Abstract
The present study aimed at investigating the impact of heat challenges on gut microbiota composition in growing pigs and its relationship with pigs’ performance and thermoregulation responses. From a total of 10 F1 sire families, 558 and 564 backcross Large White × Créole pigs were raised and phenotyped from 11 to 23 wk of age in temperate (TEMP) and in tropical (TROP) climates, respectively. In TEMP, all pigs were subjected to an acute heat challenge (3 wk at 29 °C) from 23 to 26 wk of age. Feces samples were collected at 23 wk of age both in TEMP and TROP climate (TEMP23 and TROP23 samples, respectively) and at 26 wk of age in TEMP climate (TEMP26 samples) for 16S rRNA analyses of fecal microbiota composition. The fecal microbiota composition significantly differed between the 3 environments. Using a generalized linear model on microbiota composition, 182 operational taxonomic units (OTU) and 2 pathways were differentially abundant between TEMP23 and TEMP26, and 1,296 OTU and 20 pathways between TEMP23 and TROP23. Using fecal samples collected at 23 wk of age, pigs raised under the 2 climates were discriminated with 36 OTU using a sparse partial least square discriminant analysis that had a mean classification error-rate of 1.7%. In contrast, pigs in TEMP before the acute heat challenge could be discriminated from the pigs in TEMP after the heat challenge with 32 OTU and 9.3% error rate. The microbiota can be used as biomarker of heat stress exposition. Microbiota composition revealed that pigs were separated into 2 enterotypes. The enterotypes were represented in both climates. Whatever the climate, animals belonging to the Turicibacter–Sarcina–Clostridium sensu stricto dominated enterotype were 3.3 kg heavier (P < 0.05) at 11 wk of age than those belonging to the Lactobacillus-dominated enterotype. This latter enterotype was related to a 0.3 °C lower skin temperature (P < 0.05) at 23 wk of age. Following the acute heat challenge in TEMP, this enterotype had a less-stable rectal temperature (0.34 vs. 0.25 °C variation between weeks 23 and 24, P < 0.05) without affecting growth performance (P > 0.05). Instability of the enterotypes was observed in 34% of the pigs, switching from an enterotype to another between 23 and 26 wk of age after heat stress. Despite a lower microbial diversity, the Turicibacter–Sarcina–Clostridium sensu stricto dominated enterotype was better adapted to heat stress conditions with lower thermoregulation variations.
Keywords: climate, enterotype, heat stress, microbiota, performance, pig
INTRODUCTION
In tropical regions and in temperate countries during summer, high temperatures cause economic losses in pig industry (St-Pierre et al., 2003). These losses are related to negative effects of heat stress (HS) on reproductive and growth performances. The microbiome plays a central role in health, nutrient digestion, and more generally in host energetic metabolism (Nicholson et al., 2012). Varying types of stress alter the microbial composition such as social stress in rats (Suzuki et al., 1983), sanitary stress in piglets (Mulder et al., 2009), and HS in poultry (Suzuki et al., 1983; Song et al., 2014) and in dairy cattle (Uyeno et al., 2010). Exposure to HS induces changes in gut microbial composition in broilers with a decrease in the relative abundance of Lactobacillus (Lan et al., 2004). If relationships between microbiota composition and performance have been reported in temperate environments (Ramayo-Caldas et al., 2016; Le Sciellour et al., 2018), no results were yet reported under HS conditions. We hypothesize that potential changes in microbiota composition under HS would help the host to cope with stressful conditions. To characterize microbial community composition, the generated data sets using marker gene (i.e., 16S rRNA) survey are generally large and complex (counting data with many zeros, large variance in data distribution, etc.) posing substantial “bid data” challenges. As a result, these data require specific multidimensional statistical methods. Recent advances in microbiota analysis allow the description of modifications in microbial abundance using appropriate generalized linear models (Robinson et al., 2010) and discriminant analyses (Lê Cao et al., 2011). The present study aimed at investigating the impact of hot environmental conditions, represented by tropical climate or by an acute HS under temperate climate, on pig fecal microbiota composition and at evaluating how these changes in microbiota composition interplay with animal performance.
MATERIALS AND METHODS
The present study was conducted in accordance with the French legislation on animal experimentation and ethics. The French Ministry of Agriculture authorized the experiment on living animals at the INRA facilities in temperate (TEMP) and tropical (TROP) climates (CE2012-9 from the Animal Care and Use Committee of Poitou-Charentes and 69-2012-2 from the Animal Care and Use Committee of French West Indies and Guyane) under the direction of Y. Billon and J. Fleury (INRA-PTEA; authorization number by the French Ministry of Agriculture and Fisheries: 17015 and 971-2011-03-7704, respectively).
Animals and Experimental Design
Data used in the present study were obtained from a backcross design in which 60 and 70 Large White (LW) dams in TEMP area (INRA-Génétique, Expérimentation et Systèmes Innovants, Poitou-Charentes, France; 46°N, 0.45°W) and in TROP area (INRA-Plateforme Tropicale d’Expérimentation sur l’Animal, Guadeloupe, French West Indies; 16°N, 61°W), respectively, were inseminated with the same 10 F1 (LW × Créole) boars. Créole breed is a local tropical breed. Full details on the experimental design are given by Rosé et al. (2017). Finally, 558 and 564 backcross pigs (castrated males and females) in 12 batches were produced in TEMP and TROP climates, respectively.
The general animal management conditions were homogenized in both climates before starting the study. We ensured the consistency of this management by sending farm technicians from the farm in TEMP area to the farm on TROP area and technicians from the farm in TROP area to the farm on TEMP area. Pigs were weaned at 28 d of age. During the post-weaning period (10 wk), the pigs were housed in pens of 22 animals in the TEMP climate and in pens of 12 animals in the TROP climate. At 10 wk of age, 60 of the 64 pigs were randomly selected and housed in pens of 10 animals of the same sex in both TEMP and TROP climates. After 1 wk of adaptation, the test period started at week 11 and ended at week 23. Whatever the climate, pigs had a free access to water and were fed ad libitum with a commercial grower diet presented as pellets. For practical reasons, the diets in both environments were formulated with different raw materials (wheat, corn, barley, and soybean meal in TEMP, and corn and soybean meal in TROP) but resulted in the same chemical and nutritional composition (16.0% vs. 16.4% crude protein and 13.9 and 13.5 MJ digestible energy/kg in TEMP and TROP area, respectively; Rosé et al., 2017). In both environments, the diets did not contain any antibiotic, pre- or pro-biotic, or any other extracts. At 23 wk of age, TEMP pigs were moved to a climatic room and submitted to a constant ambient temperature of about 29 °C (29.0 ± 1.3 °C) for 3 consecutive weeks.
Measurements and Samples
The live body weights (BW) were recorded every 2 wk from 11 to 23 wk of age. The average daily body weight gain (ADG) was calculated from the BW difference between the beginning and the end of each 2-wk periods. The overall ADG was also calculated from the BW at 11 and 23 wk of age. Feeding stations (ACEMA 128, ACEMO, Pontivy, France) were used to record daily feed intake. The feed intake of half of the pigs was recorded during weeks 11 to 12, 15 to 16, and 19 to 20, whereas the feed intake of the remaining pigs was recorded during weeks 13 to 14, 17 to 18, and 21 to 22. Average daily feed intake (ADFI) between 11 and 23 wk of age was then estimated based on these measurements as suggested previously (Schulze et al., 2001). When the feed consumption was not recorded, pigs had free access to a conventional feed dispenser. Feed conversion ratio (FCR) was calculated as the ratio between ADFI and ADG between weeks 11 and 23. Rectal temperature (RT) and skin temperature (ST) were measured at weeks 19 and 23 in TEMP and TROP and at weeks 24 and 26 in TEMP. Digital thermometers (Microlife Corp., Paris, France) were used to measure RT, and ST was measured on the back at the P2 site using a skin surface thermocouple probe (type K, model 88002K-IEC; Omega Engineering Inc., Stamford, CT) connected to a microprocessor-based handheld thermometer (model HH-21; Omega Engineering Inc.). Fecal samples were taken directly from the rectum on week 23 in both climates, and on week 26 in TEMP (Fig. 1). These samples were immediately stored in barcoded tubes and snap-frozen within 5 min in liquid nitrogen before storage at −80 °C until DNA extraction.
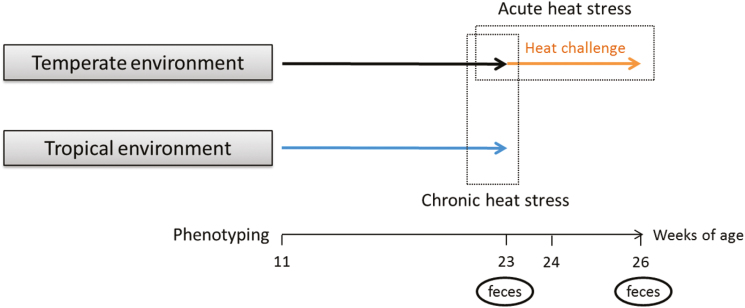
Figure 1.
Experimental design: pigs were raised under temperate (n = 558) or tropical (n = 564) environments between 11 and 26 wk of age.
The following abbreviations will be used in the current article: TEMP23, TROP23, and TEMP26 will refer to data collected at 23 wk of age on TEMP and TROP and at 26 wk of age on TEMP climate, respectively. The chronic HS will refer to the comparison between TEMP23 and TROP23. The acute HS will refer to the comparison between TEMP23 and TEMP26.
DNA Sequencing
The DNA extraction was performed with the Zimo kit from 50 mg of feces sample according to a method previously described by Verschuren et al. (2018). The V3–V4 region of the 16S rRNA gene has been amplified by PCR before sequencing on an Illumina MiSeq platform. The amplification used F460 and R460 primers (F460: CTTTCCCTACACG ACGCTCTTCCGATCTA CGGRAGGCAGCAG, R460: GGAGTTCAGA CGT GTGCTCTTCCGATCTTA CCAGGGTA TCTAATCCT) and 30 cycles of PCR with an annealing temperature of 65 °C. The resulted 2 × 250 bp paired-end sequences were cleaned internally for length, homopolymers, and undetermined nucleotides. Sequencing and sequence cleaning have been performed in the GeT-PlaGE platform (Toulouse, France).
The VSEARCH software (Rognes et al., 2016) was used to remove chimeras from the sequences data set and then to de novo cluster the resulting sequences into operational taxonomic units (OTU) with a threshold of 0.97 similarity. The abundance of each OTU in each sample was recorded. Rare OTU, representing less than 0.01% of the sequences from all samples, were removed. The Usearch software (9.2.64_i86linux32 version) was then used for taxonomic assignation based on SILVA database. The PICRUSt pipeline was applied on the 16S rRNA sequencing data (Langille et al., 2013). The resulting abundance of KEGG (Kyoto Encyclopedia of Genes and Genomes) genes orthologues in each sample was further categorized into biological pathways.
Calculations and Statistical Analyses
Statistical analyses were performed under the software R version 3.2.5. The effects of the chronic HS on production and thermoregulation traits were analyzed using an ANOVA model with the effect of climate (TEMP vs. TROP), sex type (castrated males vs. females), and sire family (n = 10) as fixed effects and the batch within each climate as a random effect. The ANOVA model used to test the effect of the acute HS on production and thermoregulation traits included the fixed effects of age (23 vs. 24 vs. 26 wk of age), sex type, sire family, and batch. The animal was included in the model as a random effect to account for within-pig covariability of repeated measurements.
The microbial diversity was assessed by the number of species and the Shannon index using the Vegan R package (Oksanen et al., 2018). The proportions of the variance explained by the environment, the batch, the sex type, or the sire family were evaluated by a principal variance component analysis (PVCA) through the Bioconductor R pvca package (Bushel, 2018). The effect of the environment (TEMP23, TROP23, and TEMP26) on the relative abundance of the bacteria at the phylum level was evaluated through Wilcoxon tests, using 2 × 2 comparisons. Generalized linear models (GLM) were then established using edgeR R package (Robinson et al., 2010) to evaluate the impact of the environment on the abundance of each OTU and of each pathway. The fixed effect of the sex was included in the model. The effect of batch within each climate was considered as a random effect. The analyzed models compared TEMP23 vs. TEMP26, and TEMP23 vs. TROP23 for the OTU, and also TEMP26 vs. TROP23 for the pathways. The P-values were adjusted for multiple tests with the Benjamini–Hochberg method. In addition, a threshold was fixed to 0.5 log2 fold change to extract the most differentially represented pathways in the studied environments.
To predict the environment in which the pigs were raised based on their microbial data, sparse partial least squares discriminant analyses (sPLS-DA) were performed by using MixOmics R package (Lê Cao et al., 2011). The sPLS-DA was performed on the OTU table previously normalized through a total sum scaling and centered log ratio transformation. This table was not rarefied and the counts varied between 7,000 and 75,234 sequences per sample. A M-fold cross-validation was performed to evaluate the error rate of the models with 10-folds and 10 times repetition.
To aggregate abundance profiles into groups with similar bacterial compositions, enterotypes were constituted according to Arumugam et al. (2011). Samples were clustered based on the relative genus abundances using Jensen–Shannon divergence distance and the Partitioning Around Medoids clustering algorithm. The results were assessed for the optimal number of clusters (enterotypes) using the Calinski–Harabasz Index. The statistical significance of the optimal clustering was evaluated by comparing the Silhouette coefficient of the optimal clustering to a distribution of Silhouette coefficients derived from a simulation from 100 different randomized tables, which modeled the null distribution with no clustering. The randomized tables were based on the original relative genus abundance table randomly reorganized by sample. Genera abundances between enterotypes were compared using Kruskal–Wallis tests. The effects of the enterotypes on performance and thermoregulatory responses measured between 11 and 23 wk of age and microbial diversity were tested using ANOVA models including the effect of enterotype, climate, sex type, sire family, and interactions. The effect of enterotypes established at 23 wk of age on pigs’ performance, thermoregulatory response, and microbial diversity during the acute HS in TEMP climate was analyzed by ANOVA. The models included the effect of the batch, sex, sire family, and interactions. The models also included random effects: the ADG between 21 and 23 wk of age, the ST and the RT between 19 and 23 wk of age, and the number of OTU and the Shannon index at 23 wk of age.
RESULTS
Animal Performance
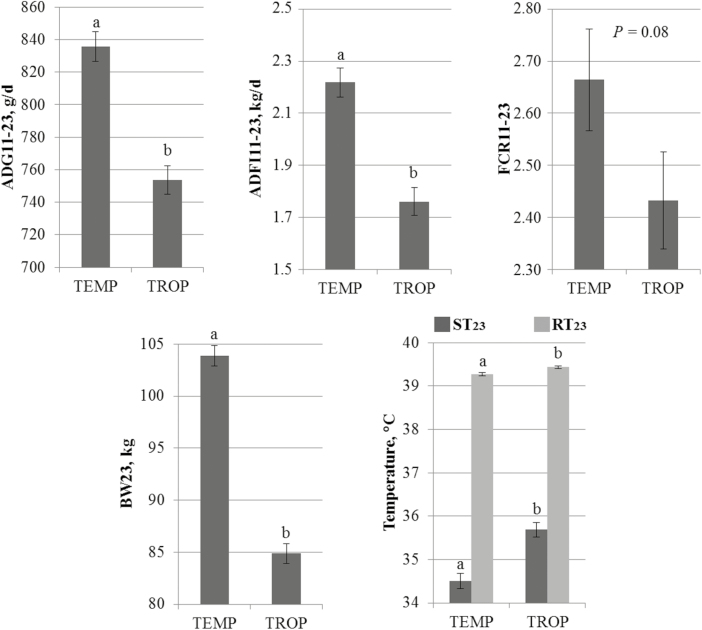
Between 11 and 23 wk of age, the daily ambient temperature averaged 25.2 and 26.3 °C in TEMP and TROP climates, respectively. On the subset of pigs with fecal samples at 23 wk of age, 520 and 531 pigs had full records for growth performance and thermoregulation responses in TEMP and in TROP, respectively. The ADG and ADFI were lower (754 vs. 836 g/d, SE = 9, P < 0.05 and 1.76 vs. 2.22 kg/d, SE = 0.05, P < 0.05, respectively) in TROP when compared with the TEMP conditions (Fig. 2). The FCR did not differ between TROP and TEMP climates (2.43 vs. 2.66, SE = 0.09, P = 0.08). At 23 wk of age, TEMP pigs were 19 kg heavier (SE = 1, P < 0.05) compared with pigs raised under TROP conditions. The RT was 0.1 °C greater (SE = 0.03, P < 0.05) in pigs raised in the TROP climate compared with pigs raised in the TEMP climate. Similarly, ST was greater in TROP than in TEMP climate (35.7 vs. 34.5 °C, SE = 0.2, P < 0.05).
Figure 2.
Effect of a chronic heat stress represented by temperate and tropical climates (TEMP and TROP, respectively) on average daily gain (ADG11–23), average daily feed intake (ADFI11–23), and feed conversion ratio (FCR11–23) between 11 and 23 wk of age, on body weight at 23 wk of age (BW23) and on skin and rectal temperatures at 23 wk of age (ST23 and RT23, respectively). Least squares means within a trait with different superscripts significantly differ (P < 0.05).
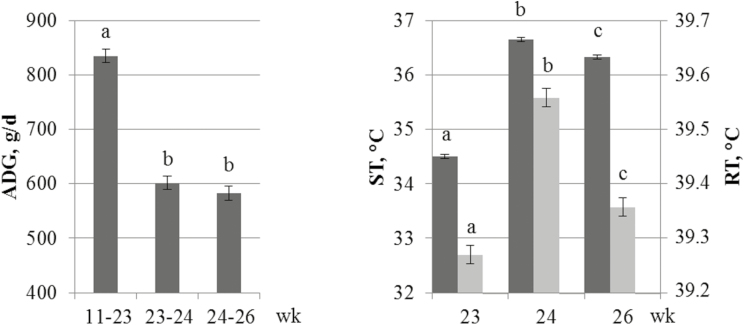
In TEMP climate, the ADG decreased from 835 to 583 g/d between weeks 23 and 24 (SE = 12, P < 0.05) and thereafter remained stable from 24 to 26 wk of age (589 g/d on average; SE = 12, P > 0.05; Fig. 3). Regarding the thermoregulation responses, both RT and ST increased from weeks 23 to 24 (39.3 to 39.6 °C, SE = 0.02, P < 0.05 and 34.5 to 36.6 °C, SE = 0.04, P < 0.05, respectively). Between weeks 24 and 26, RT and ST were reduced from 36.6 to 36.3 °C (SE = 0.02, P < 0.05) and from 39.6 to 39.4 °C (SE = 0.04, P < 0.05), respectively.
Figure 3.
Effect of an acute heat challenge applied from weeks 24 to 26 on average daily gain (ADG), skin temperature (ST), and rectal temperature (RT). Least squares means within a trait with different superscripts significantly differ (P < 0.05).
Impact of an HS on Microbiota Composition
In the fecal samples collected in the present study, the number of sequences ranged from 1 to 75,234 with an average value of 21,194 ± 11,539 sequences. Only the 1,583 samples containing more than 7,000 sequences were retained for further analyses (526 samples in TEMP23 and TEMP26 and 531 in TROP23). After filtration for the rare OTU, a total of 1,688 OTU remained in our data set. The average Prevotella abundance was 5.6% ± 5.1% and average Ruminococcus abundance was 0.3% ± 0.2%. The Mitsuokella and Treponema genera abundance reached 0.1% ± 0.1% and 0.5% ± 0.6%, respectively.
Fecal samples from the TEMP26 group presented a greater richness compared with those of the TEMP23 group (P < 0.05), but no difference in Shannon index (P = 0.13). Fecal samples from the TROP23 group did not differ from those of the TEMP23 and TEMP26 groups in the observed counts (P = 0.63 and 0.75, respectively), but presented a lower Shannon index (P < 0.05). Based on the PVCA, the environment (TEMP23, TEMP26, or TROP23) explained 20% of the total variance of the microbial composition; the sire family accounted for 3.9%, the batch for 3.2%, and the sex type for 0.8% of the total variance. The microbiota composition was dominated by Firmicutes and Bacteroidetes phyla (91.7%, 88.3%, and 87.5% for TEMP23, TEMP26, and TROP23, respectively). The relative abundance of Firmicutes, Proteobacteria, and Spirochaetes was greater (P < 0.05) under TROP climate when compared with TEMP climate (Table 1). The relative abundance of Actinobacteria and Bacteroidetes was greater (P < 0.05) in TEMP23 than in TEMP26 and TROP23 environments.
Table 1.
Relative percentage at phylum level in samples collected in temperate climate before (TEMP23) and after (TEMP26) an acute heat challenge and in tropical climate (TROP23)
| Phylum | TEMP23 | TEMP26 | TROP23 |
|---|---|---|---|
| Firmicutes | 66.85a | 70.75b | 68.73c |
| Bacteroidetes | 24.90a | 17.60b | 18.80c |
| Unclassified | 5.61a | 8.14b | 7.27c |
| Spirochaetes | 1.40a | 2.11b | 3.66c |
| Proteobacteria | 0.79a | 0.77a | 1.21b |
| Fibrobacteres | 0.25a | 0.40b | 0.14c |
| Actinobacteria | 0.19a | 0.20b | 0.17c |
| Tenericutes | 0.01a | 0.03b | 0.01c |
| Fusobacteria | <0.01 | <0.01 | <0.01 |
| Deferribacteres | <0.01 | <0.01 | <0.01 |
a–cMean percentage within a row with different superscripts significantly differ (P < 0.05) in a Kruskal–Wallis test.
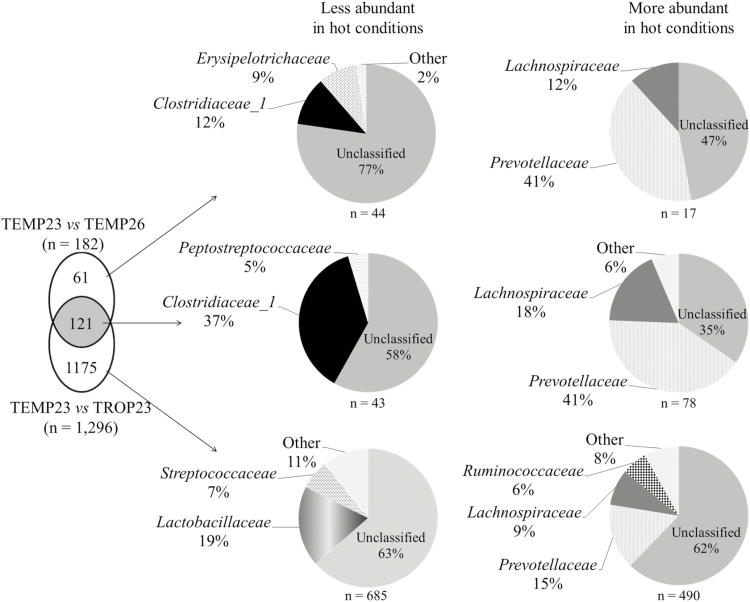
Using the GLM from edgeR package, the comparison of the microbiota from TEMP23 and TROP23 highlighted 1,296 OTU with significantly different abundance between the climates: 728 OTU significantly more abundant in TEMP23 and 568 OTU more abundant in TROP23 (P < 0.05; Fig. 4). The corresponding analysis for the comparison between TEMP23 and TEMP26 revealed 87 OTU significantly more abundant in TEMP23 and 95 OUT more abundant in TEMP26 (P < 0.05). Based on these comparisons, we could extract the OTU systematically more abundant in hot conditions (either under TROP climate or after a heat challenge under TEMP climate). These 78 OTU belonged mainly to the Prevotellaceae family (41% of the OTU) and the Lachnospiraceae family (18%). On the contrary, 37% of the 43 OTU always more abundant under TEMP23 came from the Clostridiaceae 1 family and 5% from the Peptostreptococcaceae family.
Figure 4.
Venn diagrams of the operational taxonomic units differentially abundant in a GLM analysis, aggregated at family level, between TEMP23 versus TEMP26 and between TEMP23 versus TROP23.
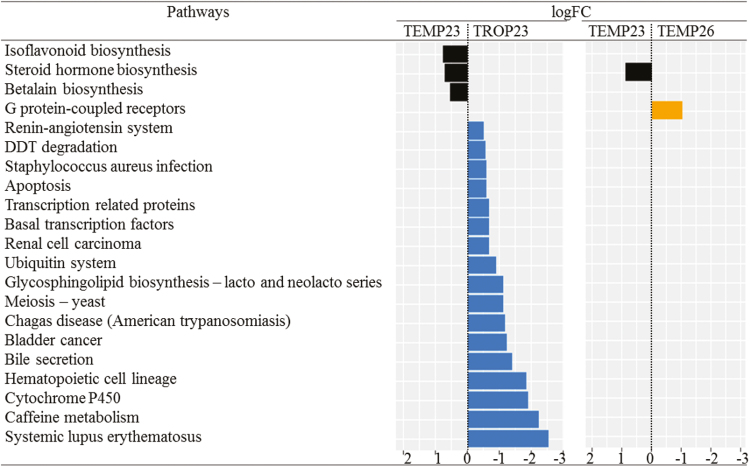
The GLM analysis extracted 3 functional pathways related to metabolism (biosynthesis of isoflavonoids, steroid hormones, and betalain) over-represented in TEMP23 compared with TROP23 (Fig. 5). In contrast, 17 pathways were over-represented in TROP23 and they were related to human diseases (5 pathways), metabolism (4 pathways), genetic information processing (3 pathways), cellular processes (2 pathways), and organismal systems (3 pathways). The steroid hormone biosynthesis pathway was over-represented in TEMP23 compared with TEMP26, and the G protein-coupled receptor pathway was over-represented in TEMP26 compared with TEMP23.
Figure 5.
Functional pathways differentially abundant between the temperate climate at 23 wk (TEMP23) and 26 wk of age (TEMP26) and the tropical climate at 23 wk of age (TROP23). Log2 fold changes (logFC) resulted from a 2 × 2 comparison using a generalized linear model analysis (false discovery rate < 0.05).
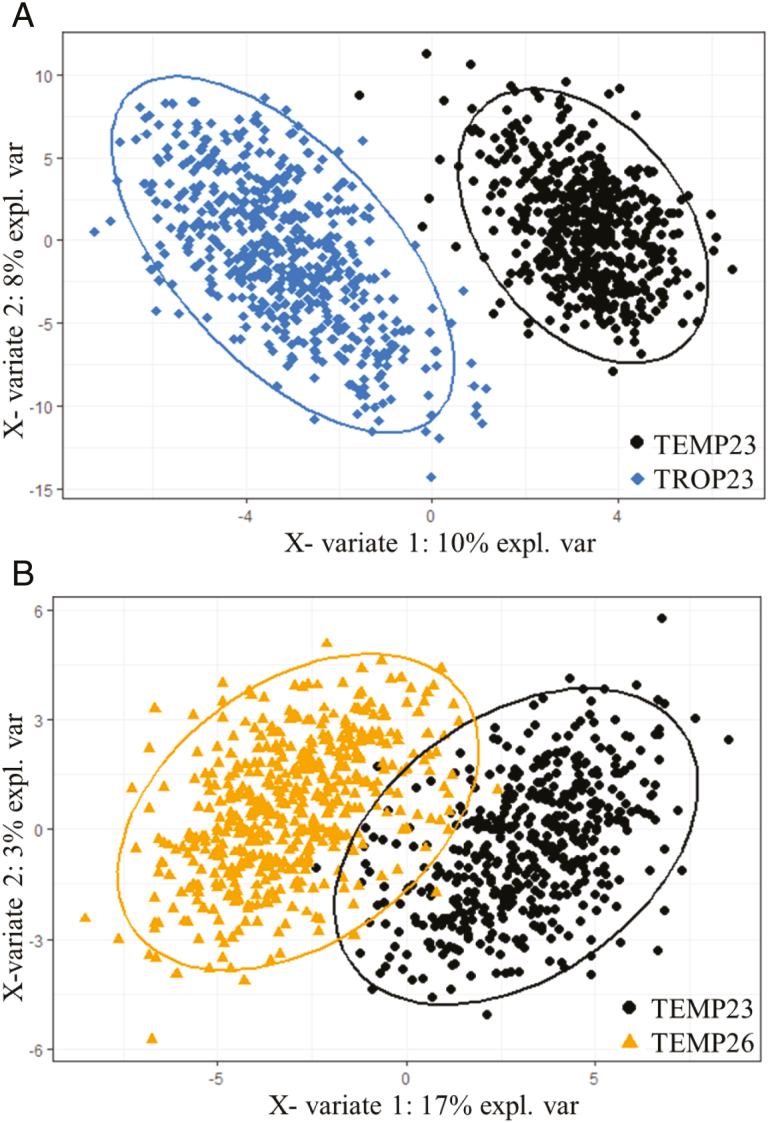
The sPLS-DA allowed the discrimination of pigs raised under 2 different environments (Fig. 6). Pigs raised under TEMP and TROP climates were discriminated with 0.2% error rate of misclassification using 36 OTU on a first component and 89 supplementary OTU on a second component. Among the OTU selected on the first and the second components, 21% belonged to the Prevotellaceae family. As well, fecal samples at TEMP23 and TEMP26 were used to discriminate pigs before and after the acute heat challenge in a multilevel sPLS-DA. The discrimination reached 7.1% error rate and used 62 OTU on 2 components. Among the OTU selected on the first and the second components, 19% belonged to the Prevotellaceae family and 14% to the Lachnospiraceae family. The OTU selected in the sPLS-DA were systematically found as differentially abundant in the GLM analysis using edgeR package. From the comparison of the 2 previous sPLS-DA, we extracted 5 OTU systematically used to discriminate pigs raised in TEMP23 from pigs raised in hot conditions. These OTU belonged to the Lactobacillaceae family (n = 1), Peptostreptococcaceae family (n = 1), and Prevotellaceae family (n = 3).
Figure 6.
Score plot of 2-component sparse partial least square discriminant analysis models showing feces samples clustering according to the environment (TEMP23: under temperate climate at week 23 of age; TEMP26: under temperate climate after a 3-wk heat challenge period; TROP23: under tropical climate at week 23 of age) with percentage of variance captured for each principal component. According to the cross-validation permutation test, the misclassification error rates with 2 components are 0.2 and 7.1% in models 1 (A) and 2 (B), respectively.
Effect of the Enterotypes on Performance and Thermoregulation
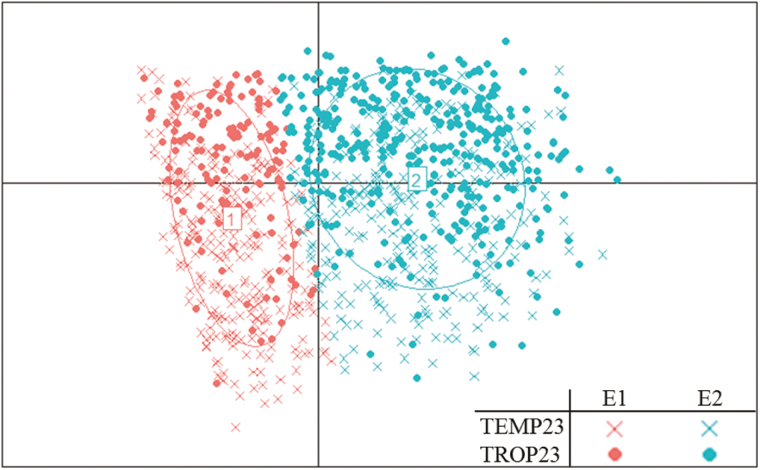
Following the methodology of Arumugam et al. (2011), the microbiota clustered into 2 enterotypes with a mean silhouette value of 0.29 (Fig. 7), whereas the mean silhouette value reached by randomized tables was 0.03. This result confirmed that the enterotypes were accurately discriminated. These 2 enterotypes were represented in each climate: 261 and 145 pigs clustered in enterotype 1 (E1) in TEMP23 and in TROP23, respectively, and 265 and 386 pigs clustered in enterotype 2 (E2) in TEMP23 and in TROP23, respectively.
Figure 7.
Distribution of the microbial enterotypes 1 (E1) and 2 (E2) in 23-wk-old pigs raised in temperate (TEMP23) or tropical (TROP23) climates.
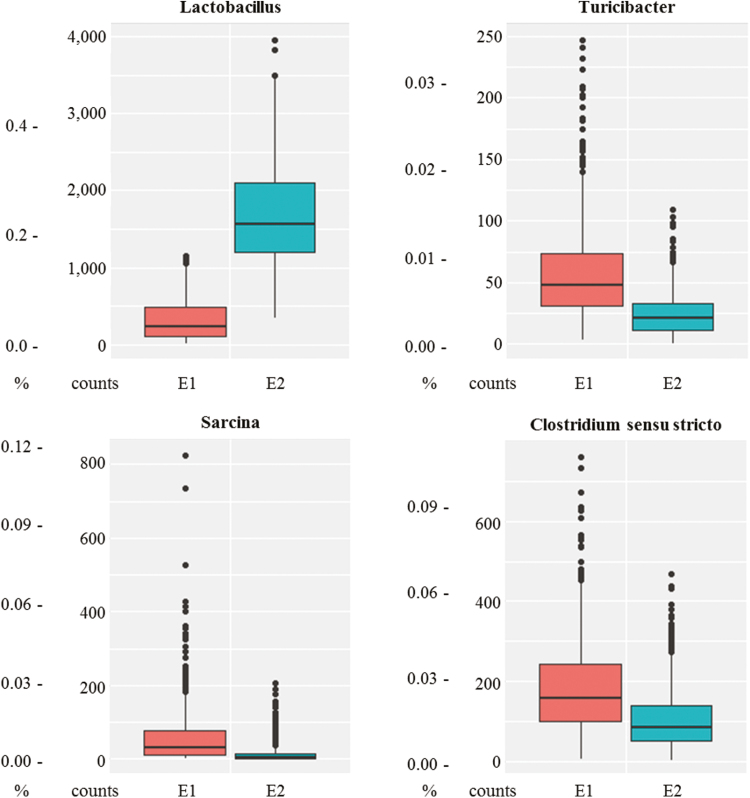
Enterotypes in our experiment differed in Lactobacillus, Turicibacter, Sarcina, and Clostridium sensu stricto abundances (P < 0.05). The abundance of these latter genera in E1 were 4.5 ± 3.7%, 0.8 ± 0.5%, 0.8 ± 1.0%, and 2.3 ± 1.6%, respectively (Fig. 8). The corresponding values in E2 were 23.4 ± 9.1%, 0.3 ± 0.2%, 0.2 ± 0.4%, and 0.1 ± 1.0%. This second enterotype was dominated by the Lactobacillus genus. According to the ANOVA, based on the number of OTU (2,586 vs. 2,742 OTU in TEMP23 and 2,600 vs. 2,698 OTU in TROP23, respectively), the alpha diversity of the samples in E2 was greater (P < 0.05) compared with the samples in E1. The Shannon index (7.18 vs. 7.25 in TEMP23 and 7.14 vs. 7.18 in TROP23, respectively) confirmed the greater diversity in E2 compared with E1 (Table 2).
Figure 8.
Abundance (% and counts) of the 4 main genera contributing to the microbial enterotypes 1 (E1 in red) and 2 (E2 in turquoise).
Table 2.
Effect of the microbial enterotype (E1 and E2) established at 23 wk of age on production traits, thermoregulatory responses, and microbial diversity (least square means) in temperate (TEMP) and tropical (TROP) climates
| Item1 | TEMP | TROP | RSD2 | Statistics3 | ||
|---|---|---|---|---|---|---|
| E 1 | E 2 | E 1 | E 2 | |||
| No. of pigs | 261 | 265 | 145 | 386 | ||
| Production and thermoregulatory traits | ||||||
| BW11, kg | 32a | 31a | 22b | 21c | 4 | E, C, F |
| BW23, kg | 104a | 104a | 86b | 85b | 10 | C, S, F |
| ADG11–23, g/d | 835a | 837a | 754b | 754b | 86 | C, S, F |
| ADFI11–23, kg/d | 2.2a | 2.2a | 1.7b | 1.8b | 0.4 | C, S, F |
| FCR11–23 | 2.69 | 2.63 | 2.37 | 2.45 | 0.47 | C, S, F |
| ST23, °C | 34.5a | 34.5a | 35.5b | 35.7c | 0.7 | E, C, S, F |
| RT23, °C | 39.2a | 39.3ab | 39.4b | 39.4b | 0.3 | C, S, F |
| Microbial diversity at 23 wk of age | ||||||
| No. of OTU | 2,586a | 2,742b | 2,600a | 2,698b | 222 | E, S |
| Shannon Index | 7.18a | 7.25b | 7.14a | 7.18a | 0.18 | E, F |
1BW11 and BW23 = body weight at 11 and 23 wk of age, ADG11–23 = average daily gain between 11 and 23 wk of age; ADFI11–23 = average daily feed intake between 11 and 23 wk of age; FCR11–23 = feed conversion ratio between 11 and 23 wk of age; ST23 = skin temperature at 23 wk of age; RT23 = rectal temperature at 23 wk of age; OTU = operational taxonomic unit.
2Residual standard deviation from an ANOVA model accounting for the enterotype (E), the climate (C), the sex type (S), the sire family (F), the batch, and the interactions E × C and E × S.
3Significant effects (P < 0.05). Batch effect was significant for all traits and is not reported in the table.
a–cLeast squares means within a row with different superscripts significantly differ (P < 0.05).
From the ANOVA analysis of the effect of enterotypes on production and thermoregulation traits recorded during the growth period in both climates, whatever the trait, the interaction between the enterotypes and the climate was not significant (P > 0.05). Pigs in E1 were 3.3 kg heavier at week 11 compared with pigs in E2 (P < 0.05; Table 2). However, the 2 enterotypes did not differ for the BW record at 23 wk of age (97.5 vs. 93.8 kg for E1 and E2, respectively; P = 0.42). Between 11 and 23 wk of age, the 2 enterotypes did not relate to different ADG (806 vs. 787 g/d for E1 and E2, respectively; P = 0.99), ADFI (2.05 vs. 1.94 kg/d for E1 and E2, respectively; P = 0.12), or FCR (2.57 vs. 2.52 for E1 and E2, respectively; P = 0.09). Regarding the thermoregulation traits, ST at 23 wk of age was 0.3 °C greater in E2 pigs compared with E1 pigs (34.9 vs. 35.2 °C, respectively; P < 0.05), but the RT did not differ between the 2 enterotypes (39.3 vs. 39.4 °C, respectively; P = 0.11). In TEMP climate, when pigs were exposed to an acute HS challenge between 23 and 26 wk of age, the animals from both E1 and E2 presented similar ADG and ST variation (Table 3). During the acute HS challenge, the rise in RT from weeks 23 to 24 was greater in pigs from E2 than in those from E1 (0.34 vs. 0.25 °C, respectively, P < 0.05). On a long-term response, between weeks 23 and 26, the RT variation remained 0.05 °C greater (P < 0.05) in E2 compared with E1.
Table 3.
Effect of the microbial enterotype (E1 and E2) established at 23 wk of age on production traits, thermoregulatory responses, and microbial diversity (least square means) after an acute heat stress in temperate climate
| Item1 | E 1 | E 2 | RSD2 | Statistics3 |
|---|---|---|---|---|
| No. of pigs | 261 | 265 | ||
| Production and thermoregulatory traits | ||||
| Short-term response (from 23 to 24 wk) | ||||
| ADG, g/d | 590 | 600 | 390 | F, ADG21–23 |
| Change in ST, °C | 2.20 | 2.11 | 0.98 | F, ST19–23 |
| Change in RT, °C | 0.25a | 0.34b | 0.45 | E, RT19–23, E × S |
| Long-term response (from 23 to 26 wk) | ||||
| ADG, g/d | 579 | 589 | 157 | F, ADG21–23, E × S |
| Change in ST, °C | 1.86 | 1.87 | 0.87 | ST19–23 |
| Change in RT, °C | 0.09a | 0.14b | 0.43 | E, RT19–23, E × S |
| Microbial diversity | ||||
| No. of OTU | ||||
| At 23 wk | 2,586a | 2,742b | 226 | E, F |
| Change from 23 to 26 wk | 12 | 41 | 201 | No. of OTU23 |
| Shannon index | ||||
| At 23 wk | 7.18a | 7.25b | 0.19 | E, F |
| Change from 23 to 26 wk | −0.02 | 0.01 | 0.15 | F, Shannon index23 |
1ST = skin temperature; OTU = operational taxonomic unit; RT = rectal temperature.
2Residual standard deviation from an ANOVA model accounting for the enterotype (E), the sex type (S), the sire family (F), the batch, and the interactions E × S. The models for short- or long-term responses also accounted for the ADG between 21 and 23 wk of age (ADG21–23), the ST and the RT between 19 and 23 wk of age (ST19–23 and RT19–23, respectively), the number of OTU and the Shannon index at 23 wk of age (no. of OTU23 and Shannon index23, respectively) for ADG, ST, RT, number of OTU, and Shannon index variations, respectively.
3Significant effects (P < 0.05). Batch effect was significant for all traits and is not reported in the table.
a–bLeast squares means within a row with different superscripts significantly differ (P < 0.05).
When pigs were exposed to an acute HS in TEMP climate, their distribution in the 2 enterotypes changed. Among the 241 pigs belonging to E1 in week 23, 199 (82.6%) remained in the same enterotype in week 26, but 42 (17.4%) switched from E1 to E2 in week 26. In contrast, 123 pigs remained in E2 from weeks 23 to 26 when 122 pigs switched from E2 to E1. Performance and thermoregulation response of pigs switching from one enterotype to another one did not differ from those pigs that remained stable in their enterotype at 23 and 26 wk of age. In particular, growth performance and RT variation after HS did not differ between pig that switched or not (results not shown).
DISCUSSION
Performance and Thermoregulatory Response to HS
The effects of tropical climate on performance from 11 to 23 wk of age have already been detailed by Rosé et al. (2017). In the subset of pigs with fecal samples included in our study, we confirmed that ADFI and ADG were significantly reduced in TROP compared with TEMP climate. These results are also in agreement with Renaudeau et al. (2006). In TROP, the combined effects of high temperature and high relative humidity possibly accentuate the ADFI reduction due to heat (Granier et al., 1998) due to the low ability of the pigs to lose heat by evaporation under high ambient humidity. In agreement with Rosé et al. (2017), feed efficiency tended to increase in TROP conditions. As suggested by Renaudeau et al. (2011), in moderate HS conditions, feed efficiency increases due to feed restriction effect.
The exposure to a 3-wk acute HS of 29 °C from 23 wk of age resulted in a significant increase in body temperature within the first week of exposure (+0.3 °C for RT) followed by a gradual decline (−0.1 °C/wk from weeks 24 to 26). This biphasic response to an acute HS is in agreement with previous studies: the decrease in RT and ST overtime during the exposition to high ambient temperature demonstrates a long-term adaptation to HS (Morrison and Mount, 1971; Renaudeau et al., 2007, 2008). According to Renaudeau et al. (2013), this acclimation is mainly related to a reduction in metabolic heat production. In addition, this latter study showed that the total amount of energy intake is reduced in hot conditions with subsequent negative effects on both the energy retained and growth performance. This result was confirmed by the reduced ADG during the 3-wk exposure to 29 °C, compared with that measured between weeks 11 and 23.
Microbiota Composition Response to HS
In accordance with previous studies conducted in pigs (Xiao et al., 2016; Holman et al., 2017), fecal samples collected in the present study were dominated by Firmicutes and Bacteroidetes. The PVCA revealed that the environment explained 20% of the total variance in microbiota composition. It led to an accurate discrimination of the climates based on the microbial abundance information. Indeed, 77% of the OTU differed between TEMP and TROP, but only 11% differed from fecal samples collected before and after acute HS in TEMP climate. In addition, the TEMP and TROP pigs could be discriminated using sPLS-DA on fecal microbiota composition with a greater accuracy (0.2% error rate) than the TEMP23 and TEMP26 pigs (7.1% error rate). The 2 climates differed in ambient temperature, humidity, and feed composition among others noncontrolled external effects. In contrast, the acute heat challenge was applied on the same pigs, which drastically reduced the external sources of variation. Similarly, heifers raised under temperate- or tropical-simulated climates differed in ruminal microbiota composition but when exposed to a temperature increase in the TEMP conditions, the microbiota composition did not vary (Tajima et al., 2007). In this latter study as in our, no difference in microbial diversity has been observed between the environmental conditions.
In our experimental conditions, Lachnospiraceae abundance in HS conditions (TEMP26 and TROP23) increased. However, contradictory results were published on the relationship between Lachnospiraceae abundance and stressful situations. Mice exposed to the water immersion restraint stress showed an increase in Lachnospiraceae (Li et al., 2017). In contrast, Lachnospiraceae abundance was negatively correlated with hematological measure of stress in squirrels (Stothart and Newman, 2017). From that, it can be hypothesized that changes in Lachnospiraceae abundance would depend on the nature of stress.
In our study, among the OTU, more abundant in TEMP23 compared with TROP23, 19% belonged to Lactobacillaceae. This family was also highly represented in OTU that discriminated the 2 climatic conditions with the sPLS-DA. Similarly, the relative abundance of Lactobacillaceae was depleted in HS broilers (Song et al., 2014). At a functional level, Lactobacillus and Clostridium possess a bile salt hydrolase which participates to the detoxification of bile acids in the gut (Krishnan et al., 2015). As a result, reduced Lactobacillus and Clostridium abundance observed in our HS conditions (TEMP26 and TROP23) could be connected to the over-represented bile secretion pathway reported in these HS conditions. According to Krishnan et al. (2015), the bile acid secretion favors the gut colonization by pathogens. The greater abundance of potentially pathogenic Prevotellaceae in TROP compared with TEMP would illustrate a greater colonization of the gut by pathogenic bacteria in HS conditions (Larsen, 2017). In human, Prevotella have been associated with chronic inflammatory diseases (Scher et al., 2013; Larsen, 2017). Therefore, we could hypothesize that Prevotellaceae bacteria may have participated to the over-representation of disease-related pathways in our experiment. Unfortunately, we did not measure traits related to diseases in TROP. Cytochromes P450 enzymes (CYP)-related pathway was also over-represented in TROP climate. In agreement, CYP expression was improved in HS mice (Bhusari et al., 2007). These CYP play an important role in gut health improvement by acting on the degradation of bile acid (Hrycay et al., 2014; Li and Apte, 2015), drugs and xenobiotics (Wilson and Nicholson, 2017). In other words, the over-representation of CYP pathway in hot conditions could be considered as a consequence of the deterioration of gut health.
As explained before, the reduced abundance of Lactobacillaceae in HS conditions could be the first step of a dysbiosis and subsequent gut inflammation (Rojo et al., 2017). From that, it can be hypothesized that nutritional strategies based on probiotics supplementation with Lactobacillus species would help pigs to cope with the effects of HS. In broilers, when Lactobacillaceae abundance decreased, a dietary supplementation with Lactobacillaceae improved the broilers’ adaptation to acute HS (Lan et al., 2004). In pigs, Lactobacillus strains are often used as a probiotic to improve the immune system and the growth performances (Hou et al., 2015; Valeriano et al., 2017) but have not been tested yet in HS conditions. Therefore, Lactobacillaceae supplementation could be investigated to improve the pigs’ adaptation to HS.
The apoptosis and ubiquitin system pathways were over-represented in TROP compared with TEMP. In HS broilers, heat shock proteins including ubiquitin were upregulated (Li et al., 2011), whereas proteins that negatively regulate apoptosis, among which the ubiquitin-specific proteinase 45, were downregulated in broilers (Li et al., 2011; Loyau et al., 2016; Wang et al., 2018) and in mammals (Parag et al., 1987; Riezman, 2004). In particular, the cellular apoptosis and protein degradation through the ubiquitin system in chronic HS conditions was demonstrated in finishing pigs (Cui et al., 2016). This could be due to an accumulation of denaturized proteins and the activation of the mitochondrial-dependent apoptosis pathway in HS pigs (Gu et al., 2015).
Enterotypes and Heat Challenge Perturbation
We classified our fecal samples collected on 23-wk-old pigs based on the relative abundance of microbial genera into 2 enterotypes, according to the methodology from Arumugam et al. (2011). Using the same mathematical methodology, Ramayo-Caldas et al. (2016) had also defined 2 enterotypes (post-weaning enterotype A [PEA] and post-weaning enterotype B [PEB]) with feces samples collected on 60-d-old pigs. The alpha diversity in the PEA enterotype was greater than in the PEB enterotype. Similarly, microbiota diversity differed between the 2 enterotypes found in the present study. Whatever the climate, E1 enterotype presented a lower diversity compared with E2. In the study by Ramayo-Caldas et al. (2016), the PEA enterotype was dominated by Ruminococcus and Treponema, whereas the PEB enterotype was dominated by Prevotella and Mitsuokella. In our experiment, the relative abundance of Prevotella and Mitsuokella corresponded for all samples to the PEA enterotype. However, the relative abundance of Ruminococcus and Treponema measured in our fecal samples situated all of them in the PEB enterotype. As a consequence, the enterotypes as defined previously (Ramayo-Caldas et al., 2016) were not applicable in our study. The discrepancy between this previous study and the present one could be related to the difference of age at sampling the feces. According to Zhao et al. (2015), the microbiota composition is not fully stabilized at 60 d of age and evolves with aging. Similarly, microbiota composition of healthy humans evolved continuously throughout time between 3 enterotypes previously defined by Arumugam et al. (2011) (Knights et al., 2014). These results suggest that the enterotypes’ definition is probably age dependent. The instability of the enterotypes is one reason why the notion of enterotype is highly controverted (Huse et al., 2012; Jeffery et al., 2012; Gorvitovskaia et al., 2016). In addition, between 23 and 26 wk of age, part of the pigs switched from an enterotype to another, and this switch was not related to changes in production traits or in HS tolerance. The evolution in microbiota composition could have been accentuated by environmental climatic conditions. Similarly, other environmental factors such as feeding management can trigger modifications of the microbiota composition within 24 h and induced a switch between enterotypes in human (Wu et al., 2011) and in piglets (Mach et al., 2015).
Pigs belonging to E1, with minimal microbial diversity, were heavier at the beginning of the experiment compared with pigs in E2. In accordance, 60-d-old pigs in PEB enterotype, with minimal microbial diversity, were heavier than the pigs in PEA enterotype (Ramayo-Caldas et al., 2016). However, we did not observe significant differences in ADG despite a cluster-related growth performance previously observed in younger pigs (Mach et al., 2015; Ramayo-Caldas et al., 2016), maybe due to the differences between the defined enterotypes in these studies compared with ours. In the present experiment, growth and feeding performances were not related to enterotypes in any climate until 23 wk of age. Regarding the impact of the enterotypes on acclimation responses to HS, pigs in the E1 enterotype presented lower ST variation in TEMP and TROP between 19 and 23 wk of age. In addition, pigs in E1 exposed to an acute HS in TEMP showed a more stable RT at short and long terms compared with pigs in E2. Therefore, we hypothesized that, despite a lower microbial diversity, the specific microbiota composition in the E1 would help the host to better cope with the heat perturbation compared with pigs in the E2. However, this hypothesis has to be validated in further investigations.
This study demonstrated that fecal microbiota composition is significantly influenced by the climatic conditions. However, it remains unclear how this shift affects microbiota functions and helps the host to better cope with HS conditions. Better understanding of the physiological role of microbial populations that are significantly affected by chronic and acute HS could help to develop targeted approaches to prevent heat distress in swine.
Footnotes
This study is part of the Feed-a-Gene Project funded by the European Union’s H2020 Program (grant 633531), and of the PigHeat project funded by the French National Agency of Research (ANR-12-ADAP-0015).
The authors thank K. Benony, D. Beramice, B. Bocage, M. Bructer, and F. Silou from the experimental unit INRA-PTEA, and F. Meslier, J. Bailly, P. Epagneaud, and C. Lebourhis from the experimental unit INRA-GenESI for animal care and sample collection, and the GeT genomic platform for 16S RNA sequencing.
LITERATURE CITED
- Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D. R., Fernandes G. R., Tap J., Bruls T., Batto J. M., et al. ; MetaHIT Consortium. 2011. Enterotypes of the human gut microbiome. Nature 473:174–180. doi: 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhusari S., Liu Z., Hearne L. B., Spiers D. E., Lamberson W. R., and Antoniou E.. 2007. Expression profiling of heat stress effects on mice fed ergot alkaloids. Toxicol. Sci. 95:89–97. doi: 10.1093/toxsci/kfl142 [DOI] [PubMed] [Google Scholar]
- Bushel P. 2018. pvca: Principal variance component analysis (PVCA). R package version 1.24.0.
- Cui Y., Hao Y., Li J., Bao W., Li G., Gao Y., and Gu X.. 2016. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: A proteomic approach. Int. J. Mol. Sci. 17:393. doi: 10.3390/ijms17050393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvitovskaia A., Holmes S. P., and Huse S. M.. 2016. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 4:15. doi: 10.1186/s40168-016-0160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier R., Massabie P., and Bouby A.. 1998. Incidence du taux d’humidité relative de l’air ambiant sur les performances zootechniques du porc à l’engrais élevé à 28°C. J. Rech. Porc. 30:331–336. [Google Scholar]
- Gu Z. T., Li L., Wu F., Zhao P., Yang H., Liu Y. S., Geng Y., Zhao M., and Su L.. 2015. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 5:11497. doi: 10.1038/srep11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman D. B., Brunelle B. W., Trachsel J., and Allen H. K.. 2017. Meta-analysis to define a core microbiota in the swine gut. mSystems 2:e00004–e00017. doi: 10.1128/mSystems.00004-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C., Zeng X., Yang F., Liu H., and Qiao S.. 2015. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. J. Anim. Sci. Biotechnol. 6:14. doi: 10.1186/s40104-015-0014-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrycay E., Forrest D., Liu L., Wang R., Tai J., Deo A., Ling V., and Bandiera S.. 2014. Hepatic bile acid metabolism and expression of cytochrome P450 and related enzymes are altered in Bsep (−/−) mice. Mol. Cell. Biochem. 389:119–132. doi: 10.1007/s11010-013-1933-y [DOI] [PubMed] [Google Scholar]
- Huse S. M., Ye Y., Zhou Y., and Fodor A. A.. 2012. A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS One 7:e34242. doi: 10.1371/journal.pone.0034242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery I. B., Claesson M. J., O’Toole P. W., and Shanahan F.. 2012. Categorization of the gut microbiota: Enterotypes or gradients? Nat. Rev. Microbiol. 10:591–592. doi: 10.1038/nrmicro2859 [DOI] [PubMed] [Google Scholar]
- Knights D., Ward T. L., McKinlay C. E., Miller H., Gonzalez A., McDonald D., and Knight R.. 2014. Rethinking “enterotypes”. Cell Host Microbe. 16:433–437. doi: 10.1016/j.chom.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S., Alden N., and Lee K.. 2015. Pathways and functions of gut microbiota metabolism impacting host physiology. Curr. Opin. Biotechnol. 36:137–145. doi: 10.1016/j.copbio.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P. T., Sakamoto M., and Benno Y.. 2004. Effects of two probiotic Lactobacillus strains on jejunal and cecal microbiota of broiler chicken under acute heat stress condition as revealed by molecular analysis of 16S rRNA genes. Microbiol. Immunol. 48:917–929. doi: 10.1111/j.1348-0421.2004.tb03620.x [DOI] [PubMed] [Google Scholar]
- Langille M. G. I., Zaneveld J., Caporaso J. G., McDonald D., Knights D., Reyes J. A., Clemente J. C., Burkepile D. E., Thurber R. L. V., Knight R., . et al. 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31:814–821. doi: 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J. M. 2017. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 151:363–374. doi: 10.1111/imm.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê Cao K. A., Boitard S., and Besse P.. 2011. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinformatics. 12:253. doi: 10.1186/1471-2105-12-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Sciellour M., Labussière E., Zemb O., and Renaudeau D.. 2018. Effect of dietary fiber content on nutrient digestibility and fecal microbiota composition in growing-finishing pigs. PLoS One 13:e0206159. doi: 10.1371/journal.pone.0206159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., and Apte U.. 2015. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv. Pharmacol. 74:263–302. doi: 10.1016/bs.apha.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang X., Wang G., Li N., and Wu C.. 2011. Expression analysis of global gene response to chronic heat exposure in broiler chickens (Gallus gallus) reveals new reactive genes. Poult. Sci. 90:1028–1036. doi: 10.3382/ps.2010-01144 [DOI] [PubMed] [Google Scholar]
- Li S., Wang Z., Yang Y., Yang S., Yao C., Liu K., Cui S., Zou Q., Sun H., and Guo G.. 2017. Lachnospiraceae shift in the microbial community of mice faecal sample effects on water immersion restraint stress. AMB Express. 7:82. doi: 10.1186/s13568-017-0383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyau T., Hennequet-Antier C., Coustham V., Berri C., Leduc M., Crochet S., Sannier M., Duclos M. J., Mignon-Grasteau S., Tesseraud S., et al. 2016. Thermal manipulation of the chicken embryo triggers differential gene expression in response to a later heat challenge. BMC Genomics 17:329. doi: 10.1186/s12864-016-2661-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach N., Berri M., Estellé J., Levenez F., Lemonnier G., Denis C., Leplat J. J., Chevaleyre C., Billon Y., Doré J., et al. 2015. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ. Microbiol. Rep. 7:554–569. doi: 10.1111/1758-2229.12285 [DOI] [PubMed] [Google Scholar]
- Morrison S. R., and Mount L. E.. 1971. Adaptation of growing pigs to changes in environmental temperature. Anim. Sci. 13:51–57. doi: 10.1017/S0003356100029421 [DOI] [Google Scholar]
- Mulder I. E., Schmidt B., Stokes C. R., Lewis M., Bailey M., Aminov R. I., Prosser J. I., Gill B. P., Pluske J. R., Mayer C.-D., . et al. 2009. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 7:79. doi: 10.1186/1741-7007-7-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., E. Holmes J. Kinross R. Burcelin G. Gibson W. Jia, and Pettersson S.. 2012. Host-gut microbiota metabolic interactions. Science 336:1262–1267. doi: 10.1126/science.1223813 [DOI] [PubMed] [Google Scholar]
- Oksanen J., Blanchet G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P. R., O’Hara R. B., Simpson G. L., Solymos P., et al. 2018. Vegan: Community ecology package. R package version 2.5–3.
- Parag H. A., Raboy B., and Kulka R. G.. 1987. Effect of heat shock on protein degradation in mammalian cells: Involvement of the ubiquitin system. EMBO J. 6:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramayo-Caldas Y., Mach N., Lepage P., Levenez F., Denis C., Lemonnier G., Leplat J. J., Billon Y., Berri M., Doré J., . et al. 2016. Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME J. 10:2973–2977. doi: 10.1038/ismej.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau D., Frances G., Dubois S., Gilbert H., and Noblet J.. 2013. Effect of thermal heat stress on energy utilization in two lines of pigs divergently selected for residual feed intake. J. Anim. Sci. 91:1162–1175. doi: 10.2527/jas.2012-5689 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Giorgi M., Silou F., and Weisbecker J. L.. 2006. Effect of breed (lean or fat pigs) and sex on performance and feeding behaviour of group housed growing pigs in a tropical climate. Asian-Australas. J. Anim. Sci. 19:593–600. doi: 10.5713/ajas.2006.593 [DOI] [Google Scholar]
- Renaudeau D., Gourdine J. L., and St-Pierre N. R.. 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi: 10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Huc E., and Noblet J.. 2007. Acclimation to high ambient temperature in Large White and Caribbean Creole growing pigs. J. Anim. Sci. 85:779–790. doi: 10.2527/jas.2006-430 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Kerdoncuff M., Anaïs C., and Gourdine J. L.. 2008. Effect of temperature level on thermal acclimation in Large White growing pigs. Animal 2:1619. doi: 10.1017/S1751731108002814 [DOI] [PubMed] [Google Scholar]
- Riezman H. 2004. Why do cells require heat shock proteins to survive heat stress? Cell Cycle 3:60–62. doi: 10.4161/cc.3.1.625 [DOI] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., and Smyth G. K.. 2010. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognes T., Flouri T., Nichols B., Quince C., and Mahé F.. 2016. VSEARCH: A versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo D., Méndez-García C., Raczkowska B. A., Bargiela R., Moya A., Ferrer M., and Barbas C.. 2017. Exploring the human microbiome from multiple perspectives: Factors altering its composition and function. FEMS Microbiol. Rev. 41:453–478. doi: 10.1093/femsre/fuw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosé R., Gilbert H., Loyau T., Giorgi M., Billon Y., Riquet J., Renaudeau D., and Gourdine J. L.. 2017. Interactions between sire family and production environment (temperate vs. tropical) on performance and thermoregulation responses in grocing pigs. J. Anim. Sci. 95:4738–4751. doi: 10.2527/jas2017.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher J. U., Sczesnak A., Longman R. S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E. G., Abramson S. B., . et al. 2013. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2:e01202. doi: 10.7554/eLife.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze V., Roehe R., Looft H., and Kalm E.. 2001. Effects of continuous and periodic feeding by electronic feeders on accuracy of measuring feed intake information and their genetic association with growth performances. J. Anim. Breed. Genet. 118:403–416. doi: 10.1046/j.1439-0388.2001.00158.x [DOI] [Google Scholar]
- Song J., Xiao K., Ke Y. L., Jiao L. F., Hu C. H., Diao Q. Y., Shi B., and Zou X. T.. 2014. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 93:581–588. doi: 10.3382/ps.2013-03455 [DOI] [PubMed] [Google Scholar]
- Stothart M., and Newman A.. 2017 A gut reaction to city life, stress-microbiome interactions in urban squirrels.http://hdl.handle.net/10214/10375. Accessed 6 September 2018.
- St-Pierre N. R., Cobanov B., and Schnitkey G.. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Suzuki K., Harasawa R., Yoshitake Y., and Mitsuoka T.. 1983. Effects of crowding and heat stress on intestinal flora, body weight gain, and feed efficiency of growing rats and chicks. Nihon Juigaku Zasshi Jpn. J. Vet. Sci. 45:331–338. doi: 10.1292/jvms1939.45.331 [DOI] [PubMed] [Google Scholar]
- Tajima K., Nonaka I., Higuchi K., Takusari N., Kurihara M., Takenaka A., Mitsumori M., Kajikawa H., and Aminov R. I.. 2007. Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe 13:57–64. doi: 10.1016/j.anaerobe.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Uyeno Y., Sekiguchi Y., Tajima K., Takenaka A., Kurihara M., and Kamagata Y.. 2010. An rRNA-based analysis for evaluating the effect of heat stress on the rumen microbial composition of Holstein heifers. Anaerobe 16:27–33. doi: 10.1016/j.anaerobe.2009.04.006 [DOI] [PubMed] [Google Scholar]
- Valeriano V. D. V., Balolong M. P., and Kang D. K.. 2017. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 122:554–567. doi: 10.1111/jam.13364 [DOI] [PubMed] [Google Scholar]
- Verschuren L. M. G., Calus M. P. L., Jansman A. J. M., Bergsma R., Knol E. F., Gilbert H., and Zemb O.. 2018. Fecal microbial composition associated with variation in feed efficiency in pigs depends on diet and sex. J. Anim. Sci. 96:1405–1418. doi: 10.1093/jas/sky060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. H., Cheng C. Y., Chen C. J., Chan H. L., Chen H. H., Tang P. C., Chen C. F., Lee Y. P., and Huang S. Y.. 2018. Acute heat stress changes protein expression in the testes of a broiler-type strain of Taiwan country chickens. Anim. Biotechnol. 30:129–145. doi: 10.1080/10495398.2018.1446972 [DOI] [PubMed] [Google Scholar]
- Wilson I. D., and Nicholson J. K.. 2017. Gut microbiome interactions with drug metabolism, efficacy and toxicity. Transl. Res. J. Lab. Clin. Med. 179:204–222. doi: 10.1016/j.trsl.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. D., Chen J., Hoffmann C., Bittinger K., Chen Y. Y., Keilbaugh S. A., Bewtra M., Knights D., Walters W. A., Knight R., . et al. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. doi: 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Estellé J., Kiilerich P., Ramayo-Caldas Y., Xia Z., Feng Q., Liang S., Pedersen A. Ø., Kjeldsen N. J., Liu C., . et al. 2016. A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 1:16161. doi: 10.1038/nmicrobiol.2016.161 [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang Y., Liu S., Huang J., Zhai Z., He C., Ding J., Wang J., Wang H., Fan W., Zhao J., and Meng H.. 2015. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One 10:e0117441. doi: 10.1371/journal.pone.0117441 [DOI] [PMC free article] [PubMed] [Google Scholar]