ABSTRACT
Approximately 4.4% of the human genome is comprised of endogenous retroviral sequences, a record of an evolutionary battle between man and retroviruses. Much of what we know about viral immunity comes from studies using mouse models. Experiments using the Friend virus (FV) model have been particularly informative in defining highly complex anti-retroviral mechanisms of the intrinsic, innate and adaptive arms of immunity. FV studies have unraveled fundamental principles about how the immune system controls both acute and chronic viral infections. They led to a more complete understanding of retroviral immunity that begins with cellular sensing, production of type I interferons, and the induction of intrinsic restriction factors. Novel mechanisms have been revealed, which demonstrate that these earliest responses affect not only virus replication, but also subsequent innate and adaptive immunity. This review on FV immunity not only surveys the complex host responses to a retroviral infection from acute infection to chronicity, but also highlights the many feedback mechanisms that regulate and counter-regulate the various arms of the immune system. In addition, the discovery of molecular mechanisms of immunity in this model have led to therapeutic interventions with implications for HIV cure and vaccine development.
Keywords: Friend retrovirus, mouse model, intrinsic immunity, innate immunity, adaptive immunity, immunotherapy
Complex Friend retrovirus immunity.
FRIEND VIRUS INTRODUCTION AND BACKGROUND
‘In 1956, at a meeting of the American Association for Cancer Research in Atlantic City, Charlotte Friend reported on the isolation of a virus that produced a fatal leukemia when inoculated into adult mice. This was a time when the concept of viruses causing cancer was still viewed with extreme skepticism and the presentation of such data by a young woman not long out of graduate school was met with disbelief and derision’ (Diamond 1994). Not only did Dr Friend prove to be correct, but Friend virus (FV) became a primary tool for studying viral leukemogenesis, oncology and host resistance to infection. In her original report, Dr Friend tested seven different strains of mice and found only two strains were susceptible to leukemia. This finding elicited a great deal of interest in determining the genetics of host susceptibility and resistance, but Mendelian studies were complicated by the fact that in most genetic crosses, susceptibility and resistance were controlled by multigenic factors (Fieldsteel, Dawson and Bostick 1961; Franker and Quilligan 1966; Mirand 1966; Mirand, Grace and Buffett 1966; Lilly 1967). In the mid 1960s, Frank Lilly mapped the first FV susceptibility trait to chromosome 17, linked to the major histocompatibility complex (MHC) of the mouse (Lilly, Boyse and Old 1964; Lilly 1966). This was only the second viral susceptibility trait ever mapped. At that time, the MHC was known to control graft acceptance and rejection between mouse strains, but the mechanisms of action were not understood, and the link to virus susceptibility and resistance was very intriguing. Then in 1974, landmark papers by Zinkernagel and Doherty elucidated the connection between the MHC and T cell responses to viral infection. Their studies suggested that discrimination between self and non-self in tissue grafts extended to T cell recognition of ‘altered self’ in virally infected cells (Zinkernagel and Doherty 1974a,b), a phenomenon now understood to be a viral peptide (non-self) bound to MHC molecules (self). In collaboration with Jack Stimpfling in 1974, Bruce Chesebro used MHC recombinant mice to map an FV resistance trait to a specific gene within the MHC, H-2Db. Within the next few years the immunological mechanisms of genetic resistance were uncovered, including roles for H-2Db-restricted T cell responses (Friedman, Lilly and Nathenson 1974; Blank, Freedman and Lilly 1976; Chesebro and Wehrly 1976a,b; Chesebro and Wehrly 1978; Chesebro and Wehrly 1979; Plata and Lilly 1979) and Rfv3-dependent B cell responses (Chesebro and Wehrly 1979; Chesebro et al. 1979; Chesebro et al. 1981).
As immunological studies moved into the era of molecular immunology, several key findings allowed the development of tools that enabled the study of FV resistance at a much more detailed and mechanistic level. The discovery that MHC molecules presented peptides to T cells (reviewed in van Bleek and Nathenson (1992); Blum, Wearsch and Cresswell (2013) and Roche and Furuta (2015)) led to the identification of the FV DbGagL peptide presented by H-2Db class I molecules (Chen et al. 1996), and the FV env122–141 peptide presented by H2-Ab class II molecules (Iwashiro et al. 1993; Shimizu et al. 1994). These peptide identifications further enabled the development of tetramers to quantify FV-specific CD4+ and CD8+ T cells (Schepers et al. 2002) as well as the development of TCR transgenic mice carrying CD8+T cells specific for DbGagL (Ohlen et al. 2002) and CD4+ TCR transgenic mice specific for FV env122–141 (Antunes et al. 2008). Use of these immunological tools has greatly advanced the discovery of immunological mechanisms of resistance to FV infection with relevance to human immunity in several chronic viral infections. In addition to the adaptive immune responses required for resistance to FV, innate and intrinsic immune responses also play important roles and will be reviewed as well.
Although FV is often referred to as a retrovirus, it is actually a complex of two viruses, replication competent Friend murine leukemia virus (F-MuLV), which is sometimes referred to as the helper virus, and a replication defective virus called spleen focus-forming virus (SFFV) (Kabat 1989). F-MuLV is classified as a simple gammaretrovirus with a standard arrangement of 2 LTRs flanking gag, pol and env genes (Troxler, Ruscetti and Scolnick 1980). An alternate CUG start site (Prats et al. 1989) is used to produce an additional protein, glycosylated gag (glycogag), which is expressed on viral particles and the surface of infected cells (Evans, Dresler and Kabat 1977; Fujisawa et al. 2001; Nitta et al. 2010). Interestingly, the defective SFFV is the predominant pathogenic component in FV infection of adult mice. SFFV encodes a defective env protein (gp55) with oncogenic properties (Li et al. 1987; Li and Baltimore 1991). The gag and pol genes of SFFV are also defective so SFFV must co-infect a cell infected with replication competent F-MuLV (helper) in order for the SFFV genome to get packaged and form transducing virions. The cellular tropism of FV is broad and FV can infect any dividing cell except hepatocytes, which do not express the mCAT-1 receptor for viral entry (Closs et al. 1993). That said, the preferred target for FV is nucleated erythroid precursors. Spread of virions is greatly enhanced by the binding of SFFV gp55 to the erythropoietin receptor and the short form of the stem cell kinase receptor (SF-Stk, previously known as Fv2) (Li et al. 1987; Persons et al. 1999; He et al. 2010). These gp55/Epo-Stk interactions deliver potent proliferative signals to nucleated erythroid progenitors significantly magnifying the erythroid target population of dividing cells and resulting in gross splenomegaly, hepatomegaly and bone marrow hypertrophy. In fact, mice with the long form of Stk (LF-Stk), which does not interact with gp55, are highly resistant to FV-induced splenomegaly and disease (Table 1). FV also infects B cells and myeloid cells, whereas infection levels of T cells are much lower (Windmann et al. 2019). In resistant mice, infected erythroid precursors are efficiently eliminated by T cells, but the infected immune cell populations can partly escape from T cell killing. Infected T and B cells subsequently accumulate in B cell follicles (Windmann et al. 2019), a specific immunological niche that cytotoxic cells usually can't excess and that also harbors the viral reservoir in HIV infected humans or SIV infected monkeys (Connick et al. 2014; Fukazawa et al. 2015).
Table 1.
Resistance (in decreasing order) of commonly used mouse strains for FV studies.
| Resistance genes | |||
|---|---|---|---|
| Mouse strain (abbreviation) | MHC1 | FV22 | Rfv3 (Apobec3)3 |
| C57BL/10 (B10) | H-2b/b | FV2r/r | Rfv3r/r |
| C57BL/6 (B6) | H-2b/b | FV2r/r | Rfv3r/r |
| (A.BY x B10)F1 (Y10) | H-2b/b | FV2s/r | Rfv3s/r |
| (A/Wy x B10)F1 (Y10.A) | H-2a/b | FV2s/r | Rfv3s/r |
| (Balb/c x B10)F1 | H-2d/b | FV2s/r | Rfv3s/r |
| A.BY | H-2b/b | FV2s/s | Rfv3s/s |
| A/Wy | H-2a/a | FV2s/s | Rfv3s/s |
| Balb/c | H-2d/d | FV2s/s | Rfv3s/s |
The most potent resistance gene in MHC is H-2D, and homozygous H-2Db/b alelles provide for the best CD8+ T cell responses. The H-2b alelle of MHC class II gene, H-2A is also resistant. H-2b/b homozygous mice are more resistant than heterozygous mice (Hasenkrug et al. 1994).
The r alelle is resistant (LF-Stk), s is susceptible (SF-Stk). FV2 susceptibility is dominant because one copy of the SF-Stk gene product allows interaction with SFFV gp55, which induces erythroproliferation, splenomegaly, and hepatomegaly
The r alelle is resistant, s is susceptible. Rfv3 resistance is dominant because one copy of Apobec3 provides enough transcription to provide Apobec-mediated functions as discussed in the Apobec3 chapter.
In susceptible mice that fail to mount rapid T helper cell, CD8+ T cell and B cell responses, the SFFV genome eventually integrates into and activates the Spi-1 transcriptional factor gene (Moreau-Gachelin, Tavitian and Tambourin 1988; Paul et al. 1989; Lavigueur and Bernstein 1991; Paul et al. 1991; Schuetze et al. 1992; Hegde, Hankey and Paulson 2012), inactivates the p53 tumor suppressor gene (Munroe, Peacock and Benchimol 1990; Johnson and Benchimol 1992; Johnson, Chung and Benchimol 1993), and produces a malignant erythroleukemia in a multistage manner (Cmarik and Ruscetti 2010). Although erythroleukemias in humans are rare, insights from FV-induced erthroleukemias have also revealed much about the development of acute myeloid leukemias in humans. In a similar manner to FV-induced erythroleukemia, at least two oncogenic events are required, one that bestows a proliferative advantage and one that disrupts normal differentiation as recently reviewed (Boddu et al. 2018; Moreau-Gachelin 2008). In addition to the Stk gene described above, a number of other non-immunological host genes involved in resistance and susceptibility to FV-induced leukemia have been described and reviewed (Chesebro, Miyazawa and Britt 1990; Hoatlin and Kabat 1995; Moreau-Gachelin 2008; Boddu et al. 2018), but this review focuses on the nature of the immune responses that result in susceptibility or resistance to FV infection.
FV has been a powerful tool for studying immunological resistance to retroviral infection because it infects adult, immunocompetent mice, and different mouse strains have varying resistance to FV infection (Table 1). Studies with such strains have been used to define not only the determinants of genetic resistance, but also the immunological mechanisms of that resistance. Whereas highly susceptible strains of mice such as Balb/c never recover from acute FV infection and develop rapid erythroleukemia, resistant mice recover from acute infection within a few weeks, but then go on to develop a life-long chronic infection. Thus, resistant mice are powerful tools to not only study mechanisms of resistance to acute infection, but also mechanisms by which retroviruses evade complete eradication and establish and maintain chronicity. Such mechanisms of immunological resistance to acute infection ranging from intrinisic immunity to innate and adaptive immunity will be discussed. Studies of chronic FV in resistant mouse strains yielded the first insights that responses by CD4+ regulatory T cells (Tregs) suppressed critical CD8+ T cell responses during infections and thereby promoted the establishment of chronic FV (reviewed in Hasenkrug, Chougnet and Dittmer (2018)). This review will also discuss the involvement of co-inhibitory molecules and other suppressive immune cell populations in the promotion of chronic FV infection.
In addition to the complex nature of FV, an additional complication was discovered in 2008 (Robertson et al. 2008a). Since in vivo passaged FV complex was always more virulent than cultured virus stocks from cloned viruses, studies requiring highly pathogenic virus were historically done using mouse-passaged swarm stocks. An unintended consequence of the use of in vivo passaged stocks was the propagation of an endemic mouse virus, lactate dehydrogenase-elevating virus (LDV). Evidence indicated that LDV was present in FV stocks as early as 1963 (Riley 1963) and may have been a component of the FV complex from its first isolation. LDV is a positive-stranded, enveloped RNA virus classified in the order Nidovirales, which also contains coronaviruses (Drosten et al. 2003; Ksiazek et al. 2003; Peiris et al. 2003). Its name derives from its capacity to rapidly infect and cytolyse a subset of macrophages that clear excess lactate dehydrogenase from the circulation (Inada and Mims 1985). LDV induces rather dramatic effects on the immune system. Among the reported effects are impaired antigen presentation by macrophages (Isakov, Feldman and Segal 1982), polyclonal B cell activation (Coutelier and Van Snick 1985), NK cell activation (Markine-Goriaynoff et al. 2002), impaired delayed-type hypersensitivity responses (Inada and Mims 1986), and inhibited cellular immune responses (Howard et al. 1969). These immunosuppressive effects of LDV allow FV titers to reach much higher levels before the infection comes under immunological control (Robertson et al. 2008a; Duley et al. 2012). Consequently, much lower doses of FV were required to establish chronic FV infections in the presence of LDV than in its absence. Furthermore, the LDV-induced polyclonal activation of B cells expands the population of dividing target cells for virus entry.
These impacts from LDV explain why in vivo passaged FV stocks were more virulent than in vitro cloned stocks. Thus, studies after 2008 must specify whether the virus stocks contained LDV or not. A prominent example of the effects of LDV is that Rfv3-mediated promotion of virus-neutralizing antibodies turned out to be highly dependent on the presence of LDV in the stock. LDV is a potent inducer of type I interferons (IFN I) via TLR7 signaling (Ammann et al. 2009), and Apobec3 (Rfv3)-mediated antibody effects are dependent on type I IFN signaling and observable only in the presence of LDV (Barrett et al. 2017).
VIRUS SENSING
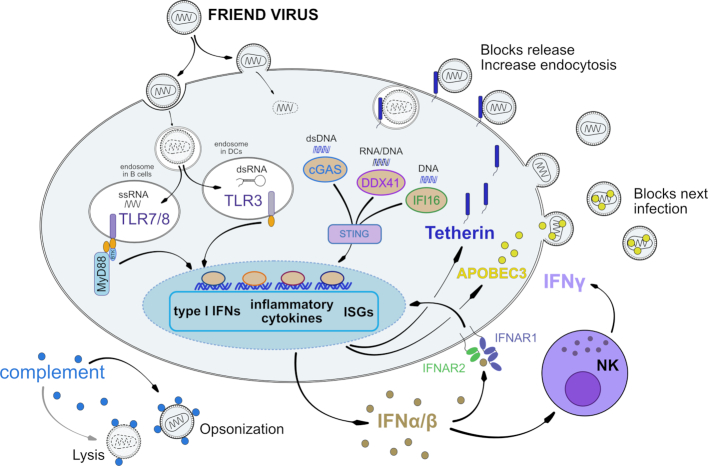
The host immune system is able to sense invading pathogens by a variety of germline-encoded receptors. These pattern recognition receptors (PRR) recognize pathogen-associated molecular patterns, which are highly conserved molecular structures of pathogens. A well-characterized family of PRRs are the Toll-like receptors (TLRs) that are located on cellular or endosomal membranes. Endosomal TLR3, TLR7/8 and TLR9 sense viral nucleic acid components like double-stranded RNA, single-stranded RNA or non-methylated CpG-containing DNA, respectively (reviewed in Akira and Takeda (2004) and Beutler (2009)). Cytosolic DNA and RNA sensors, such as cyclic GMP-AMP synthase (cGAS), retinoic acid inducible gene I (RIG-I) and Melanoma Differentiation-Associated protein 5 (MDA5), also detect replication intermediates of many viruses (Gao et al. 2013; Sun et al. 2013). Virus sensing induces the production of IFN I, which in turn leads to the expression of hundreds of interferon-stimulated genes (ISGs) eliciting an antiviral state in the infected as well as surrounding cells.
Many in vitro studies revealed that multiple PRRs such as TLR3, TLR7/8 and cGAS are able to sense murine retroviruses. This is possible because in the retroviral replication cycle single-stranded and double-stranded (hairpin RNA loops) viral RNAs as well as viral DNA and even RNA/DNA hybrids exist. During acute FV infection, TLR3 is an important sensor involved in the control of viral replication (Gibbert et al. 2014). Up to 10 days post FV infection, mice deficient in TLR3 had significantly higher viral loads compared to wild-type mice (Gibbert et al. 2014). During acute FV infection, TLR3 signaling was critical for the activation of myeloid dendritic cells (DCs), which subsequently resulted in increased cytotoxicity of CD8+ T cells and natural killer (NK) cells mediating the reduction in viral loads. In contrast, TLR7 and myeloid differentiation primary response 88 (MyD88), an important adaptor molecule for the signaling pathway of all TLR family members, except TLR3, were not required for viral control during initial FV infection. However, after 2 weeks of FV infection MyD88−/− mice failed to control the virus due to an impaired humoral immune response (Browne and Littman 2009; Kane et al. 2011; Gibbert et al. 2014). MyD88 and TLR7-dependent sensing of murine leukemia viruses (MLV) was crucial for the development of neutralizing antibody responses, but T cell responses were only partially dependent on this pathway (Browne and Littman 2009; Kane et al. 2011). TLR3 is mainly expressed by myeloid DCs, indicating that antigen presenting cells are primarily activated by TLR3, whereas TLR7 is highly expressed by different B cell subsets, and thus the humoral immune response depends on TLR7 signaling. Indeed, another study showed that TLR7 is an important sensor in FV infection that facilitates the induction of germinal center B cells (Browne 2011). Also, CD4+ helper T cell responses are diminished in FV-infected TLR7−/− mice, whereas CD8+ T cell responses are TLR7 independent (Browne 2011). In a subsequent study, the same authors suggested that TLR7 sensing might also be critical during early FV infection because TLR7 sensing influenced IgM antibody production, but the differences in viral loads between TLR7−/− and wild-type mice were rather small (Browne 2013).
Recent work implies that retroviral reverse transcripts are also detected by cellular DNA sensors such as cyclic GMP-AMP synthase (cGAS), DExD/H-box helicase 41 (DDX41) and interferon-inducible protein 16 (IFI16). IFI16, DDX41 and cGAS interact with and signal through STING, which results in the transcription of several genes including type I IFNs. It was already shown, that cGAS and DDX41 are required for the cytosolic DNA exonuclease three prime repair exonuclease (TREX)1-dependent expression of type I IFNs in MLV infection (Gao et al. 2013; Stavrou et al. 2015). While cGAS senses double-stranded viral DNA, DDX41 seems to sense viral RNA/DNA hybrids generated at the first step of murine leukemia virus reverse transcription, and both sensors were required for potent anti-viral innate immunity (Stavrou et al. 2018a). The RNA sensor zinc-finger antiviral protein induces the degradation of MLV transcripts by the exosome (Lee et al. 2013), whereas other cytosolic RNA sensors like Rig-I or MDA5 do not affect replication of MLV or FV (Gibbert et al. 2010; Lee et al. 2013).
In conclusion, many DNA- and RNA-sensors are able to recognize replication intermediates of murine retroviruses (Fig. 1). For some of these PRRs their individual role in host innate and adaptive immune responses were defined. However, it is likely that additional pathways exist that efficiently sense murine retroviruses and initiate protective immune responses. As many of these sensors are completely independent, countermeasures by the virus may be compensated for by other PRRs. Targeting these different PRRs during retroviral infections might improve host immune responses and control viral replication, which offers novel approaches for antiretroviral immunotherapies (Olbrich et al. 2002; Gibbert et al. 2010).
Figure 1.
Sensing of FV and induction of intrinsic and innate immunity. After virus entry, a number of different viral nucleic acid products can be sensed and IFN I responses are initiated. Although the IFN I response is limited by FV due to an unknown viral mechanisms, it induces the expression and activity of the restriction factors APOBEC3 and Tetherin as well as anti-viral NK cell responses. Complement binding can directly lead to virus lysis or increase virus uptake leading to cellular degradation and improved antigen presentation.
INTRINSIC IMMUNE RESPONSES
Type I Interferons
During many viral infections, the initial host response is the induction of IFN I. The type I IFN family consists of numerous IFNα subtypes, IFNβ, IFNε, IFNκ and IFNω that all bind to the same Interferon-α/β-receptor (IFNAR), which is composed of the two subunits IFNAR1 and IFNAR2. The type I IFN response is initially induced by sensing of viral nucleic acids by various pattern recognition receptors leading to the expression of the early IFN I subtypes IFNβ and IFNα4 (Erlandsson et al. 1998; Marie, Durbin and Levy 1998). The binding of IFN to its receptor results in the activation of the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) signalling pathway, which induces the transcription of several hundred genes, so called IFN-stimulated genes (ISG). These gene products have various biological functions and some of them can directly suppress viral replication, viral protein translation, or viral infectivity (Clemens and Elia 1997; Stark et al. 1998). ISGs that have been shown to directly restrict retrovirus replication are APOBEC, Tetherin and Tripartite motifs (TRIMs) (reviewed in Malim and Bieniasz (2012)).
IFN I also inhibit cell or tumor proliferation or regulate innate and adaptive immune responses. They improve host immune responses by the activation of natural killer (NK) cells (Trinchieri et al. 1981; Salazar-Mather, Ishikawa and Biron 1996), stimulation of the antigen presentation machinery (Epperson et al. 1992; Hermann et al. 1998), including maturation of DCs (Le Bon et al. 2003), and augmentation of CD8+ T cell (Honda et al. 2005; Le Bon et al. 2006a,b) and B cell differentiation (Le Bon et al. 2001; Le Bon et al. 2006b). In contrast, they can also counteract overwhelming immune responses by the induction of anti-inflammatory IL-10 (Aman et al. 1996) or the surface expression of PD-1 (Terawaki et al. 2011) to limit the risk of tissue damage by the host immune system.
Many viruses evolved different mechanisms to evade the host IFN response to promote their replication and persistence (Randall and Goodbourn 2008). During acute FV infection the type I IFN response is quite weak and no systemic induction of type I IFN is detectable. However, low level expression of IFNA mRNA could be found in spleen cells at 72 hours post FV infection (Gerlach et al. 2006). The IFNα subtypes α1 and α6 were expressed at higher levels in splenocytes during acute FV infection compared to α4 and α9 (Gerlach et al. 2009). Furthermore, mRNA of IFNα-induced ISGs was detected in spleens and bone marrow from FV-infected mice (Gerlach et al. 2009; Gibbert et al. 2012; Halemano et al. 2013a) indicating that local expression of IFNα and a subsequent ISG response is elicited during acute FV infection. It was previously reported that Moloney murine leukemia virus-based retroviral replicating vectors (RRV) did not induce measurable type I IFN responses. Furthermore, Moloney RRV even suppressed IFN I induction by other viral vectors, indicating that they actively counter-regulate type I IFN responses (Lin et al. 2014). This is also likely for FV infection, but the exact molecular mechanism for poor induction of type I IFNs by FV remains elusive. The exogenous application of IFNα subtypes or IFN-inducing TLR ligands during FV infection resulted in efficient viral control (Gerlach et al. 2009; Gibbert et al. 2010; Gibbert et al. 2012; Halemano et al. 2013a). In these experiments, the subtypes IFNα1, α4, α9 and α11 were shown to have the strongest anti-FV activity in vivo (Gerlach et al. 2009; Gibbert et al. 2012). Harper et al. showed that IFNα treatment inhibits acute FV replication at least in part through the anti-viral effector molecule Apobec3 (Harper et al. 2013) (Fig. 1).
Apobec3
The APOBEC3 (A3) proteins are deoxycytidine deaminases that gained major attention when it was discovered that members of this family are counteracted by the HIV-1 protein Vif (Sheehy et al. 2002). HIV-1 Vif binds and targets various A3 members for degradation via the ubiquitin-proteasome pathway (Sheehy, Gaddis and Malim 2003; Stopak et al. 2003). In humans, there are 7 hA3 members (A-H) (Arjan-Odedra et al. 2012). By contrast, mice only encode one APOBEC3 gene (mA3), making it straightforward to derive mA3 knockout (KO) mice (Okeoma et al. 2007; Santiago et al. 2008). Mouse APOBEC3 is a double-domain deaminase, with the N-terminal half being evolutionarily similar to hA3C, hA3F and other ‘Z2’ domains with the C-terminal half bearing more similarity to hA3H (Refsland and Harris 2013). Studies in FV infection revealed that mA3 KO mice had ∼10-fold higher levels of infectious virus compared to B6 WT mice at 7 dpi (Santiago et al. 2008; Takeda et al. 2008). The potent in vivo inhibitory activity of mA3 was consistent across multiple murine retroviruses tested (Okeoma et al. 2007; Low et al. 2009; Jones, Mehta and Okeoma 2012). Importantly, mA3 is the molecular counterpart of a classical resistance gene, Recovery from Friend virus gene 3 or Rfv3 (Santiago et al. 2008; Takeda et al. 2008; Santiago et al. 2011).
Mouse A3 produced by an infected cell gets incorporated into F-MuLV particles and inhibits replication in the next target cell (Browne and Littman 2008). The anti-viral mechanism of A3 is deaminase-independent, as hypermutated reverse transcripts are rarely detected (Browne and Littman 2008; Smith et al. 2011; Barrett et al. 2014; Boi et al. 2018) and deaminase-dead mA3 retains the ability to restrict MLV in vivo (Stavrou et al. 2018b). The mechanism involves the steric inhibition of reverse transcription by decreasing the activity and fidelity of the reverse transcriptase itself (Boi et al. 2014). Interestingly, in vivo, mA3 does not decrease the total number of FV particles released at 7 dpi despite the reduction in infectious viremia (Smith et al. 2011). This substantial release of noninfectious particles acts as antigen to help prime the B cell response (see chapter 5.4).
Whereas HIV-1 encodes the Vif protein to counteract the antiviral effects of APOBEC3G (Sheehy et al. 2002), FV encodes the glyco-Gag protein (Evans, Dresler and Kabat 1977), which functions as an antagonist of mA3 (Kolokithas et al. 2010). Glyco-Gag is a glycosylated form of Gag that originates in an upstream CUG initiation codon and is essential for MLV replication in vivo (Chun and Fan 1994; Corbin et al. 1994; Stavrou et al. 2013). Glyco-gag-deficient CasFr and Moloney MLV are severely impaired for replication in WT mice but replicate to similar levels as WT virus in mA3 KO mice (Kolokithas et al. 2010; Stavrou et al. 2013). Like HIV-1 Vif, glyco-Gag directly binds to mA3 in vitro but unlike Vif, glyco-Gag does not appear to promote mA3 degradation (Kolokithas et al. 2010). In contrast, glyco-Gag-deficient MLV had more unstable capsids and stimulated IFNβ responses more strongly than WT virus, suggesting that glyco-Gag may shield MLV DNA from being sensed in the cytoplasm during capsid uncoating (Stavrou et al. 2013).
IFNα administration into FV/LDV-infected mice resulted in the upregulation of many known restriction factors and decreased FV replication, but genetic inactivation of only mA3 almost completely abrogated this therapeutic effect (Halemano et al. 2013a). Thus, mA3 acted as the major effector molecule of exogenously-administered IFNα therapy. Interestingly, in an IFNAR KO background, mA3 still reduced early infectious viremia, though not via antibody-mediated mechanisms (Barrett et al. 2017). Furthermore, this reduction of infectious viremia occurred in the presence or absence of LDV. Thus, in resistant B6 mice, mA3 restriction of acute FV infection did not require type I IFN signaling. This is not surprising, since murine retroviruses seem to suppress endogenous IFN I production, minimizing IFN signaling during acute infection (Lin et al. 2014). However, in an mA3 deficient background, the loss of IFNAR increased acute FV viremia (Barrett et al. 2017). These findings suggest that the low amount of virus-induced type I IFN triggered antiviral effectors other than mA3 that contributed to inhibiting acute FV replication in vivo. One important type I IFN-regulated factor is Tetherin/BST2.
Tetherin
Tetherin/BST2 is a 28-to-36 kD protein that restricts virion release. Tetherin is counteracted by the HIV-1 Vpu protein, which enhances virion release (Neil, Zang and Bieniasz 2008; Van Damme et al. 2008). The relevance of Tetherin in retroviral infections in vivo was initially confirmed in a study comparing Moloney MLV (in the context of IFNα treatment) and LP-BM5 (murine AIDS) infection levels in B6 WT versus Tetherin KO mice (Liberatore and Bieniasz 2011). In the FV infection model, genetic ablation of Tetherin in B6 mice had no effect on viremia until 14 dpi, a timeframe when adaptive immune responses have already come into play (Li et al. (2014). Interestingly, at 14 dpi, Tetherin KO mice exhibited weaker NK cell, CD4+ T cell and CD8+ T cell responses, which inversely correlated with FV infection levels (Li et al. 2014). Since Tetherin had no effect on viremia at early time points (3–7 dpi) in contrast to mA3 (Li et al. 2016), it was proposed that Tetherin may be acting as an immunomodulator of cell-mediated immunity.
Tetherin inhibits virion release by ‘tethering’ virion and cellular membranes through its two ends: an N-terminal transmembrane domain and a C-terminal GPI-anchor (Perez-Caballero et al. 2009). The aggregation of virions on the cell surface is quite striking (Neil, Zang and Bieniasz 2008), and in vitro, Tetherin+ cells infected with Vpu-deficient HIV-1 were more susceptible to NK cell-mediated antibody-dependent cellular cytotoxicity (Arias et al. 2014). Notably, this ‘stapling’ of virion particles subsequently leads to the reuptake of virions for subsequent degradation through the endolysosomal pathway (Neil et al. 2006), a critical step for MHC II presentation in myeloid DCs, a known target for FV infection (Balkow et al. 2007). As early as 3 dpi, Tetherin KO myeloid DCs exhibited lower activation levels than WT DCs (Li et al. 2016), suggesting that the mechanism by which Tetherin influences T cell immunity may be linked to antigen presentation. In addition, internalization of tethered virions probably leads to an improved viral sensing by endosomal sensors in myeloid cells, resulting in an upregulation of cytokines, such as IL-15, that are required to facilitate NK cell activation and functionality (see chapter 4.2).
Interestingly, a special mouse strain, the NZW/LacJ, harbors a single nucleotide polymorphism in the Tetherin start site that results in a truncated protein lacking the N-terminal endocytosis motif (Barrett et al. 2012). The NZW Tetherin variant is expressed at higher levels on the cell surface due to a defect in endocytosis. In a backcross study, mice that were homozygous for the NZW Tetherin variant exhibited higher viremia and weaker NK cell responses at 7 dp FV infection compared to mice expressing B6 Tetherin (Barrett et al. 2012). Altogether, these results suggested that endocytosis-competent Tetherin promoted antigen presentation by inducing the reuptake of virions into DCs. Such reuptake of virions may be necessary for efficient MHC presentation, as unlike free proteins, virion capsid proteins in lattice-like structures may be more resistant to protease degradation. Many details for how endocytosis-competent Tetherin promotes antigen presentation remain unclear. Of note, humans also express an endocytosis-defective NZW-like protein due to translation from downstream start sites (Cocka and Bates 2012). Details on how this Tetherin variant may affect cell-mediated immune responses against HIV-1 remain incomplete.
Other retroviral restriction factors
Some restriction factors other than APOBEC3 and Tetherin with potent activity against MLV were identified in vitro. Interestingly, potent inhibition of FV in vitro by these factors did not necessarily translate to inhibition in vivo. Ribonuclease L, APOBEC1 and SAMHD1 KO mice exhibited similar FV viremia levels as WT mice (Behrendt et al. 2013; Li et al. 2013; Barrett et al. 2014). The reasons for the differential activities of antiretroviral proteins in vitro versus in vivo remain unclear. One possibility is that these factors may have evolved to more potently counteract other virus families (e.g. Ribonuclease L KO mice are more susceptible to West Nile virus (Samuel et al. 2006)), and the residual activity against retroviruses are just in vitro overexpression artefacts. Alternatively, genetic and environmental modifiers may explain why multiple restriction factors with antiretroviral activity were retained throughout mammalian evolution.
INNATE IMMUNE RESPONSES
The complement system
The complement system is comprised of more than 40 proteins and plays an important role in innate immunity. Upon activation of the classical, lectin or the alternative pathway, a proteolytic cascade is initiated that induces lysis of invading pathogens or infected cells (Holers 2014). Alternatively, deposition of complement fragments on the pathogen's surface enhances clearance by phagocytosis (Carroll and Isenman 2012). Besides its role in innate immunity, the complement system bridges the innate and adaptive immune response and is involved in antibody maturation, memory B-cell formation and modulation of T cell responses (Carroll and Isenman 2012; West, Kolev and Kemper 2018). Retroviruses can activate the complement system by either direct or Ab-independent interaction with C1q, the first component of the classical pathway (Ebenbichler et al. 1991; Stoiber et al. 1994). In addition, interactions of retroviruses with the mannose-binding lectin, the initial trigger of the lectin pathway, were described (Thielens, Tacnet-Delorme and Arlaud 2002; Ji, Gewurz and Spear 2005). After seroconversion, pathogen-specific antibodies further trigger complement activation by initiating the classical complement pathway. As complement proteins are expressed in nearly all compartments of the host, invading pathogens are confronted with this first line of immune defense from the very beginning of an infection. To avoid complement-mediated virolysis retroviruses have adapted two main strategies. Similar to the host cell, viruses bind fluid-phase regulators of complement activation (RCAs), such as factor H (fH), on their surface to avoid destruction by activated complement. This defense strategy was shown for human- and simian-derived retroviruses (Freissmuth, Dierich and Stoiber 2003; Miller-Novak et al. 2018), but also for F-MuLV (Housiaux, Hill and Petersen 1988). In addition, incorporation of membrane-anchored RCAs such as CD55 or CD59 into the viral membrane during the budding process shields against complement attacks. Several retroviruses found in primates take advantage of this defense mechanism (Montefiori et al. 1994; Saifuddin et al. 1995; Spear et al. 1995; Stoiber et al. 1996). As shown for Mouse Mammary Tumor viruses (MMTV) and F-MuLV, murine retroviruses adapt CD55 and CR1-related gene/protein/Y (Crry), an RCA specific for rodents (H.S. unpublished observation). As fH and Crry regulate the complement system by distinct mechanisms, retroviral virolysis is avoided at different activation levels of the complement cascade (Laskowski et al. 2016).
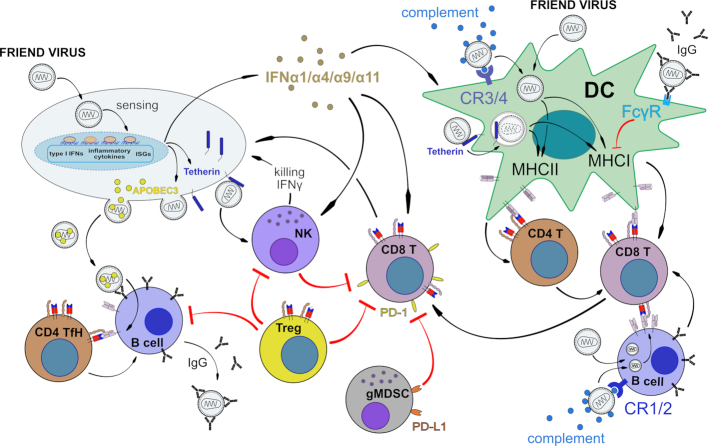
Although interfering at several steps of the cascade, retroviruses cannot avoid complement activation, which results in deposition and accumulation of complement fragments on the viral surface, a process referred to as opsonization (Holers 2014). Similar to other retroviruses, FV turns complement-opsonization to its advantage. C3-fragments deposited on the viral surface allow interactions with complement-receptor (CR) expressing cells, such as CR1-positive erythrocytes, CR3 and CR4 on macrophages, monocytes and DCs or CR2-positive B cells (Groenewegen, Voogd and Freedman 1979; Isitor and Adogwa 1992; Banki et al. 2006; Banki et al. 2010; Bila et al. 2011). Binding of FV to CRs may help the virus to disseminate in the host and results in complement-mediated enhancement of infection. On the other hand, follicular DC (FDC) trap opsonized retroviruses in the germinal center by binding with CR2. Binding of native antigens to the FDC network is crucial for the development of B cell responses during the germinal center reaction (El Shikh and Pitzalis 2012), but retroviruses bound to FDCs also represent an important viral reservoir (Banki et al. 2005; Heesters et al. 2015). In HIV infection uptake of complement-opsonized virions by DCs expressing CR3 or CR4 efficiently bypasses SAMHD1 restriction (Posch et al. 2015). As murine SAMHD1 is known as intrinsic inhibitor of FV in vitro (Behrendt et al. 2013), it is possible that complement may be involved in an analogous manner to HIV.
Complement-mediated enhancement of infection may not always be advantageous for the virus. Complement-opsonization of HIV has been shown to promote MHC I presentation by DCs suggesting that complement directs retroviruses towards MHC I recognition (Tjomsland et al. 2013). Indeed, depending on opsonization either with complement protein or IgG, HIV associates with different MHC compartments in infected DCs (Wilflingseder et al. 2007; Posch et al. 2012). Thus, an accumulation of virus in DCs increases their capacity to present antigen to virus-specific CD8+ T cells (Banki et al. 2010). Studies using the FV mouse model indicated that the enhancing role of complement on DC-mediated CD8+ T cell induction also occurred in vivo and identified complement as natural adjuvant for a DC-induced expansion and differentiation of specific cytotoxic T cells against FV (Banki et al. 2010). In addition, targeting of CR on DCs with CD11c-specific single-chain antibody fragments (scFv) fused to immunodominant viral antigens can be used to induce FV-specific T cell responses in vivo (Ejaz et al. 2012). Similar to DCs, polyclonal activation of B cells promotes their infection with opsonized FV both in vitro and in vivo. This enhanced infection correlated with an increased potency of B cells to activate FV-specific CD8+ T cells (Bila et al. 2011). In contrast to complement, IgG-opsonization of retroviruses—through the binding to Fcγ-receptors—reduces the capacity of DCs to activate virus-specific CD8+ T cells (Posch et al. 2012; Banki et al. 2019).
Thus, complement has two sides of a coin in FV infection. On one side, FV takes advantage of opsonization with complement proteins, which enhances virus distribution and increases infection levels (Fig. 1). On the other side, complement is a natural adjuvant for the induction of FV-specific CD8+ T cell responses, which is crucial to control acute FV infection (Dittmer, Race and Hasenkrug 1999; Dittmer et al. 2004; chapter 5.3).
NK and NKT cell responses
Natural killer (NK) cells are innate immune cells that protect against tumors and many virus infections. NK cells express activating and inhibitory receptors that facilitate the killing of transformed or virus-infected cells. Several antiviral effector functions of NK cells, such as the release of cytotoxic granules, cytokine production and antibody dependent cellular cytotoxicity, have been described. Many studies suggested that NK cells play critical roles in retroviral immunity, as retroviruses evolved strategies to escape NK cell recognition (Jost and Altfeld 2013). Notably, selective depletion of NK cells during the initial phase (3 dpi) of FV infection led to increased viral loads, highlighting a critical role for NK cells in early retrovirus control in vivo (Littwitz et al. 2013).
NK cell responses during FV infection were heavily influenced by the dose of the virus inoculum. At low or medium doses of FV (up to 20 000 SFFU), NK cells were only partially activated and had limited antiviral functions. By contrast, high-dose FV infection (at least 40 000 SFFU) of B6 mice resulted in a higher production of the NK cell stimulating cytokines IL-15 and IL-18 by macrophages and DCs (Littwitz-Salomon, Schimmer and Dittmer 2017a). NK cells activated by high-dose infection produced IFNγ as early as 3 dpi and killed FV-transformed tumor cells in vitro and in vivo. These antiretroviral and antitumor activities were reported to be linked to the NK cell activating receptor NKG2D (Ogawa et al. 2011). In addition to the virus inoculum dose, type I IFNs and viral co-infections may also drive NK cell activation. Murine retroviruses actively suppress type I IFN expression (Lin et al. 2014), but therapeutic administration of IFNα improved NK cell function and virus control in FV-infected mice (Gerlach et al. 2009; Gibbert et al. 2012). If NK cells were pre-activated by another virus infection (e.g. MCMV), superinfection with FV was also more efficiently controlled (Francois et al. 2013).
The antiviral impact of NK cells appears to be limited to very early FV infection. Selective depletion of NK cells from 7 to 15 dpi did not affect viral loads (Littwitz et al. 2013). One possible explanation is that FV is efficiently controlled by cytotoxic CD8+ T cells at this time point (Joedicke et al. 2014b). Interestingly, depletion of NK cells at 20 to 30 dpi resulted in reduced viral loads and significantly improved FV-specific CD8+ T cell responses (Littwitz et al. 2013). These data suggested that NK cells have an inhibitory role in the transition phase between acute and chronic FV infection. Such regulatory effects of NK cells on CD8+ T cell responses have also been described in LCMV-infected mice (Waggoner et al. 2011; Lang et al. 2012). The underlying mechanism(s) appeared to involve the upregulation of inhibitory ligands as well as the downregulation of activating ligands on T cells by type I IFNs (Crouse et al. 2014; Xu et al. 2014). The molecular mechanism of CD8+ T cell suppression by NK cells during FV infection remains to be determined.
During the initial phase of FV infection (3 dpi), a subpopulation of IL-10-producing CD4+ regulatory T cells (Tregs) limited the activation and antiviral functions of NK cells (Littwitz-Salomon et al. 2018a). At later time points after virus inoculation (∼12dpi), Tregs responded to FV infection with activation and expansion (Hasenkrug, Chougnet and Dittmer 2018). These virus-induced Tregs suppress NK cells even more efficiently than the IL-10-producing Tregs. The mechanism of action involved the consumption of available IL-2 that resulted in decreased NK cell activation (Littwitz-Salomon et al. 2015). This Treg suppression could be overcome in FV-infected mice through the therapeutic administration of IL-2 that was specifically directed to NK cells by monoclonal antibodies.
The role of certain NK cell/NK cell-like subsets in FV infection remains under active investigation. Persistent FV infection in B6 mice could induce memory-like NK cells (Littwitz-Salomon et al. 2018b), consistent with other infection models. Memory-like NK cells show improved antiviral activity upon a secondary encounter with FV antigen and thus have features of memory T cells. CD1d-restricted NKT cells are also activated during FV infection (Littwitz-Salomon, Schimmer and Dittmer 2017b). Infection increased the NKT cell cytotoxic potential against FV transformed tumor cells, and treatment with the exogenous activator αGalCer led to increased NKT cell numbers and improved FV control. Further studies should shed more insight on how memory-like NK cells and NKT cells participate in retroviral control and pathogenesis. To conclude, NK cells serve as important players in FV immunity (Fig. 1), exemplifying the tight interplay between the innate and the adaptive arms of the immune system in regulating retroviral infection.
ADAPTIVE IMMUNE RESPONSES
Dendritic cells
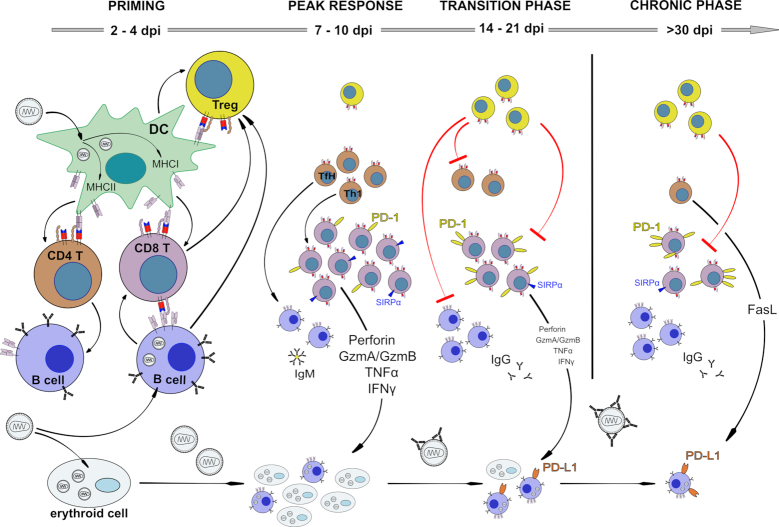
DC are an important link between the innate and adaptive immune system. They take up antigens or become infected by viruses, which matures them and enables them to efficiently present antigen to T cells. FV can productively infect CD11c+ DCs in vitroand in vivo(Balkow et al. 2007) (Fig. 2). Infection levels are increased by complement opsonization of the virus (Banki et al. 2010). TLR3 sensing of FV infection efficiently activates DCs to express co-stimulatory molecules for T cell interaction, a mechanism that required IFN I production (Gibbert et al. 2014). Infected DC present antigen to FV-specific CD8+ T cells and prime T cell responses in vitro and in vivo including their functional maturation into cytotoxic T cells (Banki et al. 2010, Li et al. 2016) (Fig. 2). In addition, DCs can also prime FV-specific helper CD4+ T cells (Li et al. 2016). However, for CD4+ T cells it has been shown that FV-infected bone marrow-derived DCs can acquire a tolerogenic phenotype (Balkow et al. 2007; Shen et al. 2018) in vitro that favors the induction of regulatory T cells rather than effector CD4+ T cells (Balkow et al. 2007).
Figure 2.
Kinetics of adaptive immune responses during FV infection. Virus-infected DCs and B cells prime CD4+ and CD8+ effector T cells, but infected DCs can also initiate Treg responses. Membrane-bound TNFα-positive CD8+ T cells and GITR expressing B cells expand Tregs. Effector T cells as well as antibody-producing B cells control acute virus replication in a complex immune response. Cytotoxic CD8+ T cells are especially effective in restricting virus spread, but become exhausted via Tregs and inhibitory receptors during the transition phase between acute and chronic infection. Infected B cells upregulate ligands for inhibitory receptors and subsequently escape from CTL killing, suppress CD8+ T cell functions and form a persisting viral reservoir. During chronic infection most CD8+ T cells, except a few SIRPα-positive ones, are dysfunctional and virus replication is kept in check by cytotoxic CD4+ T cells and most likely neutralizing antibodies.
Taken together, FV-infected DCs play an important role in FV immunity and link the innate response to specific T cell activation.
CD4+ T cell responses
CD4+ T cells are a heterogenous population of T cells that orchestrate the immune system with their helper and regulatory properties. Moreover, they can also mediate direct anti-FV functions (Hasenkrug, Brooks and Dittmer 1998; Iwashiro et al. 2001b; Nair et al. 2010). Although less studied than their CD8+ counterparts, several CD4+ T cell subsets are induced in response to FV infection (Iwashiro et al. 1993; Antunes et al. 2008; Pike et al. 2009; Nair et al. 2010; Danelli, Donnarumma and Kassiotis 2018). Mouse CD4+ T cells of the H2-Ab haplotype target numerous epitopes encoded by F-MLV gag, pol and env (Messer, Lavender and Hasenkrug 2014). However, most studies of CD4+ T cell responses to FV have focused on a dominant epitope from the F-MuLV envelope glycoprotein (env124–138) (Iwashiro et al. 1993). CD4+ T cells reactive to the F-MuLV env124–138 epitope, presented by H2-Ab, have been detected using a variety of techniques with different sensitivities, including peptide-specific cytokine release assays and peptide-MHC II tetramer binding. However, even improved tetramer reagents capture only a fraction of the F-MuLV-reactive CD4+ T cell pool (Thorborn et al. 2014a). The use of TCR-transgenic CD4+ T cells bearing Ab/env124–138-reactive receptors permitted accurate monitoring of the kinetics of their response to FV infection, independently of their ability to secrete cytokines or bind peptide-MHC tetramers, which may be compromised by Tregs or intrinsic TCR downregulation, respectively (Nair et al. 2010; Ploquin, Eksmond and Kassiotis 2011). Priming of TCR transgenic F-MuLV-reactive CD4+ T cells can be detected 3–4 days after FV infection, peaking on day 7 and declining thereafter (Thorborn et al. 2014a; Merkenschlager et al. 2016). CD4+ T cell kinetics can be delayed in mice with increased susceptibility to FV infection (Nair et al. 2010), but the peak of the CD4+ T cell response to FV infection appears to precede the peak of the CD8+ T cell response.
F-MuLV-specific CD4+ T cells are critical to the control of FV infections (Marques et al. 2008; Pike et al. 2009). Early studies highlighted an important role for CD4+ T cells in providing help for the antibody response (Super et al. 1998; Hasenkrug 1999). Indeed, F-MuLV-reactive CD4+ T cells demonstrate robust follicular helper (Tfh) differentiation, which can be much stronger than that of CD4+ T cells responding to other viral infections (Ploquin, Eksmond and Kassiotis 2011; Danelli, Donnarumma and Kassiotis 2018). F-MuLV-reactive CD4+ T cells also promote cellular immunity. Although dispensable for priming of F-MuLV-reactive cytotoxic CD8+ T cells, CD4+ T cell help promotes survival of cytotoxic effector CD8+ T cells (Nair et al. 2010). Moreover, F-MuLV-reactive CD4+ T cells exhibit direct antiviral activity, independently of other arms of adaptive immunity (Hasenkrug, Brooks and Dittmer 1998; Iwashiro et al. 2001b). CD4+ T cells have long been shown to mediate rejection of FV-induced transplantable tumors, including those lacking MHC II expression (Greenberg, Cheever and Fefer 1981; Greenberg, Kern and Cheever 1985). This anti-tumoral effect is largely attributable to IFN-γ production by Th1-differentiated cells, although studies with FV infection highlighted a protective effect of virus-specific CD4+ T cells independent of IFN-γR-signaling in the host (Pike et al. 2009). FV-specific CD4+ T cells have also been shown to acquire cytotoxic activity against MHC II-expressing targets during chronic FV infection (Malyshkina et al. 2017). This cytotoxic activity is FasL-dependent, whereas granzyme-dependent cytotoxicity, which can develop in response to F-MuLV env124–138 immunization delivered by heterologous viral vectors (Donnarumma et al. 2016), appears suppressed by CD4+ Tregs in the natural FV infection (Manzke et al. 2013; Malyshkina et al. 2017). Indeed, Tregs have been shown to be strongly activated and proliferative during FV infection, and suppress all functional subsets of FV-specific effector CD4+ and CD8+ T cells in both the acute and chronic phases of infection (Nair et al. 2010; Manzke et al. 2013; Moore et al. 2019).
Recent studies defined TCR avidity as a major determinant of the CD4+ T cell response to and protection against FV infection, and explored its role in selection, expansion and maintenance of distinct CD4+ T cell clonotypes (Thorborn, Young and Kassiotis 2014b; Merkenschlager and Kassiotis 2015). These studies utilized FV-specific, TCRβ chain-transgenic mice. Such mice have a semi-polyclonal TCR repertoire with an increased frequency of Ab/env124–138-reactive CD4+ T cell clonotypes (Antunes et al. 2008; Ploquin, Eksmond and Kassiotis 2011). Importantly, the functional avidity of each TCR in this system is determined by the particular TCRα chain used, with TCR Vα2 family chains (encoded by Trav14 gene segments) and Vα3 family chains (encoded by Trav9 gene segments) creating clonotypes with higher and lower functional avidity, respectively (Antunes et al. 2008; Ploquin, Eksmond and Kassiotis 2011). Surprisingly, despite the enormous potential of recombinatorial somatic TCR repertoire formation, the ability of Trav14 gene segments to generate high-avidity Ab/env124–138-reactive TCRs is, at least in part, germline encoded (Young et al. 2012). This finding underscores the importance of polymorphic germline TCR gene segment attributes that cannot be recreated by random somatic recombination and selection.
Formation of high-avidity Ab/env124–138-reactive TCRs requires the presence of appropriate Trav14 alleles in the germline (Young et al. 2012). However, germline Trav14 alleles alone are not sufficient to enrich the repertoire in high-avidity TCRs. All TCRs have to succeed through thymic selection, where excessive self-reactivity is a disadvantage. This is particularly problematic for TCRs reactive with retroviral antigens, given that mammalian genomes comprise numerous endogenous retroviruses, potentially expressing self-peptides of retroviral origin and mediating both positive and negative thymic selection. Indeed, thymic selection of Ab/env124–138-reactive CD4+ T cells is heavily shaped by Emv2, a single-copy endogenous ecotropic MLV in the genome of B6 mice, bearing high similarity with F-MuLV (Young et al. 2012). However, TCR cross-reactivity between the env124–138 epitope variants encoded by F-MuLV or Emv2 is inversely proportional to avidity for either variant, with TCRs with the highest avidity for F-MuLV env being the least cross-reactive with Emv2 env (Young et al. 2012). These studies provided proof of principle that negative selection by Emv2-encoded self-peptides can in fact enhance, instead of diminishing, representation of clonotypes with high avidity for Ab/env124–138, by removing competing clonotypes, albeit at the expense of cross-reactivity.
A long-term increase in the frequency of antigen-reactive lymphocytes through clonal expansion and selection is a hallmark of immunological memory and the aim of vaccination. Although there is general consensus that CD4+ T cells are clonally selected during the immune response on the basis of TCR signal strength in response to antigen, the underlying factors responsible for differences in TCR sensitivity to antigen stimulation are still a matter of debate (Merkenschlager and Kassiotis 2015). TCR binding kinetics to antigenic peptide-MHC (pMHC) complexes have traditionally been considered the dominant factor, determining the strength of T cell activation. However, studies in the FV model (Merkenschlager et al. 2016), as well as independently in other models (Persaud et al. 2014), have emphasized the importance of TCR self-reactivity in setting TCR responsiveness to antigens. Experiments with Ab/env124–138-reactive TCR-transgenic mice correlated differences in functional avidity and biological response with self-reactivity rather than affinity to antigen (Merkenschlager et al. 2016). More surprisingly, they further uncovered B cell presentation of antigen as a powerful T cell-extrinsic factor that can reverse TCR sensitivity-based hierarchies during antigen-mediated clonal selection, thereby preserving diversity of the antigen-selected TCR repertoire (Merkenschlager et al. 2016). In contrast, Tregs represent another T cell-extrinsic factor that was shown to restrict the TCR repertoire of Ab/env124–138-reactive CD4+ T cells (Fontaine et al. 2018). Therefore, the clonotypic evolution of the response to FV is shaped by the relative balance and kinetics of B cell and Treg activity. TCR interactions with distinct antigen-presenting cell (APC) subsets, as well as regulation by Tregs, determines not only the clonotypic composition of Ab/env124–138-reactive CD4+ T cells, but also their functional differentiation. In addition to the powerful effect of T cell-extrinsic factors, such as cytokines, effector CD4+ T cell differentiation is also influenced by TCR avidity in a T cell-intrinsic manner (Linterman and Vinuesa 2010; Lo and Allen 2013; Thorborn, Young and Kassiotis 2014b). The integration of multiple T cell-extrinsic and T cell-intrinsic variables affecting CD4+ T cell differentiation creates the necessary diversity of functional T cell responses, tailored to the nature of the antigen.
In contrast to the typically balanced Th1-Tfh response in other viral infections, the CD4+ T cell response to FV infection was recently shown to be heavily skewed towards Tfh differentiation (Danelli, Donnarumma and Kassiotis 2018). Biased Tfh differentiation in response to FV infection was clearly promoted by TCR signal strength, as measured by a reporter for TCR signaling and experimentally manipulated with altered epitopes, but was not affected by TCR clonotypic avidity (Danelli, Donnarumma and Kassiotis 2018). Notably, the difference in TCR signal strength experienced by Th1 and Tfh Ab/env124–138-reactive CD4+ T cells, was not due to differences in the avidities of their respective TCR repertoires, but rather a consequence of their differentiation and interaction with distinct subsets of APC (Danelli, Donnarumma and Kassiotis 2018). This central role for APCs also underlies the induction of distinct ratios of Th1, Tfh or cytotoxic CD4+ T cells by different vaccine vectors or immunization regimens in the FV model (Donnarumma et al. 2016; Shen et al. 2018).
Although naïve Ab/env124–138-reactive CD4+ T cells have not been observed to differentiate into Tregs in the natural course of FV infection (Antunes et al. 2008; Myers et al. 2013), thymically-derived Tregs of highly polyclonal TCR specificities are strongly activated and expanded by FV infection (Hasenkrug, Chougnet and Dittmer 2018). Indeed, it were studies in this model that first implicated Tregs in the regulation of the adaptive immunity to an infection (Iwashiro et al. 2001a). This expansion involves Tregs that do not react with FV antigens but rather self-antigens and is fueled by at least two separate mechanisms. First, natural thymus-derived Tregs, which constitutively express the high affinity IL-2 receptor, are polyclonally activated by IL-2 secreted by F-MuLV-reactive CD4+ T cells and by GITR ligand expressed on activated B cells (Myers et al. 2013; Moore et al. 2017). Second, thymus-derived Tregs bearing Vβ5 family TCRβ chains specifically react with a retroviral superantigen encoded by endogenous mouse mammary tumor virus 9 (MMTV9) (Myers et al. 2013; Joedicke et al. 2014a). This mode of Treg expansion accounts for about one in every 10 Tregs and is modulated by membrane-bound TNF-α signals delivered by F-MuLV-reactive CD8+ T cells (Myers et al. 2013; Joedicke et al. 2014a). Further details on the Treg response in FV infection were recently reviewed (Hasenkrug, Chougnet and Dittmer 2018). Together, these studies highlight the complex cellular interactions that are responsible for shaping common, as well as unique attributes of the FV-specific effector CD4+ T cell response (Fig. 2).
CD8+ T cell responses
CD8+ T cells play a central role in protection against acute infections with most, if not all viruses. CD8+ T cell responses have been extensively studied in mice infected with FV as a model to help us understand the functions of these cells, the kinetics of the responses and the mechanisms by which the immune system controls those responses. Early mapping of FV resistance genes showed that the MHC I gene, H-2Db, was essential for a high recovery phenotype on mice, suggesting that class I-restricted CD8+ T cell responses were important for recovery (Chesebro, Miyazawa and Britt 1990). Further experiments using CD8+ T cell depletions confirmed that these cells were indeed critical for recovery, even in highly resistant B6 mice (Robertson et al. 1992; Hasenkrug 1999; Robertson et al. 2008a). The most predominant expansion of CD8+ T cells after FV infection is found in the organs with the highest viral replication, the spleen and bone marrow (Zelinskyy et al. 2009a). A large proportion of the FV-activated CD8+ T cells recognize the H-2Db-restricted immunodominant FV GagL epitope (DbGagL) (Chen et al. 1996) and the study of this immunodominant response has been facilitated by the development of tetramers that stain the DbGagL-specific CD8+ T cells (Schepers et al. 2002) and transgenic T cells bearing a TCR specific for the DbGagL epitope (Ohlen et al. 2001). Staining of the TCR and activation markers on CD8+ T cells during FV infection indicates that there are also CD8+ T cells responding to other epitopes that the DbGagL.
In FV-infected H-2b resistant mice, activated virus-specific CD8+ T cells can be detected as early as 7 days post infection (Robertson et al. 1992; Zelinskyy et al. 2006). The peak expansion of FV-specific CD8+ T cells occurs within the next few days to a week, depending on the mouse strain and whether there is any co-infection with other viruses (Robertson et al. 2008a). There is clear evidence that activation of the FV-specific CD8+ T cells is driven by DC (Banki et al. 2010), but virus-infected B cells have also been shown to be important antigen-presenting cells for the CD8+ T cell response (Bila et al. 2011; Moore et al. 2019), particularly when the virus is opsonized with complement (Bila et al. 2011). As in many viral infections the priming of the CD8+ T cell response seems to be CD4+ T cell independent during acute FV infection (Nair et al. 2010).
Mice depleted of CD8+ T cells during acute infection are unable to efficiently control virus replication and develop severe splenomegaly or even leukemia (Robertson et al. 1992; Hasenkrug 1999; Robertson et al. 2008a; Joedicke et al. 2014b). The anti-viral activity of the CD8+ T cells is clearly associated with their ability to produce cytotoxic molecules. Activated FV-specific CD8+ T cells produce the granzymes A and B as well as perforin (Zelinskyy et al. 2005), and execute efficient killing of GagL-presenting target cells in vivo (Zelinskyy et al. 2009a). Interestingly, the expression of any one of the three mentioned cytotoxic molecules seems to be sufficient to mediate anti-FV cytotoxicity, but mice lacking all three molecules rapidly succumb to infection (Zelinskyy et al. 2004). Thus, the exocytosis pathway is the central mechanism of the antiviral activity of CD8+ T cells. However, in mice infected with very low doses of FV or the weakly replicating F-MuLV helper virus, CD8+ T cells do not switch on the exocytosis pathway, but rather control the low level infection using the Fas ligand-Fas pathway of target cell killing (Zelinskyy et al. 2007; Zelinskyy, Werner and Dittmer 2013). In addition to direct killing, FV-activated CD8+ T cells also produce pro-inflammatory cytokines, especially IFNγ, TNFα and IL-2 (Zelinskyy et al. 2009b). There is also experimental evidence that production of IFNγ as well as TNFα directly contribute to FV control (Iwashiro et al. 2001b; Stromnes et al. 2002; Myers et al. 2013).
During the late phase of acute FV infection (between 2 to 3 weeks pi) the functional activity of CD8+ T cells dramatically changes. The cells are not only reduced in numbers (contraction phase), but start to develop a severe dysfunction. They gradually lose their ability to produce granzymes and perforin and subsequently the expression of IFNγ, TNFα and IL-2 (Zelinskyy et al. 2005; Zelinskyy et al. 2009b). As a consequence most FV-specific CD8+ T cells appear functionally exhausted and are very inefficient in killing peptide-labeled targets in vivo (Zelinskyy et al. 2009a). However, a small subset of functional CD8+ T cells are preserved even in chronic infection and uniquely express the cell surface protein SIRPα (Myers et al. 2019). CD8+ T cell exhaustion contributes to the subsequent development of FV chronicity (Dietze et al. 2011), similar to what has been described for chronic LCMV infection (Barber et al. 2006). Not surprisingly then, experimental depletion of exhausted CD8+ T cells during chronic infection has no observable effect on chronic FV loads (Hasenkrug, Brooks and Dittmer 1998).
Studies using the FV model have defined several cell populations and molecules that contribute to the development of CD8+ T cell dysfunction and subsequent viral chronicity. Similar to what has been discovered in the LCMV model (Barber et al. 2006), inhibitory receptors expressed on effector CD8+ T cells play an important role in functional exhaustion. Several inhibitory receptors are upregulated on CD8+ T cells during chronic FV infection (Zelinskyy et al. 2011; Dietze et al. 2013), but the most dominant suppressive activity seems to be mediated by the programmed cell death protein 1 (PD-1), which is termed an inhibitory receptor. During chronic infection PD-1 expression can be used as a surrogate marker for exhausted CD8+ T cells and blocking the signaling of this receptor with antibodies can reactivate exhausted T cells (Barber et al. 2006; Dietze et al. 2013). Blocking the PD-1/PDL-1 axis results in the expansion of functional CD8+ T cells that express SIRPα (Myers et al. 2019). Increased expression of co-inhibitory molecules such as PD-1 is likely to prevent immunopathogenic effects from over-stimulated CD8+ T cells responses. The upregulation of PD-1 is initiated during acute FV infection (Zelinskyy et al. 2011). Results indicate that it is not the simple expression of co-inhibitory or co-stimulatory molecules that dictate the function of the effector cell, but whether the balance of the signals from these molecules is more inhibitory or stimulatory. In the inflammatory milieu present during acute infections, the balance tilts toward high function, but as the infection clears and inflammation declines, the balance tilts toward inhibition and protection from immunopathology.
As FV infection develops, subpopulations of virus-infected cells such as B cells and granulocytic cells start to express high levels of the PD-1 ligand (PD-L1) (Akhmetzyanova et al. 2015). This enhanced expression of PD-L1 protects these cells from cytolytic T cell killing and they seed a reservoir of chronically infected cells. At the same time, frequent contacts of virus-specific CD8+ T cells with these protected targets result in a functional impairment of the effector CD8+ T cells due to PD-L1-PD-1 interaction (Akhmetzyanova et al. 2015). It has recently been shown that the PD-1/PD-L1 pathway is not only involved in T cell exhaustion, but also influences the proliferation and apoptosis of CD8+ T cells during acute FV infection (David et al. 2019).
In addition to these mechanisms of inhibition, there are also specific cell types that contribute to CD8+ T cell dysfunction in chronic FV infection. The important role of Tregs in viral chronicity was first described in the FV model (Iwashiro et al. 2001a; Dittmer et al. 2004). Tregs become activated and expand during the late phase of acute FV infection (Zelinskyy et al. 2006) and subsequently suppress the proliferation and function, including cytotoxicity and cytokine production, of effector CD8+ T cells (Zelinskyy et al. 2009b; Zelinskyy et al. 2009a). Not surprisingly, experimental depletion of Tregs during the acute FV infection augments the cytotoxic CD8+ T cell response and improves virus control (Zelinskyy et al. 2009a; Moore et al. 2019). Treg ablation during chronic infection reactivates exhausted CD8+ T cells, including both induction of cytotoxicity and cytokine production, and also results in reduced viral loads (Dietze et al. 2011). Interestingly, the activation and expansion of Tregs is partly driven by activated effector CD8+ T cells themselves via their expression of membrane-bound TNFα that binds to the TNF receptor 2 on thymus-derived Tregs (Myers et al. 2013; Joedicke et al. 2014a). So far the exact molecular mechanism by which Tregs exert their immunosuppressive influence on CD8+ T cells remains unknown (Hasenkrug, Chougnet and Dittmer 2018). Both of the mechanisms of T cell exhaustion that we describe here, PD-1/PD-L1 expression and Treg expansion, are independent events and blocking both pathways simultaneously has a synergistic effect on CD8+ T cell mediated virus control (Dietze et al. 2013).
Recently, we discovered a third mechanism of T cell suppression in the FV model which is mediated by myeloid derived suppressor cells (MDSC) (Drabczyk-Pluta et al. 2017). Suppression of T and B cell responses by MDSCs had been described before in another mouse retrovirus model (reviewed in O´Connor, Rastad and Green 2017). We found that granulocytic MDSC restrict the functional activity of FV-specific CD8+ T cells and that this suppression involved arginase 1, PD-L1, and the ATP dephosphorylating enzyme CD39. In addition, NK cells were also able to suppress CD8+ T cell responses during the later phase of FV infection, but the molecular mechanisms remains to be determined (Littwitz et al. 2013). Thus, CD8+ T cell responses are strongly modulated/restricted during FV infection (Fig. 2). Such tight control is necessary as overactive CD8+ T cells can be more dangerous for the host than the virus infection itself. The other edge of the sword though, is that suppression of the CD8+ T cell response can allow chronic infection to develop. From an evolutionary point of view, chronic infections often produce less severe selective pressures than immunopathology. In the case of FV, chronically infected mice have a normal reproduction rate and only very few animals develop leukemia late in life (Hasenkrug, Brooks and Dittmer 1998).
B cell responses and antibodies
In 1963, it was shown that FV-infected mice mounted antibody responses against FV virions and FV leukemia cells (Old, Boyse and Lilly 1963). The importance of these antibodies was demonstrated in studies showing that passive antibody transfers of FV-specific antibodies could be protective against FV-induced leukemia (Wheelock et al. 1972). However, passive antibody transfers only protected mouse strains that were also able to mount essential cell-mediated immune responses. Thus, depletion of either CD4+ or CD8+ T cells ablated antibody-mediated protection (Hasenkrug, Brooks and Chesebro 1995a). In 1979, it was found that the ability of different mouse strains to mount virus-neutralizing antibody responses was controlled by a single autosomal, non-MHC gene. The gene was termed Rfv-3 (Recovery from Friend virus gene 3) and was shown to be essential for control of viremia and prevention of leukemia (Chesebro and Wehrly 1979; Chesebro et al. 1979; Britt and Chesebro 1983). Further studies revealed that mice depleted of CD4+ T-cells in vivo showed undetectable FV-neutralizing antibody responses, high viremia and reduced survival times. Thus, CD4+ T-helper cells were required for the Rfv-3-controlled FV-neutralizing antibody responses, including IgM responses (Super et al. 1998). These T helper cells are now known to be Follicular T helper or Tfh cells (Kassiotis and O'Garra 2009), and FV infections induce a strongly biased differentiation of CD4+ T cells into Tfh cells (Danelli, Donnarumma and Kassiotis 2018). Microsatellite mapping experiments undertaken in an effort to determine the identity of the Rfv-3 gene showed that it mapped to a 20 centimorgan region of chromosome 15 (Hasenkrug et al. 1995b), and further studies narrowed the location to a 0.83 centimorgan region (Super et al. 1999; Kanari et al. 2005). The identified region was very gene rich though, containing at least 61 genes unlinked to the MHC, the immunoglobulin loci or T cell receptor loci. One gene of particular interest, Mouse APOBEC3 (see chapter 3.2), exhibited striking functional polymorphisms that correlated with the Rfv3 phenotype of inbred mouse strains. Rfv3 resistant mice such as B6 express a truncated mA3 protein lacking exon 5 that is more resistant to retroviral protease (Abudu et al. 2006; Li et al. 2012). Rfv3 susceptible mice such as BALB/c, A.BY, A/WySn and 129P2 express a full-length mA3 at 10-fold lower mRNA levels than in Rfv3 resistant mice (Takeda et al. 2008; Okeoma, Petersen and Ross 2009; Sanville et al. 2010; Santiago et al. 2011; Halemano et al. 2013a). Targeted genetic inactivation of mA3 combined with genetic backcross studies demonstrated that mA3 indeed controlled neutralizing antibody responses to FV infection and encoded the Rfv-3 phenotype (Santiago et al. 2008).
Mouse APOBEC3 promoted germinal center B cell development during acute FV infection (Santiago et al. 2010). At this stage of B cell development, a direct and an indirect model may explain how mA3 influences Nab responses. The indirect effect is linked to the unique mechanism by which mA3 to restricts acute FV replication. It was shown that although mA3 decreased titers of infectious virus during acute FV infection, plasma viral RNA loads were maintained (Smith et al. 2011). These findings indicated that mA3 restriction promoted neutralizing antibody responses by maintaining high concentrations of virions with native B cell epitopes, but in the context of low virion infectivity. The direct effect of mA3 on antibody responses is through somatic hypermutation. Somatic hypermutation is an integral process in the affinity maturation of antibodies important for the development of the highly avid antibodies necessary for protection from viral infections. Somatic hypermutation occurs via the enzymatic activity of activation-induced deaminase (AID), and enzyme evolutionarily related to mA3. However, in contrast to AID, which prefers to mutate deoxycytidines preceded by a purine, all APOBEC3 members, including mA3, preferentially mutate deoxycytidines preceded by a pyrimidine (Chelico, Pham and Goodman 2009). Studies showed that mA3 complemented AID in driving immunoglobulin gene somatic hypermutation during retrovirus infection. This revealed a novel mechanism through which mA3 promoted the neutralizing antibody responses essential for recovery from retroviral infection (Halemano et al. 2014). Apobec-mediated somatic hypermutation may also be relevant in HIV-1 vaccine development, as most broadly-neutralizing antibodies that achieve cross-clade HIV-1 neutralization are heavily mutated. Highly mutated immunoglobulin genes are more likely to have improbable protein structures that promote neutralization breadth (Wiehe et al. 2018).
The main target of FV-specific neutralizing antibodies is the F-MuLV Env protein. Isolation of FV-specific neutralizing monoclonal antibodies suggested multiple mechanisms of inhibition, such as inhibiting the expansion of virus-producing cells, promoting NK cell-mediated ADCC and complement-dependent neutralization in vitro (reviewed in Halemano et al. (2013b)). Sequence comparisons of monoclonal antibodies from Rfv3-resistant versus Rfv3-susceptible mice suggested that protective neutralizing antibodies are associated with the IgG2c subclass, specific VH gene families, increased binding affinity to virions and higher somatic mutations (Halemano et al. 2014). In passive transfer studies, the neutralizing activity of the antibodies was shown to be dependent on activating Fcγ receptors, but not on complement (Halemano et al. 2015).
The ability of B cells to become activated and respond to infection is strongly modulated by Tregs, which provide a level of homeostatic suppression of B cells. Such B cell modulation reduces the potential to generate autoimmune antibodies and ensures that only specific responses associated with danger signals from pathogens elicit antibody production. In mice infected with FV, which induces a robust expansion of Tregs, depletion of Tregs led to elevated activation, proliferation and class switching of B cells (Moore, Messer and Hasenkrug 2018). In addition, Treg depletion enhanced the kinetics and production levels of virus-specific and virus-neutralizing antibodies and also reduced FV viremia. Thus, similar to T cell responses, specific B cell responses to viral infections must overcome a threshold level of Treg-mediated suppression before the antibody response can be mounted (Moore, Messer and Hasenkrug 2018) (Fig. 2).
Antibody production during FV infection is obviously a very important function of B cells, but studies also indicate that antigen presentation by B cells is important for stimulating CD8+ cytotoxic T lymphocyte responses. For example, complement opsonization of FV enhances the infection of B cells in vitro and increases their ability to activate FV-specific CD8+ T cells (Bila et al. 2011). Recent studies have shown that while FV infection of DC can reduce their APC capacity (Balkow et al. 2007; Shen et al. 2018), the infection of B cells produced a stimulatory effect as evidenced by increased expression of costimulatory molecules required for APC function. Furthermore, FV-infected B cells had significantly better APC function than uninfected B cells from the same mouse as measured by their capacity to prime CD8+ T cell activation and proliferation in vitro (Moore et al. 2019). As discussed in the chapter on CD4+ T cells, antigen presentation by B cells is also important in the generation of high avidity, FV-specific CD4+ T cells (Ploquin, Eksmond and Kassiotis 2011). The antigen presentation function of B cells falls under Treg-mediated suppression in a similar manner as described for antibody production by B cells. Thus, depletion of Tregs, even in naïve mice, strongly upregulates costimulatory molecule expression on B cells and enhances their APC function for CD8+ T cell priming (Moore et al. 2019).
As discussed earlier, FV infections induce the activation and proliferation of Tregs with immunosuppressive activity for both T cells and B cells. Unexpectedly, the FV-induced Treg response is dependent on B cells, which provide essential signals for Treg expansion during FV infection (Fig. 2). Treg responses are greatly diminished in B cell-deficient mice but can be restored by adoptive transfers of B cells at the time of infection. The feeble Treg responses in B cell-deficient mice are associated with enhanced virus-specific CD8+ T cell responses and accelerated virus control during the first 2 weeks of infection. In vitro experiments demonstrated that B cells promote Treg activation and proliferation through a glucocorticoid-induced receptor superfamily member 18 (GITR) ligand-dependent mechanism. Thus, B cells play paradoxically opposing roles during FV infection. They provide proliferative signals to immunsuppressive Tregs, which slows early virus control through suppression of T cell and B cell responses, and they also produce virus-specific antibodies, which are essential for long-term virus control (Moore et al. 2017).
Another interesting interaction between lymphocyte subsets responding to FV infections is that the expansion of Tfh cells, which is necessary to promote B cell responses, is enhanced by the presence of Tregs. Tregs have been shown to act as an IL-2 sink in FV infection (Littwitz-Salomon et al. 2015) and low IL-2 concentrations promote Tfh differentiation and germinal center formation (Ballesteros-Tato et al. 2012; Danelli, Donnarumma and Kassiotis 2018). Thus, on one hand, FV-induced expansion of Tregs enhances Tfh differentiation thereby promoting antibody responses, but on the other hand, the B cell response is suppressed by FV-induced Tregs as discussed above. The complex dual nature of these intercellular interactions reveals a delicately balanced system evolved to react but not overreact to pathogenic challenges.
IMMUNOTHERAPIES IN FV INFECTION
In the last decades the FV model has been extensively used to design and test novel concepts of immunotherapy against infectious diseases. The FV model provides the opportunity to not only study immunotherapeutics that enhance viral immunity during an acute infection, but also during an established chronic viral infection (Table 2). These two phases of infection present very distinct immunological environments with unique requirements for successful immunotherapy.
Table 2.
Approaches for immunotherapy during acute and chronic FV infection.
| Therapeutic | Improved immune response | Reference |
|---|---|---|
| Treg depletion | CD4+ T cells, CD8+ T cells, NK cells, B cells | (Zelinskyy et al. 2009a) |
| (Dietze et al. 2011) | ||
| (Manzke et al. 2013) | ||
| (Littwitz-Salomon et al. 2015) | ||
| (Littwitz-Salomon et al. 2018a) | ||
| (Moore et al. 2018) | ||
| MDSC depletion | CD8+ T cells | (Drabczyk-Pluta et al. 2017) |
| PD-1/PDL-1 blockade | CD8+ T cells | (Dietze et al. 2013) |
| (Akhmetzyanova et al. 2015) | ||
| Tim-3 blockade | CD8+ T cells | (Dietze et al. 2013) |
| Interferon-alpha subtypes | CD8+ T cells, NK cells | (Gerlach et al. 2009) |
| (Gibbert et al. 2012) | ||
| TLR ligands | CD8+ T cells, B cells | (Olbrich et al. 2002) |
| (Gibbert et al. 2010) | ||
| (Kraft et al. 2005) | ||
| GITR agonist and blockade | CD8+ T cells, Tregs | (Dittmer et al. 2004) |
| (He et al. 2004) | ||
| CD137 agonist | CD8+ T cells, CD4+ T cells | (Robertson et al. 2008b) |
| (Malyshkina et al. 2017) | ||
| (Malyshkina et al. 2019) | ||
| IL-15/IL-18 cytokines | NK cells | (Littwitz-Salomon, Schimmer and Dittmer 2017a) |
| IL-10 blockade | NK cells | (Littwitz-Salomon et al. 2018a) |
| CD122-directed IL-2 | NK cells | (Littwitz-Salomon et al. 2015) |
| CD1d activator αGalCer | NK T cells | (Littwitz-Salomon, Schimmer and Dittmer 2017b) |
| IFNγ blockade | Antibodies, CD8+ T cells | (Stromnes et al. 2002) |
| (Duley et al. 2012) |
Elucidation of the various molecular and cellular effectors elicited during each phase of FV infection has informed targeted therapies to improve virus control through various modalities. In the acute phase, one of the first anti-viral effectors is type I IFNs, which are sometimes used to treat viral infections in the clinic. Since FV induces only a very weak endogenous type I IFN response upon infection, exogenous IFNα was thought to be an interesting approach of immunotherapy and also an interesting model to test the hypothesis that different IFNα subtypes would have varying efficacies in virus control. Indeed, certain IFNα subtypes were able to restrict acute FV replication in vivo(Gerlach et al. 2009; Gibbert et al. 2012). The mechanisms of action were most likely the direct induction of anti-viral restriction factors (see chapter 3.1), but also the immunomodulatory properties of IFNα. Mouse IFNα4 was able to augment early FV-specific CD8+ T cell responses, whereas IFNα1 and α11 mainly activated NK cell responses (Gerlach et al. 2009; Gibbert et al. 2012). Similar findings were made when IFN responses were induced by TLR ligands rather than IFNα injections. Here TLR-9 (Olbrich et al. 2002) and TLR-3 ligands (Gibbert et al. 2010) mediated IFN-dependent direct anti-viral activity as well as stimulatory effects on virus-specific T cells. Beside IFNα subtypes, other cytokines that enhance NK cell responses were shown to restrict initial FV replication, including IL-2 specifically targeted to NK cells (Littwitz-Salomon et al. 2015) and IL-15/IL-18 (Littwitz-Salomon, Dittmer and Sutter 2016; Littwitz-Salomon, Schimmer and Dittmer 2017a). In contrast to IFNα, these effects exclusively relied on the activity of NK cells and no other immunological mechanisms were involved. Therapeutic effects during early FV infection could also be achieved by stimulating NKT cells with their exogenous activator αGalCer (Littwitz-Salomon, Schimmer and Dittmer 2017b). Antibody-mediated blockade of the glucocorticoid-induced tumor necrosis factor receptor on Tregs during acute FV infection produced faster Th1 responses and reduced virus loads and pathology. Interestingly, this immunotherapeutic also improved long-term CD8+ T cell functionality (He et al. 2004).
Although IFNγ is generally considered a potent antiviral cytokine, experiments in FV-infected mice co-infected with LDV demonstrated that LDV-induced IFNγ was actually detrimental to control of acute FV. Delivery of IFNγ-blocking antibodies during acute FV resulted in significantly reduced virus levels and better CD8+ T cell responses in the absence of IFNγ (Duley et al. 2012). This negative effect of IFNγ on CD8+ T cells was indirect, not involving IFNγ receptors on the CD8+ T cells. Another effect of the IFNγ was to promote FV infection of B cells. Interestingly, the detrimental effects of IFNγ on early FV-specific immune responses were replaced at about 2 weeks post-infection by a requirement for IFNγ to maintain virus control, especially in terms of developing high titer, class-switched antibody responses (Stromnes et al. 2002). These experiments illustrate that the timing of immunomodulatory therapeutics with respect to the disease course can be absolutely critical for efficacy.
The virus-specific CD8+ T cell response is critical for early FV control and first appears at around 1 week post FV infection. Since CD8+ T cells are under the control of suppressive Tregs as well as MDSCs it is not surprising that depletion of these suppressors enhanced CD8+ T cell responses and reduced levels of acute FV infection (reviewed in (Hasenkrug, Chougnet and Dittmer 2018)). These experiments showed that a number of different target molecules or cell types of the immune system can be manipulated by immunotherapy to influence the outcome of an acute retroviral infection.
Although mice with resistant genetic backgrounds are able to bring acute FV infections under control, a chronic, low-level infection ensues. The combination of chronic antigen stimulation and suppression by Tregs and MDSCs establishes an immunosuppressive milieu that presents a complicated therapeutic situation. During the chronic phase of infection, the cytotoxic and cytokine-mediated antiviral activities of effector CD8+ T cells are largely incapacitated (Zelinskyy et al. 2005; Zelinskyy et al. 2009a), although recent results indicate that a small subset of CD8+ T cells expressing SIRPα remains functional (Myers et al. 2019). Thus, immunotherapy in chronic viral infection mainly aims to restore the functionality of virus-specific T cells or expand the functional subsets. This concept is also very relevant for chronic human infections with viruses like HIV or HBV and for the treatment of cancers. We have shown that blocking inhibitory signals by Treg depletion (Dietze et al. 2011), PD-1 antibody blockade (Dietze et al. 2013), or MDSC depletion (Drabczyk-Pluta et al. 2017), results in reactivation of CD8+ T cells and significant reduction in chronic FV set points. Combination therapies targeting PD-1 and Tregs at the same time further improved this checkpoint immunotherapy (Dietze et al. 2013). In addition to enhancing control of chronic virus by modulating suppression and co-inhibitory molecules, it was also shown that immunostimulatory therapies could be useful. Agonistic antibodies targeting the co-stimulatory molecule CD137 reactivated FV-specific CD4+ and CD8+ effector T cells (Malyshkina et al. 2017; Malyshkina et al. 2019) and made them refractory to Treg suppression (Robertson et al. 2008b). They could even reprogram Tregs into effector T cells that kill FV-transformed tumor cells (Akhmetzyanova et al. 2016). Nanoparticle vaccines delivering FV antigen and molecules for TLR stimulation were able to induce potent T cell responses that restricted chronic FV infection (Knuschke et al. 2014). This successful therapeutic vaccination was dependent on type I IFN induction (Knuschke et al. 2018) and could be further improved by Treg depletion (Knuschke et al. 2016).
Taken together, the results from several decades of research on FV immunity have provided a detailed picture of the different phases of retroviral immunity, from acute infection to chronicity (Fig. 2). Unexpected interactions between the intrinsic, innate and adaptive immunity have been revealed during acute infection (Fig. 3). For example, intrinsic restriction factors such as Apobec and Tetherin were shown to influence NK cell, B cell and T cell responses and complement opsonization of virus was shown to strongly influence FV-specific T cell responses. Such findings are important for the development of immunotherapies and vaccines. These areas of research were also informed by novel discoveries of immune regulation during chronic FV infection. Host responses to chronic infections involve a complex state of both immune effector functions to control virus and counter-regulatory mechanisms to avert immmunopathology. Some of these mechanisms, such as Treg-mediated suppression, were first described in the FV model. These discoveries have let to therapeutic manipulations to tip the immunological balance towards immune mechansims that significantly reduce chronic infections. However, even in our most successful immunotherapy experiments with chronically FV-infected mice, we were not able to achieve viral clearence, a goal that is also still unmet in HIV research. Thus, future studies will have to concentrate on novel combination therapies that target different mechanisms of viral persistence and immune evasion to provide a proof of concept for retroviral cure or functional cure.
Figure 3.
The complex interplay of innate and adaptive immunity in FV infection. Virus sensing initiates IFN I responses, which stimulate and activate NK cells, CD8+ T cells and DCs. CD4+ and CD8+ T cell priming by FV-infected DCs is more efficient when complement-opsonized virus was taken up by the DCs, but IgG-opsonized virus inhibits T cell induction. Infected B cells can also initiate CD8+ T cell responses, which is also more efficient after infection with complement opsonized virus. The expression of APOBEC3 after virus sensing augments B cell/antibody responses, whereas the expression of Tetherin facilitates CD4+ and CD8+ T cell responses by enhancing antigen presentation. Effector CD8+ T cell responses can be suppressed by virus-activated NK cells, Tregs as well as gMDSCs.
The complex virus-host interactions during acute FV infections and the transition to a chronic infection have proven to be highly relevant to persistent infections in humans. The ability to study and treat all phases of infection make the FV model an attractive one for developing and testing therapeutics for their effects on both the virus and the intricate interactions between various components of the immune system.
Contributor Information
Ulf Dittmer, Institute for Virology, University Clinics Essen, University of Duisburg-Essen, Virchowstr. 179, 45147 Essen, Germany.
Kathrin Sutter, Institute for Virology, University Clinics Essen, University of Duisburg-Essen, Virchowstr. 179, 45147 Essen, Germany.
George Kassiotis, Retroviral Immunology, The Francis Crick Institute, 1 Midland Road, London NW1 1AT, UK; Department of Medicine, Faculty of Medicine, Imperial College London, St Mary's Hospital, Praed St, Paddington, London W2 1NY, UK.
Gennadiy Zelinskyy, Institute for Virology, University Clinics Essen, University of Duisburg-Essen, Virchowstr. 179, 45147 Essen, Germany.
Zoltán Bánki, Division of Virology, Medical University of Innsbruck, Peter-Mayrstr. 4b, A-6020 Innsbruck, Austria.
Heribert Stoiber, Division of Virology, Medical University of Innsbruck, Peter-Mayrstr. 4b, A-6020 Innsbruck, Austria.
Mario L Santiago, University of Colorado School of Medicine, 12700E 19th Ave, Aurora, CO 80045, USA.
Kim J Hasenkrug, Laboratory of Persistent Viral Diseases, Rocky Mountain Laboratories, NIAID, NIH, 903S 4th Street, Hamilton, MT 59840, USA.
FUNDING
This work was supported by the DFG (DI 714/20–1) and the RTG 1949 for U.D. and K.S. This work was also supported by The Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institute of Health, USA, Project ZA1000753 for K.J.H. and the Austrian Science Fund (FWF) research projects I-2550 (to H.S. and Z.B.) and P-21 508 (to Z.B.). This work was supported by the Francis Crick Institute (FC001099 to G.K.), which receives its core funding from Cancer Research UK, the UK Medical Research Council, and the Wellcome Trust. Funding for M.L.S. comes from NIH R01 AI116603 and R01 AI134220.
Conflict of interest. None declared.
REFERENCES
- Abudu A, Takaori-Kondo A, Izumi Tet al.. Murine retrovirus escapes from murine APOBEC3 via two distinct novel mechanisms. Curr Biol. 2006;16:1565–70. [DOI] [PubMed] [Google Scholar]
- Akhmetzyanova I, Drabczyk M, Neff CPet al.. PD-L1 expression on retrovirus-infected cells mediates immune escape from CD8+ T cell killing. PLoS Pathog. 2015;11:e1005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmetzyanova I, Zelinskyy G, Littwitz-Salomon Eet al.. CD137 agonist therapy can reprogram regulatory T cells into cytotoxic CD4+ T cells with antitumor activity. J Immunol. 2016;196:484–92. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- Aman MJ, Tretter T, Eisenbeis Iet al.. Interferon-alpha stimulates production of interleukin-10 in activated CD4+ T cells and monocytes. Blood. 1996;87:4731–6. [PubMed] [Google Scholar]
- Ammann CG, Messer RJ, Peterson KEet al.. Lactate dehydrogenase-elevating virus induces systemic lymphocyte activation via TLR7-dependent IFNalpha responses by plasmacytoid dendritic cells. PLoS One. 2009;4:e6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes I, Tolaini M, Kissenpfennig Aet al.. Retrovirus-specificity of regulatory T cells is neither present nor required in preventing retrovirus-induced bone marrow immune pathology. Immunity. 2008;29:782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias JF, Heyer LN, von Bredow Bet al.. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci USA. 2014;111:6425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjan-Odedra S, Swanson CM, Sherer NMet al.. Endogenous MOV10 inhibits the retrotransposition of endogenous retroelements but not the replication of exogenous retroviruses. Retrovirology. 2012;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkow S, Krux F, Loser Ket al.. Friend retrovirus infection of myeloid dendritic cells impairs maturation, prolongs contact to naive T cells, and favors expansion of regulatory T cells. Blood. 2007;110:3949–58. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Leon B, Graf BAet al.. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki Z, Kacani L, Rusert Pet al.. Complement dependent trapping of infectious HIV in human lymphoid tissues. AIDS. 2005;19:481–6. [DOI] [PubMed] [Google Scholar]
- Banki Z, Posch W, Ejaz Aet al.. Complement as an endogenous adjuvant for dendritic cell-mediated induction of retrovirus-specific CTLs. PLoS Pathog. 2010;6:e1000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki Z, Werner R, Riepler Let al.. Fcgamma receptor Type I (CD64)-mediated impairment of the capacity of dendritic cells to activate specific CD8 T cells by IgG-opsonized Friend virus. Viruses. 2019;11:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki Z, Wilflingseder D, Ammann CGet al.. Factor I-mediated processing of complement fragments on HIV immune complexes targets HIV to CR2-expressing B cells and facilitates B cell-mediated transmission of opsonized HIV to T cells. J Immunol. 2006;177:3469–76. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust Det al.. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. [DOI] [PubMed] [Google Scholar]
- Barrett BS, Guo K, Harper MSet al.. Reassessment of murine APOBEC1 as a retrovirus restriction factor in vivo. Virology. 2014;468-470:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett BS, Harper MS, Jones STet al.. Type I interferon signaling is required for the APOBEC3/Rfv3-dependent neutralizing antibody response but not innate retrovirus restriction. Retrovirology. 2017;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett BS, Smith DS, Li SXet al.. A single nucleotide polymorphism in tetherin promotes retrovirus restriction in vivo. PLoS Pathog. 2012;8:e1002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt R, Schumann T, Gerbaulet Aet al.. Mouse SAMHD1 has antiretroviral activity and suppresses a spontaneous cell-intrinsic antiviral response. Cell Rep. 2013;4:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bila C, Oberhauser V, Ammann CGet al.. Complement opsonization enhances friend virus infection of B cells and thereby amplifies the virus-specific CD8+ T cell response. J Virol. 2011;85:1151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank KJ, Freedman HA, Lilly F. T-lymphocytes response to Friend virus-induced tumour cell lines in mice of strains congenic at H–2. Nature. 1976;260:250–2. [DOI] [PubMed] [Google Scholar]
- Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddu P, Benton CB, Wang Wet al.. Erythroleukemia-historical perspectives and recent advances in diagnosis and management. Blood Rev. 2018;32:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boi S, Ferrell ME, Zhao Met al.. Mouse APOBEC3 expression in NIH 3T3 cells mediates hypermutation of AKV murine leukemia virus. Virology. 2018;518:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boi S, Kolokithas A, Shepard Jet al.. Incorporation of mouse APOBEC3 into murine leukemia virus virions decreases the activity and fidelity of reverse transcriptase. J Virol. 2014;88:7659–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt WJ, Chesebro B. Use of monoclonal anti-gp70 antibodies to mimic the effects of the Rfv-3 gene in mice with Friend virus-induced leukemia. J Immunol. 1983;130:2363–7. [PubMed] [Google Scholar]
- Browne EP, Littman DR. Myd88 is required for an antibody response to retroviral infection. PLoS Pathog. 2009;5:e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne EP, Littman DR. Species-specific restriction of apobec3-mediated hypermutation. J Virol. 2008;82:1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne EP. Toll-like receptor 7 controls the anti-retroviral germinal center response. PLoS Pathog. 2011;7:e1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne EP. Toll-like receptor 7 inhibits early acute retroviral infection through rapid lymphocyte responses. J Virol. 2013;87:7357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelico L, Pham P, Goodman MF. Stochastic properties of processive cytidine DNA deaminases AID and APOBEC3G. Philos Trans R Soc Lond B Biol Sci. 2009;364:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Qin H, Chesebro Bet al.. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J Virol. 1996;70:7773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B, Miyazawa M, Britt WJ. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–99. [DOI] [PubMed] [Google Scholar]
- Chesebro B, Wehrly K, Cloyd Met al.. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981;112:131–44. [DOI] [PubMed] [Google Scholar]
- Chesebro B, Wehrly K, Doig Det al.. Antibody-induced modulation of Friend virus cell surface antigens decreases virus production by persistent erythroleukemia cells: influence of the Rfv-3 gene. Proc Natl Acad Sci USA. 1979;76:5784–8.293683 [Google Scholar]
- Chesebro B, Wehrly K. Identification of a non-H-2 gene (Rfv-3) influencing recovery from viremia and leukemia induced by Friend virus complex. Proc Natl Acad Sci USA. 1979;76:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B, Wehrly K. Rfv-1 and Rfv-2, two H-2-associated genes that influence recovery from Friend leukemia virus-induced splenomegaly. J Immunol. 1978;120:1081–5. [PubMed] [Google Scholar]
- Chesebro B, Wehrly K. Studies on the role of the host immune response in recovery from Friend virus leukemia. I. Antiviral and antileukemia cell antibodies. J Exp Med. 1976a;143:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B, Wehrly K. Studies on the role of the host immune response in recovery from Friend virus leukemia. II. Cell-mediated immunity. J Exp Med. 1976b;143:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun R, Fan H. Recovery of Glycosylated gag Virus from mice Infected with a Glycosylated gag-Negative mutant of Moloney Murine Leukemia Virus. J Biomed Sci. 1994;1:218–23. [DOI] [PubMed] [Google Scholar]
- Clemens MJ, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17:503–24. [DOI] [PubMed] [Google Scholar]
- Closs EI, Borel Rinkes IH, Bader Aet al.. Retroviral infection and expression of cationic amino acid transporters in rodent hepatocytes. J Virol. 1993;67:2097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cmarik J, Ruscetti S. Friend spleen focus-forming virus activates the Tyrosine Kinase sf-Stk and the transcription factor PU.1 to cause a multi-stage Erythroleukemia in mice. Viruses. 2010;2:2235–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocka LJ, Bates P. Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 2012;8:e1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick E, Folkvord JM, Lind KTet al.. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol. 2014;193:5613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin A, Prats AC, Darlix JLet al.. A nonstructural gag-encoded glycoprotein precursor is necessary for efficient spreading and pathogenesis of murine leukemia viruses. J Virol. 1994;68:3857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier JP, Van Snick J. Isotypically restricted activation of B lymphocytes by lactic dehydrogenase virus. Eur J Immunol. 1985;15:250–5. [DOI] [PubMed] [Google Scholar]
- Crouse J, Bedenikovic G, Wiesel Met al.. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40:961–73. [DOI] [PubMed] [Google Scholar]
- Danelli L, Donnarumma T, Kassiotis G. Correlates of follicular helper bias in the CD4 T cell response to a retroviral antigen. Front Immunol. 2018;9:1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David P, Megger DA, Kaiser Tet al.. The PD-1/PD-L1 pathway affects the expansion and function of cytotoxic CD8(+) T cells during an acute retroviral infection. Front Immunol. 2019;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. Charlotte Friend: March 11, 1921-January 13, 1987. Biogr Mem Natl Acad Sci. 1994;63:127–48. [PubMed] [Google Scholar]
- Dietze KK, Zelinskyy G, Gibbert Ket al.. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc Natl Acad Sci USA. 2011;108:2420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze KK, Zelinskyy G, Liu Jet al.. Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8+ T cells and efficiently reduces chronic retroviral loads. PLoS Pathog. 2013;9:e1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer U, He H, Messer RJet al.. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. [DOI] [PubMed] [Google Scholar]
- Dittmer U, Race B, Hasenkrug KJ. Kinetics of the development of protective immunity in mice vaccinated with a live attenuated retrovirus. J Virol. 1999;73:8435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnarumma T, Young GR, Merkenschlager Jet al.. Opposing development of cytotoxic and follicular helper CD4 T cells controlled by the TCF-1-Bcl6 nexus. Cell Rep. 2016;17:1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabczyk-Pluta M, Werner T, Hoffmann Det al.. Granulocytic myeloid-derived suppressor cells suppress virus-specific CD8(+) T cell responses during acute Friend retrovirus infection. Retrovirology. 2017;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C, Gunther S, Preiser Wet al.. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. [DOI] [PubMed] [Google Scholar]
- Duley AK, Ploquin MJ, Eksmond Uet al.. Negative impact of IFN-gamma on early host immune responses to retroviral infection. J Immunol. 2012;189:2521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenbichler CF, Thielens NM, Vornhagen Ret al.. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J Exp Med. 1991;174:1417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz A, Ammann CG, Werner Ret al.. Targeting viral antigens to CD11c on dendritic cells induces retrovirus-specific T cell responses. PLoS One. 2012;7:e45102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Shikh ME, Pitzalis C. Follicular dendritic cells in health and disease. Front Immunol. 2012;3:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson DE, Arnold D, Spies Tet al.. Cytokines increase transporter in antigen processing-1 expression more rapidly than HLA class I expression in endothelial cells. J Immunol. 1992;149:3297–301. [PubMed] [Google Scholar]
- Erlandsson L, Blumenthal R, Eloranta MLet al.. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr Biol. 1998;8:223–6. [DOI] [PubMed] [Google Scholar]
- Evans LH, Dresler S, Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol. 1977;24:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel AH, Dawson PJ, Bostick WL. Quantitative aspects of Friend leukemia virus in various murine hosts. Proc Soc Exp Biol Med. 1961;108:826–9. [DOI] [PubMed] [Google Scholar]
- Fontaine M, Vogel I, Van Eycke YRet al.. Regulatory T cells constrain the TCR repertoire of antigen-stimulated conventional CD4 T cells. Embo J. 2018;37:398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois S, Peng J, Schwarz Tet al.. NK cells improve control of friend virus infection in mice persistently infected with murine cytomegalovirus. Retrovirology. 2013;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franker CK, Quilligan JJ Jr.. Genetic aspects of resistance to Friend leukemia virus. Proc Soc Exp Biol Med. 1966;121:1090–3. [DOI] [PubMed] [Google Scholar]
- Freissmuth D, Dierich MP, Stoiber H. Role of complement in the pathogenesis of SIV infection. Front Biosci. 2003;8:s733–9. [DOI] [PubMed] [Google Scholar]
- Friedman M, Lilly F, Nathenson SG. Cell surface antigen induced by Friend murine leukemia virus is also in the virion. J Virol. 1974;14:1126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa R, McAtee FJ, Favara Cet al.. N-terminal cleavage fragment of glycosylated Gag is incorporated into murine oncornavirus particles. J Virol. 2001;75:11239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Lum R, Okoye AAet al.. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Wu J, Wu YTet al.. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach N, Gibbert K, Alter Cet al.. Anti-retroviral effects of type I IFN subtypes in vivo. Eur J Immunol. 2009;39:136–46. [DOI] [PubMed] [Google Scholar]
- Gerlach N, Schimmer S, Weiss Set al.. Effects of type I interferons on Friend retrovirus infection. J Virol. 2006;80:3438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbert K, Dietze KK, Zelinskyy Get al.. Polyinosinic-polycytidylic acid treatment of Friend retrovirus-infected mice improves functional properties of virus-specific T cells and prevents virus-induced disease. J Immunol. 2010;185:6179–89. [DOI] [PubMed] [Google Scholar]
- Gibbert K, Francois S, Sigmund AMet al.. Friend retrovirus drives cytotoxic effectors through Toll-like receptor 3. Retrovirology. 2014;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbert K, Joedicke JJ, Meryk Aet al.. Interferon-alpha subtype 11 activates NK cells and enables control of retroviral infection. PLoS Pathog. 2012;8:e1002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PD, Cheever MA, Fefer A. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1 + 2- lymphocytes. J Exp Med. 1981;154:952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PD, Kern DE, Cheever MA. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- T cells. Tumor eradication does not require participation of cytotoxic T cells. J Exp Med. 1985;161:1122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Voogd J, Freedman SL. The parasagittal zonation within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. J Comp Neurol. 1979;183:551–601. [DOI] [PubMed] [Google Scholar]
- Halemano K, Barrett BS, Heilman KJet al.. Requirement for Fc effector mechanisms in the APOBEC3/Rfv3-dependent neutralizing antibody response. J Virol. 2015;89:4011–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halemano K, Barrett BS, Li SXet al.. Fv1 restriction and retrovirus vaccine immunity in Apobec3-deficient 129P2 mice. PLoS One. 2013a;8:e60500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halemano K, Guo K, Heilman KJet al.. Immunoglobulin somatic hypermutation by APOBEC3/Rfv3 during retroviral infection. Proc Natl Acad Sci USA. 2014;111:7759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halemano K, Harper MS, Guo Ket al.. Humoral immunity in the Friend retrovirus infection model. Immunol Res. 2013b;55:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper MS, Barrett BS, Smith DSet al.. IFN-alpha treatment inhibits acute Friend retrovirus replication primarily through the antiviral effector molecule Apobec3. J Immunol. 2013;190:1583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ, Brooks DM, Chesebro B. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc Natl Acad Sci USA. 1995a;92:10492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ, Brooks DM, Dittmer U. Critical role for CD4(+) T cells in controlling retrovirus replication and spread in persistently infected mice. J Virol. 1998;72:6559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ, Chougnet CA, Dittmer U. Regulatory T cells in retroviral infections. PLoS Pathog. 2018;14:e1006776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ, Sprangrude GJ, Nishio Jet al.. Recovery from Friend disease in mice with reduced major histocompatibility complex class I expression. J. Virol. 1994;68:2059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ, Valenzuela A, Letts VAet al.. Chromosome mapping of Rfv3, a host resistance gene to Friend murine retrovirus. J Virol. 1995b;69:2617–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ. Lymphocyte deficiencies increase susceptibility to friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J Virol. 1999;73:6468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesters BA, Lindqvist M, Vagefi PAet al.. Follicular dendritic cells retain infectious HIV in cycling endosomes. PLoS Pathog. 2015;11:e1005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Hankey P, Paulson RF. Self-renewal of leukemia stem cells in Friend virus-induced erythroleukemia requires proviral insertional activation of Spi1 and hedgehog signaling but not mutation of p53. Stem Cells. 2012;30:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Messer RJ, Sakaguchi Set al.. Reduction of retrovirus-induced immunosuppression by in vivo modulation of T cells during acute infection. J Virol. 2004;78:11641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann P, Rubio M, Nakajima Tet al.. IFN-alpha priming of human monocytes differentially regulates gram-positive and gram-negative bacteria-induced IL-10 release and selectively enhances IL-12p70, CD80, and MHC class I expression. J Immunol. 1998;161:2011–8. [PubMed] [Google Scholar]
- He S, Ni S, Hegde Set al.. Activation of the N-terminally truncated form of the Stk receptor tyrosine kinase Sf-Stk by Friend virus-encoded gp55 is mediated by cysteine residues in the ecotropic domain of gp55 and the extracellular domain of Sf-Stk. J Virol. 2010;84:2223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoatlin ME, Kabat D. Host-range control of a retroviral disease: friend erythroleukemia. Trends Microbiol. 1995;3:51–7. [DOI] [PubMed] [Google Scholar]
- Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol. 2014;32:433–59. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi Het al.. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. [DOI] [PubMed] [Google Scholar]
- Housiaux PJ, Hill DF, Petersen GB. Nucleotide sequence of a gene for 5S ribosomal RNA from Pseudomonas aeruginosa. Nucleic Acids Res. 1988;16:2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Mergenhagen SE, Notkins ALet al.. Inhibition of cellular immunity and enhancement of humoral antibody formation in mice infected with lactic dehydrogenase virus. Transplant Proc. 1969;1:586–8. [PubMed] [Google Scholar]
- Inada T, Mims CA. Live lactate dehydrogenase-elevating virus (LDV) induces suppressor T cells that inhibit the development of delayed hypersensitivity to LDV. J Gen Virol. 1986;67:2103–12. [DOI] [PubMed] [Google Scholar]
- Inada T, Mims CA. Pattern of infection and selective loss of Ia positive cells in suckling and adult mice inoculated with lactic dehydrogenase virus. Arch Virol. 1985;86:151–65. [DOI] [PubMed] [Google Scholar]
- Isakov N, Feldman M, Segal S. Acute infection of mice with lactic dehydrogenase virus (LDV) impairs the antigen-presenting capacity of their macrophages. Cell Immunol. 1982;66:317–32. [DOI] [PubMed] [Google Scholar]
- Isitor GN, Adogwa AO. A case of calf craniopagus in Trinidad. Vet Rec. 1992;130:401–2. [DOI] [PubMed] [Google Scholar]
- Iwashiro M, Kondo T, Shimizu Tet al.. Multiplicity of virus-encoded helper T-cell epitopes expressed on FBL-3 tumor cells. J Virol. 1993;67:4533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashiro M, Messer RJ, Peterson KEet al.. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc Natl Acad Sci USA. 2001a;98:9226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashiro M, Peterson K, Messer RJet al.. CD4(+) T cells and gamma interferon in the long-term control of persistent friend retrovirus infection. J Virol. 2001b;75:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Gewurz H, Spear GT. Mannose binding lectin (MBL) and HIV. Mol Immunol. 2005;42:145–52. [DOI] [PubMed] [Google Scholar]
- Joedicke JJ, Myers L, Carmody ABet al.. Activated CD8+ T cells induce expansion of Vbeta5+ regulatory T cells via TNFR2 signaling. J Immunol. 2014a;193:2952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joedicke JJ, Zelinskyy G, Dittmer Uet al.. CD8+ T cells are essential for controlling acute friend retrovirus infection in C57BL/6 mice. J Virol. 2014b;88:5200–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P, Benchimol S. Friend virus induced murine erythroleukaemia: the p53 locus. Cancer Surv. 1992;12:137–51. [PubMed] [Google Scholar]
- Johnson P, Chung S, Benchimol S. Growth suppression of Friend virus-transformed erythroleukemia cells by p53 protein is accompanied by hemoglobin production and is sensitive to erythropoietin. Mol Cell Biol. 1993;13:1456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Mehta HV, Okeoma CM. A novel role for APOBEC3: susceptibility to sexual transmission of murine acquired immunodeficiency virus (mAIDS) is aggravated in APOBEC3 deficient mice. Retrovirology. 2012;9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–94. [DOI] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. [DOI] [PubMed] [Google Scholar]
- Kanari Y, Clerici M, Abe Het al.. Genotypes at chromosome 22q12-13 are associated with HIV-1-exposed but uninfected status in Italians. AIDS. 2005;19:1015–24. [DOI] [PubMed] [Google Scholar]
- Kane M, Case LK, Wang Cet al.. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity. 2011;35:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiotis G, O'Garra A. Establishing the follicular helper identity. Immunity. 2009;31:450–2. [DOI] [PubMed] [Google Scholar]
- Knuschke T, Bayer W, Rotan Oet al.. Prophylactic and therapeutic vaccination with a nanoparticle-based peptide vaccine induces efficient protective immunity during acute and chronic retroviral infection. Nanomedicine. 2014;10:1787–98. [DOI] [PubMed] [Google Scholar]
- Knuschke T, Rotan O, Bayer Wet al.. Combination of nanoparticle-based therapeutic vaccination and transient ablation of regulatory T cells enhances anti-viral immunity during chronic retroviral infection. Retrovirology. 2016;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuschke T, Rotan O, Bayer Wet al.. Induction of Type I Interferons by Therapeutic Nanoparticle-Based Vaccination Is Indispensable to Reinforce Cytotoxic CD8(+) T Cell Responses During Chronic Retroviral Infection. Front Immunol. 2018;9:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokithas A, Rosenke K, Malik Fet al.. The glycosylated Gag protein of a murine leukemia virus inhibits the antiretroviral function of APOBEC3. J Virol. 2010;84:10933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AR, Arndt T, Hasenkrug KJet al.. Effective treatment of retrovirus-induced suppression of antibody responses with CpG oligodeoxynucleotides. J Gen Virol. 2005;86:3365–8. [DOI] [PubMed] [Google Scholar]
- Ksiazek TG, Erdman D, Goldsmith CSet al.. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. [DOI] [PubMed] [Google Scholar]
- Lang PA, Lang KS, Xu HCet al.. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA. 2012;109:1210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski J, Renner B, Le Quintrec Met al.. Distinct roles for the complement regulators factor H and Crry in protection of the kidney from injury. Kidney Int. 2016;90:109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigueur A, Bernstein A. p53 transgenic mice: accelerated erythroleukemia induction by Friend virus. Oncogene. 1991;6:2197–201. [PubMed] [Google Scholar]
- Le Bon A, Durand V, Kamphuis Eet al.. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006a;176:4682–9. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Etchart N, Rossmann Cet al.. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–15. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Schiavoni G, D'Agostino Get al.. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–70. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Thompson C, Kamphuis Eet al.. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006b;176:2074–8. [DOI] [PubMed] [Google Scholar]
- Lee H, Komano J, Saitoh Yet al.. Zinc-finger antiviral protein mediates retinoic acid inducible gene I-like receptor-independent antiviral response to murine leukemia virus. Proc Natl Acad Sci USA. 2013;110:12379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore RA, Bieniasz PD. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc Natl Acad Sci USA. 2011;108:18097–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hakata Y, Takeda Eet al.. Two genetic determinants acquired late in Mus evolution regulate the inclusion of exon 5, which alters mouse APOBEC3 translation efficiency. PLoS Pathog. 2012;8:e1002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JP, Baltimore D. Mechanism of leukemogenesis induced by mink cell focus-forming murine leukemia viruses. J Virol. 1991;65:2408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JP, Bestwick RK, Spiro Cet al.. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987;61:2782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F, Boyse EA, Old LJ. Genetic basis of susceptibility to viral Leukaemogenesis. Lancet. 1964;2:1207–9. [DOI] [PubMed] [Google Scholar]
- Lilly F. Susceptibility to two strains of Friend leukemia virus in mice. Science. 1967;155:461–2. [DOI] [PubMed] [Google Scholar]
- Lilly F. The inheritance of susceptibility to the Gross leukemia virus in mice. Genetics. 1966;53:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AH, Burrascano C, Pettersson PLet al.. Blockade of type I interferon (IFN) production by retroviral replicating vectors and reduced tumor cell responses to IFN likely contribute to tumor selectivity. J Virol. 2014;88:10066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Seminars in Immunopathology. 2010;32:183–96. [DOI] [PubMed] [Google Scholar]
- Li SX, Barrett BS, Guo Ket al.. Tetherin/BST-2 promotes dendritic cell activation and function during acute retrovirus infection. Sci Rep. 2016;6:20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Barrett BS, Harper MSet al.. Ribonuclease L is not critical for innate restriction and adaptive immunity against Friend retrovirus infection. Virology. 2013;443:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Barrett BS, Heilman KJet al.. Tetherin promotes the innate and adaptive cell-mediated immune response against retrovirus infection in vivo. J Immunol. 2014;193:306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littwitz-Salomon E, Akhmetzyanova I, Vallet Cet al.. Activated regulatory T cells suppress effector NK cell responses by an IL-2-mediated mechanism during an acute retroviral infection. Retrovirology. 2015;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littwitz-Salomon E, Dittmer U, Sutter K. Insufficient natural killer cell responses against retroviruses: how to improve NK cell killing of retrovirus-infected cells. Retrovirology. 2016;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littwitz-Salomon E, Malyshkina A, Schimmer Set al.. The cytotoxic activity of natural killer cells is suppressed by IL-10(+) regulatory T cells during acute retroviral infection. Front Immunol. 2018a;9:1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littwitz-Salomon E, Nguyen T, Schimmer Set al.. Friend retrovirus infection induces the development of memory-like natural killer cells. Retrovirology. 2018b;15:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littwitz-Salomon E, Schimmer S, Dittmer U. Dose of retroviral infection determines induction of antiviral NK cell responses. J Virol. 2017a;91:e01122–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littwitz-Salomon E, Schimmer S, Dittmer U. Natural killer T cells contribute to the control of acute retroviral infection. Retrovirology. 2017b;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littwitz E, Francois S, Dittmer Uet al.. Distinct roles of NK cells in viral immunity during different phases of acute Friend retrovirus infection. Retrovirology. 2013;10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A, Okeoma CM, Lovsin Net al.. Enhanced replication and pathogenesis of Moloney murine leukemia virus in mice defective in the murine APOBEC3 gene. Virology. 2009;385:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WL, Allen PM. Self-awareness: how self-peptide/MHC complexes are essential in the development of T cells. Mol Immunol. 2013;55:186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Bieniasz PD. HIV Restriction Factors and Mechanisms of Evasion. Cold Spring Harb Perspect Med. 2012;2:a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyshkina A, Littwitz-Salomon E, Sutter Ket al.. Fas Ligand-mediated cytotoxicity of CD4+ T cells during chronic retrovirus infection. Sci Rep. 2017;7:7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyshkina A, Littwitz-Salomon E, Sutter Ket al.. Chronic retroviral infection of mice promotes tumor development, but CD137 agonist therapy restores effective tumor immune surveillance. Cancer Immunol Immunother. 2019;68:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke N, Akhmetzyanova I, Hasenkrug KJet al.. CD4+ T cells develop antiretroviral cytotoxic activity in the absence of regulatory T cells and CD8+ T cells. J Virol. 2013;87:6306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. Embo J. 1998;17:6660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markine-Goriaynoff D, Hulhoven X, Cambiaso CLet al.. Natural killer cell activation after infection with lactate dehydrogenase-elevating virus. J Gen Virol. 2002;83:2709–16. [DOI] [PubMed] [Google Scholar]
- Marques R, Antunes I, Eksmond Uet al.. B lymphocyte activation by coinfection prevents immune control of friend virus infection. J Immunol. 2008;181:3432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager J, Kassiotis G. Narrowing the gap: preserving repertoire diversity despite clonal selection during the CD4 T cell response. Front Immunol. 2015;6:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager J, Ploquin MJ, Eksmond Uet al.. Stepwise B-cell-dependent expansion of T helper clonotypes diversifies the T-cell response. Nature Communications. 2016;7:10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer RJ, Lavender KJ, Hasenkrug KJ. Mice of the resistant H-2(b) haplotype mount broad CD4(+) T cell responses against 9 distinct Friend virus epitopes. Virology. 2014;456-457:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Novak LK, Das J, Musich TAet al.. Analysis of complement-mediated lysis of Simian immunodeficiency virus (SIV) and SIV-infected cells reveals sex differences in vaccine-induced immune responses in Rhesus Macaques. J Virol. 2018;92:e00721–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirand EA, Grace JT, Buffett RF. Passive and active immunity to Friend virus disease. Nature. 1966;209:696–8. [DOI] [PubMed] [Google Scholar]
- Mirand EA. Erythropoietic response of animals infected with various strains of Friend virus. Natl Cancer Inst Monogr. 1966;22:483–503. [PubMed] [Google Scholar]
- Montefiori DC, Cornell RJ, Zhou JYet al.. Complement control proteins, CD46, CD55, and CD59, as common surface constituents of human and simian immunodeficiency viruses and possible targets for vaccine protection. Virology. 1994;205:82–92. [DOI] [PubMed] [Google Scholar]
- Moore TC, Gonzaga LM, Mather JMet al.. B cell requirement for robust regulatory T cell responses to Friend retrovirus infection. MBio. 2017;8:e01122-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TC, Messer RJ, Gonzaga LMet al.. Effects of Friend virus infection and regulatory T cells on the Antigen presentation function of B cells. MBio. 2019;10:e02578–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TC, Messer RJ, Hasenkrug KJ. Regulatory T cells suppress virus-specific antibody responses to Friend retrovirus infection. PLoS One. 2018;13:e0195402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Gachelin F, Tavitian A, Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988;331:277–80. [DOI] [PubMed] [Google Scholar]
- Moreau-Gachelin F. Multi-stage Friend murine erythroleukemia: molecular insights into oncogenic cooperation. Retrovirology. 2008;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe DG, Peacock JW, Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol Cell Biol. 1990;10:3307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L, Joedicke JJ, Carmody ABet al.. IL-2-independent and TNF-alpha-dependent expansion of Vbeta5+ natural regulatory T cells during retrovirus infection. J Immunol. 2013;190:5485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LM, Tal MC, Torrez Dulgeroff LBet al.. A functional subset of CD8(+) T cells during chronic exhaustion is defined by SIRPalpha expression. Nat Commun. 2019;10:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SR, Zelinskyy G, Schimmer Set al.. Mechanisms of control of acute Friend virus infection by CD4+ T helper cells and their functional impairment by regulatory T cells. J Gen Virol. 2010;91:440–51. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Eastman SW, Jouvenet Net al.. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006;2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–30. [DOI] [PubMed] [Google Scholar]
- Nitta T, Kuznetsov Y, McPherson Aet al.. Murine leukemia virus glycosylated Gag (gPr80gag) facilitates interferon-sensitive virus release through lipid rafts. Proc Natl Acad Sci USA. 2010;107:1190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MA, Rastad JL, Green WR. The Role of Myeloid-Derived Suppressor Cells in Viral Infection. Viral Immunol. 2017;30::82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Tsuji-Kawahara S, Yuasa Tet al.. Natural killer cells recognize friend retrovirus-infected erythroid progenitor cells through NKG2D-RAE-1 interactions in vivo. J Virol. 2011;85:5423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlen C, Kalos M, Cheng LEet al.. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlen C, Kalos M, Hong DJet al.. Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. J Immunol. 2001;166:2863–70. [DOI] [PubMed] [Google Scholar]
- Okeoma CM, Lovsin N, Peterlin BMet al.. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature. 2007;445:927–30. [DOI] [PubMed] [Google Scholar]
- Okeoma CM, Petersen J, Ross SR. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection. J Virol. 2009;83:3029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich AR, Schimmer S, Heeg Ket al.. Effective postexposure treatment of retrovirus-induced disease with immunostimulatory DNA containing CpG motifs. J Virol. 2002;76:11397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L, Boyse EA, Lilly F. Formation of cytoxic antibody against Leukemias induced by Friend virus. Cancer Res. 1963;23:1063–8. [PubMed] [Google Scholar]
- Paul R, Schuetze S, Kozak SLet al.. A common site for immortalizing proviral integrations in Friend erythroleukemia: molecular cloning and characterization. J Virol. 1989;63:4958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Schuetze S, Kozak SLet al.. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor Pu.1. J Virol. 1991;65:464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Lai ST, Poon LLet al.. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D, Zang T, Ebrahimi Aet al.. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud SP, Parker CR, Lo WLet al.. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol. 2014;15:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons DA, Paulson RF, Loyd MRet al.. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23:159–65. [DOI] [PubMed] [Google Scholar]
- Pike R, Filby A, Ploquin MJet al.. Race between retroviral spread and CD4+ T-cell response determines the outcome of acute Friend virus infection. J Virol. 2009;83:11211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata F, Lilly F. Viral specificity of H-2-restricted T killer cells directed against syngeneic tumors induced by Gross, Friend, or Rauscher leukemia virus. J Exp Med. 1979;150:1174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploquin MJ, Eksmond U, Kassiotis G. B cells and TCR avidity determine distinct functions of CD4+ T cells in retroviral infection. J Immunol. 2011;187:3321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch W, Cardinaud S, Hamimi Cet al.. Antibodies attenuate the capacity of dendritic cells to stimulate HIV-specific cytotoxic T lymphocytes. J Allergy Clin Immunol. 2012;130:1368–74.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch W, Steger M, Knackmuss Uet al.. Complement-opsonized HIV-1 overcomes restriction in dendritic cells. PLoS Pathog. 2015;11:e1005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats AC, De Billy G, Wang Pet al.. CUG initiation codon used for the synthesis of a cell surface antigen coded by the murine leukemia virus. J Mol Biol. 1989;205:363–72. [DOI] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. [DOI] [PubMed] [Google Scholar]
- Refsland EW, Harris RS. The APOBEC3 family of retroelement restriction factors. Curr Top Microbiol Immunol. 2013;371:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley V. Enzymatic determination of transmissible replicating factors associated with mouse tumors. Ann NY Acad Sci. 1963;100:762–90. [PubMed] [Google Scholar]
- Robertson MN, Spangrude GJ, Hasenkrug Ket al.. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992;66:3271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Ammann CG, Messer RJet al.. Suppression of acute anti-friend virus CD8+ T-cell responses by coinfection with lactate dehydrogenase-elevating virus. J Virol. 2008a;82:408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Messer RJ, Carmody ABet al.. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J Immunol. 2008b;180:5267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifuddin M, Parker CJ, Peeples MEet al.. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med. 1995;182:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Mather TP, Ishikawa R, Biron CA. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J Immunol. 1996;157:3054–64. [PubMed] [Google Scholar]
- Samuel MA, Whitby K, Keller BCet al.. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J Virol. 2006;80:7009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Benitez RL, Montano Met al.. Innate retroviral restriction by Apobec3 promotes antibody affinity maturation in vivo. J Immunol. 2010;185:1114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Montano M, Benitez Ret al.. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science. 2008;321:1343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Smith DS, Barrett BSet al.. Persistent Friend virus replication and disease in Apobec3-deficient mice expressing functional B-cell-activating factor receptor. J Virol. 2011;85:189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanville B, Dolan MA, Wollenberg Ket al.. Adaptive evolution of Mus Apobec3 includes retroviral insertion and positive selection at two clusters of residues flanking the substrate groove. PLoS Pathog. 2010;6:e1000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers K, Toebes M, Sotthewes Get al.. Differential kinetics of antigen-specific CD4+ and CD8+ T cell responses in the regression of retrovirus-induced sarcomas. J Immunol. 2002;169:3191–9. [DOI] [PubMed] [Google Scholar]
- Schuetze S, Paul R, Gliniak BCet al.. Role of the PU.1 transcription factor in controlling differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1992;12:2967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JDet al.. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–50. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–7. [DOI] [PubMed] [Google Scholar]
- Shen L, Tenzer S, Hess Met al.. Friend virus limits adaptive cellular immune responses by imprinting a maturation-resistant and T helper type 2-biased immunophenotype in dendritic cells. PLoS One. 2018;13:e0192541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Uenishi H, Teramura Yet al.. Fine structure of a virus-encoded helper T-cell epitope expressed on FBL-3 tumor cells. J Virol. 1994;68:7704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Guo K, Barrett BSet al.. Noninfectious retrovirus particles drive the APOBEC3/Rfv3 dependent neutralizing antibody response. PLoS Pathog. 2011;7:e1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear GT, Lurain NS, Parker CJet al.. Host cell-derived complement control proteins CD55 and CD59 are incorporated into the virions of two unrelated enveloped viruses. Human T cell leukemia/lymphoma virus type I (HTLV-I) and human cytomegalovirus (HCMV). J Immunol. 1995;155:4376–81. [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BRet al.. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. [DOI] [PubMed] [Google Scholar]
- Stavrou S, Aguilera AN, Blouch Ket al.. DDX41 recognizes RNA/DNA retroviral reverse transcripts and is critical for in vivo control of Murine leukemia virus infection. MBio. 2018a;9:e00923-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou S, Blouch K, Kotla Set al.. Nucleic acid recognition orchestrates the anti-viral response to retroviruses. Cell Host Microbe. 2015;17:478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou S, Nitta T, Kotla Set al.. Murine leukemia virus glycosylated Gag blocks apolipoprotein B editing complex 3 and cytosolic sensor access to the reverse transcription complex. Proc Natl Acad Sci USA. 2013;110:9078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou S, Zhao W, Blouch Ket al.. Deaminase-dead mouse APOBEC3 is an in vivo retroviral restriction factor. J Virol. 2018b;92:e00168-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber H, Pinter C, Siccardi AGet al.. Efficient destruction of human immunodeficiency virus in human serum by inhibiting the protective action of complement factor H and decay accelerating factor (DAF, CD55). J Exp Med. 1996;183:307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber H, Thielens NM, Ebenbichler Cet al.. The envelope glycoprotein of HIV-1 gp120 and human complement protein C1q bind to the same peptides derived from three different regions of gp41, the transmembrane glycoprotein of HIV-1, and share antigenic homology. Eur J Immunol. 1994;24:294–300. [DOI] [PubMed] [Google Scholar]
- Stopak K, de Noronha C, Yonemoto Wet al.. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol Cell. 2003;12:591–601. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Dittmer U, Schumacher TNet al.. Temporal effects of gamma interferon deficiency on the course of Friend retrovirus infection in mice. J Virol. 2002;76:2225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu J, Du Fet al.. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Super HJ, Brooks D, Hasenkrug Ket al.. Requirement for CD4(+) T cells in the Friend murine retrovirus neutralizing antibody response: evidence for functional T cells in genetic low-recovery mice. J Virol. 1998;72:9400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Super HJ, Hasenkrug KJ, Simmons Set al.. Fine mapping of the friend retrovirus resistance gene, Rfv3, on mouse chromosome 15. J Virol. 1999;73:7848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda E, Tsuji-Kawahara S, Sakamoto Met al.. Mouse APOBEC3 restricts Friend leukemia virus infection and pathogenesis in vivo. J Virol. 2008;82:10998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki S, Chikuma S, Shibayama Set al.. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186:2772–9. [DOI] [PubMed] [Google Scholar]
- Thielens NM, Tacnet-Delorme P, Arlaud GJ. Interaction of C1q and mannan-binding lectin with viruses. Immunobiology. 2002;205:563–74. [DOI] [PubMed] [Google Scholar]
- Thorborn G, Ploquin MJ, Eksmond Uet al.. Clonotypic composition of the CD4+ T cell response to a vectored retroviral antigen is determined by its speed. J Immunol. 2014a;193:1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorborn G, Young GR, Kassiotis G. Effective T helper cell responses against retroviruses: are all clonotypes equal? J Leukoc Biol. 2014b;96:27–37. [DOI] [PubMed] [Google Scholar]
- Tjomsland V, Ellegard R, Burgener Aet al.. Complement opsonization of HIV-1 results in a different intracellular processing pattern and enhanced MHC class I presentation by dendritic cells. Eur J Immunol. 2013;43:1470–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Santoli D, Granato Det al.. Antagonistic effects of interferons on the cytotoxicity mediated by natural killer cells. Fed Proc. 1981;40:2705–10. [PubMed] [Google Scholar]
- Troxler DH, Ruscetti SK, Scolnick EM. The molecular biology of Friend virus. Biochim Biophys Acta. 1980;605:305–24. [DOI] [PubMed] [Google Scholar]
- van Bleek GM, Nathenson SG. Presentation of antigenic peptides by MHC class I molecules. Trends Cell Biol. 1992;2:202–7. [DOI] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura Cet al.. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LKet al.. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481:394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EE, Kolev M, Kemper C. Complement and the regulation of T cell responses. Annu Rev Immunol. 2018;36:309–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock EF, Toy ST, Caroline NLet al.. Suppression of established Friend virus leukemia by statolon. IV. Role of humoral antibody in the development of a dormant infection. J Natl Cancer Inst. 1972;48:665–73. [PubMed] [Google Scholar]
- Wiehe K, Bradley T, Meyerhoff RRet al.. Functional relevance of improbable antibody mutations for HIV broadly neutralizing antibody development. Cell Host Microbe. 2018;23:759–65.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilflingseder D, Banki Z, Garcia Eet al.. IgG opsonization of HIV impedes provirus formation in and infection of dendritic cells and subsequent long-term transfer to T cells. J Immunol. 2007;178:7840–8. [DOI] [PubMed] [Google Scholar]
- Windmann S, Otto L, Hrycak CPet al.. Infection of B cell follicle-resident cells by Friend retrovirus occurs during acute infection and is maintained during viral persistence. MBio. 2019;10:e00004-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HC, Grusdat M, Pandyra AAet al.. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40:949–60. [DOI] [PubMed] [Google Scholar]
- Young GR, Ploquin MJ, Eksmond Uet al.. Negative selection by an endogenous retrovirus promotes a higher-avidity CD4+ T cell response to retroviral infection. PLoS Pathog. 2012;8:e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G, Balkow S, Schimmer Set al.. Independent roles of perforin, granzymes, and Fas in the control of Friend retrovirus infection. Virology. 2004;330:365–74. [DOI] [PubMed] [Google Scholar]
- Zelinskyy G, Balkow S, Schimmer Set al.. The level of friend retrovirus replication determines the cytolytic pathway of CD8+ T-cell-mediated pathogen control. J Virol. 2007;81:11881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G, Dietze K, Sparwasser Tet al.. Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathog. 2009b;5:e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G, Dietze KK, Husecken YPet al.. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009a;114:3199–207. [DOI] [PubMed] [Google Scholar]
- Zelinskyy G, Kraft AR, Schimmer Set al.. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur J Immunol. 2006;36:2658–70. [DOI] [PubMed] [Google Scholar]
- Zelinskyy G, Myers L, Dietze KKet al.. Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute friend retrovirus infection but are highly cytotoxic and control virus replication. J Immunol. 2011;187:3730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G, Robertson SJ, Schimmer Set al.. CD8+ T-cell dysfunction due to cytolytic granule deficiency in persistent Friend retrovirus infection. J Virol. 2005;79:10619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G, Werner T, Dittmer U. Natural regulatory T cells inhibit production of cytotoxic molecules in CD8(+) T cells during low-level Friend retrovirus infection. Retrovirology. 2013;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974a;251:547–8. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974b;248:701–2. [DOI] [PubMed] [Google Scholar]