ABSTRACT
Background
Plant-based diets may help improve measures of body fat, blood cholesterol, glucose metabolism, and inflammation. However, limited evidence suggests that the health effects of reducing animal products may depend on the quality of plant-based foods consumed as caloric replacements.
Objective
This study examined how temporarily restricting consumption of meat, dairy, and egg (MDE) products for religious purposes influences cardiometabolic health biomarkers and whether any effects of MDE restriction on biomarkers are modified by concurrent shifts in calories, fish, and distinct plant-based foods.
Design
This study followed a sample of 99 individuals in the United States with varying degrees of adherence to Orthodox Christian (OC) guidance to abstain from MDE products during Lent, the 48-d period prior to Easter. Dietary composition was estimated from FFQs and 7-d food records; measures of body fat, blood lipids, glucose metabolism, and inflammation were collected prior to and at the end of Lent.
Results
Each serving decrease in MDE products was associated with an average −3.7% (95% CI: −5.5%, −2.0%; P < 0.0001) and −3.6% (95% CI: −5.8%, −1.3%; P = 0.003) change in fasting total and LDL blood cholesterol, respectively, which were partly explained by minor weight loss. However, the total/HDL cholesterol ratio did not significantly decrease due to an average −3.2% (95% CI: −5.8%, −0.6%; P = 0.02) change in HDL cholesterol. No associations between MDE restrictions and shifts in measures of body fat, glucose, insulin, or C-reactive protein were observed. The data could not provide evidence that changes in cardiometabolic health biomarkers in relation to MDE restriction were modified by concurrent shifts in calories, fish, or plant-based foods.
Conclusion
Temporary MDE restrictions practiced by this sample of OCs in the United States during Lent had minimal effects on cardiometabolic disease risk factors. Further research among larger samples of OCs is needed to understand how nutritionally distinct and complex combinations of plant-based foods may modify the health effects of religious fasting from MDE products.
Keywords: plant-based diets, vegetarian, vegan, cardiovascular health, metabolic health, blood cholesterol, glucose, insulin, Orthodox Christians, Lent
Introduction
Plant-based diets are allegedly more protective than omnivorous diets against cardiometabolic diseases (1, 2). It remains questionable, however, whether restriction of animal products meaningfully improves health measures if not coupled with increased intake of healthy, whole, and nutrient- and fiber-rich plant-based foods, such as whole grains, legumes, nuts, fruits, and vegetables. Nutritionally distinct plant-based food choices could account for inconsistent health measures and outcomes across vegetarian and vegan populations (3, 4). Diets relatively low in animal products but high in refined grains, potatoes, and sugar-sweetened beverages and desserts, for example, have been associated with a higher risk of developing type 2 diabetes and coronary heart disease relative to diets higher in both animal products and healthy plant-based foods (5, 6). As randomized clinical trials involving plant-based diet interventions generally incorporate guidelines to minimize processed foods and added sugars, they do not address scenarios of replacing animal products with unhealthy plant-based foods. Yet, such scenarios may be increasingly common among individuals who, whether for health, ethical, environmental, or religious motives, elect to restrict animal products and have access to a growing array of packaged and ultra-processed vegan and vegetarian food products, the healthfulness of which are unclear.
The objective of this study, therefore, was to capture the intraindividual cardiometabolic health response to restricting animal products among individuals with free choice of replacement foods. This study followed a sample of Antiochian and Greek Orthodox Christians (OCs) in the United States during Lent, a 48-d period preceding Easter when OCs are encouraged to “fast” from meat, dairy, and egg (MDE) products. The Lenten OC MDE fast differs from strict low-fat vegan diets shown to improve health markers in intervention studies (7–9), as it allows shellfish, may restrict olive oil (but not other oils) on weekdays, and permits free choice of vegan foods, regardless of refined grain or added sugar content. Furthermore, there are no explicit limitations on calories, although voluntary reductions in ad libitum energy intake during MDE fasting periods are common (10–14). Among OCs in Greece (12, 13, 15) and Egypt (16), similar forms of MDE fasting were associated with reductions in BMI and total and LDL cholesterol. However, these studies did not relate the improvements in health measures to specific changes in dietary composition, and no known studies have quantitatively examined shifts in health biomarkers in relation to MDE fasting among OCs in the United States, where dietary patterns and Lenten fasting regimens may differ from those of Greek and Egyptian OC populations.
Thus, the primary aim of this study was to examine if MDE restrictions, as practiced by OCs in the United States, are associated with improvements in measures of body fat, blood lipids, glucose metabolism, and inflammation (hypothesis 1). The secondary aim was to test if the magnitude of changes in cardiometabolic health markers in relation to OC MDE restrictions is modified by accompanying dietary shifts in calories, fish, and replacement plant-based foods (hypothesis 2).
Methods
Study design and participants
This prospective study followed participants during a 6-wk period on their normal, unrestricted diet between mid-January and early March 2016 and the subsequent 6-wk MDE-restricted period of OC Lent lasting from early March to late April 2016 (mean follow-up time: 40 d; range: 34–48 d). Participants were recruited through oral and written announcements from 8 Antiochian OC churches and 1 Greek OC church in the southern region of the United States. Non-OCs were invited to join the study in an effort to recruit individuals with a range of adherence to OC Lenten dietary restrictions. Eligibility was limited to men and nonpregnant, nonlactating women between the ages of 18 and 75 y who were born or raised in the United States or Canada. All participants provided informed consent, and study protocols were approved by the Institutional Review Board of the University of Washington.
The sample size was determined by the feasibility of recruitment (convenience sample), which lasted from September 2015 until 1 wk prior to Lent, ensuring that each participant had time to record 7 d of his or her pre-Lenten diet. Of the 141 volunteers who consented, 120 volunteers initiated study protocols, 114 completed the first (pre-Lenten) dietary assessments, and 107 finished the second round of dietary assessments during the OC Lenten period (see Supplemental Figure 1 for participant flowchart). The stated reasons for participants not continuing with the study, if given, were generally related to time constraints.
Diet and nutrition assessment
Diet was measured before and during Lent using validated 124-item self-administered FFQs (9) and 7-d undocumented food records (FRs), which were self-administered and not reviewed together by study staff and participant prior to data entry (17, 18). Each participant was instructed to complete one 7-d FR and 1 FFQ before Lent, a second 7-d FR in the middle of Lent, and a second FFQ at the end of Lent. See the Supplemental Materials for further description of the undocumented FR and details on the methods of collection, entry, and analyses of the FR data compared with the FFQ data.
FFQs were reviewed for errors by the researcher and processed by the Nutrition Assessment Shared Resource of the Fred Hutchinson Cancer Research Center, which used the 2015 version of the University of Minnesota Nutrition Data Systems for Research (NDSR) software (19) to obtain measures of average daily consumption of major food groups, macro- and micronutrients, and calories. The food variables that were generated by this software used the MyPyramid Equivalent Database (MPED) (11), which measures meat in ounces (1 oz ∼28.35 g); eggs, grains, nuts, and soy products in ounce equivalents (oz-eq); fruit, vegetables, dairy, and legumes (not including soy products) in cup equivalents (cup-eq); and discretionary oils and total added sugars in grams (see Supplemental Materials for approximate gram conversions of MPED oz-eq and cup-eq servings of food groups). Estimates of macro- and micronutrients were measured by their mass (e.g., grams), and energy intake was measured in kilocalories.
Measurement of cardiometabolic health biomarkers
Anthropometric measurements and capillary blood were collected before and at the end of Lent. Standing height without shoes was measured using a portable stadiometer (Seca-217; Seca). Weight and body fat percentage were measured using a full-body bioelectric impedance scale (HBF-514C; Omron). BMI was calculated by dividing weight in kilograms by squared height in meters (kg/m2). Capillary blood was collected from a finger prick following an ≥8-h period without food or caloric beverages. Approximately 3 drops (40 µL) of whole blood were used immediately for the assessment of blood lipids and fasting glucose using an Alere Cholestech LDX point-of-care device (20, 21) with Alere LDX Lipid and Glucose Analyzer cassettes. The Alere Cholestech LDX point-of-care device measures total cholesterol, HDL cholesterol, triglycerides, and blood glucose directly but calculates LDL cholesterol using the Friedewald formula: [total cholesterol – HDL cholesterol – (triglycerides/5)] (22). Because the point-of-care device is designed to identify individuals with elevated blood lipids, it was not able to detect triglyceride levels <45 mg/dL. Consequently, for participants with triglyceride levels below that level (n = 17 before Lent and n = 15 during Lent), the minimal detectable value of 45 mg/dL was imputed as the participants’ triglyceride value and LDL cholesterol was calculated manually using the Friedewald formula. Likewise, the minimum value of detection of 100 mg/dL for total cholesterol was used to calculate LDL cholesterol for 1 participant with low total cholesterol.
Additional capillary blood was collected in BD Microtainer tubes with serum separators. Blood was allowed to clot for ∼30 to 90 min and centrifuged at ambient temperature at 2000 × g. Serum was aliquoted and shipped on dry ice to the University of Washington Biological Anthropology and Biodemography Laboratory and kept frozen at −80°C. High-sensitivity C-reactive protein (CRP) was measured in this laboratory using an in-house enzyme-linked immunosorbent assay (23). The interassay CVs for low, medium, and high levels of control for this assay were 16.7%, 7.7%, and 11.6%, respectively; the intra-assay CVs for low, medium, and high CVs were 5.1%, 5.7%, and 10.5%, respectively. Insulin was measured by the Northwest Lipid Metabolism and Diabetes Research Laboratories (University of Washington, Seattle, WA) using a 2-site immune-enzymometric assay on a Tosoh 2000 autoanalyzer (24); the interassay CVs for low, medium, and high levels of control samples for this assay were 2.8%, 2.5%, and 2.0%, respectively. Using the measures of serum insulin obtained by the Northwest Lipid Metabolism and Diabetes Research Laboratories and glucose measured by the Alere Cholestech LDX point-of-care device, a measure of the HOMA-IR was calculated as follows: glucose (mg/dL) × insulin (μU/mL)/405 (25).
Covariates
Across the course of the study, participants completed 3 surveys to collect information about demographics; self-rated health status and existing health conditions; medication and supplement use; tobacco, alcohol, and caffeine consumption; and general dietary preferences. Physical activity levels prior to and during Lent were estimated using the validated questionnaire developed for the Aerobics Center Longitudinal Study (26, 27), which surveyed participants on their average weekly frequency, duration, and speed (if relevant) of engagement in 15 common activities (e.g., walking, household chores, yardwork). Activity-specific metabolic equivalent values (METs) representing the ratio of the metaTBLbolic cost of performing the activity to a standardized resting metabolic rate (28) were multiplied by the minutes per week of reported engagement in each activity. METs for each activity were summed and divided by 60 to obtain a measure of weekly MET hours in each data collection period.
Statistical analyses
The final sample size for analyses using FFQ data was 99 after excluding individuals who had unreliably low (<500 kcal for females and <800 kcal for males) or high (>4500 kcal for females and >5000 kcal for males) estimates of “usual” energy intake in either time period (n = 2) (29), reported being on a strict weight-loss regimen during the study period (n = 2), made dietary changes during the Catholic period of Lent occurring prior to OC Lent (n = 1), already restricted all MDE products at baseline (n = 2), or reported a change in cholesterol- or diabetes-related medications during the study period (n = 1). However, sample sizes varied for some biomarker models due to missing body fat measures (n = 2), unreliable LDL cholesterol estimates due to triglyceride levels >400 mg/dL (25) (n = 1), and missing insulin measures at 1 or both time points (n = 16). CRP models excluded individuals reporting changes in regular use of anti-inflammatory medications (n = 1), individuals whose acute use of anti-inflammatory medications (24 h prior to the health assessment) differed between the 2 collection periods (n = 13), or individuals missing CRP measurements in either collection period (n = 3). No participants had CRP levels indicative of current infection (>10 mg/L).
All measures of food and nutrient intake were standardized to a 2000-kcal diet by dividing each participant's estimated consumption (in oz-eq, cup-eq, or grams) of each food group before Lent or during Lent by his or her total caloric intake during the respective time period and multiplying that number by 2000. Composite measures of participants’ MDE consumption before Lent (MDE1 score) and during Lent (MDE2 score) were created by adding the number of servings/2000 kcal of MDE consumed in each period. For the purposes of this score, 1 MDE “serving” was defined as 3 oz (∼85 g) of unprocessed meat, 2 oz (∼57 g) of processed meat, 1 cup-eq of dairy (∼245 g milk or yogurt or ∼48 g cheese), or 2 oz-eq of eggs (∼2 eggs). The difference between the MDE1 and MDE2 scores was used as a composite measure of the change in MDE servings (MDEΔ score) during Lent. Similarly, the measure of change in fish and plant-based foods was calculated by taking the difference in pre-Lenten and Lenten standardized estimates of each food group.
For descriptive purposes, demographic characteristics, baseline measures of health biomarkers, the proportion of individuals in suboptimal ranges for health biomarkers, and pre-Lenten and Lenten dietary characteristics were tabulated by tertiles of MDEΔ score, with tertile 3 representing the greatest degree of MDE reduction. One-way ANOVA tests were used to assess differences in average pre-Lenten (baseline) and Lenten dietary characteristics and baseline health biomarker measures across MDEΔ tertiles. Chi-squared tests were used to test the difference across MDEΔ tertiles in the proportion of individuals with suboptimal baseline biomarker levels (see Supplemental Figure 2).
To test the change in each health biomarker relative to MDE reductions (hypothesis 1), the natural log-transformed measures of BMI, body fat, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, glucose, insulin, HOMA-IR, and CRP at the end of Lent were separately regressed on MDEΔ score controlling for the baseline measure of each respective biomarker. Model 1 included adjustment for age, sex, baseline BMI, average weekly METs, change in weekly METs, baseline MDE score, baseline calories, change in calories, and the number of days following the MDE restricted diet prior to the Lenten health assessment; Bonferroni-adjusted α values of 0.05/10 = 0.005 were used to assess statistical significance. Predicted marginal changes in health biomarkers within each MDEΔ tertile were estimated by regressing the untransformed health biomarker on MDEΔ tertile (categorical), controlling for the same covariates as in model 1 and using Stata's margins command (30).
Model 2 added adjustment for change in weight to model 1. Finally, model 3 tested hypothesis 2 by further including (one at a time) main and interactive effects (with MDEΔ score) and changes in calories, fish, whole grains, refined grains, legumes, soy products, nuts and seeds, fruits and vegetables, discretionary oils, or added sugars, further controlling for baseline consumption of each respective food. Bonferroni-adjusted α values of 0.05/100 = 0.0005 were used to assess statistical significance in model 3.
All analyses included participants taking cholesterol, hypertension, or diabetes medications as long as they reported consistent use of medications throughout the study (n = 14). However, sensitivity analyses were performed by reanalyzing model 1, excluding individuals taking medications to control cholesterol, blood pressure, or blood sugar. Sensitivity analyses of CRP were also performed by reanalyzing model 1, excluding individuals regularly taking statins, metformin, and low-dose anti-inflammatory medications (n = 24).
Two sets of post hoc tests were estimated with model 2. One analysis assessed the change in total/HDL cholesterol ratio in relation to MDEΔ score. The other explored the differential effects of reductions in meat compared with dairy or eggs on shifts in total and LDL blood cholesterol by replacing MDEΔ score with its separate components and using Wald tests to evaluate the difference in the β-coefficients for changes in meat, dairy, and eggs. Several diagnostic tests were also performed. To test for influential outliers, leave-one-out analyses were conducted for model 3 analyses that produced main or interactive effects with P values below the unadjusted α level of 0.05. Multicollinearity resulting from 2 correlated food variables being added together to the model, along with a highly correlated interaction term for those variables, can lead to an increase in standard errors and loss of stability in the coefficients (29). To assess the potential loss of stability in coefficients due to multicollinearity (29), the variance inflation factors (VIFs) for all model 3 covariates, including interaction terms, were performed. VIFs for all covariates were <10 with the exception of the CRP model in which the main and interactive effects for soy products were 10.2 and 10.3, respectively.
All statistical analyses were run first with the FFQ data and then repeated using the 7-d FR data (n = 94). All analyses were conducted using StataSE 14.2 (StataCorp).
Results
Sample demographics and baseline health and dietary characteristics
The adults in this study sample were 19–73 years old (mean = 46.7 years); 42% were male, and 94% reported their race as “white or Caucasian” (Table 1). There were no differences across MDEΔ tertiles in age, sex distribution, education level, or income. Four participants did not self-identify as OCs, and 77% of OCs converted as adults.
TABLE 1.
Baseline demographic, lifestyle, and dietary characteristics of study participants by MDEΔ tertile (n = 99)1
| Characteristic | Entire sample (n = 99) | Tertile 1 (n = 33)(mean MDEΔ score: −0.28; range: 1.89 to −1.30) | Tertile 2 (n = 33)(mean MDEΔ score: −1.76; range: −1.32 to −2.15) | Tertile 3 (n = 33)(mean MDEΔ score: −3.13; range: −2.21 to −4.88) |
|---|---|---|---|---|
| Age, y | 46.7 (19.1, 73.2) | 48.1 (26.2, 73.2) | 45.5 (19.1, 67.8) | 46.6 (24.2, 67.6) |
| Average weekly MET hours2 | 20.1 (0, 128.4) | 22.7 (1.1, 128.4) | 16.7 (0, 54) | 20.7 (1.2, 52.3) |
| Male | 42.4 | 39.4 | 39.4 | 48.5 |
| Self-reported race as “White or Caucasian” | 93.9 | 97.0 | 90.9 | 93.9 |
| Annual income ≥$100,0003 | 44.7 | 40.6 | 46.9 | 46.7 |
| 4-y college degree or higher | 91.8 | 97.0 | 87.9 | 90.6 |
| Orthodox Christian | 96.0 | 87.9a | 100.0b | 100.0b |
| Convert | 76.8 | 69.0 | 87.9 | 72.7 |
| Self-rated health “good” to “excellent” | 89.9 | 90.9 | 87.9 | 90.9 |
| Reporting mostly sedentary occupations | 52.5 | 57.6 | 54.5 | 45.5 |
| Current smokers | 8.1 | 9.1 | 3.0 | 12.1 |
| Consuming >7 alcohol drinks/wk | 24.2 | 24.2 | 18.2 | 30.3 |
| Taking medications for dyslipidemia | 6.1 | 6.1 | 3.0 | 9.1 |
| Taking medications for hypertension | 10.1 | 15.2 | 3.0 | 12.1 |
| Taking medications for diabetes | 3.0 | 3.0 | 3.0 | 3.0 |
| Regularly taking anti-inflammatory medications | 10.1 | 18.2a | 0b | 12.1a |
| Vegetarian or pescetarian | 5.1 | 9.1 | 6.1 | 0 |
1Values are presented as mean (minimum, maximum) or percentages. Different superscript letters represent a statistically significant difference between tertiles at an α level of 0.05.
MDEΔ, change in servings of meat, dairy, and eggs; MET, metabolic equivalent value.
2Metabolic equivalent values for moderate to vigorous physical activities.
3Based on the 32 in tertile 1, 32 in tertile 2, and 30 in tertile 3 who responded to the question on household income.
At baseline (pre-Lent), 66% of the sample was considered overweight, 28% had elevated total cholesterol levels (≥200 mg/dL), 58% had LDL cholesterol levels ≥100 mg/dL, and approximately one-fourth of the sample had fasting glucose and HOMA-IR levels within ranges associated with higher risk for metabolic syndrome and type 2 diabetes (Table 2 and Supplemental Figure 2). The proportion of individuals with suboptimal biomarker values at baseline did not differ across MDEΔ tertiles.
TABLE 2.
Baseline health measures and proportions of participants with suboptimal biomarker levels by MDEΔ tertile (n = 99)1
| Characteristic | Entire sample (n = 99) | Tertile 1 (n = 33) | Tertile 2 (n = 33) | Tertile 3 (n = 33) |
|---|---|---|---|---|
| BMI, kg/m2 | ||||
| Mean (min, max) | 27.3 (18.1, 43.8) | 28.3 (19.7, 42.3) | 27.3 (18.7, 43.8) | 26.4 (18.1, 40.9) |
| % above optimal (≥25 m/kg2) | 65.7 | 69.7 | 63.6 | 63.6 |
| Body fat percentage2 | ||||
| Mean (min, max) | 34.0 (7.2, 56.7) | 35.5 (16.7, 56.7) | 34.6 (14.6, 55.2) | 32.0 (7.2, 46.1) |
| % above optimal (age and sex specific) | 70.1 | 66.7 | 75.0 | 68.8 |
| Total cholesterol, mg/dL | ||||
| Mean (min, max) | 184.1 (103.0, 275.0) | 183.5 (114.0, 275.0) | 184.4 (103.0, 247.0) | 184.5 (128.0, 251.0) |
| % above optimal (≥200 mg/dL) | 28.3 | 30.3 | 30.3 | 24.2 |
| LDL cholesterol, mg/dL3 | ||||
| Mean (min, max) | 109.0 (49.0, 189.8) | 110.7 (53.2, 189.8) | 106.3 (49.0, 159.2) | 110.1 (55.6, 179.0) |
| % above optimal (≥100 mg/dL) | 58.2 | 59.4 | 60.6 | 54.5 |
| HDL cholesterol, mg/dL | ||||
| Mean (min, max) | 55.3 (18.0, 98.0) | 53.5 (28.0, 84.0) | 57.1 (18.0, 98.0) | 55.4 (20.0, 84.0) |
| % below optimal [≤40 (men) or ≤50 (women)] | 23.5 | 25.0 | 27.3 | 18.2 |
| Triglycerides, mg/dL | ||||
| Mean (min, max) | 99.2 (45.0, 271.0) | 97.7 (45.0, 271.0) | 104.5 (45.0, 261.0) | 95.4 (45.0, 253.0) |
| % above optimal (≥100 mg/dL) | 35.4 | 36.4 | 42.4 | 27.3 |
| Total cholesterol/HDL cholesterol | ||||
| Mean (min, max) | 3.7 (2.0, 9.4) | 3.7 (2.1, 6.8) | 3.6 (2.0, 7.5) | 3.8 (2.0, 9.4) |
| % above optimal (≥4.5) | 19.2 | 24.2 | 18.2 | 15.2 |
| Glucose, mg/dL | ||||
| Mean (min, max) | 94.3 (68.0, 166.0) | 93.5 (68.0, 116.0) | 94.2 (80.0, 166.0) | 95.2 (68.0, 156.0) |
| % above optimal (≥100 mg/dL) | 29.3 | 36.4 | 24.2 | 27.3 |
| Insulin, μU/mL4 | ||||
| Mean (min, max) | 10.0 (1.6, 99.3) | 8.4 (2.8, 26.2) | 13.3 (1.6, 99.3) | 8.4 (2.1, 24.9) |
| % above optimal (≥12 μU/mL) | 18.1 | 10.7 | 18.5 | 25.0 |
| HOMA-IR4 | ||||
| Mean (min, max) | 2.5 (0.3, 27.5) | 2.0 (0.5, 7.5) | 3.5 (0.3, 27.5) | 2.1 (0.4, 7.1) |
| % above optimal (≥2.5) | 24.1 | 25.0 | 22.2 | 25.0 |
| C-reactive protein, mg/L5 | ||||
| Mean (min, max) | 1.4 (0.1, 4.9) | 1.5 (0.1, 4.9) | 1.3 (0.1, 4.2) | 1.5 (0.1, 4.9) |
| % above optimal (>3 mg/L) | 10.1 | 15.4 | 10.3 | 7.4 |
1Optimal ranges based on standard reference values for BMI (10); total, LDL, and HDL cholesterol (11–13); triglycerides (14); glucose (15); insulin (16, 17); and HOMA-IR (18–20). To convert mg/dL to mmol/L, multiply total, LDL, and HDL cholesterol by 0.0259; triglycerides by 0.0113; and glucose by 0.056. To convert mg/dL to mmol/L, multiply total, LDL, or HDL cholesterol by 0.0259; triglycerides by 0.0113; and glucose by 0.055. HOMA-IR = glucose (mg/dL) × insulin (μU/mL)/405; MDEΔ, change in servings of meat, dairy, and eggs.
2Excluding 1 individual in tertile 2 and 1 individual in tertile 3 with missing body fat measurements at 1 or both time points.
3Excluding 1 individual in tertile 2 with an unreliable LDL measurement at the Lenten follow-up due to triglycerides >400 mg/dL.
4Excluding 5 individuals in tertile 1, 6 individuals in tertile 2, and 5 individuals in tertile 3 with missing insulin measures at 1 or both time points.
5Excluding 7 individuals in tertile 1, 4 individuals in tertile 2, and 6 individuals in tertile 3 with missing C-reactive protein measures or inconsistent use of anti-inflammatory medication.
Five individuals reported regularly following a vegetarian (n = 3) or pescetarian (n = 2) diet. Two of the vegetarians still changed their dairy and egg consumption enough to be categorized in the second MDEΔ tertile. With the exception of significantly higher baseline consumption of total meat and eggs and lower intake of nuts in the third MDEΔ tertile (the tertile that experienced the greatest amount of change in MDE consumption), there were no other significant differences in baseline consumption of total calories or animal- or plant-based foods (Table 3). However, individuals in tertile 3 did consume slightly more animal protein, saturated fat, and trans fat and less plant protein and carbohydrates than those in tertile 1 at baseline (Supplemental Table 2).
TABLE 3.
FFQ estimates of animal- and plant-based food intake before and during Lent (standardized to 2000 kcal/d) by MDEΔ tertiles (n = 99)1
| Pre-Lent | Lent | |||||
|---|---|---|---|---|---|---|
| Characteristic | Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 |
| Kilocalories (males) | 2528 (1358, 4170) | 2329 (1153, 4237) | 2170 (1147, 3875) | 1964 (1313, 3137) | 1715 (1125, 2446) | 1594 (818, 2823) |
| Kilocalories (females) | 1942 (960, 3490) | 2083 (910, 3478) | 1895 (1223, 2966) | 1699 (774, 2719) | 1578 (678, 2958) | 1325 (672, 2396) |
| Animal-based foods | ||||||
| All meat, oz | 3.7 (0.2, 8.1)a | 3.6 (0.3, 7.6)b | 5.1 (2.3, 9.7)b | 2.4 (0.1, 8.1)a | 0.5 (0.05, 1.5)b | 0.3 (0, 0.6)b |
| Red meat | 1.9 (0.1, 4.7) | 1.9 (0.1, 6.0) | 2.6 (0.6, 7.7) | 1.0 (0, 3.2)a | 0.3 (0.02, 0.7)b | 0.2 (0, 0.4)b |
| Poultry | 1.3 (0.0, 4.7) | 1.2 (0.1, 2.7) | 1.6 (0.5, 3.4) | 1.1 (0.0, 3.2)a | 0.1 (0, 0.6)b | 0.1 (0, 0.3)b |
| Processed meat | 0.5 (0, 2.1) | 0.5 (0.04, 1.4) | 0.8 (0.05, 2.0) | 0.3 (0, 2.2)a | 0.03 (0, 0.2)b | 0.01 (0, 0.1)b |
| Fish and shellfish | 0.9 (0, 2.7) | 0.8 (0.02, 2.2) | 1.0 (0.1, 2.1) | 1.0 (0, 3.7) | 1.2 (0, 4.7) | 1.6 (0.3, 4.3) |
| All dairy, cup-eq | 1.2 (0.2, 2.4) | 1.0 (0.5, 1.6) | 1.3 (0.4, 2.5) | 1.4 (0.1, 4.4)a | 0.5 (0.04, 1.4)b | 0.3 (0.1, 0.7)b |
| Milk | 0.5 (0.03, 2.0) | 0.4 (0.1, 0.6) | 0.6 (0.1, 1.4) | 0.7 (0.03, 2.2)a | 0.2 (0.02, 0.5)b | 0.1 (0, 0.3)b |
| Yogurt | 0.2 (0, 0.9) | 0.1 (0, 0.5) | 0.2 (0, 0.8) | 0.2 (0, 1.4)a | 0.04 (0, 0.3)b | 0.02 (0, 0.2)b |
| Cheese | 0.5 (0.03, 1.1) | 0.5 (0.1, 0.9) | 0.6 (0.2, 1.2) | 0.5 (0.06, 1.1)a | 0.3 (0, 0.9)b | 0.2 (0, 0.5)b |
| Eggs, oz-eq | 0.5 (0.03, 1.5)a | 0.6 (0.1, 1.6)a | 1.0 (0.1, 4.0)b | 0.4 (0.02, 1.7) | 0.4 (0.01, 1.8) | 0.3 (0, 1.7) |
| Plant-based foods | ||||||
| Whole grain, oz-eq | 1.4 (0.1, 3.2) | 1.9 (0.3, 5.6) | 1.3 (0, 3.4) | 1.6 (0, 5.5) | 2.1 (0.3, 5.3) | 2.3 (0.1, 5.9) |
| Refined grain, oz-eq | 3.6 (0.7, 6.9) | 4.3 (1.9, 6.8) | 3.4 (1.0, 6.4) | 4.1 (0.9, 6.8) | 5.1 (2.6, 8.6) | 4.8 (1.9, 8.7) |
| Legumes, cup-eq | 0.1 (0, 0.5) | 0.2 (0, 0.3) | 0.1 (0.02, 0.5) | 0.2 (0, 0.6)a | 0.3 (0.02, 0.7)b | 0.4 (0.02, 1.1)b |
| Soy products, oz-eq | 0.3 (0, 1.7) | 0.3 (0, 1.1) | 0.2 (0, 0.6) | 0.4 (0, 2.0) | 0.7 (0, 2.1) | 0.6 (0, 2.1) |
| Nuts and seeds, oz-eq | 1.9 (0, 4.9)a | 1.3 (0.2, 4.6)a,b | 0.9 (0.02, 2.1)b | 1.9 (0.02, 4.9) | 2.4 (0.3, 7.4) | 2.3 (0.1, 6.3) |
| Fruit, cup-eq2 | 1.2 (0.1, 3.7) | 0.8 (0.2, 1.6) | 0.7 (0.1, 2.0) | 1.2 (0.1, 3.4) | 1.0 (0.2, 2.3) | 1.3 (0.04, 3.4) |
| Vegetables, cup-eq3 | 2.0 (0.7, 4.3) | 1.7 (0.3, 3.9) | 2.1 (0.8, 5.9) | 2.4 (0.7, 5.3) | 2.4 (0.5, 5.9) | 3.0 (1.2, 6.6) |
| White potatoes, cup-eq | 0.4 (0.02, 0.9) | 0.4 (0.1, 1.1) | 0.4 (0.1, 0.7) | 0.4 (0.03, 0.8) | 0.5 (0.1, 1.1) | 0.5 (0.1, 1.4) |
| Discretionary oils, g | 30.5 (14.9, 44.1) | 29.0 (21.0, 43.0) | 27.0 (14.2, 46.4) | 32.4 (16.4, 50.2) | 35.8 (23.0, 63.2) | 38.0 (19.0, 61.8) |
1Values are presented as mean (5th, 95th percentile). Different superscripts indicate significant differences between tertiles in 1-factor ANOVA tests using Bonferroni-adjusted α levels of 0.017 to account for 3 pairwise comparisons for each food. 1 oz meat or fish ∼28.35 g; 1 oz-eq dairy ∼245 g milk or yogurt, ∼48 g cheese; 1 oz-eq eggs ∼1 egg; 1 oz-eq whole or refined grains ∼28.35 cooked cereals, rice, and pasta, ∼1 slice of bread, ∼1 small muffin; 1 oz-eq soy products ∼28.35 g soy nuts, ∼62.5 g tofu; 1 oz-eq legumes ∼175 g (cooked). 1 oz-eq nuts and seeds ∼14–16 g; 1 cup-eq fruit, vegetable, or potatoes ∼ 50–250 g, depending on water content. cup-eq, cup-equivalent; MDEΔ, change in servings of meat, dairy, and egg products between baseline and Lent; oz-eq, ounce-equivalent.
2Excluding fruit juice.
3Excluding white potatoes.
Hypothesis 1: MDE restrictions and reductions in cardiometabolic risk biomarkers
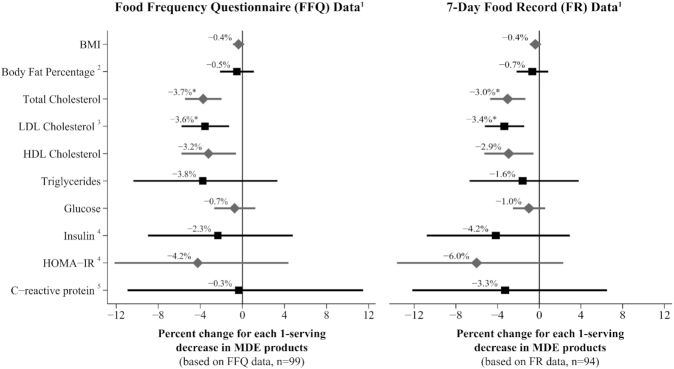
Each serving decrease in MDE consumption during Lent was associated with an average −3.7% change in total cholesterol (95% CI: −5.5%, −2.0%; P < 0.0001) and −3.6% change in LDL cholesterol (95% CI: −5.8%, −1.3%; P = 0.003) in model 1 (Figure 1; Supplemental Table 3). Marginal predictive models estimated that total and LDL cholesterol decreased by ∼22.0 mg/dL and 14.3 mg/dL, respectively, in the third MDEΔ tertile (Table 4). HDL cholesterol also changed by an average of −3.2% (95% CI: −5.8%, −0.6%; P = 0.02) (Figure 1). This change was not significant at the Bonferroni-adjusted α level, but marginal prediction models suggested that the estimated 6.6-mg/dL reduction in HDL cholesterol in the third MDEΔ tertile was significant (Table 4). There was no evidence that self-reported physical activity was significantly associated with changes in blood lipids (data not shown).
FIGURE 1.
Percent change in cardiometabolic health markers for each 1-serving decrease in meat, dairy, and egg (MDE) products (based on model 1).1Multiple linear regression results for the association between the change in health biomarkers and change in MDEΔ score [as measured by FFQs or 7-d food records (FRs)], controlling for natural log-transformed baseline biomarker value, baseline MDE score, baseline calories, change in calories, age, sex, baseline BMI, average metabolic equivalent values (METs), change in METs, and number of days on the MDE restricted diet at the time of the Lenten biomarker collection. Confidence intervals are calculated using robust standard errors. 2Excluding 2 individuals with missing body fat measurements. 3Excluding 1 individual with an unreliable LDL measure at the Lenten follow-up due to triglycerides >400 mg/dL. 4Excluding 16 individuals with missing insulin measures at 1 or both time points. 5Excluding 17 individuals with missing C-reactive protein measures or inconsistent use of anti-inflammatory medication. *Significant at a Bonferroni-adjusted α level of 0.005. HOMA-IR = glucose (mg/dL) × insulin (μU/mL)/405.
TABLE 4.
Marginal (predicted) change in cardiometabolic health biomarkers across tertiles of MDEΔ score (n = 99)1
| MDEΔ Tertile 1 | MDEΔ Tertile 2 | MDEΔ Tertile 3 | ||||
|---|---|---|---|---|---|---|
| Characteristic | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| Weight, kg | −0.2 (−0.8, 0.5) | 0.65 | −0.9 (−1.6, −0.3) | 0.008 | −1.7 (−2.3, −1.0) | <0.0001 |
| BMI | −0.03 (−0.3, 0.2) | 0.77 | −0.3 (−0.6, −0.1) | 0.009 | −0.5 (−0.8, −0.3) | <0.0001 |
| Body fat percentage2 | −0.5 (−1.2, 0.1) | 0.10 | −1.1 (−1.8, −0.3) | 0.006 | −1.2 (−2.2, −0.3) | 0.01 |
| Total cholesterol | −3.2 (−9.0, 2.5) | 0.26 | −17.6 (−24.1, −11.2) | <0.0001 | −22.0 (−28.4, −15.7) | <0.0001 |
| LDL cholesterol3 | −3.9 (−8.5, 0.6) | 0.09 | −13.5 (−19.0, −7.9) | <0.0001 | −14.3 (−19.4, −9.2) | <0.0001 |
| HDL cholesterol | 0.03 (−2.7, 2.8) | 0.98 | −3.2 (−5.8, −0.6) | 0.02 | −6.6 (−9.6, −3.6) | <0.0001 |
| Triglycerides | 7.6 (−11.0, 26.3) | 0.42 | −6.0 (−17.2, 5.1) | 0.28 | −3.7 (−18.3, 10.8) | 0.61 |
| Glucose | 3.1 (−0.5, 6.8) | 0.09 | −2.2 (−6.9, 2.5) | 0.35 | 0.6 (−3.0, 4.2) | 0.75 |
| Insulin4 | −0.2 (−2.5, 2.1) | 0.87 | −4.3 (−8.6, −0.1) | 0.05 | −0.9 (−3.6, 1.7) | 0.49 |
| HOMA-IR4 | 0.1 (−0.7, 0.9) | 0.78 | −1.4 (−2.7, −0.03) | 0.04 | −0.2 (−1.0, 0.66) | 0.68 |
| C-reactive protein5 | 0.2 (−0.2, 0.6) | 0.24 | −0.1 (−0.3, 0.1) | 0.24 | 0.004 (−0.3, 0.3) | 0.98 |
1Results of marginal predictions for change in health biomarkers within each MDEΔ tertile (as designated by FFQ data), controlling for natural log-transformed baseline biomarker value, baseline MDE score, baseline calories, change in calories, age, sex, baseline BMI, average metabolic equivalent values (METs), change in METs, and number of days on the MDE restricted diet at the time of the Lenten biomarker collection. CI, confidence interval; MDEΔ, change in servings of meat, dairy, and eggs. HOMA-IR = glucose (mg/dL) × insulin (μU/mL)/405.
2Excluding 2 individuals with missing body fat measurements. Confidence intervals are calculated using robust standard errors.
3Excluding 1 individual with an unreliable LDL measure at the Lenten follow-up due to triglycerides >400 mg/dL.
4Excluding 16 individuals with missing insulin measures at 1 or both time points.
5Excluding 17 individuals with missing C-reactive protein measures or inconsistent use of anti-inflammatory medication.
Although BMI was not linearly associated with MDEΔ score, participants in the second and third MDEΔ tertiles lost a predicted 0.9 and 1.7 kg of body weight, respectively (Table 4). Adjusting for weight loss in model 2 slightly attenuated the association between MDEΔ score and total cholesterol (β: −3.2%; 95% CI: −4.8%, −1.6%; P = 0.0002) (Table 5). The association between MDEΔ score and LDL cholesterol (β: −2.8%; 95% CI: −5.2%, −0.4%; P = 0.02), on the other hand, was no longer significant at the Bonferroni-adjusted α level when adjusting for weight loss. At any given level of MDE reduction, each kilogram of weight loss during Lent was associated with an average −0.7% (95% CI: 0.2%, 1.2%; P = 0.005) change in total blood cholesterol and an average −0.9% (95% CI: −1.6%, −0.2%; P = 0.01) change in LDL cholesterol. Controlling for weight loss did not alter the estimated relation between shifts in HDL cholesterol and MDEΔ score.
TABLE 5.
Model 2: percent change in total, LDL, and HDL cholesterol in relation to a 1-serving decrease in MDE products when controlling for weight loss (n = 99)1
| Characteristic | β (95% CI), % | P | Adjusted R2 |
|---|---|---|---|
| Total cholesterol | |||
| 1 serving MDE reduction | −3.2 (−4.8, −1.6) | 0.00022 | 0.71 |
| Weight loss, kg | −1.6 (−2.6, −0.5) | 0.005 | |
| LDL cholesterol3 | |||
| 1 serving MDE reduction | −2.8 (−5.2, −0.4) | 0.02 | 0.72 |
| Weight loss, kg | −2.0 (−3.5, −0.5) | 0.01 | |
| HDL cholesterol | |||
| 1 serving MDE reduction | −3.2 (−5.9, −0.5) | 0.02 | 0.78 |
| Weight loss, kg | −0.09 (−1.6, 1.5) | 0.90 |
1Multiple linear regression results for the association between the change in health biomarkers and change in MDEΔ score (as measured by FFQ), controlling for weight change in addition to the natural log-transformed baseline biomarker value, baseline MDE score, baseline calories, change in calories, age, sex, baseline BMI, average METs, change in METs, and number of days on the MDE restricted diet at the time of the Lenten biomarker collection. Confidence intervals were calculated using robust standard errors. CI, confidence interval; MDE, meat, dairy, and eggs; MDEΔ, change in servings of meat, dairy, and eggs; MET, metabolic equivalent value.
2Significant at a Bonferroni-adjusted α-level of 0.005.
3Excluding 1 individual with an unreliable LDL measure at the Lenten follow-up due to triglycerides >400 mg/dL.
Neither MDEΔ score nor weight loss were associated with changes in other cardiometabolic health biomarkers. The sensitivity analyses excluding individuals taking medications for hypercholesterolemia, hypertension, or diabetes did not produce different results (Supplemental Table 4). The findings from model 1 and model 2 when using the FFQ data were corroborated by the 7-d FR data (Figure 1; Supplemental Tables 5 and Supplemental Table 6).
Hypothesis 2: Testing for modification by concurrent shifts in calories, fish, and plant-based foods
Analyses using the FFQ data provided no evidence that the observed reductions in total and LDL blood cholesterol were modified by concurrent shifts in calories, fish, added sugars, oils, or other major plant-based food categories (Table 6). There were no significant interactions between MDEΔ score and calories or plant-based foods in the BMI, body fat percentage, HDL cholesterol, triglycerides, glucose, insulin, HOMA-IR, or CRP models in either the analyses of the FFQ data (Supplemental Table 7) or the FR data (Supplemental Table 8) that were not accounted for by 1 or several influential outliers (data not shown). Significant but antagonistic interactions arose between MDEΔ score and legume intake in the total cholesterol model and between MDEΔ score and whole-grain intake in the LDL cholesterol model when using the 7-d FR data (Supplemental Table 8), which further examination revealed may have been due to nonlinear relations among these variables (data not shown).
TABLE 6.
Model 3: percent change in total and LDL cholesterol in relation to MDEΔ score and concurrent changes in calories, fish, and plant-based foods1
| Characteristic | Total cholesterol (n = 99) | LDL cholesterol (n = 98)2 | ||
|---|---|---|---|---|
| β (95% CI), % | P | β (95% CI), % | P | |
| MDEΔ score (1 serving reduction) | −3.7 (−5.7, −1.6) | 0.001 | −3.3 (−6.0, −0.4) | 0.03 |
| Kilocalories (100-kcal decrease) | −0.2 (−1.1, 0.8) | 0.72 | −0.2 (−1.5, 1.1) | 0.74 |
| Interaction | 0.1 (−0.2, 0.5) | 0.45 | 0.1 (−0.3, 0.5) | 0.60 |
| MDEΔ score (1 serving reduction) | −3.2 (−4.9, −1.5) | 0.00033 | −2.9 (−5.5, −0.2) | 0.03 |
| Fish (1-oz increase) | 2.9 (−0.5, 6.3) | 0.09 | 1.7 (−3.5, 7.2) | 0.51 |
| Interaction term | −0.1 (−1.5, 1.3) | 0.88 | 0.1 (−2.0, 2.2) | 0.94 |
| MDEΔ score (1 serving reduction) | −3.2 (−5.0, −1.3) | 0.001 | −2.7 (−5.1, −0.2) | 0.03 |
| Whole grain (1-oz-eq increase) | 0.5 (−2.9, 3.9) | 0.78 | 0.9 (−3.9, 5.9) | 0.72 |
| Interaction term | 0.2 (−1.1, 1.5) | 0.76 | 0.3 (−1.6, 2.2) | 0.78 |
| MDEΔ score (1 serving reduction) | −3.1 (−4.8, −1.5) | 0.00043 | −3.6 (−6.0, −1.2) | 0.004 |
| Refined grain (1-oz-eq increase) | −0.7 (−4.0, 2.7) | 0.67 | −2.1 (−7.0, 3.1) | 0.42 |
| Interaction term | 0.2 (−1.2, 1.6) | 0.81 | 1.4 (−0.6, 3.3) | 0.17 |
| MDEΔ score (1 serving reduction) | −2.4 (−4.3, −0.4) | 0.02 | −2.4 (−5.1, 0.4) | 0.09 |
| Legumes (1-cup-eq increase) | −7.7 (−26.3, 15.5) | 0.48 | −23.9 (−46.2, 7.7) | 0.12 |
| Interaction term | 0.5 (−5.7, 7.2) | 0.87 | 8.3 (−2.3, 19.9) | 0.13 |
| MDEΔ score (1 serving reduction) | −2.5 (−4.2, −0.7) | 0.006 | −1.6 (−4.3, 1.2) | 0.27 |
| Soy products (1-oz-eq increase) | 1.7 (−5.4, 9.4) | 0.64 | 1.2 (−11.3, 15.4) | 0.86 |
| Interaction term | −2.0 (−4.7, 0.8) | 0.16 | −2.6 (−7.3, 2.3) | 0.29 |
| MDEΔ score (1 serving reduction) | −3.1 (−4.9, −1.2) | 0.002 | −2.5 (−5.1, 0.1) | 0.06 |
| Nuts and seeds (1-oz-eq increase) | 0.4 (−2.0, 2.8) | 0.74 | −0.7 (−4.5, 3.2) | 0.71 |
| Interaction term | −0.2 (−1.1, 0.7) | 0.65 | 0.2 (−1.3, 1.7) | 0.80 |
| MDEΔ score (1 serving reduction) | −3.0 (−5.0, −1.0) | 0.004 | −3.0 (−5.5, −0.4) | 0.02 |
| Fruit and vegetables (1-cup-eq increase) | −1.0 (−3.1, 1.2) | 0.36 | −2.3 (−5.1, 0.5) | 0.10 |
| Interaction term | 0.2 (−0.7, 1.1) | 0.69 | 0.8 (−0.3, 1.8) | 0.15 |
| MDEΔ score (1 serving reduction) | −2.5 (−4.4, −0.6) | 0.01 | −2.1 (−4.7, 0.7) | 0.14 |
| Oil (10-g increase) | −0.8 (−3.8, 2.3) | 0.62 | −2.9 (−7.7, 2.1) | 0.25 |
| Interaction term | −0.4 (−1.5, 0.8) | 0.53 | 0.3 (−1.5, 2.2) | 0.72 |
| MDEΔ score (1 serving reduction) | −3.0 (−4.7, −1.3) | 0.001 | −2.5 (−5.0, 0.0) | 0.05 |
| Added sugar (10-g increase) | −0.3 (−1.9, 1.3) | 0.72 | −0.4 (−2.7, 2.0) | 0.75 |
| Interaction term | −0.1 (−0.7, 0.5) | 0.78 | −0.1 (−0.9, 0.8) | 0.89 |
1Multiple linear regression results for the association between the change in health biomarkers and change in MDEΔ score (as measured by FFQ), testing for interactions between MDEΔ score and non-MDE foods. Models control for weight change in addition to the natural log-transformed baseline biomarker value, baseline MDE score, baseline calories, change in calories, age, sex, baseline BMI, average METs, change in METs, and number of days on the MDE restricted diet at time of biomarker collection. Confidence intervals were calculated using robust standard errors. CI, confidence interval; cup-eq, cup-equivalent; MDE, meat, dairy, and eggs; MDEΔ, number of meat, dairy, and egg servings given up during Lent; MET, metabolic equivalent value; oz-eq, ounce-equivalent.
2Excluding 1 individual with an unreliable LDL measure at the Lenten follow-up due to triglycerides >400 mg/dL.
3Significant at a Bonferroni-adjusted α level of 0.0005.
Post hoc analyses
The post hoc analysis with model 2 confirmed that, given the reductions in both LDL and HDL cholesterol, the total/HDL cholesterol ratio did not change significantly in relation to MDEΔ score (data not shown).
Regressing the change in total, LDL, and HDL cholesterol on meat, dairy, and eggs as separate variables produced evidence that the change in dairy largely accounted for the relation between MDEΔ score and shifts in total and LDL cholesterol (Supplemental Table 9). Each cup-eq decrease in total dairy consumption was associated with an average −5.9% (95% CI: −8.7%, −3.7%; P < 0.0001) change in total blood cholesterol and a −6.7% (95% CI: −9.8%, −3.6%; P < 0.0001) change in LDL cholesterol. Wald tests confirmed that the β-coefficients for the effect of dairy reductions on total and LDL cholesterol were significantly greater than those for the change in meat (P = 0.03 and 0.01, respectively) and the change in eggs (P = 0.01 and 0.02, respectively), neither of which were independently associated with total or LDL cholesterol. The 7-d FR data confirmed a significant difference between the β-coefficient for meat and dairy in the total cholesterol (P = 0.02) and LDL cholesterol (P = 0.0005) models but did not confirm a significant difference between the coefficients for dairy and eggs (Supplemental Table 10). There was no evidence that the effects of dairy had a greater impact on HDL cholesterol levels than meat or eggs.
Discussion
The data from this study indicate that the decreases in MDE consumption that some OCs in the United States make during Lent are associated with minor reductions in total and LDL cholesterol levels but not with changes in triglycerides, glucose metabolism, or CRP. However, the relation between MDE restrictions and total and LDL cholesterol appears to be partly explained by modest weight loss during Lent. These data were unable to provide evidence that the relation between MDE restrictions and blood lipids was modified by concurrent shifts in calories, fish, discretionary oils, added sugars, or other plant-based foods.
The health implications of the current findings are not straightforward. Blood lipids, particularly LDL cholesterol, have been linked to cardiovascular disease risk (31, 32), and plant-based diets have received attention for their ability to help lower blood lipids (3, 33). Yet, it is not clear that the reductions in LDL cholesterol observed in this study can be interpreted as beneficial given concurrent decreases in HDL cholesterol and lack of significant change in the total/HDL cholesterol ratio. In fact, cholesterol ratios may better predict cardiovascular disease risk than any single cholesterol value (34, 35). Furthermore, reductions in saturated fat and increases in carbohydrates may increase blood concentrations of small, dense LDL particles (36, 37), which are more susceptible to oxidation and more atherogenic than large buoyant LDL particles (35, 38, 39). Thus, even if MDE restrictions were maintained, the observed reductions in both LDL and HDL cholesterol, along with no observed changes in other health biomarkers, suggest that the OC practice of intermittently fasting from MDE products could have no or only a limited benefit on long-term cardiovascular health.
The suggestion that dairy might be the animal product driving the results of this study was unexpected. Although dairy, particularly cheese, is a major source of saturated fat in the typical diet in the United States (40), there has been no consistent evidence that either full or low-fat dairy has adverse effects on cardiometabolic disease risk factors or outcomes (41–44). In addition, most individuals in this study reported consuming approximately only 1 serving/2000 kcal of dairy each day and 0.5 servings/2000 kcal of cheese. Perhaps, because meat consumption declined across all MDEΔ tertiles during the study period, the change in dairy may be what distinguishes individuals who adhere to the OC guidelines more strictly from those who are more selective in which foods they restrict during Lent.
Previous research (14) found that this sample of nonmonastic OCs in the United States may not have been consuming the same amount of fruits, vegetables, legumes, and whole grains as OCs, particularly OC monastics, in other parts of the world (12, 13, 15, 45), and they may have been consuming more processed foods. Notwithstanding, this OC sample experienced, on average, a 113-kcal decrease, a 3.7-g/2000-kcal reduction in saturated fat, and a 3.8-g/2000-kcal increase in fiber in relation to each MDE serving decrease (14). They also experienced reductions in total and LDL blood cholesterol levels comparable to those observed among Greek and Egyptian OCs during MDE fasting periods (10, 12, 15, 16). Other studies of OC fasting have not detected significant changes in glucose or insulin (10, 12, 15, 16), and triglycerides were actually higher during the MDE fast in 1 population of OC monastics (12).
The results of this study also align with intervention studies that report decreases in both LDL and HDL cholesterol (but not triglycerides) (46) upon initiating regimented vegetarian or vegan diets. Improvements in glucose metabolism are not consistently observed in response to adopting a plant-based diet (47) but have been reported in 16- to 22-wk low-fat vegan intervention trials (9, 48). It is possible that the Lenten MDE diet and restriction period is not long or strict enough to elicit changes in glucose metabolism, or perhaps the current study population did not have sufficiently elevated blood glucose or insulin prior to Lent. However, it is also possible that ad libitum consumption of fat, sugar, and refined grains during Lent prevented any beneficial shifts in glucose metabolism among this study sample.
The lack of significant interactions with calories or specific plant-based foods contrasts with evidence that even mild caloric reduction can lead to improvements in cardiometabolic health markers (49) and that replacing saturated fats with refined carbohydrates can increase triglycerides (37, 50). There is otherwise little research with which to compare our null findings. One study reported a trend toward decreased total and LDL blood cholesterol among a group (n = 12) randomized to a 21-d whole-foods vegan diet but not among the comparison group (n = 11) randomized to a vegan diet that did not restrict processed foods (51); however, those findings could also be explained by the lower total and LDL choleseterol levels at baseline among the unrestricted vegan group. In the current sample, there may have been too much variability in the foods chosen as MDE replacements for significant interactions to be detected. Small increases in refined grains, for example, may have minimal impacts on biomarkers (in the short term) if also combined with reductions in calories or small increases in healthy plant-based foods.
In addition to limited statistical power and the variability and complexity of concurrent dietary changes, null changes in some health biomarkers and lack of significant interactions could also be a consequence of the short duration of the Lenten MDE restricted diet, daily variation in biomarkers (52–57), and reporting errors and biases in both retrospective FFQs and prospective FRs (29, 58, 59). The composite measure of MDE products does not capture differential effects that subcategories of meat and dairy products may have on health biomarkers, and the dietary instruments were not sensitive to variation in nutritional quality across brands or sources (e.g., organic compared with nonorganic) of similar food items. This study was unable to control for any variation in genes (60, 61), gut microbiota (62), or other biological and neurological factors that can influence responses to dietary shifts. This study did not control for changes in meal timing or meal frequency that could influence weight, blood lipids, and glucose and insulin metabolism independent of reduced MDE consumption (63). Because OCs may restrict or alter other behaviors during Lent, this study's findings may not be generalizable to populations that omit MDE products from the diet for nonreligious purposes. This study's findings also may not be generalizable to OC populations that realize the Lenten fasting practice differently. Finally, this study examined only a limited number of cardiometabolic health measures, although OC Lenten fasts may affect other facets of health.
Limitations notwithstanding, this study contributes to the scant literature on the health effects of Lenten dietary restrictions practiced by OCs, presents the first quantitative data on shifts in health biomarkers among an OC population in the United States, and presents comprehensive analyses of the relations between dietary and health biomarker shifts during a voluntary religious fast from MDE products. It is one of few studies to explore how the health effects of changing consumption of one group of foods may depend on corresponding changes in consumption of other food groups. Finally, the varying degrees of MDE restriction observed in this sample offered a unique opportunity to explore dose-response relationships.
In conclusion, this study suggests that restrictions on MDE products made by OCs in the United States during Lent may be associated with modest reductions in total and LDL blood cholesterol. However, combined with potential decreases in HDL cholesterol, these shifts may have minimal benefit on cardiometabolic health. There was no evidence that reductions in total or LDL cholesterol were modified by concurrent shifts in calories, fish, or any single plant-based food group. No changes in measures of body fat, triglycerides, glucose metabolism, or inflammation were observed in this study during Lent. Larger sample sizes are needed to further examine the complex changes that occur in dietary composition and cardiometabolic health markers, as well as long-term health outcomes, in response to the kind of intermittent vegetarianism or veganism practiced by OCs in the United States and worldwide.
Supplementary Material
Acknowledgments
We would like to acknowledge and thank Dr. Bettina Shell-Duncan for her counsel on the design, framing, and data collection methods for this study. We would also like to thank Eleanor Brindle for guidance on the biomarker collection and assay procedures used for this study and Cori Mar for feedback on the study's analytical approach.
The authors’ contributions were as follows—All authors: contributed to the development of the research design and statistical analysis plan; HJB: conducted the research and analyzed the data; HJB: wrote the first draft of the paper and had the primary responsibility for the final content; and all authors: read and approved the final manuscript.
HJB and KO have no conflicts of interest to disclose. MK has received reimbursements for travel, honoraria for speaking, and a research grant from dairy-related organizations, including the Dairy Research Institute/Dairy Management Inc., Dairy Farmers of Canada, Dairy Australia, CNIEL (France), and NZO (The Netherlands). However, no industry funding was received to conduct the work described in this manuscript.
Notes
Partial support for this research came from a National Science Foundation Doctoral Dissertation Research Improvement Grant (HJB and KO, BCS-1540282), a Shanahan Endowment Fellowship and a Eunice Kennedy Shriver National Institute of Child Health and Human Development training grant (T32 HD007543) awarded to the Center for Studies in Demography and Ecology at the University of Washington, and crowdfunding donations through Experiment.com (HJB).
Supplemental Materials, Supplemental Tables 1–10, and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CRP, C-reactive protein; cup-eq, cup-equivalent; FR, food record; MDE, meat, dairy, and eggs; MET, metabolic equivalent value; MPED, MyPyramid Equivalent Database; OCs, Orthodox Christians; oz-eq, ounce-equivalent; VIF, variance inflation factor.
References
- 1. Kahleova H, Levin S, Barnard N. Cardio-metabolic benefits of plant-based diets. Nutrients. 2017;9(8):848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahleova H, Levin S, Barnard ND. Vegetarian dietary patterns and cardiovascular disease. Prog Cardiovasc Dis. 2018;61(1):54–61. [DOI] [PubMed] [Google Scholar]
- 3. Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–9. [DOI] [PubMed] [Google Scholar]
- 4. Kwok CS, Umar S, Myint PK, Mamas MA, Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;176(3):680–6. [DOI] [PubMed] [Google Scholar]
- 5. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB, Hu FB. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnard ND, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Green A, Ferdowsian H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89(5):1588S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnard ND, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Jaster B, Seidl K, Green AA, Talpers S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777–83. [DOI] [PubMed] [Google Scholar]
- 9. Kahleova H, Tura A, Hill M, Holubkov R, Barnard N. A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: a 16-week randomized clinical trial. Nutrients. 2018;10(2):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koufakis T, Karras S, Antonopoulou V, Angeloudi E, Zebekakis P, Kotsa K. Effects of Orthodox religious fasting on human health: a systematic review. Eur J Nutr. 2017;56(8):2439–55. [DOI] [PubMed] [Google Scholar]
- 11. Sarri KO, Linardakis MK, Bervanaki FN, Tzanakis NE, Kafatos AG. Greek Orthodox fasting rituals: a hidden characteristic of the Mediterranean diet of Crete. Br J Nutr. 2004;92(2):277–84. [DOI] [PubMed] [Google Scholar]
- 12. Papadaki A, Vardavas C, Hatzis C, Kafatos A. Calcium, nutrient and food intake of Greek Orthodox Christian monks during a fasting and non-fasting week. Public Health Nutr. 2008;11(10):1022–9. [DOI] [PubMed] [Google Scholar]
- 13. Basilakis A, Kiprouli K, Mantzouranis S, Konstantinidis T, Dionisopoulou M, Hackl J, Balogh D. Nutritional study in Greek-Orthodox monasteries: effect of a 40-day religious fasting. Aktuelle Ernährungsmedizin. 2002;27(4):250–5. [Google Scholar]
- 14. Bethancourt HJ, Kratz M, O'Connor K. Spiritually-motivated restrictions on animal products have a limited impact on consumption of healthy plant-based foods. Br J Nutr. 2019;:1–33. [DOI] [PubMed] [Google Scholar]
- 15. Sarri KO, Tzanakis NE, Linardakis MK, Mamalakis GD, Kafatos AG. Effects of Greek Orthodox Christian Church fasting on serum lipids and obesity. BMC Public Health. 2003;3(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elshorbagy A, Jerneren F, Basta M, Basta C, Turner C, Khaled M, Refsum H. Amino acid changes during transition to a vegan diet supplemented with fish in healthy humans. Eur J Nutr. 2017;56(5):1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwan ML, Kushi LH, Song J, Timperi AW, Boynton AM, Johnson KM, Standley J, Kristal AR. A practical method for collecting food record data in a prospective cohort study of breast cancer survivors. Am J Epidemiol. 2010;172(11):1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolar AS, Patterson RE, White E, Neuhouser ML, Frank LL, Standley J, Potter JD, Kristal AR. A practical method for collecting 3-day food records in a large cohort. Epidemiology. 2005;16(4):579–83. [DOI] [PubMed] [Google Scholar]
- 19. Nutrition Coordinating Center: University of Minnesota. NDSR Software Food and Nutrient Database. [Internet]. Available from: http://www.ncc.umn.edu/ndsr-database-page/. [Google Scholar]
- 20. Gialamas A, Laurence CO, Yelland LN, Tideman P, Worley P, Shephard MD, Tirimacco R, Willson KJ, Ryan P, Gill J et al.. Assessing agreement between point of care and laboratory results for lipid testing from a clinical perspective. Clin Biochem. 2010;43(4–5):515–18. [DOI] [PubMed] [Google Scholar]
- 21. Shemesh T, Rowley KG, Shephard M, Piers LS, O'Dea K. Agreement between laboratory results and on-site pathology testing using Bayer DCA2000+ and Cholestech LDX point-of-care methods in remote Australian Aboriginal communities. Clin Chim Acta. 2006;367(1–2):69–76. [DOI] [PubMed] [Google Scholar]
- 22. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499. [PubMed] [Google Scholar]
- 23. Brindle E, Fujita M, Shofer J, O'Connor KA. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods. 2010;362(1–2):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marcovina S, Bowsher RR, Miller WG, Staten M, Myers G, Caudill SP, Campbell SE, Steffes MW. Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clin Chem. 2007;53(4):711–16. [DOI] [PubMed] [Google Scholar]
- 25. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–19. [DOI] [PubMed] [Google Scholar]
- 26. Kohl HW, Blair SN, Paffenbarger RS, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127(6):1228–39. [DOI] [PubMed] [Google Scholar]
- 27. Kohl H, Blair S, Paffenbarger R, Macera C, Kronenfeld J. The Aerobics Center Longitudinal Study Physical Activity Questionnaire. Med Sci Sports Exerc. 1997;29(Suppl 6):S10–S14. [Google Scholar]
- 28. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DRJ, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. [DOI] [PubMed] [Google Scholar]
- 29. Willett W. Nutritional Epidemiology. 3rd ed New York: Oxford University Press; 2013. [Google Scholar]
- 30. Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308–31. [Google Scholar]
- 31. NCEP Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106(25):3143. [PubMed] [Google Scholar]
- 32. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al.. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–e23. [DOI] [PubMed] [Google Scholar]
- 33. Yokoyama Y, Levin SM, Barnard ND. Association between plant-based diets and plasma lipids: a systematic review and meta-analysis. Nutr Rev. 2017;75(9):683–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet North Am Ed. 2014;384(9943):607–17. [DOI] [PubMed] [Google Scholar]
- 36. Faghihnia N, Tsimikas S, Miller ER, Witztum JL, Krauss RM. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. 2010;51(11):3324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siri PW, Krauss RM. Influence of dietary carbohydrate and fat on LDL and HDL particle distributions. Curr Atheroscler Rep. 2005;7(6):455–9. [DOI] [PubMed] [Google Scholar]
- 38. Verhoye E, Langlois Michel R. Circulating oxidized low-density lipoprotein: a biomarker of atherosclerosis and cardiovascular risk?. Clin Chem Lab Med. 2009;47(2):128–37. [DOI] [PubMed] [Google Scholar]
- 39. Hirayama S, Miida T. Small dense LDL: an emerging risk factor for cardiovascular disease. Clin Chim Acta. 2012;414:215–24. [DOI] [PubMed] [Google Scholar]
- 40. Huth PJ, Park KM. Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr. 2012;3(3):266–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32(4):269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr. 2013;52(1):1–24. [DOI] [PubMed] [Google Scholar]
- 43. Lovegrove JA, Givens DI. Dairy food products: good or bad for cardiometabolic disease?. Nutr Res Rev. 2016;29(2):249–67. [DOI] [PubMed] [Google Scholar]
- 44. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karras SN, Koufakis T, Petróczi A, Folkerts D, Kypraiou M, Mulrooney H, Naughton DP, Persynaki A, Zebekakis P, Skoutas D et al.. Christian Orthodox fasting in practice: a comparative evaluation between Greek Orthodox general population fasters and Athonian monks. Nutrition. 2019;59:69–76. [DOI] [PubMed] [Google Scholar]
- 46. Wang F, Zheng J, Yang B, Jiang J, Fu Y, Li D. Effects of vegetarian diets on blood lipids: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(10):e002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4(5):373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barnard ND, Cohen J, Jenkins DJA, Turner-McGrievy G, Gloede L, Jaster B, Seidl K, Green AA, Talpers S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777–83. [DOI] [PubMed] [Google Scholar]
- 49. Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: an update. Ageing Res Rev. 2017;39(Suppl C):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–55. [DOI] [PubMed] [Google Scholar]
- 51. Bloomer R, Gunnels T, Schriefer J. Comparison of a restricted and unrestricted vegan diet plan with a restricted omnivorous diet plan on health-specific measures. Healthcare. 2015;3(3):544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mogadam M, Ahmed SW, Mensch AH, Godwin ID. Within-person fluctuations of serum cholesterol and lipoproteins. Arch Intern Med. 1990;150(8):1645–8. [PubMed] [Google Scholar]
- 53. Widjaja A, Morris RJ, Levy JC, Frayn KN, Manley SE, Turner RC. Within- and between-subject variation in commonly measured anthropometric and biochemical variables. Clin Chem. 1999;45(4):561–6. [PubMed] [Google Scholar]
- 54. Bogaty P, Dagenais GR, Joseph L, Boyer L, Leblanc A, Bélisle P, Brophy JM. Time variability of C-reactive protein: implications for clinical risk stratification. PLoS One. 2013;8(4):e60759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carlsen S, Hyltoft Petersen P, Skeie S, Ø Skadberg, Sandberg S. Within-subject biological variation of glucose and HbA1c in healthy persons and in type 1 diabetes patients. Clin Chem Lab Med. 2011;49(9):1501–7. [DOI] [PubMed] [Google Scholar]
- 56. Bookstein L, Gidding SS, Donovan M, Smith FA. Day-to-day variability of serum cholesterol, triglyceride, and high-density lipoprotein cholesterol levels. Arch Intern Med. 1990;150:1653–7. [PubMed] [Google Scholar]
- 57. Demacker PNM, Schade RWB, Jansen RTP, Van ’t Laar A. Intra-individual variation of serum cholesterol, triglycerides and high density lipoprotein cholesterol in normal humans. Atherosclerosis. 45(3):259–66. [DOI] [PubMed] [Google Scholar]
- 58. Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire?. Cancer Epidemiol Biomark Prev. 2005;14(12):2826–8. [DOI] [PubMed] [Google Scholar]
- 59. Thompson FE, Subar AF. Dietary assessment methodology. In: Coulston AM, Boushey C, Ferruzzi MGeds. Nutrition in the prevention and treatment of disease. New York: Elsevier/Academic; 2013, 5–48. [Google Scholar]
- 60. Lopez-Miranda J, Ordovas JM, Mata P, Lichtenstein AH, Clevidence B, Judd JT, Schaefer EJ. Effect of apolipoprotein E phenotype on diet-induced lowering of plasma low density lipoprotein cholesterol. J Lipid Res. 1994;35(11):1965–75. [PubMed] [Google Scholar]
- 61. Sarkkinen E, Korhonen M, Erkkilä A, Ebeling T, Uusitupa M. Effect of apolipoprotein E polymorphism on serum lipid response to the separate modification of dietary fat and dietary cholesterol. Am J Clin Nutr. 1998;68(6):1215–22. [DOI] [PubMed] [Google Scholar]
- 62. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M et al.. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
- 63. St-Onge M-P, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;135(9):e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.