ABSTRACT
Background
A very limited amount of research has examined intermittent fasting (IF) programs, such as time-restricted feeding (TRF), in active populations.
Objective
Our objective was to examine the effects of TRF, with or without β-hydroxy β-methylbutyrate (HMB) supplementation, during resistance training (RT).
Methods
This study employed a randomized, placebo-controlled, reduced factorial design and was double-blind with respect to supplementation in TRF groups. Resistance-trained females were randomly assigned to a control diet (CD), TRF, or TRF plus 3 g/d HMB (TRFHMB). TRF groups consumed all calories between 1200 h and 2000 h, whereas the CD group ate regularly from breakfast until the end of the day. All groups completed 8 wk of supervised RT and consumed supplemental whey protein. Body composition, muscular performance, dietary intake, physical activity, and physiological variables were assessed. Data were analyzed prior to unblinding using mixed models and both intention-to-treat (ITT) and per protocol (PP) frameworks.
Results
Forty participants were included in ITT, and 24 were included in PP. Energy and protein intake (1.6 g/kg/d) did not differ between groups despite different feeding durations (TRF and TRFHMB: ∼7.5 h/d; CD: ∼13 h/d). Comparable fat-free mass (FFM) accretion (+2% to 3% relative to baseline) and skeletal muscle hypertrophy occurred in all groups. Differential effects on fat mass (CD: +2%; TRF: −2% to −4%; TRFHMB: −4% to −7%) were statistically significant in the PP analysis, but not ITT. Muscular performance improved without differences between groups. No changes in physiological variables occurred in any group, and minimal side effects were reported.
Conclusions
IF, in the form of TRF, did not attenuate RT adaptations in resistance-trained females. Similar FFM accretion, skeletal muscle hypertrophy, and muscular performance improvements can be achieved with dramatically different feeding programs that contain similar energy and protein content during RT. Supplemental HMB during fasting periods of TRF did not definitively improve outcomes. This study was prospectively registered at clinicaltrials.gov as NCT03404271.
Keywords: intermittent fasting, intermittent energy restriction, energy restriction, fat loss, muscle mass, resistance exercise, weight training, body composition, muscular strength, protein
Introduction
Intermittent fasting (IF) is a broad term encompassing eating patterns with regularly occurring periods of food abstention longer than a typical overnight fast (1). In contrast to traditional methods of continuous energy restriction, IF programs utilize intermittent energy restriction by interspersing periods of less restricted or unrestricted feeding with periods of severely limited energy intake. Several forms of IF have been described, including time-restricted feeding (TRF), alternate-day fasting (ADF), alternate-day modified fasting, and periodic fasting (2). The vast majority of existing research in humans has focused on weight loss and health effects induced by IF in overweight and obese adults. Cumulatively, this research has demonstrated that IF programs are viable, albeit not inherently superior, alternatives to traditional continuous energy restriction for weight loss and health improvement (3–5).
Although an increasing body of research has reported the physiological effects of IF, a very limited number of controlled trials have taken place in active or exercising individuals (6–8). Two previous investigations reported the effects of TRF in adult males performing resistance training (RT) (7, 8). Although Tinsley et al. (7) observed an apparent attenuation in lean soft tissue (LST) accretion during 8 wk of TRF, this result was confounded by the TRF group self-selecting a protein intake lower than the control diet (CD) (1.0 compared with 1.4 g/kg/d) and suboptimal for active individuals (9, 10). Nonetheless, comparable improvements in muscular performance were observed in both groups. Moro et al. (8) prescribed higher protein intake (1.9 g/kg/d) in TRF and CD and found that, although both groups maintained fat-free mass (FFM) and demonstrated similar muscular performance, TRF produced significant reductions in fat mass (FM) and differential effects on physiological markers.
The prevalence of IF eating patterns in active individuals and the paucity of existing research in this population indicate the need for further research. Additionally, as the aforementioned investigations were conducted in male participants only, and potential sex differences in responses to IF have been reported (11, 12), an examination of IF plus RT in females is warranted. Furthermore, since IF programs necessitate prolonged periods without amino acid-induced stimulation of muscle protein synthesis and suppression of muscle protein breakdown (13), it has been questioned whether modification of fasting periods to allow ingestion of amino acids or their metabolites may be beneficial for lean mass maintenance or accretion during IF (14). Due to its ability to influence muscle protein balance (15), the leucine metabolite β-hydroxy β-methylbutyrate (HMB) has been hypothesized to exert favorable effects on skeletal muscle in exercising and/or energy-restricted individuals (16–18). However, this has not been sufficiently examined in the context of IF. Therefore, the purpose of this study was to compare the physiological and performance effects of TRF, with or without HMB supplementation during fasting periods, to a CD requiring breakfast consumption during progressive RT in resistance-trained females. Primary outcomes of interest were FM reduction, FFM accretion, and skeletal muscle hypertrophy in response to RT. Secondary outcomes of interest included muscular performance (e.g. strength and endurance), as well as commonly evaluated physiological variables such as resting metabolic rate, substrate utilization, vascular function, and concentrations of glucose, insulin, blood lipids, and cortisol.
Methods
Overview
This study employed a randomized, placebo-controlled, reduced factorial design. The experiment was double-blind with respect to HMB and placebo supplements and single-blind when possible with respect to the assigned dietary program. The study was prospectively registered at clinicaltrials.gov as NCT03404271 and was approved by the Texas Tech University Institutional Review Board (IRB2017-912). The following primary outcome measures were specified a priori: FM, FFM, body fat percentage (BF%), muscle thickness of the elbow flexor muscles (MTEF), and muscle thickness of the knee extensor muscles (MTKE). Secondary outcome measures specified a priori included metrics of muscular performance, resting metabolic rate, blood markers, blood pressure, arterial stiffness, physical activity level, and questionnaire responses. Data collection occurred from January to August 2018 at Texas Tech University in Lubbock, Texas, USA. The procedures followed were in accordance with the ethical standards of the Texas Tech University Institutional Review Board.
Participants
Healthy female participants aged between 18 and 30 y were recruited via posters, e-mail announcements, and word of mouth. Participants were required to have prior RT experience, defined as reporting ≥1 y of RT at a frequency of 2 to 4 sessions per week and with weekly training of major upper- and lower-body muscle groups. Additionally, participants were screened for BF% using multifrequency bioelectrical impedance analysis (MFBIA; mBCA 514/515, Seca). The original target BF% range for participants was 15 to 29%; however, due to data from our lab indicating overestimations of body fat via MFBIA compared with a 4-component (4C) model in resistance-trained females (19), individuals with ≤33% body fat at screening were considered eligible. Individuals were excluded if they did not meet the aforementioned criteria or were pregnant, trying to become pregnant, currently breastfeeding, cigarette smokers, allergic to dairy protein, or had a pacemaker or other electrical implant. Eligible participants were stratified based on BF% at screening (15–21% or >21%) and habitual breakfast consumption (≥5 d/wk compared with <5 d/wk), and then randomly assigned to 1 of the 3 study groups [CD plus placebo (CD), TRF plus placebo (TRF), or TRF plus HMB (TRFHMB)] using sequences produced from a random sequence generator (http://www.random.org) and based on a 1:1:1 allocation ratio. Each participant within a given stratum was allocated in a sequential manner to the first available group assignment at the time of baseline [i.e., week 0 (W0)] testing using the random integer sequence for that stratum. The generation of random sequences and implementation of stratified randomization were performed by the primary investigator (GMT).
Nutrition and supplementation program
TRF and TRFHMB participants were instructed to consume all calories between 1200 h and 2000 h each day, and CD participants were instructed to consume breakfast as soon as possible after waking and to continue to eat at self-selected intervals throughout the remainder of the day. In addition to the assigned eating schedule, participants were provided with a minimal amount of dietary advice based on protein intake goals, the results of their weighed diet records, and the results of W0 metabolism testing. Specifically, participants were instructed to consume the provided whey protein supplement (Elite 100% Whey, Dymatize Enterprises, LLC) on both training and nontraining days in order to achieve a protein intake ≥1.4 g/kg/d. Supplemental whey protein was selected as a feasible method for increasing dietary protein intake to align with recommendations for lean mass accretion or retention in exercising individuals (9). The energy content of supplemental protein was ∼200–250 kcal/d. In all groups, target energy intake was prescribed by multiplying resting energy expenditure (REE), assessed via indirect calorimetry, by an activity factor of 1.5 and then subtracting 250 kcal. The goal of the small caloric reduction was to promote fat loss while still providing adequate nutritional support for muscular hypertrophy. Prior to commencement of the intervention, as well as during 2 separate weeks during the intervention, weighed diet records were completed on selected weekday and weekend days. Each participant was provided with a food scale and instructed how to properly weigh and record food items. The resultant dietary records were manually analyzed by reviewing nutrition fact labels and utilizing the USDA Food Composition Databases (https://ndb.nal.usda.gov/ndb/).
In a double-blind manner, TRF and TRFHMB participants received placebo (calcium lactate) or calcium HMB supplements, respectively. HMB and placebo capsules were produced by the same manufacturer (Metabolic Technologies, Inc.), were identical in appearance and taste, and were matched for calcium (102 mg), phosphorus (26 mg), and potassium (49 mg) content. TRF and TRFHMB participants were instructed to ingest 2 capsules on 3 occasions each day: upon waking, midmorning while still fasting, and prior to bed, for a total dose of 3 g/d. This dosing strategy (3 g/d, split into 3 doses) has been utilized in the majority of previous studies examining the effects of HMB on body composition and physical performance in active individuals (20). CD participants also received the placebo capsules for consumption at breakfast, lunch, and dinner using a unique supplement code to maintain blinding of researchers with respect to the supplements used in TRF and TRFHMB. All researchers were blinded to the supplement assignments of the TRF groups until after data collection and statistical analysis were completed, at which time the study sponsor provided supplement codes for unblinding. Additionally, trainers supervising the RT program were asked not to discuss group assignment with participants in order to maintain blinding with respect to the assigned dietary program. Participants were discouraged from consuming any additional sports supplements beyond those provided by study investigators, with the exception of common multivitamin/mineral supplements.
RT program and physical activity monitoring
All groups completed 8 wk of supervised RT in conjunction with the assigned dietary and supplementation programs. Training took place within the research laboratories under direct supervision. RT sessions were completed on 3 nonconsecutive days each week (i.e., Mondays, Wednesdays, and Fridays), and 2 different upper- and lower-body sessions were alternated (Table 1). Participants were instructed to train to momentary muscular exhaustion during each set, and the load was adjusted as necessary to ensure compliance with the specified repetition range. The weights and repetitions completed for each set of each exercise were recorded to allow calculation of RT volume. Sessions took place between 1200 and 1800 h. TRF and TRFHMB participants who performed RT sessions between 1200 and 1300 h were asked to shift their feeding window 1 h earlier (i.e., 1100 to 1900 h) on training days to ensure that RT did not take place in the fasted state. Following each RT session, participants from each group were provided with 25 g whey protein (Elite 100% Whey, Dymatize Enterprises, LLC).
TABLE 1.
Resistance training program1
| Upper body A2 | Lower body A | ||||
|---|---|---|---|---|---|
| Exercise | W0–W4 | W4–W8 | Exercise | W0–W4 | W4–W8 |
| Bentover DB rows | 4 × 8–12, 120 s | 5 × 6–8, 180 s | BB deadlift | 4 × 8–12, 120 s | 5 × 6–8, 180 s |
| DB bench press | 4 × 8–12, 120 s | Hip sled | 4 × 8–12, 120 s | 5 × 6–8, 180 s | |
| BB shoulder press | 4 × 8–12, 120 s | Lunges with DB | 4 × 8–12, 120 s | ||
| DB flyes | 4 × 8–12, 90 s | Leg curls | 4 × 8–12, 90 s | ||
| Preacher curls | 4 × 8–12, 90 s | Leg extensions | 4 × 8–12, 90 s | ||
| Triceps extension | 4 × 8–12, 90 s | ||||
| Upper body B | Lower body B | ||||
| Exercise | W0–W4 | W4–W8 | Exercise | W0–W4 | W4–W8 |
| BB bench press | 4 × 8–12, 120 s | 5 × 6–8, 180 s | BB back squat | 4 × 8–12, 120 s | 5 × 6–8, 180 s |
| Bentover DB rows | 4 × 8–12, 120 s | Stiff-leg deadlift | 4 × 8–12, 120 s | 5 × 6–8, 180 s | |
| DB shoulder press | 4 × 8–12, 120 s | Lunges with DB | 4 × 8–12, 120 s | ||
| DB curls | 4 × 8–12, 90 s | Leg curls | 4 × 8–12, 90 s | ||
| Skullcrushers | 4 × 8–12, 90 s | Leg extensions | 4 × 8–12, 90 s | ||
| Inverted rows (BW) | 4 × 8–12, 90 s | ||||
Exercise prescription shown as: sets × repetition range, rest interval. BB, barbell; BW, bodyweight; DB, dumbbell; W0, week 0; W4, week 4; W8, week 8.
Participants completed sessions in the following order: upper body A, lower body A, upper body B, lower body B. These 4 sessions were alternated in this order throughout the intervention, with adjustment of load as often as necessary to ensure momentary muscular exhaustion in the specified repetition range.
Participants were asked not to perform any RT outside of the study intervention and to avoid other high-intensity exercise. In order to objectively assess free-living physical activity levels during the course of intervention, each participant was provided with an accelerometer (ActiGraph GT9X Link; Actigraph Inc.) prior to the commencement of the study intervention, during the first half of the intervention [i.e., W0 to week 4 (W4)] and during the second half of the intervention [i.e., W4 to week 8 (W8)]. Participants were instructed to wear the devices during waking hours, whenever they were not bathing or sleeping, for ≥4 d. The accelerometer was set to record accelerations at a sampling rate of 30 Hz, and accelerations were converted into activity counts per 1-min epoch length during postdata processing. The activity counts data were screened to determine wear time for each monitoring day where nonwear time was defined as a period with ≥60 min of consecutive zero activity counts (i.e., no movement), with an allowance ≤2 min of interruption with activity counts <100 per minute (21). Physical activity energy expenditure (PAEE; kcal/min) was estimated for each minute of wear time using the Freedson's prediction equation (22) for activity counts >1951 counts/min and the Williams Work-Energy equation for activity counts ≤1951 counts/min (23). Daily PAEE was averaged across valid days of each participant where a valid day was defined as a day with ≥10 h of wear time. Lastly, although the estimated nonwear time was assumed to be nonwaking hours, average daily PAEE was adjusted by average wear time for each participant using a least-square adjustment method (24) due to the possibility of misclassification influencing daily PAEE.
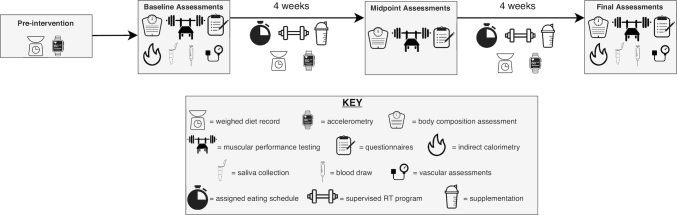
Overview of laboratory assessments
At W0, W4, and W8, participants completed 2 testing sessions: 1) a morning assessment conducted after an overnight fast to assess body composition, resting metabolic rate, substrate utilization, vascular measures, and subjective factors; and 2) an afternoon assessment of muscular performance, conducted in the nonfasted state (Figure 1). For morning assessments, participants reported to the laboratory after abstention from eating, drinking, exercising, and utilizing caffeine or nicotine for ≥8 h. Participants were interviewed to confirm adherence to these preassessment restrictions. The actual abstention from exercise was ≥14 h due to the scheduling of exercise sessions. Participants reported to the laboratory wearing athletic clothing, and all metal and accessories were removed from the body prior to testing. Each participant voided her bladder and provided a urine sample. Urine samples were assessed for urine specific gravity using a digital refractometer (PA201X-093, Misco) to ensure adequate hydration. Additionally, a standard urinary human chorionic gonadotropin test was performed to confirm that each participant was not pregnant. Finally, urine samples were frozen at −80°C for assessment of urinary HMB content after study unblinding. After voiding, each participant's body mass (BM) and height were determined via a digital scale with stadiometer (Seca 769). Blood draws were performed at Texas Tech University Student Health Services after an overnight fast, and participants completed at-home saliva collections for assessment of the cortisol awakening response (CAR).
FIGURE 1.
Study timeline and assessments. RT, resistance training.
Body composition assessment
Body composition was assessed using a modified 4C model (25, 26) produced from DXA and bioimpedance spectroscopy (BIS) data. DXA scans were performed on a Lunar Prodigy scanner (General Electric) with enCORE software (v. 16.2). The scanner was calibrated using a quality control block each morning prior to use, and positioning of participants was conducted according to the manufacturer's recommendations. Each participant was able to fit within the scanning dimensions. DXA bone mineral content (BMC) was divided by 0.9582 to yield an estimate of bone mineral (27). Additionally, body volume was estimated from DXA LST, FM, and BMC using the equation developed by Wilson et al. (25) for General Electric DXA scanners:
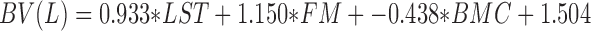 |
(1) |
BIS was utilized to obtain total body water estimates. BIS utilizes Cole modeling (28) and mixture theories (29) to predict body fluids rather than regression equations used by other impedance methods [e.g., bioelectrical impedance analysis (30)]. The BIS device used in the present study (SFB7, ImpediMed) employs 256 measurement frequencies ranging from 4 to 1000 kHz. Each participant remained supine for ≥5 min immediately prior to assessment using the manufacturer's recommended hand-to-foot electrode arrangement. Duplicate assessments were performed, with the values averaged for analysis. Assessments were reviewed for quality assurance through visual inspection of Cole plots.
The 4C equation of Wang et al. (31) was utilized for estimation of whole-body FM:
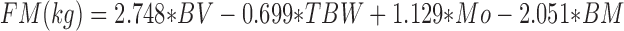 |
(2) |
FFM was calculated as BM − FM, and BF% was calculated as (FM/BM) × 100.
In addition to whole-body composition estimates, MTEF and MTKE were evaluated via ultrasonography (Logiq e, General Electric) at W0 and W8. Elbow flexor measurements took place at 66% of the distance from the acromion of the scapula to the cubital fossa, and knee extensor measurements took place at 50% of the distance from the anterior superior iliac spine to the superior border of the patella (32, 33). These distances were measured while the participant was standing, and measurement distances at W0 were recorded for use at W8. All assessments took place on the right side of the body. In the supine position, the participant's arm was abducted to ∼80° with the arm supported for elbow flexor measurements. For knee extensor measurements, a foam pad was placed beneath the knee to allow ∼10° bend at the knee joint. For all assessments, transmission gel was generously applied to the marked measurement location, and minimal pressure was applied by the transducer in order to avoid tissue compression. Three single transverse images were taken at each location, with values averaged for analysis. The gain and depth of the transducer were kept consistent for all measurements at a given site. Ultrasound images were blinded for analysis and analyzed by a single blinded researcher using ImageJ (v. 1.52a; NIH). The reliability of the researcher analyzing ultrasound images was determined through blinded analysis of 28 randomly selected ultrasound images on 2 occasions. This exercise produced minimum differences of 0.07 cm for MTEF and 0.14 cm for MTKE.
Muscular performance assessment
Assessments of muscular performance took place between 1200 and 1800 h in the nonfasted state, and participants were instructed to follow their preferred food and fluid intake patterns prior to testing. The assessment began with a 5-min warm-up period using a self-selected pace on a stationary bicycle. This warm-up period was followed by assessment of countermovement vertical jump (CMVJ) performance, testing on a mechanized squat device, and muscular strength and endurance assessment on the bench press and hip sled exercises. The CMVJ and hip sled assessments were not performed at the 4-wk assessment.
For the CMVJ tests, participants completed 8 trials while wearing their own footwear. Approximately 30 s rest separated each trial. Ground reaction force (GRF) data were obtained during the CMVJ using 2 force platforms sampled at 1 kHz (OPT464508; Advanced Mechanical Technology, Inc.). Participants stood motionless with each foot positioned on a force platform and their hands on their hips before initiating the CMVJ with a countermovement action using self-selected depth and jumping with maximum effort to achieve the highest vertical displacement possible. No instructions were provided for the landing phase except to land with each foot contacting its respective force platform from take-off and to stop downward motion and return to a motionless standing position. The raw GRF data from the 2 force platforms were smoothed using a fourth-order low-pass Butterworth digital filter with a 30 Hz cut-off frequency. The smoothed GRF from the 2 force platforms was then summed along the vertical axis to obtain the vertical GRF acting at the body's center of mass. The start of the CMVJ was defined as the time when bodyweight was reduced by 2.5% (34). Take-off was defined as the time when the summed vertical GRF decreased below a 20 N threshold (35). Jump time was then calculated as the time elapsed between the start of the CMVJ and take-off, expressed in units of seconds. Vertical jump height was calculated using the impulse-momentum relation and expressed in units of meters.
Isometric and isokinetic squats were performed using a mechanized squat device (Exerbotics eSq) (36, 37). At the first assessment, each participant's preferred foot positioning was determined using a custom grid overlaid on the foot platform of the squat device. This foot positioning was recorded and utilized for all visits. No weight belts, knee wraps, or other aids were utilized during testing. Prior to testing, the participant's range of motion for isokinetic testing was determined. The range of motion was set to 90° between the thigh and lower leg at the bottom of the repetition and ∼170° at the top of the repetition, as determined by a goniometer. The isometric testing included maximal effort pushes at 120° and 150° knee angles. Each participant was instructed to push against the device as hard and fast as possible while attempting to complete a squat movement. Two isometric pushes were performed at each knee angle, and each effort lasted ∼2 to 3 s. After the isometric testing, a 3-repetition maximum isokinetic force production test was completed. Prior to testing, participants observed the movement of the machine and received verbal instruction regarding proper performance of the assessment. Each of the repetitions during the maximal isokinetic force production test consisted of a 4-s eccentric phase, followed by an approximately half-second pause at the 90° knee position and a 4-s concentric phase. During testing, the force signal was sampled from the load cell at 1 kHz (MP100; Biopac Systems, Inc.), stored on a personal computer, and processed off-line using custom-written software (LabVIEW, Version 11.0; National Instruments). The scaled force signal was low-pass filtered, with a 10-Hz cut-off (zero-phase lag, fourth-order Butterworth filter). All subsequent analyses were conducted on the scaled and filtered force signal. For the isometric force production tests, the rate of force development (RFD) over specific time intervals (i.e., 30, 50, 100, and 200 ms) was calculated by manually specifying the onset of force production within the custom LabVIEW program. For each repetition of the maximum isokinetic force production test, isokinetic peak forces were determined as the highest mean 25-ms epoch for both concentric and eccentric testing (i.e., PFCON and PFECC).
Resistance exercise performance for the bench press and hip sled (leg press) exercises was evaluated via the 1-repetition maximum (1RM) and repetitions to failure (RTF) with 70% of the W0 1RM. The 1RM testing protocol was based on the recommendations of the National Strength and Conditioning Association (38). Briefly, after completing warm-up sets, participants completed 2 to 3 repetitions using a load estimated to be near maximal. 1RM attempts then commenced, with the goal of obtaining the 1RM in 3 to 5 attempts. Three minutes of rest were allowed between attempts. The maximal weight lifted with proper form was recorded as 1RM. After the 1RM was obtained, a 3-min rest period was allowed before RTF were completed using 70% of the W0 1RM. For all participants, the bench press was tested before the hip sled in order to allow recovery of the lower body following the mechanized squat testing.
Metabolic and physiological measures
REE and substrate utilization were assessed via indirect calorimetry (TrueOne 2400, ParvoMedics). Gas and flow calibrations were performed each morning according to the manufacturer's specifications, and the preassessment procedures of Compher et al. (39) were utilized. Participants were instructed to remain motionless but awake during the assessment, which took place in a climate-controlled room with the lights dimmed. The first 5 min of each test were discarded, and the assessment continued until there was a period of 5 consecutive minutes with a CV for REE of ≤5%. The average CV for REE in the present study was 3.2% ± 1.1% (mean ± SD).
Brachial blood pressure was measured using an automated cuff-based sphygmomanometer (HEM-907, Omron Healthcare). From this measurement, mean blood pressure and diastolic blood pressure were used to calibrate ensemble-averaged pressure waveforms measured at the left radial artery using applanation tonometry (SphygmoCor PVx, AtCor Medical). A general transfer function was also used to synthesize a central aortic waveform from the radial artery measurement. Wave separation analysis of the aortic pressure waveform allowed estimation of aortic pulse wave velocity, an index of arterial stiffness. Each participant remained supine for ≥10 min prior to vascular assessment. Duplicate measurements were obtained and averaged for analysis.
Blood samples, collected by certified health professionals, were transported via courier to a local clinical laboratory for analysis (University Medical Center Health System). Testing was performed using standard instrumentation (Cobas 6000, Roche Diagnostics) and following procedures outlined by the manufacturer. Total cholesterol, triglycerides, and HDL cholesterol were assessed using enzymatic colorimetric assays (40–42), and VLDL and non-HDL cholesterol were calculated (VLDL = triglycerides/5 (43); non-HDL = total cholesterol – HDL). LDL cholesterol was calculated using the Martin–Hopkins equation (44). Glucose was measured using an enzymatic UV test (45), and insulin was assessed via an electrochemiluminescence immunoassay (46). Results of the clinical laboratory analyses were provided to study investigators.
Each participant was familiarized with the saliva collection procedures at study commencement. Saliva collection took place using the passive drool method, with allows saliva to be transferred from the mouth to a small vial according to the manufacturer's recommendations (47). Three saliva samples were collected during both the preintervention period and the final week of the intervention for assessment of CAR [the characteristic increase in cortisol concentrations upon waking (48)]. These samples were collected at the participant's home 0, 30, and 45 min after waking. The importance of collecting the saliva sample exactly as instructed was strongly emphasized to research participants. Each participant was provided with a reminder sign to place by the bedside and instructed to set an alarm for saliva collection timepoints. Upon obtaining the sample, each participant was instructed to place the vial in the freezer until it could be transported to the laboratory. Upon delivery to the lab, each vial of saliva was stored at −80°C until shipment to a saliva testing facility for analysis (Salimetrics LLC). For the analysis, samples were thawed to room temperature, vortexed, and then centrifuged for 15 min at ∼1500 × g immediately before performing the assay. Samples were tested for salivary cortisol using a high-sensitivity enzyme immunoassay (Cat. No. 1–3002). The sample test volume was 25 μL of saliva per determination. The assay has a lower limit of sensitivity of 0.007 μg/dL, a standard curve range from 0.012–3.0 μg/dL, an average intra-assay CV of 4.60%, and an average interassay CV of 6.00%, which meets the manufacturer's criteria for accuracy and repeatability in Salivary Bioscience, and exceeds the applicable NIH guidelines for Enhancing Reproducibility through Rigor and Transparency.
Questionnaires
As part of the screening procedures, participants were interviewed using a lifestyle questionnaire to determine typical eating and exercise habits. Participants completed follow-up lifestyle questionnaires at subsequent research visits. Additionally, participants completed the Mood and Feelings Questionnaire (49), the Pittsburgh Sleep Quality Index (50), the Three-Factor Eating Questionnaire Revised 18-item version (51), and a menstrual cycle questionnaire at each morning laboratory assessment session.
Statistical analysis
An a priori power analysis was performed (G*Power, v. 3.1.9.2) using an effect size (ES) estimated from a previous investigation of TRF and RT (8). FM was specified as the primary dependent variable for the power analysis, and the ES used for the power analysis was the observed ES for FM reduction in TRF minus the ES for FM reduction in the control group. Using this ES (d = 0.46), a α error probability of 0.05, and power of 0.8, it was estimated that 15 participants were needed to detect significant changes in FM. When the power analysis was performed using an ES for muscular performance improvement from the same study (d = 0.25), the software estimated that 36 participants are needed to detect significant changes. Therefore, in order to promote adequate power for less sensitive measures and accounting for a 10% attrition rate, the target sample size was 40.
All data analysis occurred prior to the unblinding of study investigators and prior to receipt of urinary HMB concentrations. Data were analyzed in the intention-to-treat (ITT) framework using a model-based likelihood method, meaning that the intervention effects were estimated from all participants who were randomly assigned into the groups regardless of whether they complied with the intervention protocol (e.g., missing at follow-up assessments or drop-outs). Additional per protocol (PP) analyses were performed by excluding participants who dropped out of the study or failed to comply with the study protocol (defined as <80% compliance with the assigned eating schedule, completing fewer than 22/24 RT sessions, or <70% compliance with capsule supplements as determined by capsule counts). For both ITT and PP analyses, a linear mixed model with restricted maximum likelihood method was used to test changes in outcome variables over time across groups (i.e., CD, TRF, and TRFHMB). The model was established based on unstructured variance-covariance structure for the repeated measure and missing values were assumed to be missing at random. The normality of residuals assumption was tested using visual examination of Q-Q plots. If the group by time interaction effect was significant, simple effects tests were performed using one-factor or repeated measures ANOVA as appropriate, followed by Sidak's pairwise comparisons using a Bonferroni adjusted α level for each test. In the absence of statistically significant group by time interactions, main effects were examined with follow-up using Sidak's pairwise comparisons. Cohen's d ES were calculated for each group by dividing the difference between W0 and W8 values by the pooled SD. A familywise α level of <0.05 was used for statistical significance, and all data analyses were performed using IBM SPSS v. 25 and Microsoft Excel v. 16.16.3. Data in the text, tables, and figures of this article represent results of the ITT analysis unless otherwise specified. Results from the PP analysis, as well as supplemental data for both analyses, are included in the online supporting material.
Results
Participants
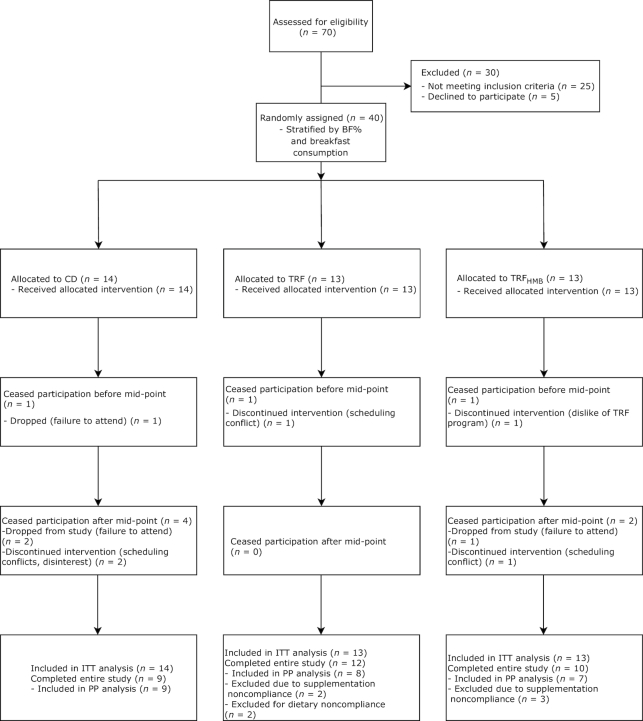
Forty participants were randomly assigned and included in the ITT analysis, and 24 participants were included in the PP analysis (Figure 2). No baseline differences were present between groups (Table 2; Supplemental Table 1). Although participants were not excluded for noncompliance in the ITT analysis, average group compliance with the assigned protocol was ≥89% for the assigned eating schedule and ≥84% for the assigned capsule supplementation based on capsule count (Supplemental Table 2). Urinary HMB concentrations increased significantly in TRFHMB from the preintervention period to the intervention, with no changes in CD or TRF (Supplemental Table 3).
FIGURE 2.
CONSORT flow chart. BF%, body fat percentage; CD, control diet; ITT, intention-to-treat; PP, per protocol; TRF, time-restricted feeding; TRFHMB, time-restricted feeding plus β-hydroxy β-methylbutyrate supplementation.
TABLE 2.
Participant characteristics1
| CD (n = 14) | TRF (n = 13) | TRFHMB (n = 13) | P (group) | |
|---|---|---|---|---|
| Age, y | 22.0 ± 2.4 | 22.1 ± 2.1 | 22.3 ± 3.4 | 0.95 |
| Body mass, kg | 64.6 ± 8.8 | 63.8 ± 8.5 | 63.2 ± 6.1 | 0.89 |
| Height, cm | 169.4 ± 7.5 | 163.6 ± 5.9 | 166.0 ± 4.8 | 0.07 |
| RT experience, y | 5.4 ± 3.0 | 5.0 ± 1.9 | 5.1 ± 2.1 | 0.85 |
| Current RT, d/wk | 2.9 ± 0.5 | 3.3 ± 0.6 | 3.0 ± 0.9 | 0.22 |
1Data are presented as mean ± SE. P values are from one-factor ANOVA. Data from intention-to-treat analysis are displayed, and data from per protocol analysis are displayed in the online supporting material. CD, control diet; RT, resistance training; TRF, time-restricted feeding; TRFHMB, time-restricted feeding plus β-hydroxy β-methylbutyrate supplementation.
Nutrition and supplementation
Prior to the intervention, there were no differences in the time of the first or last eating occasion of the day, nor the total duration of the feeding window (Supplemental Table 4). During the intervention, the time of the first eating occasion was later in TRF and TRFHMB compared with CD, whereas the time of the last eating occasion was later in CD. These differences resulted in a significantly longer feeding window for CD (13.2 ± 1.6 h/d) compared with TRF (7.5 ± 0.6 h/d) and TRFHMB (7.6 ± 0.7 h/d). Within the feeding windows, the meal frequency did not differ between groups before or during the intervention.
Analysis of weighed diet records prior to intervention commencement indicated that all groups reported an average energy intake that was comparable to W0 REE. During the intervention, reported energy intake increased in all groups (mean change from 23 to 194 kcal/d depending upon the group), with no differences between groups (Table 3;Supplemental Table 5). The magnitude of increase in energy intake approximated the average daily calories consumed from the provided whey protein supplements (∼200–250 kcal/d for all participants). Despite this increase in energy intake, reported caloric consumption remained only slightly greater than W0 and W8 REE and was below the original energy intake goal of REE × 1.5 – 250 kcal/d. Protein consumption in all groups increased from habitual intakes of 1.1 to 1.2 g/kg/d, as estimated during the preintervention period, to an intake of 1.6 g/kg/d in all groups during the intervention. Carbohydrate and fat intake generally did not change during the intervention.
TABLE 3.
Nutrient intake1
| Group2 | Preintervention3 | During intervention | Δ | Δ (%) | P (group) | P (time) | P (I) | |
|---|---|---|---|---|---|---|---|---|
| Energy, kcal | CD | 1384 ± 117 | 1570 ± 111 | 186 | 13 | 0.91 | 0.01 | 0.62 |
| TRF | 1430 ± 121 | 1624 ± 107 | 194 | 14 | ||||
| TRFHMB | 1466 ± 111 | 1489 ± 112 | 23 | 2 | ||||
| Protein | ||||||||
| g | CD | 67 ± 7 | 98 ± 7 | 31 | 46 | 0.49 | <0.0001 | 0.87 |
| TRF | 79 ± 8 | 105 ± 6 | 26 | 33 | ||||
| TRFHMB | 77 ± 8 | 102 ± 7 | 25 | 32 | ||||
| % | CD | 20 ± 2 | 27 ± 2 | 7 | 35 | 0.67 | <0.0001 | 0.48 |
| TRF | 23 ± 2 | 27 ± 2 | 4 | 17 | ||||
| TRFHMB | 21 ± 2 | 28 ± 2 | 7 | 33 | ||||
| g/kg | CD | 1.1 ± 0.1 | 1.6 ± 0.1 | 0.5 | 45 | 0.58 | <0.0001 | 0.82 |
| TRF | 1.2 ± 0.1 | 1.6 ± 0.1 | 0.4 | 33 | ||||
| TRFHMB | 1.2 ± 0.1 | 1.6 ± 0.1 | 0.4 | 33 | ||||
| Carbohydrate | ||||||||
| g | CD | 158 ± 15 | 165 ± 17 | 7 | 4 | 0.58 | 0.90 | 0.83 |
| TRF | 167 ± 16 | 157 ± 16 | −10 | −6 | ||||
| TRFHMB | 146 ± 16 | 145 ± 17 | −1 | −1 | ||||
| % | CD | 47 ± 3 | 42 ± 2 | −5 | −11 | 0.24 | 0.045 | 0.58 |
| TRF | 45 ± 3 | 39 ± 2 | −6 | −13 | ||||
| TRFHMB | 40 ± 3 | 39 ± 2 | −1 | −3 | ||||
| g/kg | CD | 2.5 ± 0.3 | 2.6 ± 0.3 | 0.1 | 4 | 0.59 | 0.82 | 0.81 |
| TRF | 2.7 ± 0.3 | 2.5 ± 0.3 | −0.2 | −7 | ||||
| TRFHMB | 2.3 ± 0.3 | 2.3 ± 0.3 | 0.0 | 0 | ||||
| Fat | ||||||||
| g | CD | 51 ± 10 | 54 ± 6 | 3 | 6 | 0.46 | 0.74 | 0.12 |
| TRF | 52 ± 11 | 64 ± 5 | 12 | 23 | ||||
| TRFHMB | 75 ± 11 | 53 ± 6 | −22 | −29 | ||||
| % | CD | 34 ± 3 | 32 ± 2 | −2 | −6 | 0.34 | 0.24 | 0.20 |
| TRF | 32 ± 3 | 34 ± 2 | 2 | 6 | ||||
| TRFHMB | 39 ± 3 | 32 ± 3 | −7 | −18 | ||||
| g/kg | CD | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.1 | 13 | 0.46 | 0.80 | 0.11 |
| TRF | 0.8 ± 0.2 | 1.0 ± 0.1 | 0.2 | 25 | ||||
| TRFHMB | 1.2 ± 0.2 | 0.8 ± 0.1 | −0.4 | −33 |
1Data are presented as mean ± SE. P values are from mixed model analysis. Data from intention-to-treat analysis are displayed, and data from per protocol analysis are displayed in the online supporting material. CD, control diet; I, group by time interaction; TRF, time-restricted feeding; TRFHMB, time-restricted feeding plus β-hydroxy β-methylbutyrate supplementation.
n = 40 (CD: n = 14; TRF: n = 13; TRFHMB: n = 13).
No preintervention differences between groups were present.
RT program and physical activity monitoring
There were no differences between groups for upper- or lower-body session volume or total RT volume (Supplemental Table 6). In all groups, volume increased from the first half of the intervention (i.e., W0 to W4) to the second half of the intervention (i.e., W4 to W8), with the magnitude of increase in group session volume ranging from 17% to 27%. During the intervention, group step counts ranged from 7166 to 9179 steps/d, with no significant differences between groups or across time (Supplemental Table 7). Group by time interactions were present for PAEE, sedentary time, and light intensity PA. Follow-up analysis indicated that differences between groups were present in the preintervention period for sedentary time and light intensity PA, but not during the first or second half of the intervention. Furthermore, no statistically significant differences between time points within a group were observed, with the exception of higher sedentary time observed in the TRF group during the first half of the intervention compared with preintervention.
Body composition
FFM, MTEF, and MTKE increased in all groups without differences between groups (Supplemental Table 8). In the ITT analysis, time main effects indicated decreases in FM and BF% for all groups combined (Figure 3). In the PP analysis, significant group by time interactions indicated reductions in FM for TRF and TRFHMB relative to CD at specific time points (Supplemental Figure 1). Although FM was significantly lower at W4 than W0 in TRF, FM at W8 did not significantly differ from W0. In contrast, FM and BF% in TRFHMB were lower at W8 than W0 in the PP analysis (Supplemental Table 8).
FIGURE 3.
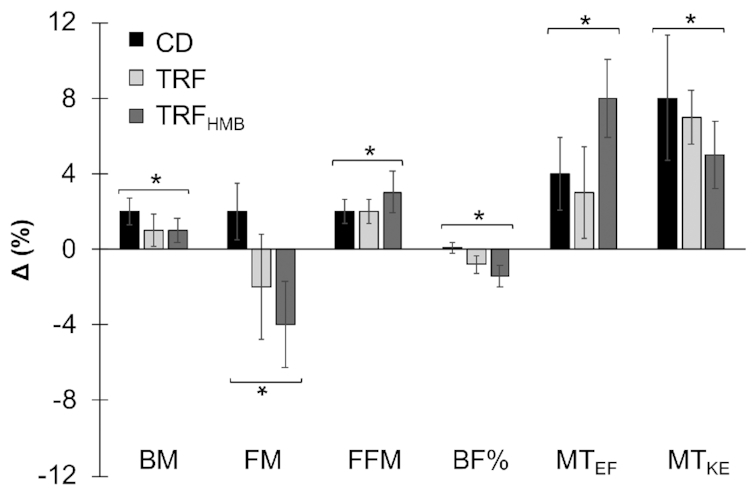
Body composition changes. Results from intention-to-treat analysis are displayed [n = 40 (CD: n = 14; TRF: n = 13; TRFHMB: n = 13)], and data from per protocol analysis are displayed in the online supporting material. Percent changes (mean ± SE) displayed as differences between W0 and W8 values relative to W0 values for each variable. Total body composition was estimated using a 4-component model, and muscle thickness was assessed via ultrasonography. Asterisks with brackets indicate significant changes in all groups (i.e., time main effects), with nonsignificant differences between groups, based on mixed model analysis and follow-up tests. BF%, body fat percentage; BM, body mass; CD, control diet; FM, fat mass; FFM, fat-free mass; MTEF, ultrasound muscle thickness of elbow flexors; MTKE, ultrasound muscle thickness of knee extensors; TRF, time-restricted feeding; TRFHMB, time-restricted feeding plus β-hydroxy β-methylbutyrate supplementation.
Muscular performance
Maximal strength and muscular endurance improved in all groups without statistically significant differences between groups (Figure 4; Supplemental Figure 2; Supplemental Table 9). In both the ITT and PP analyses, several RFD variables were improved in all groups, with more consistent improvements observed in the ITT analysis (Supplemental Table 10). A trend (P = 0.06) for a time main effect for increased jump height was also observed in the ITT analysis, although the ES in CD (d = 0.63) and TRFHMB (d = 0.65) appeared larger than TRF (d = 0.00), and no significant effects were present in the PP analysis (Supplemental Table 11).
FIGURE 4.
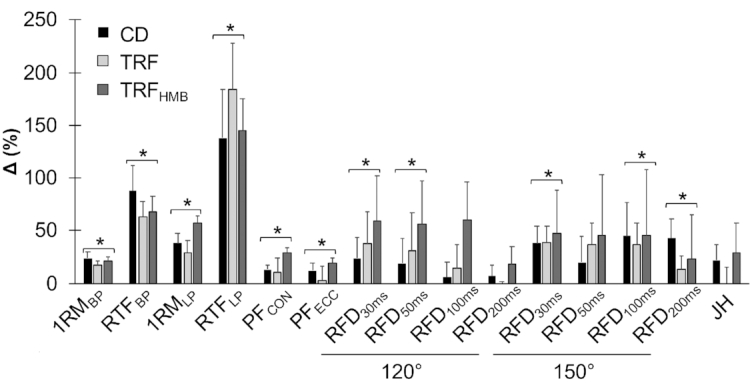
Muscular performance changes. Results from intention-to-treat analysis are displayed [n = 40 (CD: n = 14; TRF: n = 13; TRFHMB: n = 13)], and data from per protocol analysis are displayed in the online supporting material. Percent changes (mean + SE) displayed as differences between W0 and W8 values relative to W0 values for each variable. Asterisks with brackets indicate significant changes in all groups (i.e., time main effects), with nonsignificant differences between groups, based on mixed model analysis and follow-up tests. Muscular strength and endurance were evaluated on the hip sled (leg press) and bench press exercises, peak forces were obtained from isokinetic squat testing, rate of force development was obtained from isometric squat testing, and jump height was calculated using force platforms. 120°, 120° knee angle for isometric squat testing; 150°, 150° knee angle for isometric squat testing; 1RMBP, 1-repetition maximum on bench press; 1RMLP, 1-repetition maximum on leg press; CD, control diet; JH, jump height; PFCON, peak concentric force on mechanized squat; PFECC, peak eccentric force on mechanized squat; RFD, rate of force development (durations over which RFD values were calculated are shown in subscripts); RTFBP, repetitions to failure on bench press; RTFLP, repetitions to failure on leg press; TRF, time-restricted feeding; TRFHMB, time-restricted feeding plus β-hydroxy β-methylbutyrate supplementation.
Metabolic and physiological variables
No significant changes in REE or RQ were observed in any group (Supplemental Table 12). Blood markers were generally unchanged by the study intervention (Supplemental Table 13). No significant changes in vascular assessments, CAR, or average cortisol concentrations were observed (Supplemental Tables 14 and 15).
Questionnaires
Overall, no major side effects or adverse events occurred during the study. At W4, 84% of participants reported no side effects. Reported side effects included both suppressed appetite (n = 1) and increased appetite with associated irritability (n = 1) in TRF, morning fatigue in TRFHMB (n = 1), nausea in CD (n = 1), and bloated stomach in CD and TRFHMB (n = 1 each). At W8, 90% of participants reported no side effects. Reported side effects included suppressed appetite (n = 1) in TRF and bloated stomach in both TRF and TRFHMB (n = 1 each). With one exception, the side effects reported at W8 were the same as reported at W4 (i.e., the same participants and same specific side effects).
No differences between groups were observed for questionnaire responses. A time main effect indicated improvement in scores for the Mood and Feelings Questionnaire at W4 and W8 compared with W0 in all groups (Supplemental Table 16). The uncontrolled eating score of the Three Factor Eating Questionnaire was reduced across time in all groups. The proportion of participants with regularly occurring menstrual cycles in each group ranged from 69 to 79% (Supplemental Table 17).
Discussion
To our knowledge, the present investigation is the first controlled trial of IF plus RT in female participants. The purpose of the trial was to compare the effects of TRF, with or without HMB supplementation during fasting periods, to a CD requiring breakfast consumption during progressive RT. The primary finding was that following a TRF program in which all calories were consumed within ∼7.5 h/d did not impair gains in FFM, skeletal muscle hypertrophy, or muscular performance compared with a CD with feeding spread over ∼13 h/d when all diets were similar in energy and protein intake.
An increasing amount of research has reported the physiological effects of IF in general (1, 3–5, 52) and TRF in particular (53–56), although a very limited number of controlled trials have taken place in active individuals (6–8). Bhutani et al. (6) found that 12 wk of ADF plus endurance exercise produced greater improvements in body composition and blood lipids than ADF or exercise alone in lightly active obese adults. Tinsley et al. (7) employed an 8-wk TRF program with a 4-h feeding period for 4 d per week and ad libitum feeding plus RT for 3 d per week in young recreationally active males. Although not statistically significant, there was an apparent attenuation in LST gains with TRF (−0.2 kg; d = −0.02) compared with the unrestricted CD (+2.3 kg; d = 0.25). However, protein intake in the TRF group was not only lower than the CD (1.0 compared with 1.4 g/kg/d), but also suboptimal for active individuals (9, 10). Despite discrepancies in protein intake and LST accretion, comparable improvements in muscular strength and endurance were observed in both groups. Finally, Moro et al. (8) examined 8 wk of TRF with an 8-h feeding period each day in conjunction with RT in resistance-trained males. Both TRF and control groups employed an identical meal frequency and were counseled to maintain habitual energy and macronutrient intake throughout the intervention, including 1.9 g/kg/d of protein. Although both groups maintained FFM and experienced similar changes in muscular performance, the TRF program produced significant reductions in FM and differential responses in inflammatory markers and circulating hormone concentrations. Although these and other data have demonstrated effects of IF on a variety of physiological markers (1, 3–5, 52, 53), these variables were generally unchanged in the present trial. This finding may be partially attributable to the healthy, active population and lack of energy restriction relative to habitual intake.
In the present investigation, adherence to TRF did not attenuate FFM accretion, skeletal muscle hypertrophy, or improvements in muscular performance. Differential effects on FM (CD: +2% relative to baseline; TRF: −2% to −4%; TRFHMB: −4% to −7%) were only observed in the PP analysis, with significant reductions in FM and BF% in TRFHMB at W8. It has been recognized that longitudinal data are needed to elucidate the impact of the daily distribution of protein intake on adaptations to RT (9). As IF necessitates prolonged periods without stimulation of muscle protein synthesis and suppression of muscle protein breakdown via dietary amino acids (13), it represents an opportunity to investigate this question. The present results reveal no detrimental effects on RT adaptations of limiting all protein and other nutrient intake to ∼7.5 h/d compared with ∼13 h/d. In the context of IF, it has also been questioned whether implementation of modified fasting periods to allow ingestion of selected amino acids or their metabolites may be beneficial for lean mass maintenance or accretion, particularly in active individuals (14). The branched-chain amino acid leucine is known to stimulate protein synthesis through the mechanistic target of rapamycin (mTOR) signaling pathway and produces metabolites that may modulate its physiological effects (14, 57, 58). Due to its ability to stimulate muscle protein synthesis and attenuate muscle proteolysis (15), it has been hypothesized that the leucine metabolite HMB may exert favorable effects on body composition and performance in exercising and/or energy-restricted individuals (16–18). However, previous investigations of supplemental HMB in active populations have produced mixed results, and a recent meta-analysis in athletes revealed no definitive advantages for body composition or muscular performance (20). The present investigation may be the first trial to directly examine the influence of supplementation with a leucine metabolite during IF and does not reveal distinct benefits of HMB supplementation.
It is worth emphasizing that the dietary advice provided in the present investigation was minimal. Specifically, each participant met briefly (<10 min) to discuss the assigned eating schedule and protein consumption target with the primary investigator at the time of group assignment. Two additional follow-up visits of similar duration allowed discussion of the results of weighed diet records. As estimated energy intake was typically below the target intake, the primary dietary feedback was to achieve a high protein intake through consumption of the provided whey protein supplement and protein-containing foods. In all groups, average protein intake increased from 1.1 to 1.2 g/kg/d in the preintervention period to 1.6 g/kg/d during the study intervention, a range consistent with optimal intake for muscular adaptations (9, 10). Although weighed diet records revealed no significant differences between groups for energy or macronutrient intake, the shortcomings of self-reported dietary intake are well-established, and resultant nutrient intake estimates should be viewed with substantial caution (59, 60). Due to the relative likelihood of behavioral modification or underreporting on diet tracking days, it is possible that both the energy and macronutrient content of the diet actually consumed by participants in all groups was higher than the estimates obtained from weighed diet records.
Strengths of the present study include rigorous body composition assessment methodology, including preassessment and procedural standardization and implementation of a 4C model compared with previous investigations utilizing DXA (7, 8). Additional strengths were the fully supervised RT program, double blinding of data collection and analysis with respect to HMB and placebo supplements, and the wide range of performance and physiological variables examined. Limitations include reliance on a limited number of weighed dietary records, an inability to objectively confirm adherence to TRF, inclusion of female participants only, and a lack of mechanistic examinations of adaptations to TRF and HMB supplementation.
In summary, similar gains in FFM, skeletal muscle hypertrophy, and muscular performance can be achieved with dramatically different feeding schedules provided that energy and protein intake are similar during a progressive RT program. In a healthy, active female population, TRF did not produce changes in physiological variables including resting metabolic rate, substrate utilization, blood lipids, glucose and insulin, blood pressure, arterial stiffness, or cortisol responses. Although a possible benefit of HMB for fat loss was observed in the PP analysis, supplementation did not definitively improve outcomes during TRF. Due to their potential to favorably influence body composition without compromising physical performance, additional examination of various IF protocols in both sedentary and active populations is warranted.
Supplementary Material
Acknowledgments
We acknowledge the following individuals for their efforts in assisting with this project: Danielle Hardin, Michael Villarreal, Danielle Salinsky, Jonah Sell, Kaitlin Hicks, Alfred Kankam, Ty Palmer, and Aleese Handley.
The authors’ contributions were as follows—GMT: designed research; GMT, MLM, AJG, YK, JUG, JRH, DNK, and MRC: conducted research; GMT, YK, JUG, and JRH: provided essential materials; AP and TAV: provided additional intellectual contributions to study; GMT and YK: performed statistical analysis; GMT: wrote the manuscript; GMT: had primary responsibility for final content; and all authors: read and approved the manuscript. All authors declare no relevant conflicts of interest.
Notes
Supported by MTI Biotech Inc. and Texas Tech University. In-kind donations were received from MTI Biotech Inc. (HMB and placebo capsule supplements) and Dymatize Enterprises (whey protein supplements). These entities did not play a role in the overall design or execution of the study, the analysis and interpretation of the data, or the presentation of the results found in this article.
Data described in this article may be made available upon request pending application and approval by study investigators and Texas Tech University.
Supplemental Tables 1–17 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ADF, alternate-day fasting; BF%, body fat percentage; BIS, bioimpedance spectroscopy; BM, body mass; BMC, bone mineral content; CAR, cortisol awakening response; CD, control diet; CMVJ, countermovement vertical jump; ES, effect size; FFM, fat-free mass; FM, fat mass; GRF, ground reaction force; HMB, β-hydroxy β-methylbutyrate; IF, intermittent fasting; ITT, intention-to-treat; LST, lean soft tissue; MFBIA, multifrequency bioelectrical impedance analysis; MTEF, muscle thickness of the elbow flexor muscles; MTKE, muscle thickness of the knee extensor muscles; PAEE, physical activity energy expenditure; PP, per protocol; REE, resting energy expenditure; RFD, rate of force development; RT, resistance training; RTF, repetitions to failure; TRF, time-restricted feeding; TRFHMB, time-restricted feeding plus HMB supplementation; W0, week 0 (baseline assessment); W4, week 4 (midpoint assessment); W8, week 8 (final assessment); 1RM, 1-repetition maximum; 4C, 4-component.
References
- 1. Tinsley G, Bounty P. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015;73(10):661–74. [DOI] [PubMed] [Google Scholar]
- 2. Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG 3rd, Leeuwenburgh C, Mattson MP. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring). 2018;26(2):254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris L, Hamilton S, Azevedo LB, Olajide J, De Brun C, Waller G, Whittaker V, Sharp T, Lean M, Hankey C et al.. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2018;16(2):507–47. [DOI] [PubMed] [Google Scholar]
- 4. Seimon RV, Roekenes JA, Zibellini J, Zhu B, Gibson AA, Hills AP, Wood RE, King NA, Byrne NM, Sainsbury A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol. 2015;418:Pt 2:153–72. [DOI] [PubMed] [Google Scholar]
- 5. Davis CS, Clarke RE, Coulter SN, Rounsefell KN, Walker RE, Rauch CE, Huggins CE, Ryan L. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292–9. [DOI] [PubMed] [Google Scholar]
- 6. Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring, Md). 2013;21:1370–9. [DOI] [PubMed] [Google Scholar]
- 7. Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017;17(2):200–7. [DOI] [PubMed] [Google Scholar]
- 8. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jäger R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, Purpura M, Ziegenfuss TN, Ferrando AA, Arent SM et al.. International Society of Sports Nutrition Position Stand: protein and exercise. J Int Soc Sport Nutr. 2017;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillips SM. Dietary protein requirements and adaptive advantages in athletes. Br J Nutr. 2012;108:Suppl 2:S158–67. [DOI] [PubMed] [Google Scholar]
- 11. Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res. 2005;13:574–81. [DOI] [PubMed] [Google Scholar]
- 12. Heilbronn L, Smith S, Martin C, Anton S, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81(1):69–73. [DOI] [PubMed] [Google Scholar]
- 13. McGlory C, Vliet S, Stokes T, Mittendorfer B, Phillips SM. The impact of exercise and nutrition on the regulation of skeletal muscle mass. J Physiol. 2019;597(5):1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tinsley GM, Givan AH, Graybeal AJ, Villarreal MI, Cross AG. β-Hydroxy β-methylbutyrate free acid alters cortisol responses, but not myofibrillar proteolysis, during a 24-h fast. Br J Nutr. 2018;119(5):517–26. [DOI] [PubMed] [Google Scholar]
- 15. Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM et al.. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol. 2013;591(11):2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR et al.. International Society of Sports Nutrition Position Stand: β-hydroxy-β-methylbutyrate (HMB). J Int Soc Sport Nutr. 2013;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasselgren PO. β-Hydroxy-β-methylbutyrate (HMB) and prevention of muscle wasting. Metabolism. 2014;63(1):5–8. [DOI] [PubMed] [Google Scholar]
- 18. Silva VR, Belozo FL, Micheletti TO, Conrado M, Stout JR, Pimentel GD, Gonzalez AM. β-Hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: a systematic review. Nutr Res. 2017;45:1–9. [DOI] [PubMed] [Google Scholar]
- 19. Graybeal AJ, Moore ML, Cruz MR, Tinsley GM. Body composition assessment in male and female bodybuilders: a 4-compartment model comparison of dual-energy X-ray absorptiometry and impedance-based devices. J Strength Cond Res. 2018;Epub ahead of print. doi:10.1519/jsc.0000000000002831. [DOI] [PubMed] [Google Scholar]
- 20. Sanchez-Martinez J, Santos-Lozano A, Garcia-Hermoso A, Sadarangani KP, Cristi-Montero C. Effects of β-hydroxy-β-methylbutyrate supplementation on strength and body composition in trained and competitive athletes: a meta-analysis of randomized controlled trials. J Sci Med Sport. 2018;21(7):727–35. [DOI] [PubMed] [Google Scholar]
- 21. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 22. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81. [DOI] [PubMed] [Google Scholar]
- 23. Williams R. Kcal Estimates from Activity Counts using the Potential Energy Method. CSA, Inc; 1998;ActiGraph 49. [Google Scholar]
- 24. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S.; discussion 9S-31S. [DOI] [PubMed] [Google Scholar]
- 25. Wilson JP, Strauss BJ, Fan B, Duewer FW, Shepherd JA. Improved 4-compartment body-composition model for a clinically accessible measure of total body protein. Am J Clin Nutr. 2013;97:497–504. [DOI] [PubMed] [Google Scholar]
- 26. Ng BK, Liu YE, Wang W, Kelly TL, Wilson KE, Schoeller DA, Heymsfield SB, Shepherd JA. Validation of rapid 4-component body composition assessment with the use of dual-energy X-ray absorptiometry and bioelectrical impedance analysis. Am J Clin Nutr. 2018;108(4):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang ZM, Deurenberg P, Guo SS, Pietrobelli A, Wang J, Pierson RN Jr, Heymsfield SB. Six-compartment body composition model: inter-method comparisons of total body fat measurement. Int J Obes Relat Metab Disord. 1998;22:329. [DOI] [PubMed] [Google Scholar]
- 28. Cole KS. Permeability and impermeability of cell membranes for ions. Cold Spring Harb Symp Quant Biol. 1940;8:110–22. [Google Scholar]
- 29. Hanai T. Electrical Properties of Emulsions, Emulsion Science. London; New York: Academic Press; 1968. [Google Scholar]
- 30. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior J-C, Pirlich M et al.. Bioelectrical impedance analysis–part I: review of principles and methods. Clin Nutr. 2004;23:1226–43. [DOI] [PubMed] [Google Scholar]
- 31. Wang ZM, Xavier P-S, Kotler DP, Wielopolski L, Withers RT, Pierson J, Heymsfield SB. Multicomponent methods: evaluation of new and traditional soft tissue mineral models by in vivo neutron activation analysis. Am J Clin Nutr. 2002;76:968–74. [DOI] [PubMed] [Google Scholar]
- 32. Jenkins ND, Miller JM, Buckner SL, Cochrane KC, Bergstrom HC, Hill EC, Smith CM, Housh TJ, Cramer JT. Test-retest reliability of single transverse versus panoramic ultrasound imaging for muscle size and echo intensity of the biceps brachii. Ultrasound Med Biol. 2015;41(6):1584–91. [DOI] [PubMed] [Google Scholar]
- 33. Bemben MG. Use of diagnostic ultrasound for assessing muscle size. J Strength Cond Res. 2002;16:103–8. [PubMed] [Google Scholar]
- 34. Meylan CM, Nosaka K, Green J, Cronin JB. Temporal and kinetic analysis of unilateral jumping in the vertical, horizontal, and lateral directions. J Sports Sci. 2010;28(5):545–54. [DOI] [PubMed] [Google Scholar]
- 35. Harry JR, Barker LA, James R, Dufek JS. Performance differences among skilled soccer players of different playing positions during vertical jumping and landing. J Strength Cond Res. 2018;32(2):304–12. [DOI] [PubMed] [Google Scholar]
- 36. Stock MS, Luera MJ. Consistency of peak and mean concentric and eccentric force using a novel squat testing device. J Appl Biomech. 2014;30:322–5. [DOI] [PubMed] [Google Scholar]
- 37. Palmer TB, Pineda JG, Durham RM. Effects of knee position on the reliability and production of maximal and rapid strength characteristics during an isometric squat test. J Appl Biomech. 2018;34(2):111–7. [DOI] [PubMed] [Google Scholar]
- 38. NSCA. Essentials of Strength Training and Conditioning. 4th ed Champaign, IL: Human Kinetics; 2016. [Google Scholar]
- 39. Compher C, Frankenfield D, Keim N, Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881–903. [DOI] [PubMed] [Google Scholar]
- 40. Roche Diagnostics GmbH. Triglycerides (TRIGL: 0005171407190c701V8.0). 2019. [Google Scholar]
- 41. Roche Diagnostics GmbH. Cholesterol Gen.2 (CHOL2: 0005168538190c701V9.0) 2019. [Google Scholar]
- 42. Roche Diagnostics GmbH. HDL (HDLC4: 0207528604190_USAV1.0). 2018. [Google Scholar]
- 43. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 44. Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310(19):2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roche Diagnostics GmbH. Glucose (GLUC3: 0205168791190c701V5.0) 2016. [Google Scholar]
- 46. Roche Diagnostics GmbH. Insulin (Elecsys Insulin: 07027559501V1.0) 2018. [Google Scholar]
- 47. Salimetrics LLC. Saliva Collection Handbook. 2015. [Google Scholar]
- 48. Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wust S, Dockray S, Smyth N, Evans P, Hellhammer DH et al.. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–32. [DOI] [PubMed] [Google Scholar]
- 49. Angold A, Costello EJ, Messer SC, Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res. 1995;5(4):237–49. [Google Scholar]
- 50. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 51. Lauzon BD, Romon M, Deschamps V, Lafay L, Borys J-M, Karlsson J, Ducimetière P, Charles MA, Group FLVSFS. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134:2372–80. [DOI] [PubMed] [Google Scholar]
- 52. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72(5):308–18. [DOI] [PubMed] [Google Scholar]
- 54. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK et al.. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metabolism. 2015;22(5):789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rittig N, Bach E, Thomsen HH, Moller AB, Hansen J, Johannsen M, Jensen E, Serena A, Jorgensen JO, Richelsen B et al.. Anabolic effects of leucine-rich whey protein, carbohydrate, and soy protein with and without beta-hydroxy-beta-methylbutyrate (HMB) during fasting-induced catabolism: a human randomized crossover trial. Clin Nutr. 2017;36(3):697–705. [DOI] [PubMed] [Google Scholar]
- 58. Duan Y, Li F, Li Y, Tang Y, Kong X, Feng Z, Anthony TG, Watford M, Hou Y, Wu G et al.. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids. 2016;48(1):41–51. [DOI] [PubMed] [Google Scholar]
- 59. Livingstone MB, Prentice AM, Strain JJ, Coward WA, Black AE, Barker ME, McKenna PG, Whitehead RG. Accuracy of weighed dietary records in studies of diet and health. BMJ (Clinical Research Ed). 1990;300(6726):708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sawaya AL, Tucker K, Tsay R, Willett W, Saltzman E, Dallal GE, Roberts SB. Evaluation of four methods for determining energy intake in young and older women: comparison with doubly labeled water measurements of total energy expenditure. Am J Clin Nutr. 1996;63(4):491–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.