Abstract
In pig breeding, selection commonly takes place in purebred (PB) pigs raised mainly in temperate climates (TEMP) under optimal environmental conditions in nucleus farms. However, pork production typically makes use of crossbred (CB) animals raised in nonstandardized commercial farms, which are located not only in TEMP regions but also in tropical and subtropical regions (TROP). Besides the differences in the genetic background of PB and CB, differences in climate conditions, and differences between nucleus and commercial farms can lower the genetic correlation between the performance of PB in the TEMP (PBTEMP) and CB in the TROP (CBTROP). Genetic correlations (rg) between the performance of PB and CB growing-finishing pigs in TROP and TEMP environments have not been reported yet, due to the scarcity of data in both CB and TROP. Therefore, the present study aimed 1) to verify the presence of genotype × environment interaction (G × E) and 2) to estimate the rg for carcass and growth performance traits when PB and 3-way CB pigs are raised in 2 different climatic environments (TROP and TEMP). Phenotypic records of 217,332 PB and 195,978 CB, representing 2 climatic environments: TROP (Brazil) and TEMP (Canada, France, and the Netherlands) were available for this study. The PB population consisted of 2 sire lines, and the CB population consisted of terminal 3-way cross progeny generated by crossing sires from one of the PB sire lines with commercially available 2-way maternal sow crosses. G × E appears to be present for average daily gain, protein deposition, and muscle depth given the rg estimates between PB in both environments (0.64 to 0.79). With the presence of G × E, phenotypes should be collected in TROP when the objective is to improve the performance of CB in the TROP. Also, based on the rg estimates between PBTEMP and CBTROP (0.22 to 0.25), and on the expected responses to selection, selecting based only on the performance of PBTEMP would give limited genetic progress in the CBTROP. The rg estimates between PBTROP and CBTROP are high (0.80 to 0.99), suggesting that combined crossbred–purebred selection schemes would probably not be necessary to increase genetic progress in CBTROP. However, the calculated responses to selection show that when the objective is the improvement of CBTROP, direct selection based on the performance of CBTROP has the potential to lead to the higher genetic progress compared with indirect selection on the performance of PBTROP.
Keywords: breeding program, correlated response, crossbred pigs, genotype by environment interactions, growing-finishing pigs
INTRODUCTION
In pig breeding, consolidation has resulted in a reduced number of global breeding programs where selection takes place in purebred pigs (PB) raised mainly in temperate climates (TEMP) under optimal environmental conditions (Knap, 2005). However, pork production typically makes use of crossbred (CB) animals raised in nonstandardized commercial farms all over the world. As the final product of pig breeding is mostly a CB animal (Hidalgo et al., 2015), the genetic correlation between the performance of PB and CB (rpc) is an important parameter to be considered by pig breeding companies applying crossbreeding schemes. Combined crossbred and purebred selection (CCPS) is recommended for traits presenting rpc estimates lower than 0.8 (Wei and van der Werf, 1994), which is the case for pigs, where the average of the reported rpc estimates is 0.63 (Wientjes and Calus, 2017).
In addition to the variable sensitivity of genotypes to changes from nucleus to commercial farming systems, differences in sensitivity to climate conditions can also lower the rpc when PB and CB are kept in different climates. Around 50% of the commercial farms are in tropical and subtropical regions (TROP; Rosé et al., 2017). Sensitivity to ambient temperature and humidity (heat stress) has been described in growing-finishing pigs (Zumbach et al., 2008; Fragomeni et al., 2016; Rosé et al., 2017). In an international context, the sensitivity to heat stress becomes especially important because pork production is spread between TROP and TEMP. Nevertheless, to the best of our knowledge, rg between the performance of PB in TEMP and CB growing-finishing pigs in TROP environments have not been reported yet. This is probably due to the scarcity of data in both CB and TROP. Therefore, the current study aimed 1) to verify the presence of genotype × environment interaction (G × E) and 2) to estimate the rpc for carcass and growth performance traits when PB and 3-way CB pigs are raised in both TEMP and TROP climates.
MATERIALS AND METHODS
Ethic Statement
Data for this study were collected as part of routine data recording in a commercial breeding program. Observations from 19 farms located in 4 different countries (Brazil, Canada, France, and the Netherlands) were used in this study. All these farms are operating in line with the regulations on protection of animals of their countries.
Dataset
To verify the presence of G × E for different climates, phenotypic records were available on 217,332 PB and 195,978 CB pigs (Table 1), across 2 climatic environments, TROP (Brazil) and TEMP (Canada, France, and the Netherlands). The PB population consisted of 2 sire lines, which were located in 12 farms. The CB population consisted of terminal 3-way CB progeny generated by crossing sires from one of the PB sire lines with commercially available 2-way maternal sow crosses, which were located in 8 farms. Pedigree records were available for all animals, up to a maximum of 17 generations. A total of 535,272 pigs were included in the pedigree file with 6,229 different sires and 30,800 different dams.
Table 1.
Number of pigs with phenotypes of each line (sire or 3-way cross) by country1
| Country | Purebred farms | Sire line 1 | Sire line 2 | Crossbred farms | CB 1 | CB 2 | Total |
|---|---|---|---|---|---|---|---|
| Brazil | 4 | 7,223 | 13,451 | 2 | 975 | 4,785 | 26,434 |
| Canada | 3 | 46,598 | 14,989 | 1 | 9,045 | — | 70,632 |
| France | 1 | 29,345 | 24,196 | — | — | — | 53,541 |
| The Netherlands | 4 | 39,134 | 42,396 | 5 | 80,044 | 101,129 | 262,703 |
| Total | 12 | 122,300 | 95,032 | 8 | 90,064 | 105,914 | 413,310 |
1CB = three-way cross between the numbered sire line and a crossbred female (Large White × Landrace).
Traits
Each growth performance trait (ADG; lipid deposition [LD], and protein deposition [PD]) and carcass trait (back fat thickness [BF] and muscle depth [MD]) was considered as a different trait depending on the group of pigs in which it was measured (i.e., the 4 groups PBTROP, CBTROP, PBTEMP, and CBTEMP) (Table 2). All animals were weighed individually at the start of the growing-finishing period (“ontest”). All PB, and all CB in Canada, had their BW (kilogram) recorded, and BF (millimeter) and MD (millimeter) ultrasonically measured at the end of the growing-finishing period (“offtest”). In Brazil, most of CB had their BW recorded, and BF ultrasonically measured at offtest. A small number, 250, of CB pigs in one farm in Brazil, had their hot carcass weight (HCW) recorded at slaughter. All CB animals in the Netherlands had their HCW recorded along with BF and MD using the Hennessy Grading Probe (Hennessy Grading Systems, Auckland, New Zealand) or the Capteur Gras Maigre (CGM, Sydel, France) at slaughter. For the pigs with BW recorded offtest (BWofftest), ADGontest (gram per day) was obtained as the difference between BWofftest and BWontest, divided by the length of the test period. For the pigs with HCW recorded at slaughter, ADG was obtained as the calculated BW (CBW) minus BWontest, divided by the length of the growing-finishing period. The formula used to obtain the CBW based on the HCW (Handboek varkenshouderij, 2004) was the following:
Table 2.
Number of observations (No.), mean (µ), standard deviation (SD), minimum (Min), and maximum (Max) for covariates1 and traits2 used to estimate variance components and genetic correlations
| TROP | TEMP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Traits | No. | μ | SD | Min | Max | No. | μ | SD | Min | Max |
| Purebreds | ||||||||||
| BWbirth, g | 1,658 | 331.5 | 660.0 | 2,670 | — | — | — | — | ||
| BWontest, kg | 29.6 | 5.6 | 10.0 | 57.0 | 32.1 | 8.4 | 9.0 | 59.0 | ||
| BWofftest, kg | 102.9 | 11.4 | 73.9 | 152.7 | 125.9 | 10.0 | 94.3 | 155.1 | ||
| ADG, g/d | 20,344 | 934.5 | 128.1 | 543.0 | 1,464 | 192,767 | 1,001 | 138.6 | 540.0 | 1,474 |
| LD, g/d | 19,987 | 145.9 | 45.3 | 29.1 | 376.6 | 188,250 | 162.9 | 53.8 | 21.3 | 450.1 |
| PD, g/d | 19,987 | 160.6 | 21.0 | 85.5 | 248.4 | 188,250 | 172.1 | 24.3 | 78.9 | 272.5 |
| BF, mm | 20,746 | 10.1 | 1.7 | 4.3 | 17.4 | 195,394 | 9.6 | 1.9 | 3.3 | 18.6 |
| MD, mm | 13,979 | 58.3 | 5.9 | 38.6 | 80.3 | 193,856 | 59.0 | 5.9 | 37.5 | 81.4 |
| Crossbreds | ||||||||||
| BWbirth, g | 1,445 | 325.7 | 450.0 | 2,350 | 1,375 | 312.4 | 440.0 | 2,350 | ||
| BWontest, kg | 24.4 | 4.8 | 10.5 | 40.4 | 25.7 | 4.7 | 10.2 | 40.8 | ||
| BWofftest, kg | 104.8 | 10.9 | 78.4 | 151.8 | 118.6 | 9.0 | 79.0 | 150.0 | ||
| HCW, kg | 93.1 | 7.9 | 68.0 | 113.9 | 92.9 | 6.6 | 72.2 | 114.0 | ||
| CBW, kg | 118.5 | 8.3 | 90.9 | 139.2 | 118.4 | 6.8 | 95.8 | 139.4 | ||
| ADG, g/d | 5,756 | 936.3 | 104.1 | 507.0 | 1,336 | 47,945 | 869.2 | 93.7 | 562.0 | 1,156 |
| LD, g/d | 5,227 | 212.3 | 57.6 | 69.3 | 459.2 | 21,205 | 219.8 | 58.8 | 53.7 | 490.0 |
| PD, g/d | 5,227 | 144.1 | 13.8 | 92.0 | 200.4 | 21,205 | 138.9 | 19.2 | 64.7 | 211.7 |
| BF, mm | 5,577 | 13.1 | 2.3 | 6.1 | 20.3 | 190,064 | 13.6 | 2.6 | 5.5 | 24.1 |
| MD, mm | — | — | — | — | — | 190,563 | 62.4 | 6.5 | 39.7 | 86.6 |
1BWbirth = body weight at birth; BWontest = body weight ontest; BWofftest = body weight offtest; HCW = hot carcass weight; CBW = calculated BW.
2LD = lipid deposition; PD = protein deposition; BF = back fat thickness; MD = muscle depth; TROP = tropical climate; TEMP = temperate climate.
Lipid deposition (gram per day) and PD (gram per day) were estimated as the increment in lipid and protein mass content during the growing-finishing period based on BW and back fat measurements (de Greef et al., 1994):
Genetic Parameters Estimation
Univariate analyses were performed to estimate the variance components and heritabilities for all traits. Genetic correlations were estimated using bivariate analyses. A linear mixed model implemented in ASReml (Gilmour et al., 2009) was used for the analyses as follows:
| [1] |
in which y is the vector of observations; X, Z, W, V, and U are known incidence matrices; b is a vector of fixed effects (Table 3); a is a vector of random additive genetic effects (breeding values), ; c is a vector of random nongenetic effects common to individuals born in the same litter, ; g is the vector of random pen effects (individuals grouped together in the same pen) ; f is the vector of random effects common to individuals performance tested in the same compartment of the barn within the same contemporary group, ; and e is a vector of residuals, . A is a matrix of average additive genetic relationships among all individuals, Ic, Ig, If, and Ie are identity matrices of the appropriate dimensions and , and are covariance matrices related to each effect. In the case of univariate analyses, the covariance matrix is a scalar with the variance component, , associated with the respective effect. In the case of bivariate analyses, the covariance matrices for PBTROP and CBTROP are given by:
Table 3.
Fixed effects included in the vector b of Eq. 1 for the traits1
| Model | Dependent trait(s)1 |
|---|---|
| Fixed effects2 | |
| A | ADG; LD; PD |
| µ + SEXj + LINEk + HYSl + COMPm + b1 × BWbirth | |
| B | BF and MD offtest |
| µ + SEXj + LINEk + HYSl + COMPm + b1 × BWofftest | |
| C | BF and MD at slaughter |
| µ + SEXj + LINEk + HYSl + COMPm + b1 × HCW |
1LD = lipid deposition; PD = protein deposition; BF = back fat thickness; MD = muscle depth.
2SEX = the sex of the pig; LINE = the line of the pig; HYS = herd–year–season = farm × year × month of birth; COMP = compartment within barn × farm; BWbirth = body weight at birth; BWofftest = body weight offtest; HCW = hot carcass weight (BF and MD were pre-adjusted for the covariate weight prior to the analysis).
For the bivariate analysis of the other combinations of groups PBTROP, CBTROP, PBTEMP, and CBTEMP, the covariance matrices are set up in the same manner. The rpc estimates in the TROP and in the TEMP , the rg between the performance of PB in both climates , the genetic correlation between the performance of PBTROP and , and the genetic correlation between the performance of PBTEMP and were estimated by:
Responses to Selection
To assess the genetic progress a breeding program can achieve for CBTROP performance for the traits studied here, we use the breeders’ equation to calculate the responses to selection. Phenotypes measured in PB or CB and in TROP or TEMP were considered as 4 different traits, similar to Wientjes and Calus (2017). Three different responses to selection were calculated: 1) the response for the CBTROP trait to direct selection based on CBTROP performance ; 2) the correlated response for the CBTROP trait to indirect selection based on PBTROP performance ; and 3) the correlated response for the CBTROP trait to indirect selection based on PBTEMP performance .
These 3 responses were calculated as follows (Falconer and Mackay, 1996):
where is the intensity of selection on CBTROP (assumed to be 1 in this study), is the square root of the heritability of the trait CBTROP, and is the genetic standard deviation of the trait CBTROP.
where is the intensity of selection on PBTROP (assumed to be 1 in this study), is the square root of the heritability of the trait PBTROP, is the genetic correlation between the performance of PBTROP and CBTROP, and is the genetic standard deviation of the trait CBTROP.
where is the intensity of selection on PBTEMP (assumed to be 1 in this study), is the square root of the heritability of the trait PBTEMP, is the genetic correlation between the performance of PBTEMP and CBTROP, and is the genetic standard deviation of the trait CBTROP.
Genetic Distances
To evaluate the genetic distance between the PB and CB located at both TROP and TEMP environments, a distance plot was produced by applying principal coordinate analysis to the additive relationship matrix using the function cmdscale in R. From each combination of population and environment, 500 animals were selected at random to be included in the distance plot.
RESULTS
Variance Components
Estimates of variance components are presented in Table 4. As expected, all traits presented medium to high heritability estimates, with the larger values for CBTROP for growth performance traits (0.38 to 0.45) and for PBTEMP for carcass traits (0.41 to 0.46). In the TROP, all traits in CB presented larger values for heritability estimates, whereas in the TEMP, PB presented larger heritability estimates for LD, BF, and MD, and CB presented larger estimates for ADG and PD. The phenotypic variance explained by the common environment among litter mates was similar in the 4 groups, being larger for growth performance traits (3% to 7%) than for carcass traits (2% to 5%). The phenotypic variance explained by the contemporary pen effect was larger for growth performance traits (4% to 19%) than for carcass traits (3% to 13%), with the larger values (0.08 to 0.19) for PB_TROP and the lower values (0.02 to 0.09) for CB_TEMP. The phenotypic variance explained by the contemporary compartment effect was larger for growth performance traits (2% to 12%) than for carcass traits (1% to 5%), with the larger values (0.04 to 0.12) for PB_TROP, and the lower values (0.01 to 0.06) for CB in both climates.
Table 4.
Additive genetic and phenotypic variances and contribution (SE) of different random effects1 to the estimation of the traits2 expressed as percentage of the phenotypic variance
| Traits | h 2 | ||||||
|---|---|---|---|---|---|---|---|
| TROP | |||||||
| Purebreds | |||||||
| ADG | 2,680 | 14,890 | 0.18 (0.02) | 0.06 (0.01) | 0.17 (0.01) | 0.12 (0.02) | 0.48 (0.02) |
| LD | 492 | 1,822 | 0.27 (0.02) | 0.06 (0.01) | 0.12 (0.01) | 0.08 (0.01) | 0.48 (0.02) |
| PD | 62.9 | 392.9 | 0.16 (0.02) | 0.05 (0.01) | 0.19 (0.01) | 0.12 (0.02) | 0.48 (0.02) |
| BF | 0.7 | 2.3 | 0.29 (0.02) | 0.05 (0.01) | 0.13 (0.01) | 0.05 (0.01) | 0.48 (0.02) |
| MD | 6.2 | 17.7 | 0.35 (0.03) | 0.03 (0.01) | 0.08 (0.01) | 0.04 (0.01) | 0.49 (0.02) |
| Crossbreds | |||||||
| ADG | 3,788 | 9,238 | 0.41 (0.06) | 0.05 (0.01) | 0.06 (0.01) | 0.03 (0.02) | 0.45 (0.05) |
| LD | 1,146 | 3,015 | 0.38 (0.06) | 0.04 (0.01) | 0.09 (0.01) | 0.02 (0.02) | 0.47 (0.05) |
| PD | 89.9 | 199.7 | 0.45 (0.06) | 0.03 (0.01) | 0.06 (0.01) | 0.05 (0.02) | 0.40 (0.05) |
| BF | 1.8 | 4.5 | 0.39 (0.06) | 0.03 (0.01) | 0.07 (0.01) | 0.02 (0.01) | 0.49 (0.05) |
| MD | — | — | — | — | — | — | — |
| TEMP | |||||||
| Purebreds | |||||||
| ADG | 2,810 | 13,381 | 0.21 (0.01) | 0.07 (0.00) | 0.12 (0.00) | 0.07 (0.00) | 0.53 (0.01) |
| LD | 862 | 2,462 | 0.35 (0.01) | 0.06 (0.00) | 0.09 (0.00) | 0.05 (0.00) | 0.45 (0.01) |
| PD | 80.5 | 366.0 | 0.22 (0.01) | 0.06 (0.00) | 0.11 (0.00) | 0.06 (0.00) | 0.54 (0.01) |
| BF | 1.3 | 2.7 | 0.46 (0.01) | 0.04 (0.00) | 0.06 (0.00) | 0.03 (0.00) | 0.40 (0.01) |
| MD | 7.7 | 18.8 | 0.41 (0.01) | 0.03 (0.00) | 0.06 (0.00) | 0.05 (0.00) | 0.44 (0.01) |
| Crossbreds | |||||||
| ADG | 1,739 | 7,563 | 0.23 (0.01) | 0.05 (0.00) | 0.07 (0.00) | 0.06 (0.01) | 0.58 (0.01) |
| LD | 1,014 | 3,170 | 0.32 (0.02) | 0.04 (0.01) | 0.04 (0.00) | 0.04 (0.01) | 0.56 (0.02) |
| PD | 59.3 | 219.6 | 0.27 (0.02) | 0.06 (0.01) | 0.07 (0.01) | 0.05 (0.01) | 0.55 (0.02) |
| BF | 2.3 | 6.0 | 0.38 (0.01) | 0.03 (0.00) | 0.03 (0.00) | 0.02 (0.00) | 0.54 (0.01) |
| MD | 6.1 | 35.8 | 0.17 (0.01) | 0.02 (0.00) | 0.02 (0.00) | 0.01 (0.00) | 0.77 (0.01) |
1 = additive genetic variance; = phenotypic variance; h2 = heritability; = variance of common litter; = variance of contemporary pen; = variance of contemporary compartment; = residual variance;
2LD = lipid deposition; PD = protein deposition; BF = back fat thickness; MD = muscle depth; TROP = tropical climate; TEMP = temperate climate. 0.00 = value lower than 0.005.
Genetic Correlations
Estimates of genetic correlations between climates and between PB and CB are presented in Table 5. Some estimates could only be obtained with restrained components, in all cases these estimates included data from PBTROP, and should be treated with caution. Estimates of (0.80 to 0.99) were higher than (0.71 to 0.81). G × E appears to be present for ADG, PD, and MD () and absent for BF and LD (). The rpc estimates of PBTROP and CBTEMP were mostly moderate and rpc estimates between PBTEMP and CBTROP were low (0.22 to 0.25) and had relatively high SE.
Table 5.
Genetic correlations (SE)1
| Traits | |||||
|---|---|---|---|---|---|
| ADG | 0.88 (0.14) | 0.73 (0.04) | 0.64* (0.25) | 0.45 (0.40) | 0.22 (0.58) |
| LD | 0.97* (0.08) | 0.78 (0.04) | 0.97* (0.10) | 0.87 (0.31) | 0.24 (0.77) |
| PD | 0.80 (0.16) | 0.79 (0.05) | 0.73* (0.23) | 0.68 (0.37) | nc |
| BF | 0.99* (0.10) | 0.81 (0.02) | 0.98* (0.08) | 0.97* (0.11) | 0.25 (0.52) |
| MD | — | 0.71 (0.02) | 0.75 (0.17) | 0.88 (0.17) | — |
1 = purebred–crossbred genetic correlation in tropical environment; = purebred–crossbred genetic correlation in temperate environment; = genotype by climate interaction in PB; = genetic correlation between the performance of PB in tropical climate and CB in temperate climate; = genetic correlation between the performance of PB in temperate climate and CB in tropical climate.
*Genetic variance components converged restrained (liable to change from positive definite to fixed at a boundary) and thus should be interpreted with caution; nc = not converged.
Responses to Selection
The calculated responses to selection are presented in Table 6. Direct selection for CBTROP leads to higher responses than indirect selection on either PBTROP or PBTEMP. Across the different traits, the direct response was between 1.2- and 2.2-fold higher than the correlated response and between 3.7- and 6.4-fold higher than .
Table 6.
Response to direct selection on CBTROP and correlated response for indirect selection based on PBTROP or PBTEMP
| Response1 | ADG, g/d | LD, g/d | PD, g/d | BF, mm |
|---|---|---|---|---|
| 39.4 | 20.9 | 6.36 | 0.84 | |
| 23 | 16.2 | 2.92 | 0.72 | |
| 6.2 | 4.8 | 1.02 | 0.23 | |
| 1.7 | 1.3 | 2.2 | 1.2 | |
| 6.4 | 4.3 | 6.2 | 3.7 |
1 = response to direct CBTROP selection, = correlated response for CBTROP performance to indirect selection on PBTROP performance, = correlated response for CBTROP performance to indirect selection on PBTEMP performance. LD = lipid deposition; PD = protein deposition; BF = back fat thickness.
Genetic Distances
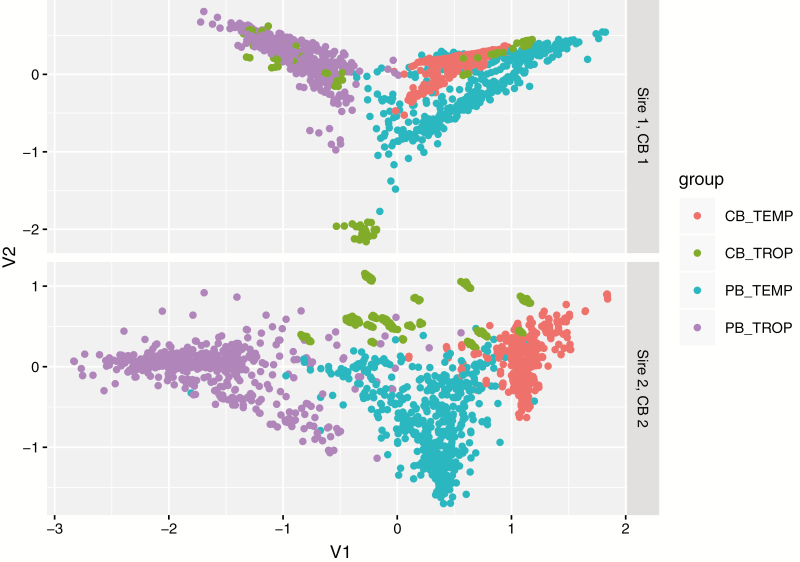
PBTEMP and CBTEMP are found close together on the distance plot (Fig. 1), as expected based on the small SE that are observed for in Table 5. For both lines 1 and 2, the average distance between points for PBTEMP and PBTROP is larger than the distance between PBTEMP and CBTEMP. This indicates that the pedigree relationships contributing to are on average smaller than the pedigree relationships for . CB pigs in TROP show different patterns for CB1 and CB2. For sire line 1, some CBTROP are found close to PBTROP, some are close to PBTEMP, whereas others are at some distance from all other groups. For sire line 2, CBTROP are separated from the other groups, but closest to PBTEMP.
Figure 1.
Distance plot based on the first (V1) and second (V2) principal components of the additive relationship matrix between purebred (PB) and crossbred (CB) pigs located in both tropical (TROP) and temperate (TEMP) environment for sire lines 1 and 2.
DISCUSSION
The performance of CB pigs is typically improved by applying selection in PB lines. In recent years, the use of data on CB offspring has come into play as well as the collection of performance data in the commercial environment. With this, the need for estimation of G × E and rpc has increased. Estimates of rpc in pigs were recently reviewed by Wientjes and Calus (2017) who reported an rpc average of 0.63. The rpc can, in theory, be decomposed into a G × E caused by differences between nucleus and commercial farms (Bijma and van Arendonk, 1998; Zumbach et al., 2007; Tusell et al., 2016; Wientjes and Calus, 2017; Godinho et al., 2018), and a genotype × genotype interaction (G × G) due to interaction of genes with the differences in genetic background of PB and CB. Here we aimed to separate these 2 components by estimating rpc of the same PB and their CB in 2 environments, as well as estimating the PB genetic correlation between these 2 environments.
Data
Even though the data used herein contained records on over 400,000 pigs, it did not yield reliable estimates for some of the correlations of interest. Some estimates of and and also the rpc estimates of PBTROP and CBTEMP for BF were obtained with components restrained. The data on PBTROP are common to all these estimates. Clearly, many records alone are not sufficient for accurate estimation of genetic correlations. In addition, the records obtained in the 2 environments should be on pigs that are closely related. Typically, the relationships are closest between PB and CB in the same environment. Relationships between PB in different environments are lower, and finally, the relationship between CB in different environments is smallest because the pedigree connection would mostly be through relationships between PB in the 2 environments. A small subset of 250 CB pigs in TROP were produced as part of an experiment where semen was collected from PB sires in TEMP and exported to Brazil to produce CB in TROP. In this experiment, the relationships from PBTEMP to CBTROP are good but the number of sires involved was too small to estimate genetic correlations based on this experiment alone. Because importing semen is both difficult and costly, due to legislation and veterinary requirements, this is a routine practice for production of PB in TROP, but not for the production of CB in the same environment. In addition, in the last years the number of live boars imported from TEMP to TROP that are used for both PB and CB production has greatly increased. With such a close link between the TEMP and TROP populations, data that are currently been generated may contribute greatly to future better correlation estimates.
Another possibility for improving the estimation of such correlations may be the use of genomic data to more accurately measure relationships between PBTEMP and CBTROP. The benefits of genomic, rather than pedigree, relationships for estimation of genetic correlations are unknown. For the estimation of breeding values with genomic relationships, it is still important to have close relatives in the training dataset if a high accuracy is required (Pszczola et al., 2012). To estimate a genetic correlation with small SE may therefore still require close relatives to be present in both PBTEMP and CBTROP, even when using a genomic relationship matrix.
Environments
Robertson (1959) suggested that G × E is important when genetic correlations (rg) are less than 0.8, and this suggestion is widely accepted in animal breeding. Estimates less than 0.8 for ADG, PD, and MD indicate G × E for these traits. G × E may be due to the climatic differences since sensitivity to heat stress has been described in pigs (Zumbach et al., 2008; Fragomeni et al., 2016; Rosé et al., 2017). There may also be differences in the farms for PB in TROP and TEMP that could contribute to G × E. First, PB in TROP were kept in open or semi-open barns. Second, the farm environments in different climates were also different in health status and management practices. Differences in health status of farms have been shown to cause G × E in pigs (Rashidi et al., 2014; Herrero-Medrano et al., 2015; Mathur, 2018).
The nucleus farms for the PBTEMP probably provide the least, and CBTROP the most challenging environment, whereas PBTROP and CBTEMP are in intermediate environments. The environment of PBTROP is better controlled than commercial Brazilian farms but considerably less well controlled than the environment provided by genetic nucleus farms in TEMP. Therefore, differences between the environment of PBTROP and CBTEMP are probably smaller than the differences between the environment of PBTEMP and CBTROP, as PBTEMP are in the better-controlled environment and CBTROP are in the less well-controlled environment.
A more challenging environment in CBTROP could also explain the larger differences between the heritability estimates of PB and CB in TROP in comparison with the same estimates in TEMP (Table 4). Under a less well-controlled environment in CBTROP, animals would be challenged to express their genetic potential and consequently the genetic variance would be higher. Differences between nucleus and commercial environments are probably smaller in the TEMP what makes these estimates more similar in this environment except for the trait MD.
Estimates of rpc Within Environment
The estimates of rpc within environments ( and ) were in the range of the literature (Wientjes and Calus, 2017), with higher values in TROP than in TEMP. The for ADG and PD were estimated without model constraints and are in the same range as estimates; the equivalent values for LD and BF were close to 1.0 but estimated with components restrained, so differences with the corresponding are uncertain. Standard errors of were higher, which is expected given the reduced number of records in TROP. For ADG, BF, and LD, the higher could be explained by the BW being recorded offtest and the back fat measurements being done ultrasonically in both PB and CB in the TROP, whereas in the TEMP, the vast majority of CB had BW calculated based on the carcass weight and BF recorded with a probe in the carcass at slaughter.
The environment of PB in TROP is more challenging than for PB in TEMP, which could also make the difference between PB and CB environments smaller in TROP than in TEMP. This may have resulted in the higher than . Moreover, selection under improved conditions has been shown to increase environmental sensitivity of genotypes (van der Waaij, 2004). Therefore, the reverse may be true in TROP whereby the challenging environment for PB may have resulted in selection for more robust PBTROP. This increased robustness could contribute to the higher when transmitted to the CB in TROP.
CCPS is recommended when the rpc is lower than 0.8; the values of the rpc estimates in this study are mostly around this value. With higher values of compared with , we should expect less benefit of having data recorded on CB in TROP than in TEMP.
Purebred–Crossbred Correlations Between Environments
The typical situation in pig production is that genetic progress is created by selection of PB in TEMP. The performance of CB in TROP would therefore depend on the genetics of PB in TEMP. When PB and CB were in different climates, the was low (0.22 to 0.25). Besides the G × G, these estimates are also lowered by G × E given the climate differences; the differences in the health status and farm management between the TROP and the TEMP, as discussed earlier, also contribute. The rpc between climates was higher when the PB were in the TROP with between 0.45 and 0.97. We speculate that an increased robustness of PBTROP would also be reflected in the higher compared to the . This could indicate a benefit for overall efficiency by selecting records on PBTROP when the objective is to improve the performance of CB in both TEMP and TROP. However, most of the pigs’ nucleus farms are in the TEMP.
Genetic Distances
Figure 1 shows data on the same number of pigs for all 4 groups. However, the points for CBTEMP are much more clustered than for the other 3 groups. The points for CBTROP, especially in the upper panel for sire line 1, are less clustered than for the other 3 groups. The SE of are the smallest (Table 5), which is to be expected given that PBTEMP and CBTEMP are found closest together on the distance plots, and the largest number of records contribute to these estimates. The large pedigree distances perhaps contribute to the size of SE of , although the major reason for that might be the smaller number of records in CBTROP. Given the smaller distance of CBTROP to PBTEMP, a smaller SE would be expected for than for but the opposite is observed. The estimated values for are however much closer to 0 than for which increases the expected SE of the estimates (Bijma and Bastiaansen, 2014). Smaller, though still rather large, values are seen for the SE of compared with the SE of , which is not supported by the distances in Figure 1. Especially in the lower panel for sire line 2, a large distance is observed between PBTROP and CBTEMP. The much larger dataset for CBTEMP will probably have contributed to this smaller SE.
CONCLUSIONS
The current dataset collected by pig breeding programs makes it difficult to obtain accurate estimates of genetic correlations between pig performance in temperate and tropical climates. This is a fact especially when the aim is to estimate rpc between PB in one climate and CB in the other climate because pigs in both climates are only distantly related. G × E between the temperate and tropical environment appears to be present for ADG, PD, and MD. In addition, rpc within TEMP and within TROP are not close to 1 for most traits. Collection of phenotypes in the TROP should be included when the objective of selection is the performance of CBTROP. On the basis of and the expected responses to selection, selection for CBTROP based on the performance of PBTEMP would compromise the genetic progress for the traits being studied. Based solely on the estimates, CCPS would not be necessary to increase genetic progress in CBTROP. However, based on the calculated responses to selection, when the objective is to improve the performance of CBTROP, direct selection based on the performance of CBTROP has the potential to lead to the higher genetic progress for growth and carcass traits.
Footnotes
This work is financially supported by the Netherlands Organisation for Scientific Research (NWO) through the LocalPork project W 08.250.102 in the Food and Business Global Challenges Program.
REFERENCES
- Bijma P., and van Arendonk J. A.. 1998. Maximizing genetic gain for the sire line of a crossbreeding scheme utilizing both purebred and crossbred information. J. Anim. Sci. 66:529–542. doi:10.1017/S135772980000970X [Google Scholar]
- Bijma P., and Bastiaansen J. W.. 2014. Standard error of the genetic correlation: How much data do we need to estimate a purebred-crossbred genetic correlation? Genet. Sel. Evol. 46:79. doi:10.1186/s12711-014-0079-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Greef K. H., Verstegen M. W. A., Kemp B., and van der Togt P. L.. 1994. The effect of body weight and energy intake on the composition of deposited tissue in pigs. Anim. Prod. 58:263–270. doi:10.1017/S1357729800042582 [Google Scholar]
- Falconer D. S. and Mackay T. F. C.. 1996. Introduction to quantitative genetics. Longman, Essex, UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragomeni B. O., Lourenco D. A. L., Tsuruta S., Gray K., Huang Y., and Misztal I.. 2016. Modeling response to heat stress in pigs from nucleus and commercial farms in different locations. J. Anim. Sci. 94:4789–4798. doi:10.2527/jas.2016-0536 [DOI] [PubMed] [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., and Thompson R.. 2009. ASReml user guide release 3.0. VSN International LTD, Hemel Hempstead, UK. [Google Scholar]
- Godinho R. M., Bergsma R., Silva F. F., Sevil lano C. A., Knol E. F., Lopes M. S., Lopes P. S., Bastiaansen J. W. M., and Guimarães S. E. F.. 2018. Genetic correlations between feed efficiency traits, and growth performance and carcass traits in purebred and crossbred pigs. J. Anim. Sci. 96:817–829. doi:10.1093/jas/skx011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handboek varkenshouderij. 2004. p. 312 The Netherlands: Wageningen UR Livestock Research, Wageningen: https://www.wur.nl/nl/Onderzoek-Resultaten/Onderzoeksinstituten/livestock-research/Producten/Show/Handboek-Varkenshouderij.htm. [Google Scholar]
- Herrero-Medrano J. M., Mathur P. K., ten Napel J., Rashidi H., Alexandri P., Knol E. F., and Mulder H. A.. 2015. Estimation of genetic parameters and breeding values across challenged environments to select for robust pigs. J. Anim. Sci. 93:1494–1502. doi:10.2527/jas.2014-8583 [DOI] [PubMed] [Google Scholar]
- Hidalgo A. M., Bastiaansen J. W., Lopes M. S., Harlizius B., Groenen M. A., and de Koning D. J.. 2015. Accuracy of predicted genomic breeding values in purebred and crossbred pigs. G3 (Bethesda) 5:1575–1583. doi:10.1534/g3.115.018119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knap P. W. 2005. Breeding robust pigs. Austral. J. Exp. Agric. 45:763–773. [Google Scholar]
- Mathur P. K. 2018. Genotype-environment interactions in pig breeding. In: Proceedings of 11th World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand p. 668. [Google Scholar]
- Pszczola M., Strabel T., Mulder H. A., and Calus M. P.. 2012. Reliability of direct genomic values for animals with different relationships within and to the reference population. J. Dairy Sci. 95:389–400. doi:10.3168/jds.2011-4338 [DOI] [PubMed] [Google Scholar]
- Rashidi H., Mulder H. A., Mathur P., Van Arendonk J. A., and Knol E. F.. 2014. Variation among sows in response to porcine reproductive and respiratory syndrome. J. Anim. Sci. 92:95–105. doi:10.2527/jas.2013-6889 [DOI] [PubMed] [Google Scholar]
- Robertson A. 1959. The sampling variance of the genetic correlation coefficient. Biometrics 15:469–485. [Google Scholar]
- Rosé R., Gilbert H., Loyau T., Giorgi M., Billon Y., Riquet J., Renaudeau D., Gourdine J.-L.. 2017. Interactions between sire family and production environment (temperate vs. tropical) on performance and thermoregulation responses in growing pigs. J. Anim. Sci. 95:4738–4751. doi.org/10.2527/jas2017.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusell L., Gilbert H., Riquet J., Mercat M. J., Legarra A., and Larzul C.. 2016. Pedigree and genomic evaluation of pigs using a terminal-cross model. Genet. Sel. Evol. 48:32. doi:10.1186/s12711-016-0211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij E. H. 2004. A resource allocation model describing consequences of artificial selection under metabolic stress. J. Anim. Sci. 82:973–981. doi:10.2527/2004.824973x [DOI] [PubMed] [Google Scholar]
- Wei M., and van der Werf J. H. J.. 1994. Maximizing genetic response in crossbreds using both purebred and crossbred information. Anim. Prod. 59:401–413. doi:10.1017/S0003356100007923 [Google Scholar]
- Wientjes Y. C. J., and Calus M. P. L.. 2017. Board invited review: The purebred-crossbred correlation in pigs: A review of theory, estimates, and implications. J. Anim. Sci. 95:3467–3478. doi:10.2527/jas.2017.1669 [DOI] [PubMed] [Google Scholar]
- Zumbach B., Misztal I., Tsuruta S., Holl J., Herring W., Long T.. 2007. Genetic correlations between two strains of Durocs and crossbreds from differing production environments for slaughter traits. J. Anim. Sci. 85:901–908. doi:10.2527/jas.2006-499 [DOI] [PubMed] [Google Scholar]
- Zumbach B., Misztal I., Tsuruta S., Sanchez J. P., Azain M., Herring W., Holl J., Long T., and Culbertson M.. 2008. Genetic components of heat stress in finishing pigs: Parameter estimation. J. Anim. Sci. 86:2076–2081. doi:10.2527/jas.2007-0282 [DOI] [PubMed] [Google Scholar]