ABSTRACT
Background
Studies have found beneficial effects of plant-based diets on weight. However, not all plant foods are necessarily beneficial.
Objectives
The aim of this study was to examine associations of changes in intake of 3 variations of plant-based diet indices (overall, healthful, and unhealthful) with weight change over 4-y intervals spanning >20 y.
Methods
Data from 3 ongoing prospective observational cohort studies in the United States were used, namely the Nurses’ Health Study (NHS), NHS2, and the Health Professionals Follow-up Study (HPFS), with 126,982 adult men and women. Self-reported diet data were collected every 4 y, and self-reported weight data were used to compute weight change every 4 y over >20 y of follow-up.
Results
On average, participants gained a mean of 0.90 kg (HPFS) to 1.98 kg (NHS2) over 4-y intervals. Different types of plant-based diet indices were associated with different amounts of weight gain. After adjusting for several potential confounders, including concomitant changes in other lifestyle factors, a 1-SD increase in intake of an overall plant-based diet index was associated with 0.04 kg less weight gain over 4-y periods (95% CI: 0.05, 0.02 kg; P < 0.001). A 1-SD increase in intake of a healthful version of a plant-based diet index (emphasizing whole grains, fruits/vegetables, nuts/legumes, vegetable oils, tea/coffee) was associated with 0.68 kg less weight gain over 4-y periods (95% CI: 0.69, 0.66 kg; P < 0.001). Conversely, a 1-SD increase in an unhealthful version of a plant-based diet index (emphasizing refined grains, potato/fries, sweets, sweetened drinks/juices) was associated with 0.36 kg more weight gain (95% CI: 0.34, 0.37 kg, P < 0.001).
Conclusion
Plant-based diets, especially when rich in healthier plant foods, are associated with less weight gain over 4-y intervals. This supports current recommendations to increase intake of healthy plant foods, and reducing intake of less-healthy plant foods and animal foods, for improved health outcomes.
Keywords: plant-based diets, weight change, prospective cohort studies, dietary pattern, epidemiology, obesity
Introduction
Overweight and obesity combined constitute the fifth leading cause of death in the world (1), posing an immense public health challenge. For many people, losing weight is difficult once gained, and for those who have lost weight, maintaining it over the long term is also challenging (2); thus, prevention of weight gain is an important public health goal.
Several studies have found plant-based diets to be associated with favorable weight outcomes (3–7). A meta-analysis of 12 randomized controlled trials (RCTs) found plant-based diets, defined as vegetarian or vegan diets, to result in significantly greater weight loss relative to a range of control diets (7). However, these interventions lasted for relatively short durations (median duration = 18 wk). Defining a plant-based diet as a vegetarian diet can also pose challenges when translating findings to public health and clinical applications. Extreme dietary changes such as complete exclusion of animal foods can be hard to adopt and adhere to for long periods. Such a diet also does not distinguish between high-quality plant foods (e.g., whole grains, nuts), which have been associated with improved weight outcomes, and low-quality plant foods [e.g., sugar-sweetened beverages (SSBs), refined grains], which have been associated with increased weight gain (8).
To overcome these limitations, we have created 3 versions of graded plant-based diets (9, 10): an overall plant-based diet index (PDI) which emphasizes consumption of all plant foods and reducing animal food intake; a healthful plant-based diet index (hPDI) emphasizing intake of healthy plant foods; and an unhealthful plant-based diet index (uPDI) emphasizing consumption of less-healthy plant foods. In the present analysis, we aimed to examine the associations of 4-y changes in these plant-based diet indices with weight change over the same period, spanning >20 y of follow-up in 3 cohorts.
Methods
Study population
We used data from 3 ongoing prospective cohort studies in the United States: the Nurses’ Health Study (NHS) initiated in 1976 with 121,701 female nurses (aged 30–55 y), NHS2 initiated in 1989 with 116,686 female nurses (aged 25–42 y), and the Health Professionals Follow-Up Study (HPFS) initiated in 1986 with 51,529 male health professionals (aged 40–75 y). Every 2 y, participants receive a follow-up questionnaire on lifestyle factors and medical history. We chose the baseline for this analysis as the follow-up cycle when detailed information on diet, weight, and key covariates were first available (1986 for NHS and HPFS, 1991 for NHS2). The study protocol was approved by the institutional review boards of the Harvard TH Chan School of Public Health and Brigham and Women's Hospital. All participants provided voluntary responses to mailed questionnaires that served as informed consent.
We excluded participants with self-reported diabetes, cardiovascular diseases (myocardial infarction, angina, coronary artery surgery, stroke, pulmonary embolism), cancer (except nonmelanoma skin cancer), respiratory diseases (emphysema, active tuberculosis, chronic bronchitis), neurodegenerative disorders (amyotrophic lateral sclerosis, Parkinson's disease, multiple sclerosis, Alzheimer's disease), gastric conditions (ulcerative colitis, gastric or duodenal ulcers, gastric surgery/intestinal bypass), chronic kidney disease, or systemic lupus erythematosus at baseline. Additional baseline exclusions were implausible energy intake (<600 or >3500 kcal/d for women and <800 or >4200 kcal/d for men), and missing data on diet change or weight change. We also excluded participants >65 y of age, as weight loss at older ages may reflect loss of lean mass. During follow-up, we censored individuals when they reported a diabetes diagnosis, and 6 y prior to diagnoses of cancer, respiratory diseases, neurodegenerative disorders, gastric conditions, chronic kidney disease, or lupus. We continued to censor individuals aged >65 y and those with missing data on diet change and weight change over the follow-up. Lastly, we excluded newly pregnant women for one 4-y cycle. There were 46,790 women in NHS, 59,217 women in NHS2, and 20,975 men in HPFS after baseline exclusions.
Diet assessment
Dietary data were collected every 4 y using a semiquantitative food-frequency questionnaire, in which participants reported how often, on average, they had consumed defined portions of ∼130 food items over the previous year through the use of 9 response categories ranging from “never or less than once/month” to “≥6 times per day.” The reliability and validity of the questionnaires have been described previously (11–14).
We created the 3 indices, PDI, hPDI, and uPDI, as follows. First, we first created 18 food groups based on nutrient and culinary similarities within the larger categories of healthy plant foods (whole grains, fruits, vegetables, nuts, legumes, vegetable oils, tea/coffee), less-healthy plant foods (fruit juices, SSBs, refined grains, potatoes, sweets), and animal foods (butter/lard, dairy, egg, fish/seafood, meat, miscellaneous animal-based foods) (Supplemental Table 1). Intake of each food group was ranked into quintiles and given positive or reverse scores. With positive scores, participants above the highest quintile of a food group received a score of 5, following through to participants below the lowest quintile who received a score of 1. With reverse scores, participants above the highest quintile of a food group received a score of 1, following through to participants below the lowest quintile who received a score of 5. To create the PDI, we gave positive scores to all plant food groups and reverse scores to animal food groups. For the hPDI, we gave positive scores to healthy plant food groups, and reverse scores to less-healthy plant food groups and animal food groups. For the uPDI we gave positive scores to less-healthy plant food groups, and reverse scores to healthy plant food groups and animal food groups. The 18 food group scores were summed to obtain the indices. Alcoholic beverages are associated in different directions with various diseases, and margarine's fatty acid composition has changed from high trans to high unsaturated fats; hence, we adjusted for them as covariates in multivariable analyses instead of including them in the indices.
Weight change
Participants reported their height in inches and weight in pounds at baseline and provided updated information on weight on the biennial questionnaires thereafter. These self-reported weight data have been previously validated in these cohorts, with a Spearman correlation coefficient of 0.96 with measured weight (15). Weight change was calculated by subtracting weight at the start of each 4-y interval from weight at the end of the 4-y interval. Weight change (in pounds) over 4-y intervals was the predeclared primary endpoint of this study. Analyses not prespecified are considered exploratory.
Assessment of covariates
The biennial questionnaires assess updated information on disease diagnoses, medication use, and several lifestyle factors including physical activity, smoking, sleep duration, and hours of sitting and television watching. Among women, updated information is also assessed on parity, menopausal status, postmenopausal hormone use, and oral contraceptive use (NHS2 only).
Statistical analysis
We used multivariable generalized linear regression models (with unstructured correlation matrix and robust variance) to examine the associations of 4-y changes in intake of the indices with concomitant 4-y weight changes. We had six 4-y cycles for NHS and HPFS (1986–2010), and five 4-y cycles for NHS2 (1991–2011). In each 4-y interval, we adjusted for age, questionnaire cycle, baseline BMI, baseline and change in physical activity, daily hours of sleep, weekly hours of sitting and watching television, change in smoking status, and changes in alcohol and margarine intake. Among women, we additionally adjusted for parity, menopausal status and postmenopausal hormone use, and oral contraceptive use. We examined associations with quintiles of change in each index, as well as with per-SD increase in each index. Changes in weight and diet were truncated at the 0.5th and 99.5th percentiles to minimize influence of outliers.
We examined potential interactions with BMI, physical activity, age, race, smoking status, and baseline carbohydrate intake. We also examined associations with joint classifications of changes in physical activity and changes in the indices, given previously documented associations of physical activity with weight change (8). We evaluated independent associations of the 3 food categories that constituted the indices (healthy plant foods, less-healthy plant foods, animal foods) with weight change by entering all 3 simultaneously into the model in place of the individual indices. The analysis was carried out separately for each cohort, and combined using meta-analysis with a fixed-effects model; we examined potential heterogeneity across the three cohorts through the use of the Cochrane Q statistic (16). Analyses were performed with SAS version 9.2 (SAS Institute Inc.), and statistical significance was set at a 2-factor P value <0.05.
Results
At baseline, on average, participants were aged 52 y in the NHS, aged 37 y in the NHS2, and aged 50 y in the HPFS, with a mean BMI of 25 kg/m2 (Table 1). On average, participants gained weight over each 4-y follow-up cycle (2.0–4.4 lb) (1 lb is equal to 0.45 kg), with mean weight gain being lowest in HPFS and highest in NHS2. Given this, inverse associations of the diet indices with weight change (i.e., negative values) should be interpreted as “less weight gain,” not as “weight loss.” In other words, most participants gained weight over time in these cohorts, and the positive and inverse (negative) associations reported in Table 1 reflect different amounts of weight gain (more weight gain or less weight gain), and not weight loss. On average, participants consumed 4–5 servings per d of animal foods, 9–11 servings per d of healthy plant foods, and 4–5 servings per d of less-healthy plant foods at baseline. Participants who increased PDI and hPDI intake during the first 4-y interval showed less mean weight gain and more favorable changes in physical activity over the same interval relative to participants who decreased intake of these indices; opposite patterns were observed for uPDI (Supplemental Table 2). Averaged across the 4-y intervals, an increase in hPDI intake was reflective of an increase in healthy plant food intake, and a decrease in less-healthy plant food and animal food intake (Supplemental Table 3).
TABLE 1.
Age-standardized characteristics of participants in the 3 cohorts1
| NHS (n = 46,790) | NHS2 (n = 59,217) | HPFS (n = 20,975) | ||||
|---|---|---|---|---|---|---|
| Baseline (1986) | 4-y change2 | Baseline (1991) | 4-y change2 | Baseline (1986) | 4-y change2 | |
| Age, y | 52 ± 7.1 | — | 37 ± 4.4 | — | 50 ± 7.7 | —- |
| Alcohol, g/d | 6.4 ± 11 | −0.14 ± 1.4 | 3.2 ± 6.2 | 0.69 ± 1.7 | 11 ± 15 | 0.26 ± 2.1 |
| Energy intake, kcal/d | 1770 ± 523 | −5.8 ± 89 | 1780 ± 540 | −7.6 ± 145 | 2009 ± 614 | −6.1 ± 109 |
| Overall plant-based diet index | 55 ± 6.4 | 0.18 ± 1.3 | 55 ± 6.6 | −0.02 ± 2.4 | 55 ± 6.5 | 0.32 ± 1.5 |
| Healthful plant-based diet index | 55 ± 7.3 | −0.03 ± 1.4 | 55 ± 7.4 | −0.01 ± 2.2 | 54 ± 7.5 | 0.45 ± 1.5 |
| Unhealthful plant-based diet index | 55 ± 7.6 | −0.52 ± 1.5 | 55 ± 7.6 | −0.77 ± 2.7 | 55 ± 7.0 | −0.36 ± 1.6 |
| Animal food intake, servings/d | 4.8 ± 2.0 | −0.11 ± 0.49 | 4.3 ± 2.0 | −0.06 ± 0.85 | 4.7 ± 2.4 | −0.07 ± 0.60 |
| Healthy plant food intake, servings/d | 11 ± 4.1 | 0.03 ± 0.79 | 9.1(4.2 | 0.12 ± 1.5 | 9.7 ± 4.1 | 0.32 ± 0.90 |
| Less-healthy plant food intake, servings/d | 4.2 ± 2.3 | −0.04 ± 0.47 | 4.4 ± 2.3 | −0.34 ± 0.79 | 4.7 ± 2.6 | −0.09 ± 0.57 |
| Physical activity, MET/wk | 14 ± 21 | 0.66 ± 5.3 | 21 ± 27 | 1.1 ± 9.3 | 22 ± 30 | 6.1 ± 8.8 |
| Sitting and watching television, h/wk | 13 ± 12 | — | 2.5 ± 0.80 | — | 11 ± 8.4 | 3.9 ± 0.51 |
| Sleep, h/d | 7.0 ± 1.0 | — | 7.0 ± 1.0 | — | 7.1 ± 0.9 | — |
| Weight, lb | 148 ± 29 | 2.6 ± 2.4 | 147 ± 33 | 4.4 ± 4.9 | 179 ± 25 | 2.0 ± 2.2 |
| BMI, kg/m2 | 25 ± 4.5 | 0.44 ± 0.46 | 25 ± 5.3 | 0.73 ± 0.87 | 25 ± 3.1 | 0.27 ± 0.34 |
| BMI category, % | ||||||
| <25 | 60 | — | 66 | — | 48 | — |
| ≥25–<30 | 28 | — | 20 | — | 44 | — |
| ≥30 | 12 | — | 13 | — | 7.6 | — |
| Smoking, % | ||||||
| Never | 46 | — | 66 | — | 51 | — |
| Past | 35 | — | 23 | — | 40 | — |
| Current | 19 | — | 12 | — | 9.1 | — |
Data are means ± SDs for continuous variables, and percentages for dichotomous variables. 1 lb = 0.45 kg.
HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent task; NHS, Nurses’ Health Study.
Averaged (mean) over the entire follow-up.
Pooling across the cohorts, after adjusting for multiple potential confounders, each 1-SD increase in PDI over 4 y was associated with a weight change of −0.09 lb (95% CI: −0.12, −0.05 lb; P < 0.001) (i.e., per 1-SD increase was associated with less weight gain over time) (Table 2). This inverse association was substantially stronger for hPDI, with each 1-SD increase over 4-y periods associated with a weight change of −1.50 lb (95% CI: −1.53, −1.46 lb; P < 0.001) (Table 3). Conversely, each 1-SD increase in uPDI over 4-y periods was associated with a weight change of 0.79 lb (95% CI: 0.75, 0.82 lb; P < 0.001) (i.e., per 1-SD increase was associated with more weight gain over time). We found similar results when we examined associations with quintiles of index change. For instance, relative to participants who did not change their hPDI intake over 4 y (and on average gained 3.1 lb), those who increased their hPDI intake (quintile 5) experienced less weight gain (−2.21 lb; 95% CI: −2.31, −2.11 lb), whereas those who decreased their hPDI intake (quintile 1) experienced more weight gain (1.86 lb; 95% CI: 1.76, 1.96 lb). There was significant heterogeneity across the cohorts, with associations being strongest for NHS2, with the exception of PDI for which associations were strongest for HPFS (P-heterogeneity < 0.05).
TABLE 2.
Weight change over 4-y periods according to quintiles of change in the overall plant-based diet index1
| Q1 | Q2 | Q3 | Q4 | Q5 | Per 1-SD increase | P value2 | |
|---|---|---|---|---|---|---|---|
| NHS (542,167 person-years) | |||||||
| Median index change | −7 | −3 | 0 | 3 | 7 | ||
| Mean weight change, lb | 2.7 | 2.7 | 2.8 | 2.5 | 2.4 | ||
| Age-adjusted model, lb | −0.16 (−0.32, 0.01) | −0.18 (−0.34, −0.02) | Ref | −0.23 (−0.38, −0.07) | −0.14 (−0.31, 0.02) | 0.00 (−0.06, 0.06) | 0.93 |
| Multivariable model, lb | −0.05 (−0.21, 0.12) | 0.03 (−0.13, 0.19) | Ref | −0.04 (−0.20, 0.11) | −0.12 (−0.28, 0.04) | −0.04 (−0.10, 0.02) | 0.21 |
| NHS2 (933,040 person-years) | |||||||
| Median index change | −7 | −3 | 0 | 3 | 7 | ||
| Mean weight change, lb | 4.4 | 4.4 | 4.4 | 4.5 | 4.3 | ||
| Age-adjusted model, lb | −0.05 (−0.21, 0.10) | −0.09 (−0.23, 0.06) | Ref | 0.15 (0.00, 0.30) | 0.04 (−0.10, 0.19) | 0.02 (−0.04, 0.07) | 0.52 |
| Multivariable model, lb | −0.04 (−0.19, 0.12) | −0.15 (−0.29, 0.00) | Ref | 0.07 (−0.08, 0.21) | −0.07 (−0.21, 0.08) | −0.03 (−0.09, 0.03) | 0.29 |
| HPFS (221,168 person-years) | |||||||
| Median index change | −7 | −2 | 1 | 3 | 7 | ||
| Mean weight change, lb | 2.2 | 2.1 | 2.0 | 1.8 | 1.5 | ||
| Age-adjusted model, lb | 0.18 (−0.04, 0.39) | 0.12 (−0.07, 0.32) | Ref | −0.21 (−0.41, 0.00) | −0.49 (−0.70, −0.29) | −0.27 (−0.35, −0.19) | <0.001 |
| Multivariable model, lb | 0.19 (−0.02, 0.40) | 0.13 (−0.07, 0.32) | Ref | −0.22 (−0.42, −0.01) | −0.55 (−0.76, −0.35) | −0.29 (−0.36, −0.21) | <0.001 |
| Pooled (fixed-effects model) | |||||||
| Age-adjusted model, lb | −0.04 (−0.14, 0.06) | −0.07 (−0.16, 0.02) | Ref | −0.07 (−0.17, 0.02)3 | −0.14 (−0.24, −0.04)3 | −0.05 (−0.09, −0.02)3 | 0.0053 |
| Multivariable model, lb | 0.01 (−0.09, 0.11) | −0.02 (−0.11, 0.07) | Ref | −0.04 (−0.13, 0.06) | −0.19 (−0.29, −0.10)3 | −0.09 (−0.12, −0.05)3 | <0.0013 |
Values are weight change in lb (95% CI). Given that most participants gained weight over 4-y intervals, inverse associations of the diet indices with weight change (i.e., negative values) should be interpreted as “less weight gain,” not as “weight loss.” We used multivariable generalized linear regression models (with unstructured correlation matrix and robust variance) to conduct this analysis. Multivariable model: adjusted for age (y, continuous), questionnaire cycle (4-y intervals), baseline BMI (kg/m2, continuous), baseline and change in physical activity (MET/wk, continuous), baseline hours of sleep per day (≤6, 7, 8, and >8 h), hours of sitting and watching television per week [baseline only in NHS and NHS2 (0–1, 2–5, 6–20, 21–40, and >40 h); and also change in HPFS (continuous)], change in smoking status (stayed never smoker, stayed former smoker, stayed current smoker, change from former to current smoker, change from never to current smoker, and change from current to former smoker), and changes in alcohol intake (continuous) and margarine intake (continuous), and among women only, baseline parity (0, 1–2, 3, ≥4 children), menopausal status and postmenopausal hormone use (premenopausal, and postmenopausal never, current, past users), and oral contraceptive use (never, current, and past users, NHS2 only). 1 lb = 0.45 kg. HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent task; NHS, Nurses’ Health Study.
P value obtained from per 1-SD increase in β coefficient.
P value for Q statistic for heterogeneity <0.05, indicating statistically significant heterogeneity in results among the 3 studies.
TABLE 3.
Weight change over 4-y periods according to quintiles of change in the hPDI and uDPI1
| Q1 | Q2 | Q3 | Q4 | Q5 | Per 1-SD increase | P value2 | |
|---|---|---|---|---|---|---|---|
| hPDI | |||||||
| NHS | |||||||
| Median index change | −8 | −4 | 0 | 3 | 8 | ||
| Mean weight change, lb | 4.3 | 3.3 | 2.8 | 2.1 | 0.69 | ||
| Age-adjusted model, lb | 1.75 (1.58, 1.91) | 0.53 (0.37, 0.69) | Ref | −0.76 (−0.91, −0.60) | −2.13 (−2.30, −1.96) | −1.40 (−1.47, −1.34) | <0.001 |
| Multivariable model, lb | 1.71 (1.54, 1.87) | 0.51 (0.36, 0.67) | Ref | −0.73 (−0.88, −0.58) | −2.10 (−2.27, −1.94) | −1.39 (−1.45, −1.33) | <0.001 |
| NHS2 | |||||||
| Median index change | −8 | −3 | 0 | 3 | 9 | ||
| Mean weight change, lb | 6.6 | 5.3 | 4.5 | 3.7 | 2.0 | ||
| Age-adjusted model, lb | 2.25 (2.10, 2.40) | 0.75 (0.60, 0.89) | Ref | −0.89 (−1.03, −0.74) | −2.53 (−2.69, −2.38) | −1.77 (−1.83, −1.71) | <0.001 |
| Multivariable model, lb | 2.19 (2.04, 2.34) | 0.66 (0.52, 0.81) | Ref | −0.88 (−1.03, −0.74) | −2.49 (−2.65, −2.34) | −1.74 (−1.80, −1.68) | <0.001 |
| HPFS | |||||||
| Median index change | −8 | −3 | 0 | 4 | 9 | ||
| Mean weight change, lb | 3.4 | 2.5 | 2.1 | 1.3 | 0.29 | ||
| Age-adjusted model | 1.46 (1.24, 1.67) | 0.52 (0.32, 0.71) | Ref | −0.78 (−0.99, −0.58) | −1.83 (−2.04, −1.62) | −1.26 (−1.34, −1.18) | <0.001 |
| Multivariable model | 1.45 (1.24, 1.66) | 0.54 (0.35, 0.74) | Ref | −0.71 (−0.91, −0.51) | −1.85 (−2.06, −1.64) | −1.25 (−1.33, −1.17) | <0.001 |
| Pooled (fixed-effects model) | |||||||
| Age-adjusted model | 1.90 (1.80, 2.00)3 | 0.62 (0.52, 0.71) | Ref | −0.82 (−0.91, −0.72) | −2.23 (−2.33, −2.13)3 | −1.52 (−1.55, −1.48)3 | <0.0013 |
| Multivariable model | 1.86 (1.76, 1.96)3 | 0.58 (0.49, 0.67) | Ref | −0.79 (−0.88, −0.70) | −2.21 (−2.31, −2.11)3 | −1.50 (−1.53, −1.46)3 | <0.0013 |
| uPDI | |||||||
| NHS | |||||||
| Median index change | −7 | −3 | 0 | 3 | 7 | ||
| Mean weight change, lb | 1.9 | 2.4 | 2.7 | 2.9 lbs | 3.3 | ||
| Age-adjusted model, lb | −0.97 (−1.13, −0.81) | −0.33 (−0.49, −0.17) | Ref | 0.25 (0.09, 0.41) | 0.84 (0.68, 1.01) | 0.66 (0.60, 0.72) | <0.001 |
| Multivariable model, lb | −0.90 (−1.06, −0.74) | −0.15 (−0.31, 0.01) | Ref | 0.44 (0.28, 0.60) | 0.88 (0.72, 1.04) | 0.66 (0.60, 0.72) | <0.001 |
| NHS2 | |||||||
| Median index change | −8 | −4 | −1 | 2 | 7 | ||
| Mean weight change, lb | 3.1 | 4.0 | 4.5 | 4.9 | 5.6 | ||
| Age-adjusted model, lb | −1.61 (−1.77, −1.46) | −0.58 (−0.72, −0.43) | Ref | 0.44 (0.29, 0.59) | 1.43 (1.27, 1.58) | 1.09 (1.03, 1.15) | <0.001 |
| Multivariable model, lb | −1.56 (−1.71, −1.40) | −0.51 (−0.65, −0.37) | Ref | 0.48 (0.34, 0.63) | 1.47 (1.32, 1.62) | 1.10 (1.04, 1.16) | <0.001 |
| HPFS | |||||||
| Median index change | −7 | −3 | 0 | 3 | 7 | ||
| Mean weight change, lb | 1.3 | 1.8 | 2.1 | 2.1 | 2.3 | ||
| Age-adjusted model, lb | −0.90 (−1.12, −0.68) | −0.28 (−0.48, −0.08) | Ref | 0.01 (−0.19, 0.21) | 0.35 (0.13, 0.57) | 0.43 (0.36, 0.51) | <0.001 |
| Multivariable model, lb | −0.93 (−1.15, −0.71) | −0.29 (−0.49, −0.10) | Ref | 0.02 (−0.18, 0.22) | 0.34 (0.12, 0.55) | 0.43 (0.36, 0.51) | <0.001 |
| Pooled (fixed-effects model) | |||||||
| Age-adjusted model, lb | −1.22 (−1.32, −1.13)3 | −0.42 (−0.52, −0.33)3 | Ref | 0.28 (0.18, 0.37)3 | 0.99 (0.88, 1.09)3 | 0.79 (0.75, 0.82)3 | <0.0013 |
| Multivariable model, lb | −1.18 (−1.28, −1.08)3 | −0.34 (−0.43, −0.24)3 | Ref | 0.36 (0.27, 0.46)3 | 1.01 (0.91, 1.11)3 | 0.79 (0.75, 0.82)3 | <0.0013 |
Values are weight change in lb (95% CI). Given that most participants gained weight over 4-y intervals, inverse associations of the diet indices with weight change (i.e., negative values) should be interpreted as “less weight gain,” not as “weight loss.” We used multivariable generalized linear regression models (with unstructured correlation matrix and robust variance) to conduct this analysis. Multivariable model: adjusted for age, questionnaire cycle, baseline BMI, change in smoking status, baseline and change in physical activity, hours of sleep, hours of sitting and watching television, change in alcohol consumption, change in margarine intake, and for women only, baseline parity, menopausal status, postmenopausal hormone use, and oral contraceptive use (NHS2 only). 1 lb = 0.45 kg. hPDI, healthful plant-based diet index; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; uPDI, unhealthful plant-based diet index.
P value obtained from per 1-SD increase in β coefficient.
P value for Q statistic for heterogeneity <0.05, indicating statistically significant heterogeneity in results among the 3 studies.
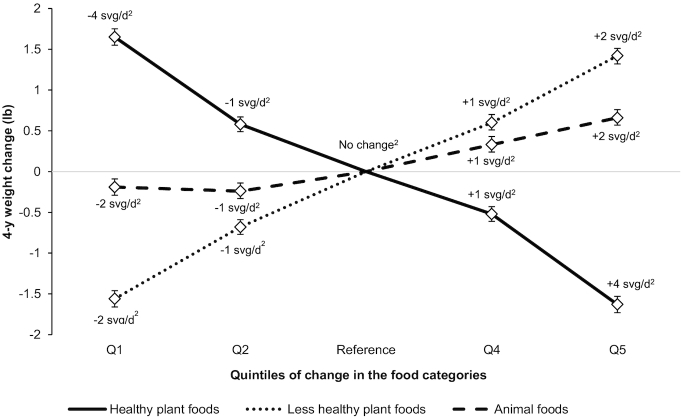
when we adjusted for additional covariates, including markers of socioeconomic status, marital status, and ethnicity [weight change per 1-SD increase in PDI: −0.08 lb (95% CI: −0.11, −0.04 lb; P < 0.001), in hPDI: −1.51 lb (95% CI: −1.54, −1.47 lb; P < 0.001), in uPDI: 0.79 lb (95% CI: 0.75, 0.82 lb; P < 0.001)]. The results were also unchanged upon further adjustment for red meat intake [weight change per 1-SD increase in PDI: −0.09 lb (95% CI: −0.12, −0.05 lb; P < 0.001), in hPDI: −1.50 lb (95% CI: −1.53, −1.46 lb; P < 0.001), in uPDI: 0.79 lb (95% CI: 0.76, 0.83 lb; P < 0.001)]. When we adjusted for SSB intake, the associations became slightly stronger for PDI (weight change per 1-SD increase: −0.20 lb; 95% CI: −0.23, −016 lb; P < 0.001), and weaker for hPDI (−1.43 lb; 95% CI: −1.47, −1.39 lb; P < 0.001) and uPDI (0.74 lb; 95% CI: 0.70, 0.78 lb; P < 0.001). When we simultaneously included the 3 food categories (animal, healthy plant, and less-healthy plant foods) in the same model in place of the individual indices, we found positive associations of animal foods and less-healthy plant foods, and an inverse association of healthy plant foods with weight change (Figure 1).
FIGURE 1.
Weight change over 4-y periods according to quintiles of change in animal foods and healthy and less-healthy plant foods. Values are weight change in lb (95% CI) pooled across the cohorts through the use of a fixed-effects model. Given that most participants gained weight over 4-y intervals, inverse associations of the diet indices with weight change (i.e., negative values) should be interpreted as “less weight gain,” not as “weight loss.” We used multivariable generalized linear regression models (with unstructured correlation matrix and robust variance) to conduct this analysis. Adjusted for age, questionnaire cycle, baseline BMI, change in smoking status, baseline and change in physical activity, hours of sleep, hours of sitting and watching television, change in alcohol consumption, change in margarine intake, and for women only, baseline parity, menopausal status, postmenopausal hormone use, and oral contraceptive use (NHS2 only). All 3 food categories were entered simultaneously into the fully adjusted model. All P values <0.001. 1 lb = 0.45 kg. NHS, Nurses’ Health Study. 2Median change in each quintile (averaged across the 3 cohorts).
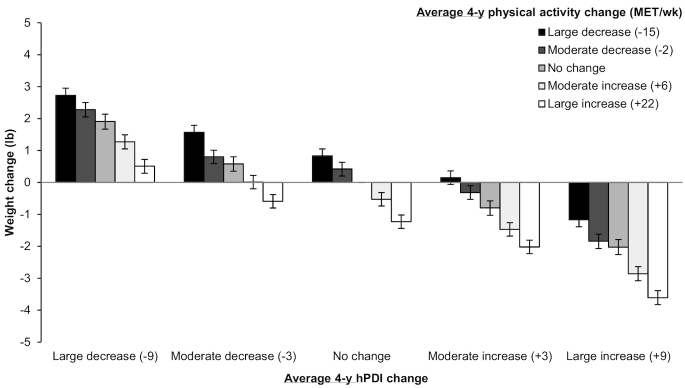
The associations of hPDI and uPDI with 4-y weight change were stronger among participants who were Caucasian, overweight/obese, younger, less active, and had lower baseline carbohydrate intake, although the associations were in the same direction and were statistically significant in all subgroups (Table 4). PDI was inversely associated with weight change in normal-weight and overweight participants, but positively associated with weight change among obese participants. Associations of PDI with weight change were stronger among more-active relative to less-active participants. For uPDI, associations were significantly stronger among never smokers compared with ever smokers. We also jointly classified participants according to changes in intake of hPDI and physical activity (Figure 2). Compared with participants who did not change their hPDI intake or physical activity levels over 4-y intervals (and gained, on average, 3.2 lb during those intervals), those who had large increases in both experienced substantially less weight gain (−3.61 lb; 95% CI: −3.83, −3.39 lb), whereas those who had large decreases in both experienced substantially more weight gain (2.73 lb; 95% CI: 2.50, 2.95 lb). Of note, even when participants decreased their physical activity levels, a large increase in hPDI intake was associated with significantly lower weight gain over 4 y relative to no change in either behavior (−1.17 lb; 95% CI: −1.39, −0.94 lb).
TABLE 4.
Weight change over 4-y periods per 1-SD increase in plant-based diet indices, stratified by selected characteristics1
| PDI | hPDI | uPDI | |
|---|---|---|---|
| Race | |||
| Caucasian | −0.09 (−0.12, −0.05)2 | −1.50 (−1.54, −1.46)2 | 0.80 (0.76, 0.83)2 |
| Black | −0.05 (−0.40, 0.30) | −1.26 (−1.67, −0.86)2 | 0.88 (0.49, 1.28)2 |
| Other3 | −0.05 (−0.22, 0.12) | −1.32 (−1.50, −1.14) | 0.56 (0.39, 0.73) |
| P-interaction 14 | 0.55 | 0.14 | 0.59 |
| P-interaction 25 | 0.90 | 0.03 | 0.01 |
| BMI, kg/m2 | |||
| <25 | −0.16 (−0.19, −0.12)2 | −0.76 (−0.79, −0.72)2 | 0.38 (0.34, 0.41)2 |
| 25–<30 | −0.08 (−0.15, −0.02)2 | −1.68 (−1.75, −1.62)2 | 0.90 (0.84, 0.97)2 |
| ≥30 | 0.22 (0.09, 0.34) | −3.05 (−3.18, −2.93)2 | 1.68 (1.56, 1.80)2 |
| P-interaction 16 | <0.0012 | <0.0012 | <0.0012 |
| P-interaction 27 | <0.001 | <0.0012 | <0.0012 |
| Age, y | |||
| <55 | −0.10 (−0.15, −0.06)2 | −1.63 (−1.67, −1.58)2 | 0.93 (0.88, 0.97)2 |
| ≥55 | −0.10 (−0.16, −0.04)2 | −1.23 (−1.29, −1.17)2 | 0.55 (0.49, 0.61)2 |
| P-interaction | 0.0262 | <0.0012 | 0.0012 |
| Physical activity levels | |||
| <median MET/wk | −0.07 (−0.12, −0.02)2 | −1.65 (−1.71, −1.60)2 | 0.88 (0.82, 0.93)2 |
| ≥median MET/wk | −0.09 (−0.14, −0.04)2 | −1.29 (−1.34, −1.24)2 | 0.67 (0.62, 0.72)2 |
| P-interaction | 0.0032 | <0.001 | <0.0012 |
| Baseline carbohydrate intake | |||
| <median g/d | −0.13 (−0.18, −0.08)2 | −1.54 (−1.60, −1.49)2 | 0.84 (0.79, 0.90)2 |
| ≥median g/d | −0.05 (−0.10, −0.00)2 | −1.42 (−1.47, −1.37)2 | 0.75 (0.70, 0.79)2 |
| P-interaction | 0.09 | 0.0012 | 0.02 |
| Smoking status | |||
| Ever smokers | −0.11 (−0.17, −0.05)2 | −1.44 (−1.50, −1.38)2 | 0.65 (0.59, 0.70)2 |
| Never smokers | −0.05 (−0.10, 0.00)2 | −1.50 (−1.55, −1.45)2 | 0.87 (0.82, 0.92)2 |
| P-interaction | 0.140 | 0.78 | <0.0012 |
Values are weight change in lb (95% CI) pooled across the cohorts using a fixed-effects model. Given that most participants gained weight over 4-y intervals, inverse associations of the diet indices with weight change (i.e., negative values) should be interpreted as “less weight gain,” not as “weight loss.” We used multivariable generalized linear regression models (with unstructured correlation matrix and robust variance) to conduct this analysis. Adjusted for age, questionnaire cycle, baseline BMI, change in smoking status, baseline and change in physical activity, hours of sleep, hours of sitting and watching television, change in alcohol consumption, change in margarine intake, and for women only, baseline parity, menopausal status, postmenopausal hormone use, and oral contraceptive use (NHS2 only). 1 lb = 0.45 kg. hPDI, healthful plant-based diet index; MET, metabolic equivalent task; NHS, Nurses’ Health Study; PDI, overall plant-based diet index; uPDI, unhealthful plant-based diet index.
P value for Q statistic for heterogeneity <0.05, indicating statistically significant heterogeneity in results among the 3 studies.
Asian, American Indian, Hawaiian, or other ancestry.
P-interaction comparing blacks with Caucasians.
P-interaction comparing all other races with Caucasians.
P-interaction comparing BMI 25–<30 with BMI <25.
P-interaction comparing BMI ≥30 with BMI <25.
FIGURE 2.
Joint classification of changes in the hPDI and changes in physical activity (compared with no change in both) in relation to 4-y weight change. Values are weight change in lb (95% CI) pooled across the cohorts using a fixed-effects model. Given that most participants gained weight over 4-y intervals, inverse associations of the diet indices with weight change (i.e., negative values) should be interpreted as “less weight gain,” not as “weight loss.” We used multivariable generalized linear regression models (with unstructured correlation matrix and robust variance) to conduct this analysis. Adjusted for age, questionnaire cycle, baseline BMI, change in smoking status, baseline and change in physical activity, hours of sleep, hours of sitting and watching television, change in alcohol consumption, change in margarine intake, and for women only, baseline parity, menopausal status, postmenopausal hormone use, and oral contraceptive use (NHS2 only). The reference group (no change in hPDI or physical activity) experienced a mean 4-y weight gain of 3.2 lb. 1 lb = 0.45 kg. hPDI, healthful plant-based diet index; MET, metabolic equivalent task; NHS, Nurses’ Health Study.
Discussion
Participants on average gained weight over 4-y intervals in 3 prospective cohorts in the United States, but we found that consumption of different types of plant-based diet indices was associated with different amounts of weight gain. Four-year increases in consumption of PDI were associated with 0.09 lb (95% CI: 0.12, 0.05 lb) less weight gain over the same period. This inverse association was substantially stronger for hPDI (1.50 lb less weight gain; 95% CI: 1.53, 1.46 lb), but uPDI was associated with 0.79 lb (95% CI: 0.75, 0.82 lb) more weight gain. The associations for hPDI and uPDI were similar in a subgroup analysis in terms of direction and statistical significance, although the magnitudes of the associations were stronger among Caucasians, low-baseline carbohydrate consumers, and younger, less-active, and overweight or obese participants. PDI was positively associated with weight change only in overweight or obese participants. This could be due to differing composition of the overall plant-based diet index consumed by these participants or some other mechanisms, and warrants further investigation.
The higher fiber content, lower saturated fat levels, and lower calorie content of several foods in a healthful plant-based diet could lower energy intake, thereby preventing weight gain. Dietary fiber in particular is thought to affect energy intake through changes in hunger and satiety cues (17); the higher amount of chewing and the viscous gel formation resulting from the absorption of water by soluble fibers may result in slower gut transit time and higher satiety. Such a diet may also influence adiposity through changes in the gut microbial environment (18, 19). An unhealthful plant-based diet, on the other hand, would have the opposite effect on the above pathways. In addition, the higher glycemic index and glycemic load of such a diet may decrease satiety and increase hunger signals (20, 21). The higher added-sugar levels of an unhealthful plant-based diet may also lead to increased energy intake through neurochemical changes in the brain (22, 23). Lastly, high intake of SSBs may promote weight gain through incomplete compensation of liquid calories at subsequent meals (24).
Several studies have found vegetarian diets to be inversely associated with weight change. Sacks et al. (3) were among the first to report on this connection, finding dramatically lower weights in a population that followed a strict vegetarian diet relative to sex- and age-matched controls, although unmeasured confounding cannot be ruled out. Nevertheless, several RCTs have found vegan and vegetarian diets to be associated with improved weight outcomes in the short term. A meta-analysis of 12 such trials found a significant reduction in weight among vegetarians relative to control groups, with a mean difference of −2.02 kg (95% CI: −2.80, −1.23 kg) (7). However, the median duration of the trials included in the above meta-analysis was only 18 wk. Small amounts of weight gain per year continued over several decades can shift normal-weight adults to overweight and obesity over their life course. Moreover, in most intervention studies, short-term weight loss has been followed by rebound and weight regain within the first 1–2 y (25). Hence, it is important to understand associations of plant-based diets with weight change over longer durations. In our study, we leveraged the long-term periodic data collected on weight, diet, and other lifestyle factors in 3 prospective cohorts to understand how changes in adherence to plant-based diets relate to weight change over 4-y intervals spanning >2 decades. Our findings are consistent with those of the EPIC-Oxford study, in which the smallest weight gains were observed in participants who changed from a meat-based diet to one with fewer animal foods over 5 y (6).
Another strength of our study is that we created variations of plant-based diets based on the quality of plant foods consumed, unlike previous studies of vegetarianism which have treated all plant foods similarly. We found that although a plant-based diet that emphasized high-quality plant foods was associated with less weight gain, a plant-based diet that emphasized low-quality plant foods was associated with substantially higher weight gain. Low-quality plant foods were also associated with higher weight gain independent of the other food categories, which is in line with a previous analysis of individual foods and weight change in these cohorts (8). Thus, it is important to consider the quality of plant foods consumed in a predominantly plant-based diet. We also found that even moderately lower intake of animal foods was associated with substantially less weight gain over 4 y. However, on average, participants in our cohorts gained weight over the course of follow-up, and it is possible that more beneficial dietary change combined with additional lifestyle changes such as increases in physical activity are needed to prevent weight gain and maintain normal weight over the long term.
For most people, losing or maintaining weight over the long term is difficult due to physiologic resistance to weight loss, an evolutionarily programmed attraction among humans to energy-dense foods, sociocultural norms that encourage intake of high-caloric foods, and a food environment that is replete with high-caloric foods available at low cost (26, 27). Additionally, obesity is associated with tremendous costs, both in terms of health and health care expenditures (28). Thus, prevention of long-term weight gain is a crucial public health goal. Our results add to the existing literature on long-term dietary changes in relation to weight change, with potential benefits from increasing intake of a healthful plant-based diet. In our previous analyses these indices predicted risk of developing type 2 diabetes (9) and coronary heart disease (10), with inverse associations for hPDI, and positive associations for uPDI. This analysis adds to our understanding of potential mechanisms through which healthful and unhealthful plant-based diets may impact cardiometabolic disease risk. This study also adds to our previous analysis in which we found the Alternate Mediterranean Diet, the Alternate Health Eating Index-2010, and the Dietary Approaches to Stop Hypertension to be associated with less weight gain over 4-y intervals (29). The hPDI is an alternative dietary pattern that can be adopted to help prevent long-term weight gain over the life course. This supports the 2015–2020 Dietary Guidelines for Americans (30), which advocate several healthful dietary patterns for improved health outcomes, including a healthy vegetarian diet.
Some degree of error in assessment is inevitable in our study due to the self-report nature of the data. Nevertheless, we have demonstrated reasonable reliability and validity of the dietary data, and a high level of concordance between self-reported and measured weight in these cohorts. Residual or unmeasured confounding cannot be ruled out, given the observational study design. However, we were able to control for numerous potential confounders, including changes in lifestyle factors. It is also possible that the associations reflect reverse causation, with weight change causing changes in diet. However, the considerable evidence from RCTs on the protective effects of plant-based diets on weight gain further supports a causal interpretation of our findings. Our study populations consisted largely of Caucasian, educated health professionals, and it would be useful to replicate these findings in other ethnic and socioeconomic groups. Given that weight loss at older ages may reflect loss of lean mass, we restricted our analysis to participants aged ≤65 y. Thus, it would be important to replicate these findings in an older population using measures of both lean body mass and adiposity.
In conclusion, increased adherence to a healthful plant-based diet, which emphasized intake of high-quality plant foods such as whole grains, fruits, and vegetables, was associated with substantially lower 4-y weight gain, whereas an unhealthful plant-based diet was associated with higher weight gain. These findings support current recommendations to increase intake of healthy plant foods, while reducing intake of less-healthy plant foods and animal foods, for improved health outcomes.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—AS, FBH, and WW: designed the research (project conception, development of overall research plan, and study oversight); AS and VM: conducted the research and analyzed the data; AS: wrote the first draft of the manuscript; VM, EBR, FS, WW, and FBH: contributed to the writing of the manuscript; FBH: had primary responsibility for final content; and all authors: read and approved the final manuscript. AS is currently employed at Analysis Group, Inc.; EBR received a research grant from the USDA/Blueberry Highbush Council; FBH received research support from California Walnut Commission and lecture fees from Metagenics and Standard Process; VM received research support from the Peanut Institute; no other conflicts of interest to declare.
Notes
This work was supported by research grants UM1 CA186107, UM1 CA176726, UM1 CA167552, and HL60712 from the National Institutes of Health. AS was supported by American Heart Association Grant #16POST29660000. The funders played no role in the study design, data collection, analysis and interpretation of data, writing of the manuscript, or the decision to submit the article for publication.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: hPDI, healthful plant-based diet index; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; PDI, overall plant-based diet index; RCT, randomized controlled trial; SSB, sugar-sweetened beverage; uPDI, unhealthful plant-based diet index.
References
- 1. WHO. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: WHO; 2009. [Google Scholar]
- 2. Ochner CN, Barrios DM, Lee CD, Pi-Sunyer FX. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiol Behav. 2013;0:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sacks FM, Castelli WP, Donner A, Kass EH. Plasma lipids and lipoproteins in vegetarians and controls. N Engl J Med. 1975;292(22):1148–51. [DOI] [PubMed] [Google Scholar]
- 4. Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, McLanahan SM, Kirkeeide RL, Brand RJ, Gould KL. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129–33. [DOI] [PubMed] [Google Scholar]
- 5. Chiu YF, Hsu CC, Chiu TH, Lee CY, Liu TT, Tsao CK, Chuang SC, Hsiung CA. Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: a matched cohort study. Br J Nutr. 2015;114(8):1313–20. [DOI] [PubMed] [Google Scholar]
- 6. Rosell M, Appleby P, Spencer E, Key T. Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int J Obes (Lond). 2006;30(9):1389–96. [DOI] [PubMed] [Google Scholar]
- 7. Huang RY, Huang CC, Hu FB, Chavarro JE. Vegetarian diets and weight reduction: a meta-analysis of randomized controlled trials. J Gen Intern Med. 2016;31(1):109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB, Hu FB. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26. [DOI] [PubMed] [Google Scholar]
- 12. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 13. Willett W. Nutritional epidemiology. 3rd ed New York: Oxford University Press; 2013. [Google Scholar]
- 14. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2016;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117(6):651–8. [DOI] [PubMed] [Google Scholar]
- 16. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. [Google Scholar]
- 17. Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59(5):129–39. [DOI] [PubMed] [Google Scholar]
- 18. Bajzer M, Seeley RJ.. Physiology: obesity and gut flora. Nature. 2006;444(7122):1009–10. [DOI] [PubMed] [Google Scholar]
- 19. Glick-Bauer M, Yeh M-C.. The health advantage of a vegan diet: exploring the gut microbiota connection. Nutrients. 2014;6(11):4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ludwig DS. Dietary glycemic index and obesity. J Nutr. 2000;130(2):S280–3. [DOI] [PubMed] [Google Scholar]
- 21. Chang KT, Lampe JW, Schwarz Y, Breymeyer KL, Noar KA, Song X, Neuhouser ML. Low glycemic load experimental diet more satiating than high glycemic load diet. Nutr Cancer. 2012;64(5):666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berthoud H-R. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71(4):478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levine AS, Kotz CM, Gosnell BA. Sugars: hedonic aspects, neuroregulation, and energy balance. Am J Clin Nutr. 2003;78(4):S834–42. [DOI] [PubMed] [Google Scholar]
- 24. Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013;14(8):606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H et al.. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–41. [DOI] [PubMed] [Google Scholar]
- 26. Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond). 2009;33 Suppl 2:S8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9(1):13–27. [DOI] [PubMed] [Google Scholar]
- 28. Hu FB. Obesity epidemiology. New York: Oxford University Press; 2008. [Google Scholar]
- 29. Fung TT, Pan A, Hou T, Chiuve SE, Tobias DK, Mozaffarian D, Willett WC, Hu FB. Long-term change in diet quality is associated with body weight change in men and women. J Nutr. 2015;145(8):1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US Department of Health and Human Services and US Department of Agriculture. [Internet]. 2015–2020 Dietary Guidelines for Americans 2015. Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.