Abstract
STUDY QUESTION
Is it possible to differentiate primary human testicular platelet-derived growth factor receptor alpha positive (PDGFRα+) cells into functional Leydig cells?
SUMMARY ANSWER
Although human testicular PDGFRα+ cells are multipotent and are capable of differentiating into steroidogenic cells with Leydig cell characteristics, they are not able to produce testosterone after differentiation.
WHAT IS KNOWN ALREADY
In rodents, stem Leydig cells (SLCs) that have been identified and isolated using the marker PDGFRα can give rise to adult testosterone-producing Leydig cells after appropriate differentiation in vitro. Although PDGFRα+ cells have also been identified in human testicular tissue, so far there is no evidence that these cells are true human SLCs that can differentiate into functional Leydig cells in vitro or in vivo.
STUDY DESIGN, SIZE, DURATION
We isolated testicular cells enriched for interstitial cells from frozen–thawed fragments of testicular tissue from four human donors. Depending on the obtained cell number, PDGFRα+-sorted cells of three to four donors were exposed to differentiation conditions in vitro to stimulate development into adipocytes, osteocytes, chondrocytes or into Leydig cells. We compared their cell characteristics with cells directly after sorting and cells in propagation conditions. To investigate their differentiation potential in vivo, PDGFRα+-sorted cells were transplanted in the testis of 12 luteinizing hormone receptor-knockout (LuRKO) mice of which 6 mice received immunosuppression treatment. An additional six mice did not receive cell transplantation and were used as a control.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Human testicular interstitial cells were cultured to Passage 3 and FACS sorted for HLA-A,B,C+/CD34−/PDGFRα+. We examined their mesenchymal stromal cell (MSC) membrane protein expression by FACS analyses. Furthermore, we investigated lineage-specific staining and gene expression after MSC trilineage differentiation. For the differentiation into Leydig cells, PDGFRα+-sorted cells were cultured in either proliferation or differentiation medium for 28 days, after which they were stimulated either with or without hCG, forskolin or dbcAMP for 24 h to examine the increase in gene expression of steroidogenic enzymes using qPCR. In addition, testosterone, androstenedione and progesterone levels were measured in the culture medium. We also transplanted human PDGFRα+-sorted testicular interstitial cells into the testis of LuRKO mice. Serum was collected at several time points after transplantation, and testosterone was measured. Twenty weeks after transplantation testes were collected for histological examination.
MAIN RESULTS AND THE ROLE OF CHANCE
From primary cultured human testicular interstitial cells at Passage 3, we could obtain a population of HLA-A,B,C+/CD34−/PDGFRα+ cells by FACS. The sorted cells showed characteristics of MSC and were able to differentiate into adipocytes, chondrocytes and osteocytes. Upon directed differentiation into Leydig cells in vitro, we observed a significant increase in the expression of HSD3B2 and INSL3. After 24 h stimulation with forskolin or dbcAMP, a significantly increased expression of STAR and CYP11A1 was observed. The cells already expressed HSD17B3 and CYP17A1 before differentiation but the expression of these genes were not significantly increased after differentiation and stimulation. Testosterone levels could not be detected in the medium in any of the stimulation conditions, but after stimulation with forskolin or dbcAMP, androstenedione and progesterone were detected in culture medium. After transplantation of the human cells into the testes of LuRKO mice, no significant increase in serum testosterone levels was found compared to the controls. Also, no human cells were identified in the interstitium of mice testes 20 weeks after transplantation.
LARGE SCALE DATA
N/A
LIMITATIONS, REASONS FOR CAUTION
This study was performed using tissue from only four donors because of limitations in donor material. Because of the need of sufficient cell numbers, we first propagated cells to passage 3 before FACS of the desired cell population was performed. We cannot rule out this propagation of the cells resulted in loss of stem cell properties.
WIDER IMPLICATIONS OF THE FINDINGS
A lot of information on Leydig cell development is obtained from rodent studies, while the knowledge on human Leydig cell development is very limited. Our study shows that human testicular interstitial PDGFRα+ cells have different characteristics compared to rodent testicular PDGFRα+ cells in gene expression levels of steroidogenic enzymes and potential to differentiate in adult Leydig cells under comparable culture conditions. This emphasizes the need for confirming results from rodent studies in the human situation to be able to translate this knowledge to the human conditions, to eventually contribute to improvements of testosterone replacement therapies or establishing alternative cell therapies in the future, potentially based on SLCs.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by Amsterdam UMC, location AMC, Amsterdam, the Netherlands. All authors declare no competing interests.
Keywords: PDGFRα, stem Leydig cell, testis, mesenchymal stromal cell, steroidogenesis
Introduction
Persistent low testosterone levels in men result in hypogonadism. At present, testosterone replacement therapy (TRT) is offered to these patients to restore their serum testosterone levels. Although TRT positively rescues many of the symptoms of hypogonadism, adverse effects have also been reported, such as polycythaemia and a higher risk of cardiovascular disease, prostate cancer and infertility (Bassil et al., 2009; Xu et al., 2013). Furthermore, TRT requires the lifelong administration of testosterone using gel or injections, which is burdensome and costly.
Stem Leydig cell (SLC) therapy, which aims to restore the biological production of testosterone by Leydig cells in the testis, might potentially be a preferred future treatment strategy for men with hypogonadism since it would maintain the balance of the hypothalamic-pituitary-testicular axis and also avoid the lifelong administration of testosterone. Evidence for the existence of SLCs was shown in rats treated with dimethanesulfonate (EDS), which destroys Leydig cells, after which new adult Leydig cells (ALCs) appeared 14 to 21 days later (Kerr et al., 1985; Jackson et al., 1986; Teerds et al., 1988). With this discovery, more knowledge has been obtained on the development of Leydig cells. SLCs are thought to have a mesenchymal origin (Hardy et al., 1989). In rodents, the Leydig cell lineage from SLCs toward ALCs is considered to entail four developmental stages. SLCs are already present in the fetal testis and differentiate prior to puberty, into progenitor Leydig cells (PLCs), followed by differentiation into immature Leydig cells (ILCs) and eventually ALCs. All these cell subtypes have their own characteristics (reviewed in Teerds and Huhtaniemi, 2015; Ye et al., 2017). Although the knowledge on Leydig cell development in rodents has increased over the past decades, knowledge on the development of human ALCs is still rather limited. Because there are indications that differences exist between rodent and human LC development, we do not know if the information on rodent LC development can directly be translated to the human situation (Teerds and Huhtaniemi, 2015).
To obtain further knowledge on the development of human ALCs, it is essential to identify and isolate human SLCs. Rodent SLCs were isolated for the first time from testes of neonatal rats by sorting for the membrane marker platelet-derived growth factor receptor alpha (PDGFRα). These isolated cells could be propagated and differentiated into testosterone producing cells in vitro (Ge et al., 2006). Since then, the identification of SLCs based on the presence of PDGFRα has been confirmed in neonatal rats, adult rats and pigs (Stanley et al., 2012; Landreh et al., 2013; Yu et al., 2017). In 2014, Landreh et al. were the first to isolate and culture PDGFRα+ cells from the human testes. The steroidogenic potential of these cells was demonstrated by production of pregnenolone and progesterone after stimulation with forskolin in vitro. Although an increase in mRNA encoding for steroidogenic acute regulatory protein (STAR) and cytochrome P450 Family 11, Subfamily A, Member 1 (CYP11A1) was found, no complete development into functional ALCs was achieved by forskolin stimulation, since no testosterone was produced (Landreh et al., 2014).
It is not yet clear whether human testicular PDGFRα+ cells are mesenchymal multipotent stem cells and whether they are able to differentiate into ALCs. The aim of this study therefore was to further characterize the human testicular PDGFRα+ cell population and investigate their mesenchymal stromal cell (MSC) characteristics. In addition, we used an in vitro differentiation culture assay and an in vivo xeno-transplantation model to assess the potential of the PDGFRα+ cells to differentiate into ALCs.
Materials and Methods
Human testicular tissue samples and animals
We obtained testicular tissue from four prostate cancer patients, aged 45 to 79 years, who were undergoing bilateral orchidectomy. Histological analysis showed the presence of full spermatogenesis in all cases. Depending on the number of cells available, we performed the various experiments on testicular interstitial cells from three or four patients. For MSC isolation, we obtained abdominal fat tissue of a patient undergoing abdominoplasty. Both tissues used for research were left over after clinical diagnostic and treatment procedures and were donated with informed consent. According to Dutch law, these spare tissues can be used for research without approval of a medical ethical committee as none of the patients had to undergo any additional intervention to obtain the material for this research. Luteinizing hormone receptor knock-out (LuRKO) mice, which have an inactivating mutation in the luteinizing hormone (LH) receptor, were obtained from the University of Turku, Finland (Zhang, 2001). Ethical approval for the animal experiments was obtained from the Animal Ethical Committee (DEC) of Amsterdam UMC, location AMC, according to European legislation. Animals were housed in groups in individual ventilated cages with food and water ad libitum.
Cell isolation and cell culture
An overview of the various methods performed is depicted in Figure 1. We isolated cells from cryopreserved–thawed testicular tissue fragments following the protocol previously described (van Pelt et al., 1996; Sadri-Ardekani et al., 2009). The interstitial cell fraction (ICF) was obtained after the first enzymatic digestion step with trypsin, hyaluronidase and collagenase (Chikhovskaya et al., 2014). We propagated the isolated ICF cells in medium as described for rat SLCs with minor modifications (Ge et al., 2006). Briefly, DMEM/F12 + glutamax (Gibco, Thermo Fisher Scientific, USA) was supplemented with 2% fetal calf serum (FCS, Gibco, Thermo Fisher Scientific, USA), 1% penicillin/streptomycin (pen/strep, Gibco, Thermo Fisher Scientific, USA), 1% insulin/transferrin/sodium selenite (ITS, Gibco, Thermo Fisher Scientific, USA), 1 nM dexamethasone (Sigma-Aldrich, USA), 10 ng/ml platelet-derived growth factor-BB (PDGF-BB, Peprotech, USA), 10 ng/ml epidermal growth factor (EGF, Peprotech, USA), 5 ng/ml human basic fibroblast growth factor (FGF2, Sigma-Aldrich, USA) and 1 ng/ml LIF (Prospec, Israel). Cells were cultured at a density of 4000 cells/cm2 at 37°C in a humidified 5% CO2 water-jacketed incubator. The medium was refreshed every 3–4 days. After reaching a maximum of 80% confluency, we passaged the propagated cells by detaching the cells using Accutase (Sigma-Aldrich, USA) and then plated the cells again at a density of 4000 cells/cm2. For propagation of PDGFRα+-sorted cells, the cells were cultured under the same conditions as the ICF cells.
Figure 1.
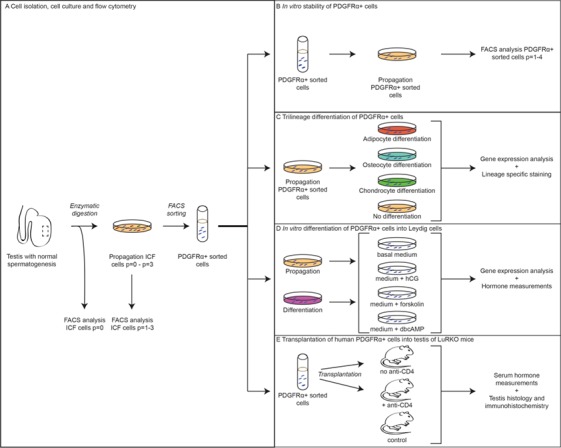
Overview of the methods performed on ICF and PDGFRα + cells. From a testicular tissue sample, interstitial cells were isolated, propagated and sorted for HLA+/CD34−/PDGFRα+. During propagation of ICF cells, flow cytometry analyses were performed at every passage (A). After sorting, the cells were used to study stability of PDGFRα expression during propagation in culture (B), trilineage differentiation potential (C), in vitro differentiation into Leydig cells (D) and in vivo differentiation into Leydig cells after transplantation into testis of LuRKO mice (E). ICF: interstitial cell fraction, PDGFRα: platelet-derived growth factor receptor alpha; LuRKO: luteinizing hormone receptor knock-out.
We isolated MSCs from fresh adipose tissue. Adipose tissue was digested with 0.25% Collagenase Type IA (Sigma-Aldrich, USA) for 60 minutes at 37°C in a shaking water bath. After placing samples upright for 5 minutes, the cells from the pellet were washed with PBS with 2% FCS (Gibco, Thermo Fisher Scientific, USA) and 1-mM EDTA (Merck Millipore, USA) and filtered through a 77-μm nylon filter. We cultured MSCs in DMEM/F12 + glutamax (Gibco, Thermo Fisher Scientific, USA) with 10% FCS (Gibco, Thermo Fisher Scientific, USA) and 1% pen/strep (Gibco, Thermo Fisher Scientific, USA). These cells were used as a positive MSC control in this study.
Flow cytometry analysis or sorting of PDGFRα+ cells
For flow cytometry analysis we used the BD FACSCanto II Flowcytometer (BD Biosciences, USA) or the BD LSRFortessa cell analyzer flow cytometer (BD Biosciences, USA) in combination with the FlowJo 10.2 software (FlowJo, LLC, USA) to analyze the data. For FACS analysis, cells were fixed in BD Cytofix/Cytoperm (BD Biosciences, USA). We stained the cells with fluorochrome-conjugated antibodies for the detection of the cell surface markers PDGFRα (BD Biosciences, USA), CD90 (eBioscience, Thermo Fisher Scientific, USA), CD73 (BD Biosciences, USA) and CD105 (BD Biosciences, USA). According to the minimal criteria for MSCs, cells need to be positive for CD90, CD73 and CD105 to describe them as MSCs (Dominici et al., 2006). To exclude non-viable cells, we used the Zombie violet fixable viability kit (Biolegend, USA) to stain dead cells before the cells were fixed.
Non-fixed ICF cells were sorted at Passage 3 using the SH800Z Cell Sorter (Sony Biotechnology, USA) for the HLA-A,B,C+/CD34−/PDGFRα+ subpopulation to exclude germ cells and endothelial cells from the sorted population. Non-viable cells were excluded by the use of 7-AAD (BD Biosciences, USA). For both analysis and sorting, we determined background fluorescence levels by using matched conjugated IgG isotype controls. The details of the antibodies that were used can be found in Supplementary Table SI.
Trilineage differentiation of PDGFRα+-sorted cells
Prior to induction of trilineage differentiation, the sorted PDGFRα+ cells were propagated to obtain a sufficient number of cells. For the induction of differentiation into adipocytes, osteocytes and chondrocytes, the cells were cultured using the StemPro adipogenesis, osteogenesis or chondrogenesis differentiation kit (Gibco, Thermo Fisher Scientific, USA), respectively, according to the protocol of the manufacturer. In parallel PDGFRα+ cells were propagated as described earlier as a negative control. As a positive control, we used MSCs derived from adipose tissue. For evaluation of the trilineage differentiation state, we used Oil Red O staining for adipocytes on day 17, Alizarin Red staining for osteocytes on Day 24 and Alcian blue for chondrocytes on Day 17 of differentiation. Pictures were taken with a Leica PMIRB-inverted microscope using Las X software (Leica Microsystems, Germany). Cells were used for gene expression analysis on Days 4, 7 and 14 of differentiation.
In vitro differentiation of PDGFRα+ cells into Leydig cells
For in vitro Leydig cell differentiation, the sorted PDGFRα+ cells were plated at a density of 20 000 cells/cm2 directly after sorting and cultured for 4 weeks in differentiation medium. As a control condition, PDGFRα+-sorted cells were propagated in parallel for 4 weeks in propagation medium as described for ICF cells. The differentiation medium was prepared according to the protocol of differentiation of rat SLCs into Leydig cells with minor adaptations (Ge et al., 2006). Briefly, the differentiation medium consisted of phenol red-free DMEM/F12 (Gibco, Thermo Fisher Scientific, USA) supplemented with 2% FCS, 1% pen/strep, 1% ITS, 10-ng/ml PDGF-BB, 70 ng/ml insulin-like growth factor-1 (R&D systems, USA), 0.75 IU/ml hCG (Organon, Netherlands), 1 nM Triiodothyronine (T3; Sigma-Aldrich, USA) and 10 nM Thyroxine (T4; Sigma-Aldrich, USA). Furthermore, 0.5 μM smoothened agonist (SAG, Merck Millipore, USA) was added, which has been shown to induce differentiation of rat testicular cells into testosterone producing cells (Li et al., 2016). The medium was refreshed every 3 to 4 days without passaging. After 4 weeks of culture, the cells were detached using Accutase and replated at a density of 17 000–21 000 cells/cm2 in 6-wells culture plates in 1.5-ml medium. In parallel, the propagated cells were replated at a similar density. After allowing the cells to attach to the plastic for 24 h, cells were washed with PBS to remove serum prior to stimulation for 24 h with either only phenol red-free DMEM/F12 with 1% pen/strep (basal medium) or with addition of 0.75 IU/ml hCG, 50 μM forskolin or 1 mM dbcAMP. We used hCG as a stimulatory factor in anticipation of cells differentiating into LH-dependent cells during the differentiation culture. Because it is known that the LH and choriogonadotropin receptor (LHCGR) can be downregulated when cells are cultured in medium containing hCG, we used forskolin or dbcAMP to circumvent the LHCGR and stimulate steroidogenesis by stimulating adenylate cyclase and cAMP-dependent protein kinase A (PKA), respectively (Habert et al., 2001; Landreh et al., 2014). After 24 h, the medium was collected and stored at −20°C until further analysis. The cells were detached from the wells and transferred to RNA extraction buffer (PicoPure RNA isolation kit, Arcturus, Thermo Fisher Scientific, USA) and stored at −80°C until used for gene expression analysis. During culturing of the cells, pictures were taken using an Olympus IX71 microscope.
Gene expression analysis
For the adipogenesis and osteogenesis differentiation assay, total RNA was isolated using the ISOLATE II RNA Mini Kit (Bioline, UK) with two times on-column DNase treatment (Bioline, UK). In the case of chondrogenesis, we used the RNeasy Fibrous Tissue mini kit (Qiagen, Germany) with three times on-column DNase treatment (Qiagen, Germany). In the case of the Leydig cell differentiation assay, total RNA was isolated using the PicoPure RNA isolation kit (Arcturus, Thermo Fisher Scientific, USA) according to the manufacturers protocol accompanied by two times on-column DNase treatment (Qiagen, Germany). To test the obtained total RNA for genomic contamination, we performed a PCR for a DNA-specific sequence of a reference gene (EEF2) and checked for DNA products on an agarose gel. No DNA product or a negligible amount of product was found. For reverse transcription to cDNA, the SensiFAST™ cDNA synthesis kit (Bioline, UK) was used according to the protocol of the manufacturer. Negative controls of all samples (−RT) for the qPCR were obtained by leaving out the reverse transcriptase to the reaction mix. We used the Roche LC480 system for qPCR. For MSC trilineage differentiation or Leydig cell differentiation assessment, 10 ng and 25 ng cDNA were used, respectively, as input for qPCR in a mix with LightCycler 480 SYBR Green I master (Roche, Switzerland) and forward and reverse primers. The PCR products were put on gel to check for absence of additional products. All primers were optimized for concentration and annealing temperature and PCR products were sequenced to confirm the correct product. A detailed list of primers can be found in Supplementary Table SII. We used the LinReg PCR program to analyze the data (Ruijter et al., 2009). For normalization of the Leydig cell differentiation assay and MSC differentiation assay, we used reference genes HEATR6 in combination with EEF2 or PLEKHA8, respectively, for which stable expression was determined by the qbase+ program (Biogazelle, Belgium).
Transplantation of human PDGFRα+ cells into testis of LuRKO mice
To investigate the differentiation potential of the sorted human HLA-A,B,C+/CD34−/PDGFRα+ cells toward Leydig cells in vivo, we xeno-transplanted these human cells into the testicular interstitium of LuRKO mice that have no functional luteinizing hormone receptor and subsequently almost no endogenous testosterone production. Human HLA-A,B,C+/CD34−/PDGFRα+ cells (110 000 ± 20 000 (mean ± SD)) were transplanted unilaterally in 12 LuRKO mice aged between 11 and 14 weeks by injecting the cell suspension through the tunica in the interstitium under whole body anesthesia using 2% isoflurane. By adding trypan blue to the cell suspension, the dispersion of the suspension became visible throughout the testes. All transplanted mice received 0.05 mg/kg temgesic (Reckitt Benckiser Healthcare, UK) analgesics subcutaneously before and 1–2 days after the surgery. Half of the transplanted mice received intraperitoneal injections of 50 μg anti-CD4 (eBioscience, Austria) on Days 0, 2 and 4 posttransplantation to suppress their immune system. Six additional mice were included as a control group and were therefore not transplanted. We injected all 18 mice three times a week with 1 IU hCG (Pregnyl, N.V. Organon, the Netherlands) to stimulate the human LHCG receptor inducing differentiation of the human transplanted cells into Leydig cells, since this dose of hCG does not induce the development of antibodies to hCG in mice (Spaliviero et al., 2004). Blood samples were taken from the buccae in all animals every 2 weeks. Serum was stored at −20°C until analysis. Twenty weeks after transplantation animals were sacrificed under total anesthesia by a mixture of CO2 and O2 followed by only CO2. Both testes were collected, and a quarter of it was fixed in 4% buffered paraformaldehyde for immunohistochemical analyses.
Hormone measurements
The testosterone concentrations in culture media and mouse serum were measured using an ELISA kit (R&D Systems, USA), according to the manufacturer instructions. This ELISA has a coefficient of variation of 2.9–4% for intra-assay precision and 5.6–6.8% for inter-assay precision. The lower limit of the assay was 30 pg/ml. We used 90 ul medium samples per well. For the standard curve, we used medium that matched with the experimental conditions. Optical density was determined using the Multiskan Ex (Thermo Fisher Scientific, USA) at 450 nm and 560 nm. Calculations were performed as described by the manufacturer using Graphpad Prism 5 (GraphPad Software, USA) with a nonlinear regression (curve fit) analysis. In addition, testosterone and androstenedione, a steroid synthesized primarily by progenitor and immature Leydig cells (reviewed in Teerds and Huhtaniemi, 2015), were measured in culture medium at the Endocrine Laboratory of Amsterdam UMC with a 2D-UPLC-MS/MS system with a lower limit of quantification of 3 pmol/l (Büttler et al., 2016). Progesterone was also measured at the Endocrine Laboratory of Amsterdam UMC with an Elecsys immunoassay (Roche Diagnostics, the Netherlands) with a lower limit of quantification of 0.2 nmol/l.
Immunohistochemical staining
Testes of transplanted and control mice were fixed, dehydrated, embedded in paraffin and serial sectioned (5 μm), and every fourth section was used for immunohistochemical analysis. In short, after removal of the paraffin, we performed heat-induced antigen retrieval (10 minutes) using 0.01 M tri-sodium citrate dehydrate buffer (pH = 6). Sections were blocked for 1 h with mouse IgG blocking reagent (Vector Laboratories, USA), after which we blocked with 5% BSA + 0.5% BSAc (Aurion, the Netherlands) for 1 h. Sections were incubated with the anti-human nucleoli primary antibody (Supplementary Table SI) overnight in a humidified box at 4°C. Endogenous peroxidase was blocked with 3% H2O2 (Millipore, USA) in methanol. As a secondary antibody, poly-HRP Goat-anti-Mouse/Rabbit (Immunologic, the Netherlands) was used with 30 minutes incubation at room temperature. The DAB bright kit (Immunologic, the Netherlands) was used to visualize the reaction product, followed by counterstaining with hematoxylin. The researcher who analyzed the sections was blinded for the origin of the sections (transplanted or not-transplanted tissue).
Statistical analysis
All data are presented as mean ± SEM using Graphpad Prism 5 program. All statistical analyses were performed with IBM SPSS Statistics 24.0 program. We performed a two-way ANOVA on the data of in vitro differentiation to Leydig cells to compare the main effects of culture conditions (after sorting, propagation and differentiation) and 24 h stimulation conditions (after sorting, basal, hCG, forskolin and dbcAMP) and the interaction effect between culture and stimulation conditions on steroidogenic gene expression. If culture conditions had a significant effect on the outcome, we performed a Tukey post-hoc test to assess which groups differ significantly from each other. If a significant effect of 24 h stimulation conditions was seen, we applied a Dunnet post-hoc test to asses which cells after stimulation have significantly changed compared to cells after sorting. For the progesterone data, a Tukey post-hoc test for stimulation conditions was performed since here no data for the control group ‘directly after sorting’ were available. A P value < 0.05 was considered statistically significant.
Results
Identification and propagation of human testicular PDGFRα+ cells
FACS analyses revealed the presence of PDGFRα+ cells in the isolated ICF cells (Fig. 2A). PDGFRα expression in ICF cells remained detectable throughout the entire culture period of three passages until sorting. Although the percentage of PDGFRα-expressing cells was low at Passage 0, the majority of the ICF cells became positive for PDGFRα from Passage 1 onward (Fig. 2B). In addition, when HLA-A,B,C+/CD34−/PDGFRα+ cells sorted at Passage 3 were further propagated in vitro, the cells remained positive for PDGFRα for at least four additional passages (Fig. 2C).
Figure 2.
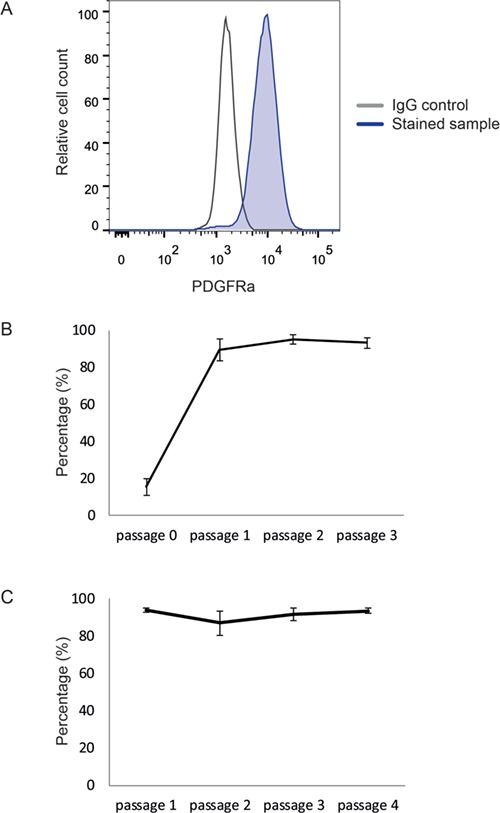
Identification and propagation of PDGFRα + cells from human testicular ICF cells. Flow cytometry analyses showing a clear PDGFRα+ cell population. The stained sample (blue) clearly separates from the IgG control (gray) (A). The percentage of PDGFRα+ cells was determined at every passage during culture of ICF cells (biological replicates n = 3) (B). The percentage of PDGFRα+ cells was determined at every passage during culture of the PDGFRα+ cells after sorting (biological replicates n = 4) (C).
Multipotent properties of human PDGFRα+ cells
FACS analysis showed that PDGFRα+ cells were positive for CD90, CD73 and CD105 proteins, characteristic for MSCs (Fig. 3A). When differentiated into the direction of adipocytes, the PDGFRα+ cells acquired lipid droplets in their cytoplasm. Although the lipid droplets were somewhat smaller in size compared to the positive control (MSCs derived from adipose tissue) (Supplementary Fig. S1A), all droplets stained positive for lipids with Oil Red O (Fig. 3B). There was a 5- to 10-fold increase in expression in CFD (adipsin) after differentiation into adipocytes (Fig. 3E). When differentiated into the direction of osteocytes, PDGFRα+ cells stained positively for calcium deposits with Alizarin Red (Fig. 3C). No differences were seen in staining between the differentiated PDGFRα+ cells and the positive control (Supplementary Fig. S1B). A 2- to 5-fold increase in the expression of SPARC was observed in the differentiated PDGFRα+ cells, but not in the differentiated MSCs. For both the differentiation into adipocytes and osteocytes, some heterogeneity was seen in the brightness of the staining between donors, with no staining for Alizarin Red in the cells of one donor. After differentiation into chondrocytes, all three PDGFRα+ samples formed typical chondrocyte colonies that stained positive with Alcian blue similar to the positive control (Fig. 3D and Supplementary Fig. S1C). Differentiated PDGFRα+ cells showed a 10- to 20-fold increase in the expression of COL10A1 (Fig. 3E). Non-differentiated, propagated PDGFRα+ cells did not show positive staining for Oil Red O, Alizarin Red or Alcian blue (data not shown). Differences in the efficiency of the differentiation between the biological samples were seen in the intensity of staining and the increase in RNA expression of the various genes.
Figure 3.
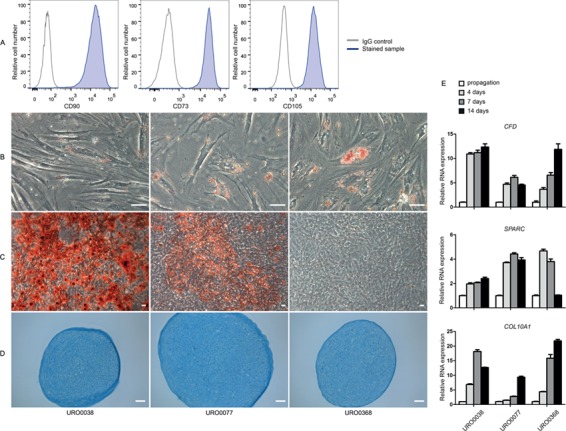
MSC characteristics of HLA-A,B,C + /CD34 − /PDGFRα + cells. PDGFRα+ cells are positive for MSC minimal criteria markers CD90, CD73 and CD105 (blue) compared to IgG controls (gray) (A). Staining for trilineage differentiation of HLA-A,B,C+/CD34−/PDGFRα+ cells into adipocytes (Oil red O) (B), osteocytes (alizarin red) (C) and chondrocytes (alcian blue) (D). Scale bar is 50 μm. Relative gene expression of CFD for adipocytes, SPARC for osteocytes and COL10A1 for chondrocytes (E). Error bars indicate technical variation (n = 3). MSC: mesenchymal stromal cell; CFD: complement factor D; SPARC: secreted protein acidic and cysteine rich; COL10A1: collagen, type X, alpha 1.
In vitro differentiation of human PDGFRα+ cells into Leydig cells
After 4 weeks of culture in differentiation medium, the sorted cells had changed from long-stretched cells into more round cells with shorter extensions. In addition, cytoplasmic granularity was seen more frequently under the differentiation condition compared to the propagation condition (Fig. 4A and B). An effect of culture conditions (after sorting versus propagation versus differentiation) was seen on RNA expression encoding for CYP11A1 (culture P = 0.045), 3β-hydroxysteroid dehydrogenase type II (HSD3B2, culture P = 0.012) and insulin-like 3 (INSL3, culture P = 0.016). Using the Tukey post-hoc test, a significantly higher expression was found in the differentiation culture compared to the propagation culture for HSD3B2 (P = 0.032) and INSL3 (P = 0.041), while no significant difference was found between culture groups for the expression of CYP11A1. There was an effect of the 24 h stimulation of the cells on RNA expression encoding for STAR (stimulation P = 0.003) and CYP11A1 (stimulation P = 0.008). Dunnet post-hoc test, comparing the various stimulation conditions with the cells directly after sorting, demonstrated a significant increased RNA expression of these two genes when cells were stimulated with forskolin (STAR: P = 0.009, CYP11A1: P = 0.049) or dbcAMP (STAR: P = 0.016, CYP11A1: P = 0.011) (Fig. 4C). Expression of mRNA of all measured enzymes, STAR, CYP11A1, HSD3B2, HSD17B3 and CYP17A1 was already detected in the HLA-A,B,C+/CD34−/PDGFRα+ cells directly after sorting prior to the differentiation culture. In addition, INSL3, a product of adult-type Leydig cells, was expressed in cells directly after sorting. There was no mRNA expression of LHCGR detected in the cells, in any of the culture conditions (data not shown).
Figure 4.
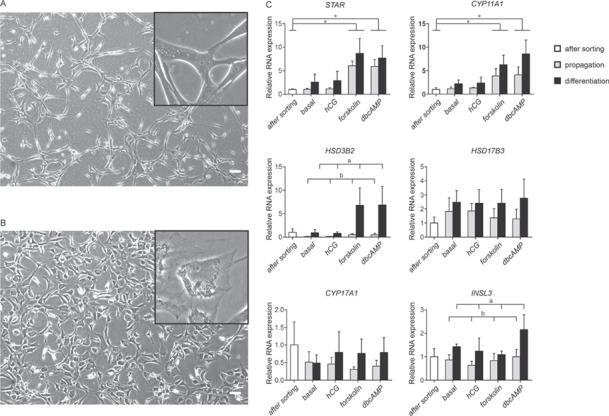
In vitro differentiation into Leydig cells. After 4 weeks of culture, the morphology of the HLA-A,B,C+/CD34−/PDGFRα+ cells differ when cultured in propagation medium (A) or when cultured in differentiation conditions (B). Scale bar is 100 μm. RNA expression analyses for Leydig cell-specific enzymes and products STAR, CYP11A1, HSD3B2, HSD17B3, CYP17A1 and INSL3 (C). Data are expressed as mean ± SEM (n = 4). Letters a and b indicate significant difference in mRNA expression between propagation and differentiation culture conditions using a Tukey post-hoc test (P < 0.05). The asterisk in the graphs (*) indicates a significant difference in mRNA expression based on 24-h stimulation conditions using a Dunnet post-hoc test (P < 0.05). STAR: steroidogenic acute regulatory protein; CYP11A1: cytochrome p450 family 11 subfamily A member 1; HSD3B2: 3β-hydroxysteroid dehydrogenase type II; HSD17B3: 17β-hydroxysteroid dehydrogenase type III; CYP17A1: cytochrome p450 family 17 subfamily A member 1; INSL3: insulin like 3.
Functionality of differentiated PDGFRα+ cells into Leydig cells
Although, based on the standard curve, low testosterone levels were calculated in some samples stimulated with forskolin, the concentrations were below the detection level of the ELISA assay. To measure testosterone and androstenedione in a more sensitive way in the culture medium, a 2D-UPLC-MS/MS system was used. Using this sensitive assay, it was still not possible to detect testosterone in the medium, independent of the culture and stimulation conditions. Androstenedione was detected in very low concentrations in two samples of one patient when cells were cultured in differentiation medium after stimulation with forskolin or dbcAMP (Fig. 5A). Progesterone was detected in culture medium (Fig. 5B). Using a two-way ANOVA, a significant increase in production of progesterone was seen under differentiation culture conditions compared to propagation culture conditions (P = 0.013). Furthermore, an effect of 24 h stimulation was seen (P = 0.009) on the production of progesterone. A Tukey post-hoc test indicated this was due to a significant difference between basal and dbcAMP (P = 0.03) and between hCG and dbcAMP (P = 0.026) stimulation.
Figure 5.
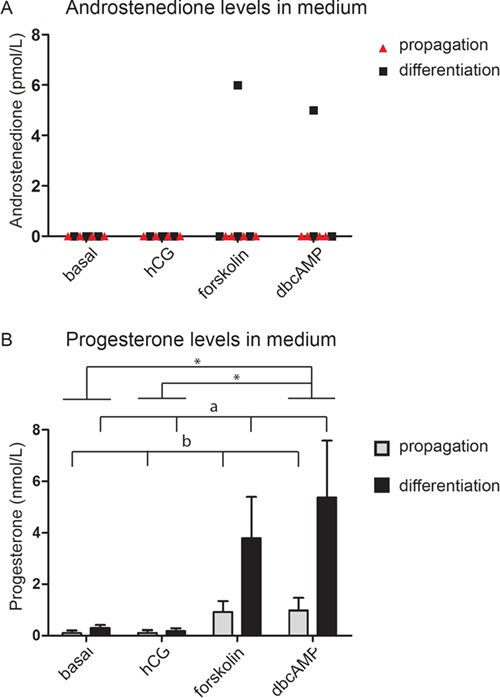
Androstenedione and progesterone production in vitro. Androstenedione levels were only detectable in two differentiation samples, stimulated with forskolin and dbcAMP (A). These two samples were both from cells from the same patient. Progesterone was found in several medium samples (B), with a significantly increased production of progesterone when cells were stimulated with dbcAMP compared to basal and hCG (asterisk (*) P < 0.05 using Tukey post-hoc test). Furthermore, a significant increased production was found when cells were cultured in differentiation medium compared to propagation medium (a and b differ significantly from each other (P < 0.05) by a two-way ANOVA).
Transplantation of human PDGFRα+ cells into testis of LuRKO mice
There was a small non-significant increase in testosterone levels observed up to Week 12 following transplantation in mice without anti-CD4 treatment. However, no differences were seen in testosterone levels compared to the control mice during the 20 weeks of follow-up after transplantation (Supplementary Fig. S2). No evidence for the presence of transplanted human cells was found in the interstitium in any of the mice 20 weeks after transplantation, including the CD4 immune suppressed mice, using anti-human nucleoli immunohistochemical staining (data not shown).
Discussion
The human testicular interstitium contains HLA-A,B,C+/CD34−/PDGFRα+ cells that can be propagated in vitro, while they maintain PDGFRα positivity. These PDGFRα+ cells show MSC characteristics by expressing CD90, CD73 and CD105 and have multipotent characteristics typical for MSCs as they are able to differentiate into adipocytes, osteocytes and chondrocytes. The sorted HLA-A,B,C+/CD34−/PDGFRα+ cells express all of the required enzymes for steroidogenesis but are not able to produce testosterone, nor after culturing cells in differentiation conditions or the addition of stimulators of steroid production. However, the cells do have steroidogenic capacity to produce progesterone, when stimulated with dbcAMP. This suggests that the interstitial HLA-A,B,C+/CD34−/PDGFRα+ cells from human testis under differentiation conditions or after 24 h stimulation partly obtain characteristics of ALCs in vitro, but additional stimulating factors are needed to differentiate the cells into functional testosterone producing Leydig cells. Next to this, although human PDGFRα+ cells might be able to differentiate in vivo into ALCs, we were not able to show this with our mouse transplantation model.
PDGFRα+ cells are known to be present in rodent testes. Already early in gonadal development, PDGF signaling via PDGFRα has an important role in migration, proliferation and differentiation of somatic cells (Brennan et al., 2003; Piprek, 2010). Also, PDGFRα+ cells have been successfully isolated from neonatal and adult rat testes, as well as from adult human testes (Ge et al., 2006; Stanley et al., 2012; Landreh et al., 2013, 2014). In the present study, we show that these PDGFRα+ cells can be not only successfully isolated from the interstitial compartment of adult human testes but also propagated in vitro while maintaining PDGFRα expression.
It is hypothesized that SLCs originate from MSCs (Hardy et al., 1989). In rodents, PDGFRα+ cells are suggested to be the SLCs (Ge et al., 2006; Stanley et al., 2012). Although it is known that MSCs are present in the human testes, it has not yet been investigated whether the testicular interstitial PDGFRα+ cells are MSCs (Gonzalez et al., 2009; Chikhovskaya et al., 2014). We show here that the sorted HLA-A,B,C+/CD34−/PDGFRα+ testicular cells express CD90, CD105 and CD73, and are able to differentiate into the MSC trilineage directions of adipocytes, osteocytes and chondrocytes, according to the minimal criteria for MSCs (Dominici et al., 2006). This indicates that HLA-A,B,C+/CD34−/PDGFRα+ testicular cells indeed have MSC characteristics and are multipotent cells, implicating that human HLA-A,B,C+/CD34−/PDGFRα+ cells have stem cell properties.
If the HLA-A,B,C+/CD34−/PDGFRα+ cell population contains SLCs, these cells should have the potential to differentiate into Leydig cells. After 4 weeks of culture under differentiation inducing conditions, the cells had changed in morphology from long stretched cells to large polyhedral cells. This change in morphology is comparable with the morphological changes described for the in vivo development of rat ALCs (Benton et al., 1995). During the development of rodent ALCs in vivo, an increase in endoplasmic reticulum size and number of lipid droplets is found (Benton et al., 1995; Haider, 2004), which might explain the increase in granularity we saw in the present study in the cytoplasm of the cells after in vitro differentiation. Our results extend the work of Landreh and co-workers in 2014 who showed an increase in STAR and CYP11A1 mRNA expression after stimulating propagated human PDGFRα+ cells with forskolin for 24 h (Landreh et al., 2014). In our study there was an increase in STAR and CYP11A1 mRNA expression when stimulated not only with forskolin but also with dbcAMP. We are the first to culture human interstitial PDGFRα+ cells in conditions to direct the differentiation into ALCs, resulting in a significant increase in the mRNA expression of HSD3B2 and INSL3.
The percentage of PDGFRα+ cells in the ICF fraction is relatively low after cell isolation. This low percentage can be a result of the destruction of cell surface markers due to the enzymatic cell isolation procedure (Autengruber et al., 2012). Indeed, when cells were treated with trypsin for subsequent passaging, lower numbers of PDGFRα+ cells were observed compared to that when using Accutase for detaching the cells. This shows the sensitivity of the PDGFRα surface marker for this type of treatments. Because of this sensitivity, we always implemented a recovery period of 24 h after cell isolation before performing FACS analysis at Passage 0, and used Accutase instead of trypsin to detach the cells for passaging.
Due to the low percentage of PDGFRα+ cells in the cell isolate immediately after isolation, propagation of the ICF cells was needed to obtain enough cells for further characterization and functional analyses. Although it cannot be ruled out that propagation affects the stem cell properties of the cells, we do not think this has influenced our results as after sorting and further propagation, the HLA-A,B,C+/CD34−/PDGFRα+ cells stayed positive for PDGFRα throughout the culture period. Furthermore, no changes in cellular morphology were seen during propagation, and after propagation, cells still showed trilineage differentiation potential, indicating the propagation did not affect the stem cell properties.
The steroidogenic enzymes characteristic for Leydig cells, including two enzymes that were not studied before in human interstitial PDGFRα+ cells (CYP17A1 and HSD17B3), were already expressed at low level in the PDGFRα+ cells immediately after sorting. Therefore, it is questionable whether the sorted cell population was a pure SLC population. Although the MSC multipotency proves the presence of cells with stem cell characteristics in the sorted population, the presence of the steroidogenic enzymes in our study indicates that the HLA-A,B,C+/CD34−/PDGFRα+-sorted population might be a mix of various developing stages toward ALCs. For human LC development, it is thought that ALCs do develop not only from progenitor cells but also through transdifferentiation of FLCs and neonatal Leydig cells via ILCs (Prince, 2001). At this point, there is still too little information on human ALC development to characterize these different LC populations independently from each other. Therefore, it might be possible that cells in several developmental stages express PDGFRα and are present in the sorted cell population.
Despite the fact that we observed an increased expression of several steroidogenic enzymes in the PDGFRα+ cells under differentiation conditions, this was not observed for all enzymes necessary to produce testosterone, and therefore, presumably, no testosterone was detected in the culture medium for any of the conditions. Even though the delta4 pathway is not the preferred pathway in human steroid synthesis, we did find progesterone production, which might be explained by the increased availability of CYP11A1 and HSD3B2, but not of CYP17A1. One can speculate that this lack of differentiation potential is due to the cryopreservation of the testicular tissue prior to cell isolation, although this is not likely since trilineage differentiation of the isolated cells was not hampered. Another explanation might be that the progenitor cells from adult human testis differentiated in vitro toward a fetal Leydig cell state. In rodents, FLCs lack the expression of Hsd17b3 and the conversion of androstenedione into testosterone is done in Sertoli cells (O’Shaughnessy et al., 2000; Shima et al., 2013). Because we did find mRNA expression of HSD17B3 in our human cells in culture, these cells most likely follow the ALC lineage. Another possibility, also suggested by Landreh and coworkers, is that there is a separate cell type in the testis responsible for the production of progesterone (Landreh et al., 2014).
Our results suggest that human testicular interstitial PDGFRα+ cells need additional components in the culture environment to induce differentiation into functional ALCs. Although an in vivo transplantation experiment will include these components, in the xeno-transplantation model performed in the present study, no testosterone-producing human ALCs were found 20 weeks after transplantation. One of the explanations for this may be the development of antibodies to long-term injections hCG, hampering hCG stimulation of ALC development. It is however unlikely that this explains the absence of testosterone-producing ALCs in our transplantation study as the dose of 1 IU hCG used in the present study is low enough to avoid generation of antibodies after 6 weeks of injections (Spaliviero et al., 2004). Since the human HLA-A,B,C+/CD34−/PDGFRα+ cells did not survive in the LuRKO mice testes, the lack of rise in serum testosterone levels is, despite the CD4-antibody immune suppression and the immune privileged environment of the testes, most likely due to rejection of the cells because of a combination of the phylogenetic distance between human and mouse and the time point of harvesting the transplanted testes (20 weeks after transplantation). Human testicular cells, which were sorted based on a different marker (p75), survived for 4.5 weeks in testes of EDS-treated adult rats without any immune suppression (Zhang et al., 2017). These cells were not only able to survive 4.5 weeks after transplantation but also able to increase testosterone production after transplantation compared to the EDS-treated control rats.
Despite the stimulation with forskolin or dbcAMP to circumvent the LH receptor, the cultured cells did not produce testosterone. This suggests that additional factors for the differentiation of human interstitial PDGFRα+ cells are needed. Factors that might be interesting in this perspective are, for example, PDGF-AA, KIT ligand, androgens, EGF, interleukins or nerve growth factor (reviewed in Teerds and Huhtaniemi, 2015; Ye et al., 2017). Another possibility is that the cells need different stimulators in different stages of ALC development or sequential medium with different supplements are needed throughout in vitro differentiation. An optimized differentiation protocol should be developed to characterize the various developmental steps from SLC to ALC in the human testis. A characteristic of rodent PLCs is the expression of Lhcgr and Hsd3b1 and the capability of these cells to produce androgens (Dong et al., 2007; Teerds and Huhtaniemi, 2015). During development, steroidogenic enzymes increase in expression. Although all steroidogenic enzymes in the human HLA-A,B,C+/CD34−/PDGFRα+-sorted population are expressed, no LHCGR was detected in both our sorted and differentiated cells. We cannot explain why LHCGR expression was not detected while some steroidogenic enzymes were already expressed in our cells, but the absence of LHCGR confirms that the cells in our study did not fully differentiate. This study indicates that Leydig cell development in humans might rely on different factors compared to rodents, which emphasizes the need for caution when translating results obtained from rodent studies to the human situation.
Conclusion
Taken together, human testicular interstitial cells that express PDGFRα, marker previously identified in rodents as the SLC marker, have MSC characteristics. The cells are multipotent, which proves their stem cell potential. Upon directed in vitro differentiation toward Leydig cells and stimulation with forskolin and dbcAMP, mRNA of some of the enzymes involved in testosterone synthesis are upregulated in human PDGFRα+ cells. The progesterone production of the cells proves that the cells have steroidogenic potential. However, the absence of detectable testosterone production by differentiated human PDGFRα+ cells in vitro shows the requirement for further improvement of the culture conditions to differentiate human PDGFRα+ interstitial cells into functional testosterone producing cells. To gain knowledge about the mechanisms of human Leydig cell dysfunction and development of a suitable therapy to increase testosterone levels, it is essential to expand the limited existing knowledge regarding the Leydig cell lineage in the normal human male testis.
Supplementary Material
Acknowledgements
We would like to thank Prof. Dr I. Huhtaniemi and Dr M. Poutanen (University of Turku) for providing the LuRKO mouse strain. We thank B. Hooibrink for help in FACS (Flow Cytometry Unit, Department of Medical Biology, Amsterdam UMC (AMC)). For the help in the animal facility, we would like to thank Dr C.L. Mulder and J.M. Bispo Serrano from the Center for Reproductive Medicine, Amsterdam UMC (AMC), University of Amsterdam and the animal caretakers of the Animal Facility of Amsterdam UMC (AMC). We thank R. van Eekelen from the Center for Reproductive Medicine, Amsterdam UMC (AMC), University of Amsterdam for his advice on statistical analyses.
Authors’ roles
All authors took part in the design of the study and critical reading and revision of the manuscript. J.E. contributed by conducting the experiments; collecting, analyzing and interpreting the data; and taking the lead in writing the manuscript. E.v.B., S.v.D. and C.d.W. contributed in conducting, collecting and analyzing the data. K.T., S.R. and A.v.P. participated in interpreting the data.
Funding
Amsterdam UMC, Academic Medical Center.
Conflict of interest
The authors report no financial or other conflict of interest relevant to this study.
Reference list
- Autengruber A, Gereke M, Hansen G, Hennig C, Bruder D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur J Microbiol Immunol (Bp) 2012;2:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag 2009;5:427–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton L, Shan L, Hardy M. Differentiation of adult Leydig cells. J Steroid Biochem Mol Biol 1995;53:61–68. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-α mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev 2003;17:800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttler RM, Peper JS, Crone EA, Lentjes EGW, Blankenstein MA, Heijboer AC. Reference values for salivary testosterone in adolescent boys and girls determined using isotope-dilution liquid-chromatography tandem mass spectrometry (ID-LC–MS/MS). Clin Chim Acta 2016;15–18. [DOI] [PubMed] [Google Scholar]
- Chikhovskaya JV, van Daalen SKM, Korver CM, Repping S, van Pelt AMM. Mesenchymal origin of multipotent human testis-derived stem cells in human testicular cell cultures. Mol Hum Reprod 2014;20:155–167. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- Dong L, Jelinsky SA, Finger JN, Johnston DS, Kopf GS, Sottas CM, Hardy MP, Ge RS. Gene expression during development of fetal and adult Leydig cells. Ann N Y Acad Sci 2007;1120:16–35. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci 2006;103:2719–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Griparic L, Vargas V, Burgee K, SantaCruz P, Anderson R, Schiewe M, Silva F, Patel A. A putative mesenchymal stem cells population isolated from adult human testes. Biochem Biophys Res Commun 2009;385:570–575. [DOI] [PubMed] [Google Scholar]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol 2001;179:47–74. [DOI] [PubMed] [Google Scholar]
- Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol 2004;233:181–241. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. 1989;124:762–770. [DOI] [PubMed] [Google Scholar]
- Jackson NC, Jackson H, Shanks JH, Dixon JS, Lendon RG. Study using in-vivo binding of 125I-labelled hCG, light and electron microscopy of the repopulation of rat Leydig cells after destruction due to administration of ethylene-1,2-dimethanesulphonate. J Reprod Fertil 1986;1–10. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Donachie K, Rommerts FFG. Selective destruction and regeneration of rat Leydig cells in vivo. Cell Tissue Res 1985;242:145–156. [DOI] [PubMed] [Google Scholar]
- Landreh L, Spinnler K, Schubert K, Häkkinen MR, Auriola S, Poutanen M, Söder O, Svechnikov K, Mayerhofer A. Human testicular peritubular cells host putative stem leydig cells with steroidogenic capacity. J Clin Endocrinol Metab 2014;99:1227–1235. [DOI] [PubMed] [Google Scholar]
- Landreh L, Stukenborg JB, Söder O, Svechnikov K. Phenotype and steroidogenic potential of PDGFRα-positive rat neonatal peritubular cells. Mol Cell Endocrinol 2013;372:96–104. [DOI] [PubMed] [Google Scholar]
- Li X, Wang Z, Jiang Z, Guo J, Zhang Y, Li C, Chung J, Folmer J, Liu J, Lian Q et al. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci U S A 2016;113:2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Baker PJ, Heikkilä M, Vainio S, McMahon AP. Localization of 17beta-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis - androstenedione is the major androgen secreted by fetal/neonatal Leydig cells. Endocrinology 2000;141:2631–2637. [DOI] [PubMed] [Google Scholar]
- van Pelt AMM, Morena AR, van Dissel-Emiliani FMF, Boitani C, Gaemers IC, de Rooij DG, Stefanini M. Isolation of the synchronized a Spermatogonia from adult vitamin A-deficient rat Testes1. Biol Reprod 1996;55:439–444. [DOI] [PubMed] [Google Scholar]
- Piprek RP. Molecular and cellular machinery of gonadal differentiation in mammals. Int J Dev Biol 2010;54:779–786. [DOI] [PubMed] [Google Scholar]
- Prince FP. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol 2001;168:213–216. [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den hoff MJB, AFM M. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 2009;37:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Mizrak SC, van Daalen SKM, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJMCH, van der Veen F et al. Propagation of human spermatogonial stem cells in vitro. JAMA 2009;302:2127–2134. [DOI] [PubMed] [Google Scholar]
- Shima Y, Miyabayashi K, Harahuchi S, Arakawa T, Otake H, Baba T, Matsuzaki S, Shishido Y, Akiyama H, Tachibana T et al. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol Endocrinol 2013;27:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaliviero JA, Jimenez M, Allan CM, Handelsman DJ. Luteinizing hormone receptor-mediated effects on initiation of spermatogenesis in gonadotropin-deficient (hpg) mice are replicated by testosterone. Biol Reprod 2004;70:32–38. [DOI] [PubMed] [Google Scholar]
- Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, Zirkin BR, Chen H. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology 2012;153:5002–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerds KJ, Huhtaniemi IT. Morphological and functional maturation of Leydig cells: from rodent models to primates. Hum Reprod Update 2015;21:310–328. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, de Rooij DG, Rommerts FFG, Wensing CJG. The regulation of the proliferation and differentiation of rat Leydig cell precursor cells after EDS administration or daily HCG treatment. J Androl 1988;9:343–351. [DOI] [PubMed] [Google Scholar]
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Li X, Li L, Chen H, Ge RS. Insights into the development of the adult Leydig cell lineage from stem Leydig cells. Front Physiol 2017;8:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Zhang P, Dong W, Zeng W, Pan C. Identification of stem Leydig cells derived from pig testicular Interstitium. Stem Cells Int 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F-P, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol 2001;15:172–183. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang J, Deng C, Jiang MH, Feng X, Xia K, Li W, Lai X, Xiao H, Ge RS et al. Transplanted human p75-positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell Death Dis 2017;8:e3123. doi: 10.1038/cddis.2017.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


