoo J., and Nyachoti C. M.. 2015. Effects of dietary threonine:lysine ratioes and sanitary conditions on performance, plasma urea nitrogen, plasma-free threonine and lysine of weaned pigs. Anim. Nutr. 1:283–288. doi: 10.1016/j.aninu.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc’h N., Lebellego L., Matte J. J., Melchior D., and Sève B.. 2009. The effect of sanitary status degradation and dietary tryptophan content on growth rate and tryptophan metabolism in weaning pigs. J. Anim. Sci. 87:1686–1694. doi: 10.2527/jas.2008-1348 [DOI] [PubMed] [Google Scholar]
- Li D., Changting X., Shiyan Q., Jinhui Z., Johnson E. W., and Thacker P. A.. 1999. Effect of dietary threonine on performance, plasma parameters and immune function of growing pigs. Anim. Feed. Sci. Technol. 78:179–188. doi: 10.1016/S0377-8401(99)00005-X [DOI] [Google Scholar]
- Libao-Mercado A. J., Leeson S., Langer S., Marty B. J., and de Lange C. F.. 2006. Efficiency of utilizing ileal digestible lysine and threonine for whole body protein deposition in growing pigs is reduced when dietary casein is replaced by wheat shorts. J. Anim. Sci. 84:1362–1374. doi: 10.2527/2006.8461362x [DOI] [PubMed] [Google Scholar]
- Libao-Mercado A. J., Zhu C. L., Fuller M. F., Rademacher M., Sève B., and de Lange C. F. M.. 2007. Effect of feeding fermentable fiber on synthesis of total and mucosal protein in the intestine of the growing pig. Livest. Sci. 109:125–128. doi: 10.1016/j.livsci.2007.01.116. [DOI] [Google Scholar]
- Lipperheide C., Diepers N., Lampreave F., Alava M., and Petersen B.. 1998. Nephelometric determination of haptoglobin plasma concentrations in fattening pigs. Zentralbl. Veterinarmed. A 45:543–550. doi: 10.1111/j.1439-0442.1998.tb00858.x [DOI] [PubMed] [Google Scholar]
- Litvak N., Rakhshandeh A., Htoo J. K., and de Lange C. F.. 2013. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. J. Anim. Sci. 91:4188–4196. doi: 10.2527/jas.2012-6160 [DOI] [PubMed] [Google Scholar]
- Llames C. R., and Fontaine J.. 1994. Determination of amino acids in feeds: Collaborative study. J. AOAC Int. 77:1362–1366. [Google Scholar]
- Makimura S., and Suzuki N.. 1982. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Nihon Juigaku Zasshi. 44:15–21. doi: 10.1292/jvms1939.44.15 [DOI] [PubMed] [Google Scholar]
- Mao X., Lai X., Yu B., He J., Yu J., Zheng P., Tian G., Zhang K., and Chen D.. 2014. Effects of dietary threonine supplementation on immune challenge induced by swine pseudorabies live vaccine in weaned pigs. Arch. Anim. Nutr. 68:1–15. doi: 10.1080/1745039X.2013.869988 [DOI] [PubMed] [Google Scholar]
- Mathai J. K., Htoo J. K., Thomson J. E., Touchette K. J., and Stein H. H.. 2016. Effects of dietary fiber on the ideal standardized ileal digestible threonine:lysine ratio for twenty-five to fifty kilogram growing gilts. J. Anim. Sci. 94:4217–4230. doi: 10.2527/jas.2016-0680. [DOI] [PubMed] [Google Scholar]
- van der Meer Y., Lammers A., Jansman A. J., Rijnen M. M., Hendriks W. H., and Gerrits W. J.. 2016. Performance of pigs kept under different sanitary conditions affected by protein intake and amino acid supplementation. J. Anim. Sci. 94:4704–4719. doi: 10.2527/jas.2016-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray W. D., Wooten H., Rakhshandeh A. R., Petry A., and Rakhshandeh A.. 2019. Immune system stimulation increases dietary threonine requirements for protein deposition in growing pigs. J. Anim. Sci. 97:735–744. doi: 10.1093/jas/sky468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne L., Le Floc’h N., Arturo-Schaan M., Foret R., Urdaci M. C., and Le Gall M.. 2012. Comparative effects of level of dietary fiber and sanitary conditions on the growth and health of weanling pigs. J. Anim. Sci. 90:2556–2569. doi: 10.2527/jas.2011-4160. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 2012. Nutrient Requirements of Swine. 11th rev. ed. National Academies Press, Washington, DC. [Google Scholar]
- Pastorelli H., van Milgen J., Lovatto P., and Montagne L.. 2012. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 6:952–961. doi: 10.1017/S175173111100228X. [DOI] [PubMed] [Google Scholar]
- Petersen H. H., Ersbøll A. K., Jensen C. S., and Nielsen J. P.. 2002. Serum-haptoglobin concentration in Danish slaughter pigs of different health status. Prev. Vet. Med. 54:325–335. doi: 10.1016/S0167-5877(02)00054-5 [DOI] [PubMed] [Google Scholar]
- Pieper R., Bindelle J., Rossnagel B., Van Kessel A., and Leterme P.. 2009. Effect of carbohydrate composition in barley and oat cultivars on microbial ecophysiology and proliferation of Salmonella enterica in an in vitro model of the porcine gastrointestinal tract. Appl. Environ. Microbiol. 75:7006–7016. doi: 10.1128/AEM.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R., Bindelle J., Malik G., Marshall J., Rossnagel B. G., Leterme P., and Van Kessel A. G.. 2012. Influence of different carbohydrate composition in barley varieties on Salmonella typhimurium var. Copenhagen colonisation in a “trojan” challenge model in pigs. Arch. Anim. Nutr. 66:163–179. doi: 10.1080/1745039X.2012.676814 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., Htoo J. K., Karrow N., Miller S. P., and de Lange C. F.. 2014. Impact of immune system stimulation on the ileal nutrient digestibility and utilisation of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br. J. Nutr. 111:101–110. doi: 10.1017/S0007114513001955. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Fjeld C. R., and Jahoor F.. 1994. Do the differences between the amino acid compositions of acute phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J. Nutr. 124:906–910. doi: 10.1093/jn/124.6.906 [DOI] [PubMed] [Google Scholar]
- Reeds P. J., and Jahoor F.. 2001. The amino acid requirements of disease. Clin. Nutr. 1:15–22. doi: 10.1054/clnu.2001.0402 [DOI] [Google Scholar]
- Ren M., Liu X. T., Wang X., Zhang G. J., Qiao S. Y., and Zeng X. F.. 2014. Increased levels of standardized ileal digestible threonine attenuate intestinal damage and immune responses in Escherichia coli K88+ challenged weaned piglets. Anim. Feed. Sci. Technol. 195:67–75. doi: 10.1016/j.anifeedsci.2014.05.013 [DOI] [Google Scholar]
- de Ridder K., Levesque C. L., Htoo J. K., and de Lange C. F.. 2012. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. J. Anim. Sci. 90:3485–3491. doi: 10.2527/jas.2011-4830. [DOI] [PubMed] [Google Scholar]
- Rudar M., Zhu C. L., and de Lange C. F.. 2016. Effect of supplemental dietary leucine and immune system stimulation on whole-body nitrogen utilization in starter pigs. J. Anim. Sci. 94:2366–2377. doi: 10.2527/jas.2015-0120. [DOI] [PubMed] [Google Scholar]
- Stein H. H., Trottier N. L., Bellaver C., and Easter R. A.. 1999. The effect of feeding level and physiological status on total flow and amino acid composition of endogenous protein at the distal ileum in swine. J. Anim. Sci. 77:1180–1187. doi: 10.2527/1999.7751180x. [DOI] [PubMed] [Google Scholar]
- Thomson L. W., Pieper R., Marshall J. K., and Van Kessel A. G.. 2012. Effect of wheat distillers dried grains with solubles or sugar beet pulp on prevalence of Salmonella enterica Typhimurium in weaned pigs. J. Anim. Sci. 90(Suppl.4):13–15. doi: 10.2527/jas.53739 [DOI] [PubMed] [Google Scholar]
- Trevisi P., Corrent E., Mazzoni M., Messori S., Priori D., Gherpelli Y., Simongiovanni A., and Bosi P.. 2015. Effect of added dietary threonine on growth performance, health, immunity and gastrointestinal function of weaning pigs with differing genetic susceptibility to Escherichia coli infection and challenged with E. coli k88ac. J. Anim. Physiol. Anim. Nutr. (Berl). 99:511–520. doi: 10.1111/jpn.12216. [DOI] [PubMed] [Google Scholar]
- Turner J. L., Dritz S. S., Higgins J. J., and Minton J. E.. 2002. Effects of Ascophyllum nodosum extract on growth performance and immune function of young pigs challenged with Salmonella typhimurium. J. Anim. Sci. 80:1947–1953. doi: 10.2527/2002.8071947x [DOI] [PubMed] [Google Scholar]
- Wang W., Zeng X., Mao X., Wu G., and Qiao S.. 2010. Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J. Nutr. 140:981–986. doi: 10.3945/jn.109.118497 [DOI] [PubMed] [Google Scholar]
- Wellington M. O., Htoo J. K., Van Kessel A. G., and Columbus D. A.. 2018. Impact of dietary fiber and immune system stimulation on threonine requirement for protein deposition in growing pigs. J. Anim. Sci. 96:5222–5232. doi: 10.1093/jas/sky381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington M. O., Htoo J. K., Van Kessel A. G., and Columbus D. A.. 2019. Estimating the optimal threonine requirement for 25–50 kg pigs fed a mixture of soluble and insoluble dietary fiber. Can. J. Anim. Sci. In press. doi: 10.1139/CJAS-2018-0218 [DOI] [Google Scholar]