Abstract
Naegleria fowleri is a thermophilic free-living amoeba that is found in warm, fresh water and causes primary amebic meningoencephalitis (PAM). The following report demonstrates the rapid and destructive clinical features of PAM in an 8-year-old male who presented with severe headaches approximately 12 days after swimming in a hot spring.
Keywords: Naegleria fowleri, PAM, primary amebic meningoencephalitis
CASE PRESENTATION
An 8-year-old male with a history of occasional migraines presented to an outside hospital (OSH) complaining of headache that began 2 days before presentation. The headache was severe (10 of 10) and described as constant, pounding pain. The headache was originally frontal and then progressed to involve his entire head. It worsened with movement. Neither acetaminophen nor ibuprofen alleviated the headache. On the day before presentation, the patient began experiencing fevers as high as 39.5°C. He also developed progressive neck-stiffness, photophobia, and multiple episodes of emesis. Subsequently, he developed acute onset delirium followed by a generalized seizure that prompted the family to call emergency services.
Upon presentation to the OSH, the patient was febrile to 40.6°C. He was initiated on antimicrobial therapy for presumed meningitis with vancomycin, ceftriaxone, and acyclovir. He was also given dexamethasone at the time of antimicrobial infusion. Lorazepam was used to effectively control his seizures. A lumbar puncture after the initiation of antibiotic therapy was performed, which produced cloudy, xanthochromic cerebrospinal fluid (CSF). Laboratory studies of the CSF revealed glucose <20 mg/dL and protein 606 mg/dL. Microscopy of the CSF revealed white blood cell count 2624 cells/μL (differential: 91% neutrophils, 4% lymphocytes, 5% monocytes) and negative Gram stain. Both culture and FilmArray Meningitis/Encephalitis (ME) panel were negative. Influenza A/B and respiratory syncytial virus by polymerase chain reaction (PCR) panel were also negative. Brain computed tomography (CT) scan without contrast and chest x-ray were normal. Medical history obtained by the facility was pertinent for the patient being fully immunized without any known ill contacts. Family denied any lifetime travel outside of the United States. However, 2 weeks before the onset of his symptoms, the patient visited his family farm in Bishop, California where he had played with snakes, lizards, and salamanders.
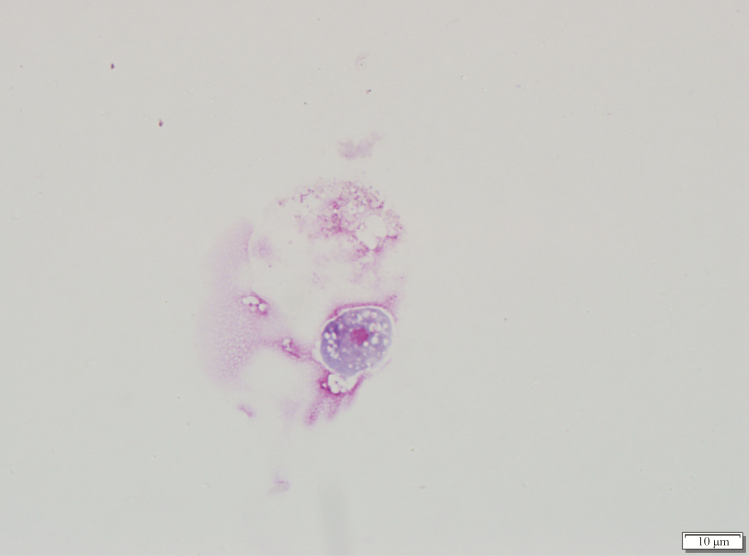
The patient was transferred to our institution the same day of presentation for a higher level of care. Re-examination of the CSF obtained from the OSH corroborated the initial findings, and fluconazole was initiated for additional antimicrobial coverage. The patient received 1 dose of fluconazole but was discontinued in favor of liposomal amphotericin B because he became comatose with persistent fevers. On day 2 of hospitalization, repeat brain CT scan without contrast showed significant cerebral edema without intracranial hemorrhage. In addition, a wet mount, trichrome stain, and Giemsa stain was performed on the CSF and revealed a potential pathogen (Figure 1). After Giemsa stain examination, the patient was started on azithromycin, posaconazole, rifampin, miltefosine, and dexamethasone in addition to liposomal amphotericin B with no improvement. The patient died on day 3 of hospitalization.
Figure 1.
Giemsa stain of the cerebrospinal fluid.
What is your diagnosis?
DISCUSSION
Giemsa stain of the CSF revealed few Naegleria fowleri trophozoites (Figure 1). Diagnosis of primary amebic meningoencephalitis (PAM) caused by N fowleri was further confirmed by targeted PCR. The patient had been on liposomal amphotericin B, and, based on Giemsa stain examination, the Centers for Disease Control and Prevention (CDC) was contacted. Additional treatment was initiated according to the CDC’s PAM antimicrobial recommendation [1–4] and included the following: azithromycin, posaconazole, rifampin, miltefosine, and dexamethasone. Clinical symptoms did not improve despite therapy, and the patient progressed to brain death on day 3 of hospitalization.
Naegleria fowleri is a free-living amoeba that inhabits both soil and fresh water. It is thermophilic and can be found in warm lakes and rivers, geothermal springs, naturally hot untreated water supplies, and warm water discharge from industrial plants. Primary amebic meningoencephalitis is most often associated with human activity in warm, fresh water that results in contaminated water entering the nasal cavity [5]. Infectivity occurs via attachment of the organism to the nasal mucosa, after which the amoeba moves along the olfactory nerve, penetrates the cribriform plate, and reaches the olfactory bulb in the central nervous system [6]. Although exposure to freshwater was initially denied, after preliminary identification and further questioning on day 2 of admission, a family member remembered that the patient and his siblings swam and submerged their heads in a hot spring, approximately 12 days before the onset of symptoms.
Onset of clinical symptoms for PAM present between 1 and 15 days from time of exposure [5]. Initial manifestations of PAM are indistinguishable from bacterial meningitis and symptomatology includes the following: fever, severe headache, photophobia, confusion, seizures, and coma [7]. Cerebrospinal fluid obtained from infected patients is often hazy with hypoglycorrhachia, elevated protein, and pleocytosis with neutrophilic predominance [7]. Rapid diagnosis of PAM can be made by microscopy or PCR. Microscopy methods include wet mount for motile trophozoites or trichrome and Giemsa stain of CSF for identification of the distinctive trophozoite morphology. Giemsa stain is the preferred microscopy method because it best highlights the amoeba’s granularity, its many vacuoles, and its large single nucleus that has a sizable karyosome and lacks peripheral chromatin (Figure 1).
Between 1962 and 2017, 143 US cases of PAM were reported to the CDC with only 4 survivors [8]. An additional patient in Mexico also survived infection [1]. The first US survivor was thought to be infected with a less virulent strain of N fowleri [9, 10]. Two other patients had full neurologic recovery, which may have been due to early diagnosis, treatment, and the inclusion of miltefosine as a novel therapeutic [2, 4]. The fourth surviving patient from the United States was also treated with miltefosine but did not receive the treatment for several days after onset of symptoms. This patient had permanent neurological sequelae [4]. The sole surviving patient from Mexico was treated immediately with amphotericin B, fluconazole, and rifampicin and had full neurologic recovery [1]. The CDC recommends initiation of antimicrobials as soon as possible; however, effective treatment for infections caused by Naegleria has not been established due to the limited number of survivors. Miltefosine has demonstrated in vitro killing activity against N fowleri when combined with an azole drug [11] and was used to successfully treat 3 patients with PAM. Regardless of treatment regimen, the mortality rate of PAM remains approximately 98% [12].
CONCLUSIONS
This case exemplifies the rapid and destructive features of N fowleri-associated PAM. Thorough patient interrogation and prompt identification of the causative agent is paramount to ensure appropriate treatment and management of these patients.
Acknowledgment
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Vargas-Zepeda J, Gómez-Alcalá AV, Vásquez-Morales JA, et al. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch Med Res 2005; 36:83–6. [DOI] [PubMed] [Google Scholar]
- 2. Linam WM, Ahmed M, Cope JR, et al. Successful treatment of an adolescent with Naegleria fowleri primary amebic meningoencephalitis. Pediatrics 2015; 135:e744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colon BL, Rice CA, Guy RK, Kyle DE. Phenotypic screens reveal posaconazole as a rapidly acting amebicidal combination partner for treatment of primary amoebic meningoencephalitis. J Infect Dis 2019; 219:1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cope JR, Conrad DA, Cohen N, et al. Use of the novel therapeutic agent miltefosine for the treatment of primary amebic meningoencephalitis: report of 1 fatal and 1 surviving case. Clin Infect Dis 2016; 62:774–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoder JS, Eddy BA, Visvesvara GS, et al. The epidemiology of primary amoebic meningoencephalitis in the USA, 1962-2008. Epidemiol Infect 2010; 138:968–75. [DOI] [PubMed] [Google Scholar]
- 6. Jarolim KL, McCosh JK, Howard MJ, John DT. A light microscopy study of the migration of Naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J Parasitol 2000; 86:50–5. [DOI] [PubMed] [Google Scholar]
- 7. Capewell LG, Harris AM, Yoder JS, et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937-2013. J Pediatric Infect Dis Soc 2015; 4:e68–75. [DOI] [PubMed] [Google Scholar]
- 8. Grace E, Asbill S, Virga K. Naegleria fowleri: pathogenesis, diagnosis, and treatment options. Antimicrob Agents Chemother 2015; 59:6677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seidel JS, Harmatz P, Visvesvara GS, et al. Successful treatment of primary amebic meningoencephalitis. N Engl J Med 1982; 306:346–8. [DOI] [PubMed] [Google Scholar]
- 10. John DT, John RA. Cytopathogenicity of Naegleria fowleri in mammalian cell cultures. Parasitol Res 1989; 76:20–5. [DOI] [PubMed] [Google Scholar]
- 11. Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol 2006; 53:121–6. [DOI] [PubMed] [Google Scholar]
- 12. Martinez AJ, Visvesvara GS. Free-living, amphizoic and opportunistic amebas. Brain Pathol 1997; 7:583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]