Abstract
Aims
To determine whether plasma apoE levels and APOE genotype are associated with all-cause and cause-specific mortality.
Methods and results
Using a prospective cohort design with 105 949 white individuals from the general population, we tested the association between plasma apoE at study enrolment and death during follow-up, and whether this was independent of APOE genotype. We confirmed the well-known association between APOE genotypes and mortality. For all-cause, cardiovascular, and cancer mortality, high levels of apoE were associated with increased risk, while for dementia-associated mortality low levels were associated with increased risk. For the highest vs. the fifth septile of plasma apoE, hazard ratios (HRs) were 1.20 (95% confidence interval 1.12–1.28) for all-cause mortality, 1.28 (1.13–1.44) for cardiovascular mortality, and 1.18 (1.05–1.32) for cancer mortality. Conversely, for the lowest vs. the fifth septile the HR was 1.44 (1.01–2.05) for dementia-associated mortality. Results were similar in analyses restricted to APOE ɛ33 carriers. Examining genetically determined plasma apoE, a 1 mg/dL increase conferred risk ratios of 0.97 (0.92–1.03) for cardiovascular mortality and 1.01 (0.95–1.06) for cancer mortality, while a 1 mg/dL decrease conferred a risk ratio of 1.70 (1.36–2.12) for dementia-associated mortality.
Conclusion
High plasma levels of apoE were associated with increased all-cause, cardiovascular, and cancer mortality, however of a non-causal nature, while low levels were causally associated with increased dementia-associated mortality.
Keywords: APOE, Apolipoprotein E, Cardiovascular, Dementia, Mortality, Survival
Introduction
The basis of human longevity and healthy ageing remains among the principal questions in biology and medicine. Life expectancy is challenged by major diseases in old age—among these, dementia and cardiovascular disease. Alzheimer disease, the most common form of dementia, is a major cause of disability in later life with an increasing global prevalence, and this presently untreatable disease deteriorates into an inevitable terminal stage.1–5 Cardiovascular disease is the leading cause of death worldwide,6 and only in Central and Western Europe has the annual number of deaths from cardiovascular disease declined.7,8 Biomarkers of lipid metabolism have been associated with increased mortality, however, the relationship of plasma levels of a crucial lipid transport protein, apolipoprotein E (apoE), with mortality is presently unknown.
ApoE plays a pivotal role in both peripheral and cerebral cholesterol metabolism. In plasma, apoE is mainly carried by triglyceride-rich lipoproteins, and serves as a ligand for members of the low-density lipoprotein (LDL) receptor family.9 In the brain, astrocyte-derived apoE is crucial for cerebral cholesterol metabolism and clearance of β-amyloid, an important pathological hallmark of Alzheimer disease.10,11 Plasma levels of apoE and other lipids and lipoproteins are under strong genetic influence by the well-known APOE polymorphism—a combination of two genetic variants (rs429358 and rs7412) giving rise to six common APOE genotypes, ɛ22, ɛ32, ɛ33, ɛ42, ɛ43, and ɛ44. Both ɛ2 and ɛ4 alleles are associated with unfavourable lipid profiles,12–19 and the ɛ4 allele is a strong genetic risk factor for Alzheimer disease,20 and by far the strongest hit in genome-wide association studies of longevity.21,22 Interestingly, low levels of plasma apoE were recently reported to be associated with increased risk of dementia,17,23,24 whereas high levels were associated with increased risk of ischaemic heart disease.25 Hence both a quantitative importance of plasma apoE levels, and a qualitative genetically determined effect, appears to be important for dementia and cardiovascular disease—two important determinants of life expectancy. A few studies have addressed the association between plasma apoE and cardiovascular mortality.26,27 Beyond that, the relationship of plasma apoE levels with all-cause and cause-specific mortality is unknown. Such associations are of substantial interest, because they may generate therapeutic and clinical considerations.
We studied the association of plasma apoE levels with all-cause and cause-specific mortality and assessed whether this was independent of isoform differences due to APOE genotype, by evaluating both APOE genotype adjusted and ɛ33 genotype stratified analyses. Using a Mendelian randomization design, we also studied genetically determined plasma apoE levels to examine if plasma apoE was causally associated with cause-specific mortality. For this purpose, we studied two large prospective cohorts of the white Danish general population, the Copenhagen General Population Study (CGPS) and the Copenhagen City Heart Study (CCHS), totalling 105 949 individuals.
Methods
Studies were approved by institutional review boards and Danish ethical committees and were conducted according to the Declaration of Helsinki, with written informed consent from participants. All individuals were white and of Danish descent. There was no overlap of individuals between studies.
Participants
We included individuals from two similar studies of the Danish general population: The Copenhagen General Population Study (CGPS) and the Copenhagen City Heart Study (CCHS). Individuals were randomly selected from the national Danish Civil Registration System to reflect the adult white population aged 20 to 100+ years. The studies combined included a total of 105 949 individuals, of whom 13 693 died during the follow-up period.
The Copenhagen General Population Study
This prospective study of the Danish general population was initiated in 2003 with the first enrolment period from 2003 to 2015 and with follow-up examinations ongoing.17,24,28,29 Data collection included a questionnaire, a physical examination, and blood sampling for biochemical and DNA analyses. We included 95 583 individuals; among these, 8321 died during follow-up.
The Copenhagen City Heart Study
This prospective study of the Danish general population was initiated in 1976–78 with follow-up examinations in 1981–83, 1991–94, and 2001–03.17,24,28,29 Participants were recruited and examined as in the CGPS. We included 10 366 individuals who gave blood for biochemical and DNA analyses at the 1991–94 or 2001–03 examinations; among these, 5372 died during follow-up.
Endpoints
Endpoints are described in detail in the Supplementary material online.
Follow-up began at the time of blood sampling (2003–13 and onwards for CGPS and 1991–94 or 2001–03 and onwards for CCHS). For all-cause mortality, follow-up ended at occurrence of death, emigration, or on 22 March 2017 (last update of the registries), whichever came first. Median follow-up was 8.5 years (range 0–25 years) for all-cause mortality, with no individuals lost to follow-up. End of follow-up for the national Danish Causes of Death Registry (31 December 2015) lags the Danish Civil Registration System (22 March 2017) by more than 1 year: as a consequence some deaths could not be classified by cause (1839 of 13 693 deaths), and cause-specific mortality follow-up was truncated accordingly on 31 December 2015.
Biochemical and genetic analyses
Apolipoprotein E was measured using nephelometry or turbidimetry (Dade Behring, Deerfield, Illinois, USA, or Dako, Glostrup, Denmark) as previously described.17,25 An ABI PRISM 7900HT Sequence Detection System (Applied Biosystems Inc., Foster City, CA, USA) and Taqman based assays were used to genotype for p.Cys130Arg (rs429358, legacy name Cys112Arg, c.388T>C) defining the ɛ4 allele and p.Arg176Cys (rs7412, legacy name Arg158Cys, c.526C>T) defining the ɛ2 allele. A total of 105 949 individuals (with 13 693 deaths) were genotyped, and for 104 153 of these individuals (with 13 312 deaths) plasma apoE measurements were obtained. Genotypes for three APOE promotor variants (rs449647, rs769446, and rs405509) in 74 560 individuals were determined similarly, as previously reported.24
Other covariates
Body mass index was measured weight in kilograms divided by measured height in metres squared. All other covariates were self-reported and described in detail in the legend to Table 1.
Table 1.
Baseline characteristics of individuals in the general population with low and high plasma apolipoprotein E
| Characteristic | Plasma apolipoprotein E |
||
|---|---|---|---|
| ≤4.5 mg/dL | >4.5 mg/dL | P-valuea | |
| Individuals, N | 67 023 | 37 130 | — |
| Women (%) | 52 | 60 | 5 × 10−128 |
| Age (years) | 56.4 ± 0.1 | 60.0 ± 0.1 | <1 × 10−300 |
| Body mass index (kg/m2) | 25.7 ± 0.0 | 26.9 ± 0.0 | <1 × 10−300 |
| Hypertension (%) | 56 | 66 | 6 × 10−254 |
| Diabetes mellitus (%) | 4 | 4 | 0.01 |
| Smoking (%) | 21 | 21 | 0.65 |
| High alcohol consumption (%) | 16 | 19 | 8 × 10−28 |
| Physical inactivity (%) | 48 | 54 | 1 × 10−69 |
| Postmenopausal (%)c | 59 | 80 | <1 × 10−300 |
| Hormonal replacement therapy (%)b | 11 | 10 | 0.01 |
| Lipid-lowering therapy (%) | 12 | 8 | 6 × 10−119 |
| Education<8 years (%) | 11 | 15 | 5 × 10−61 |
| APOE genotype (%) | <1 × 10−300 | ||
| ɛ22 | 0 | 2 | |
| ɛ32 | 4 | 28 | |
| ɛ33 | 58 | 51 | |
| ɛ42 | 1 | 6 | |
| ɛ43 | 32 | 13 | |
| ɛ44 | 4 | 1 | |
Values are presented as number, mean ± standard error of the mean, or percentage and are from the day of enrolment (2003–13 for the Copenhagen General Population Study and 1991–94 or 2001–03 for the Copenhagen City Heart Study). Missing data on categorical and continuous covariates (<0.3%) were imputed from age, sex, and population. Hypertension was defined as use of antihypertensive medication, systolic blood pressure of ≥140 mmHg, and/or diastolic blood pressure of ≥90 mmHg. Diabetes mellitus was defined as self-reported disease, use of insulin or oral hypoglycaemic agents, and/or non-fasting plasma glucose level of >11 mmol/L (>198 mg/dL). Smoking was defined as current smoking. High alcohol consumption was defined as >14/21 U per week for women/men [1 U = 12 g alcohol, equivalent to 1 glass of wine or spirit or 1 beer (33 cl)]. Physical inactivity was defined as ≤4 h per week of light physical activity in leisure time. Women reported menopausal status and use of hormonal replacement therapy. Lipid-lowering therapy was primarily statins (yes/no), and the cut-off for low education was 8 years.
P for differences by the Kruskal–Wallis equality-of-populations rank test or by the Pearson’s χ2 test, as appropriate.
In women only.
Statistical analysis
We used Stata/S.E. version 13.1 (Stata Corp., College Station, TX, USA). P-values were two-sided and values <0.0001 are given as powers of 10. Statistical analyses are described in detail in the Supplementary material online.
Results
During up to 25 years of follow-up, 13 693 individuals in the CGPS and CCHS died. Baseline characteristics of individuals with low (≤4.5 mg/dL) and high (>4.5 mg/dL) plasma apoE are shown in Table 1, and sex stratified results are shown in Table 2. The overall mean value of plasma apoE for the population was 4.3 mg/dL (Supplementary material online, Figure S1). We found no interaction between seven groups of plasma apoE and study (P for interaction = 0.26). Consequently, all further analyses were performed on the studies combined.
Table 2.
Baseline characteristics for women and men in the general population with low and high plasma apolipoprotein E
| Characteristic | Plasma apolipoprotein E |
|||||
|---|---|---|---|---|---|---|
| Women |
Men |
|||||
| ≤4.5 mg/dL | >4.5 mg/dL | P-valuea | ≤4.5 mg/dL | >4.5 mg/dL | P-valuea | |
| Individuals, N | 35 087 | 22 314 | —b | 31 936 | 14 816 | 5 × 10−128 b |
| Age (years) | 55.0 ± 0.1 | 61.2 ± 0.1 | <1 × 10−300 | 57.9 ± 0.1 | 58.3 ± 0.1 | 0.13 |
| Body mass index (kg/m2) | 25.0 ± 0.0 | 26.4 ± 0.0 | 8 × 10−282 | 26.4 ± 0.0 | 27.6 ± 0.0 | 3 × 10−240 |
| Hypertension (%) | 48 | 63 | 5 × 10−270 | 64 | 72 | 3 × 10−60 |
| Diabetes mellitus (%) | 3 | 3 | 0.01 | 5 | 5 | 1 × 10−4 |
| Smoking (%) | 20 | 19 | 0.02 | 21 | 23 | 0.003 |
| High alcohol consumption (%) | 13 | 16 | 2 × 10−13 | 19 | 24 | 9 × 10−29 |
| Physical inactivity (%) | 53 | 57 | 3 × 10−22 | 43 | 50 | 2 × 10−36 |
| Postmenopausal (%)c | 59 | 80 | <1 × 10−300 | — | — | — |
| Hormonal replacement therapy (%)c | 11 | 10 | 0.01 | — | — | — |
| Lipid-lowering therapy (%) | 10 | 8 | 6 × 10−20 | 15 | 8 | 4 × 10−106 |
| Education<8 years (%) | 10 | 15 | 9 × 10−88 | 13 | 14 | 0.001 |
| APOE genotype (%) | <1 × 10−300 | <1 × 10−300 | ||||
| ɛ22 | 0 | 2 | 0 | 2 | ||
| ɛ32 | 4 | 26 | 4 | 30 | ||
| ɛ33 | 57 | 53 | 60 | 47 | ||
| ɛ42 | 1 | 6 | 1 | 6 | ||
| ɛ43 | 34 | 13 | 31 | 14 | ||
| ɛ44 | 5 | 1 | 3 | 1 | ||
Values are presented as number, mean ± standard error of the mean, or percentage and are from the day of enrolment (2003–13 for the Copenhagen General Population Study and 1991–94 or 2001–03 for the Copenhagen City Heart Study). Missing data and definitions of variables were identical to the descriptions given in the legend to Table 1.
P for differences by the Kruskal–Wallis equality-of-populations rank test or by the Pearson’s χ2 test, as appropriate.
One test for men and women with low and high apoE.
In women only.
APOE genotype and all-cause and cause-specific mortality
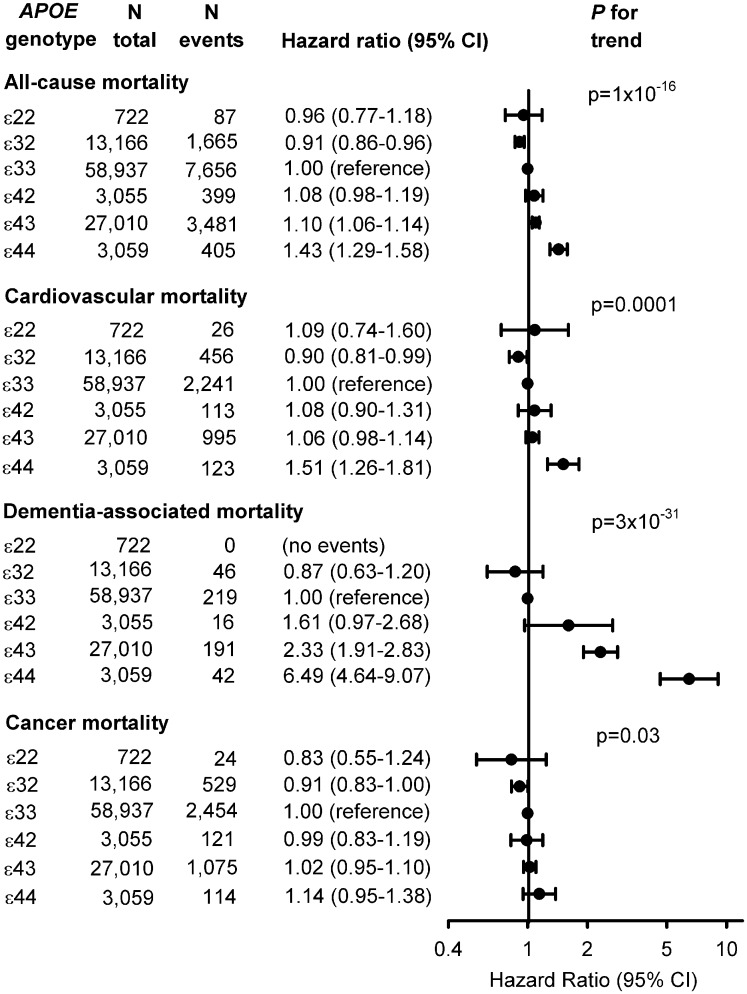
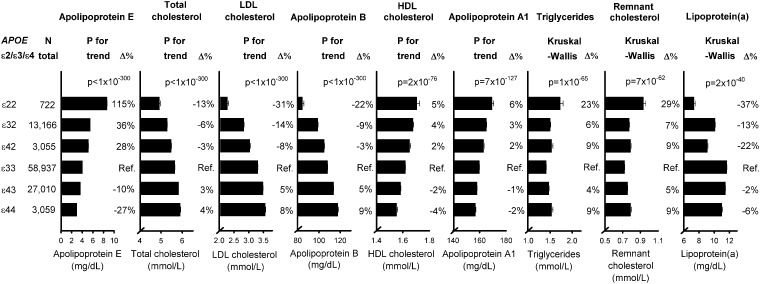
The Kaplan–Meier survival curves for overall survival for the six common APOE genotypes showed an increased mortality risk for individuals carrying the ɛ44 genotype relative to ɛ33 individuals (Supplementary material online, Figure S2), and with similar patterns in women and men separately (Supplementary material online, Figure S3). Median survival decreased from 86.9 years in ɛ32 carriers to 86.4 in ɛ33 carriers to 86.3 in ɛ22 carriers to 86.1 in ɛ42 carriers to 85.9 in ɛ43 carriers through to 83.7 years in ɛ44 carriers. All-cause, cardiovascular, and dementia-associated mortality increased correspondingly from ɛ32 to ɛ33/ɛ22 to ɛ42/ɛ43 to ɛ44 (Figure 1). Hazard ratios (HRs) for all-cause mortality were 1.10 (1.06–1.14) for ɛ43 carriers and 1.43 (1.29–1.58) for ɛ44 carriers vs. ɛ33 carriers (Figure 1), with similar patterns in women and men separately (Supplementary material online, Figure S4). For the six APOE genotypes, total cholesterol, LDL cholesterol, and apolipoprotein B increased from ɛ22 to ɛ32 to ɛ42 to ɛ33 to ɛ43 to ɛ44 (P for trends < 1 × 10−300), whereas plasma apoE, high-density lipoprotein (HDL) cholesterol, and apolipoprotein AI levels decreased from ɛ22 to ɛ32 to ɛ42 to ɛ33 to ɛ43 to ɛ44 (P for trends ≤ 2 × 10−76) (Figure 2). For triglycerides and remnant cholesterol U-shaped curves were observed with the highest levels in ɛ22 and ɛ44 carriers (P = 1 × 10−65 and 7 × 10−62), whereas the highest lipoprotein(a) level was observed in ɛ33 carriers (P = 2 × 10−40) (Figure 2).
Figure 1.
Risk of all-cause, cardiovascular, dementia-associated, and cancer mortality as a function of APOE genotype. Cox regression models were adjusted for age (time scale), body mass index, smoking, hypertension, diabetes, lipid-lowering therapy, alcohol consumption, physical inactivity, education, postmenopausal status, hormonal replacement therapy, LDL cholesterol, HDL cholesterol, and plasma triglycerides. APOE, APOE ɛ2/ɛ3/ɛ4 genotype; CI, confidence interval.
Figure 2.
Plasma levels of lipids, lipoproteins, and apolipoproteins as a function of APOE genotype. Geometric mean ± standard errors of the mean are given for apolipoprotein E, triglycerides, and lipoprotein(a); arithmetic mean ± standard errors of the mean are given for total cholesterol, LDL cholesterol, apolipoprotein B, HDL cholesterol, apolipoprotein A1, and remnant cholesterol. APOE ɛ2/ɛ3/ɛ4 genotypes are ordered according to decreasing plasma apoE levels. Differences in plasma levels of lipids, lipoproteins, and apolipoproteins are given in percent (Δ%); ɛ33 serves as the reference. P for trend (from ɛ22 to ɛ32 to ɛ42 to ɛ33 to ɛ43 to ɛ44) or the Kruskal–Wallis analysis of variance are given. APOE, APOE ɛ2/ɛ3/ɛ4 genotype; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Ref, reference.
Plasma levels of apolipoprotein E and all-cause and cause-specific mortality
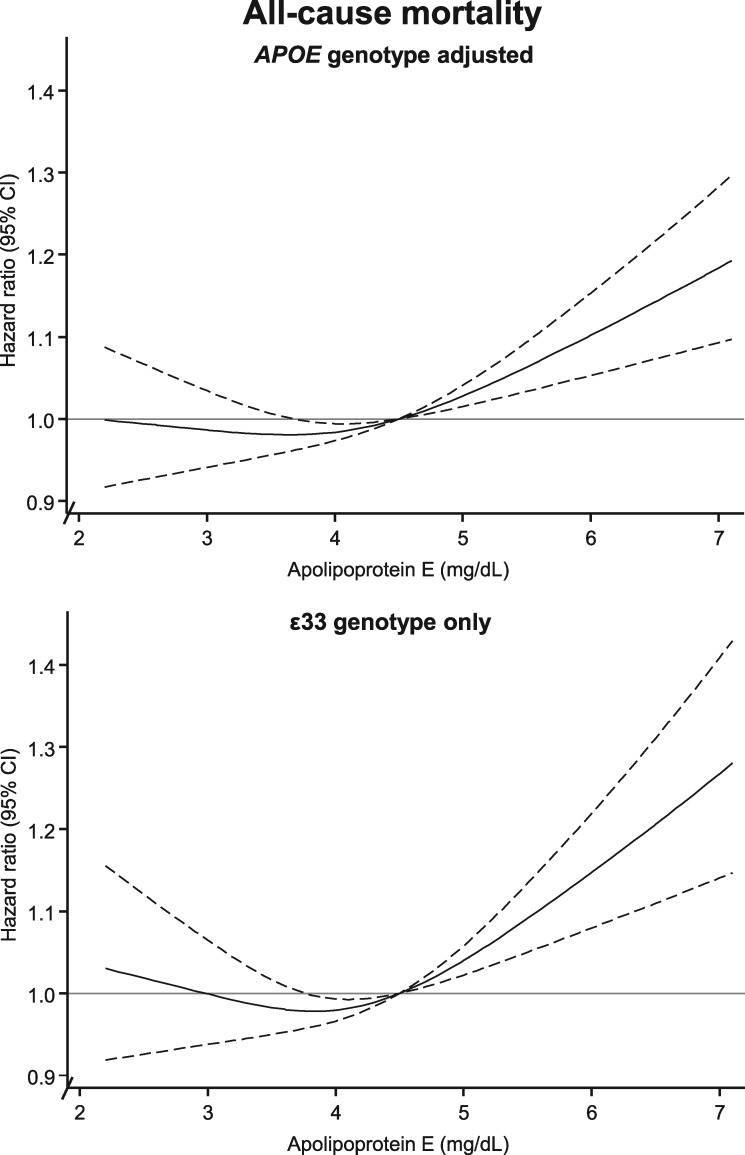
Multifactorially adjusted (including LDL cholesterol, HDL cholesterol, and triglycerides) restricted cubic spline Cox regression models evaluated risk of all-cause mortality by plasma apoE levels, further adjusted for APOE genotype (Figure 3, upper panel) or in ɛ33 carriers only (Figure 3, bottom panel). All-cause mortality increased with increasing levels of apoE relative to the reference of 4.5 mg/dL. There was no sign of non-linearity for all-cause and cause-specific mortality (P ≥ 0.30). We found interaction between seven groups of plasma apoE and sex on all-cause and cardiovascular mortality (P for interaction = 0.02 and 0.006), but no interaction for seven groups of plasma apoE and sex on dementia-associated and cancer mortality (P for interaction = 0.10 and 0.12). The associations between high levels of apoE and all-cause, cardiovascular, and cancer mortality appeared augmented in men (Supplementary material online, Figures S5–S7); otherwise largely similar patterns were observed in women and men, although overall with attenuation in women compared with in men.
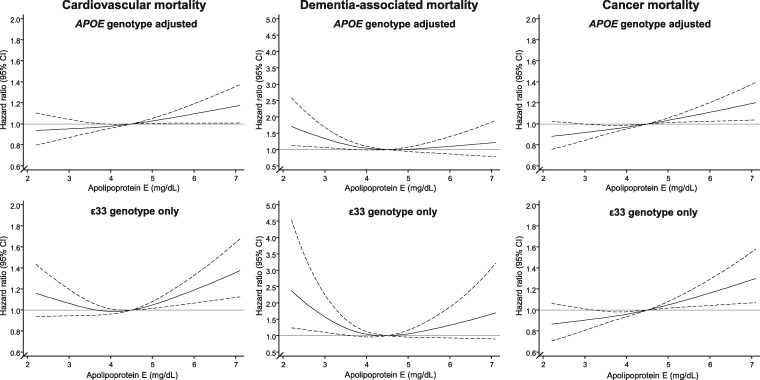
Figure 3.
Multifactorially adjusted hazard ratios for all-cause mortality according to plasma levels of apolipoprotein E in individuals in the general population. Solid lines are multifactorially adjusted hazard ratios, whereas dashed lines indicate 95% confidence intervals derived from restricted cubic splines with three knots and with the reference defined as the plasma level of apolipoprotein E with lowest overall mortality (4.5 mg/dL). Graphs are truncated at the level of 2.1 mg/dL and 7.1 mg/dL, due to statistically unstable estimates at extreme low and high levels thus only including 98 350 individuals from the Copenhagen General Population Study and the Copenhagen City Heart Study in these analyses (56 264 in the ɛ33 genotype stratified analyses). Cox regression models were adjusted for age (time scale), sex, body mass index, smoking, hypertension, diabetes, lipid-lowering therapy, alcohol consumption, physical inactivity, education, postmenopausal status, hormonal replacement therapy, LDL cholesterol, HDL cholesterol, and plasma triglycerides (both panels), and further adjustment for APOE genotype in the upper panel while analyses were restricted to ɛ33 carriers in bottom panels. 95% CI, 95% confidence interval; APOE, APOE ɛ2/ɛ3/ɛ4 genotype; ɛ33, APOE wildtype carriers.
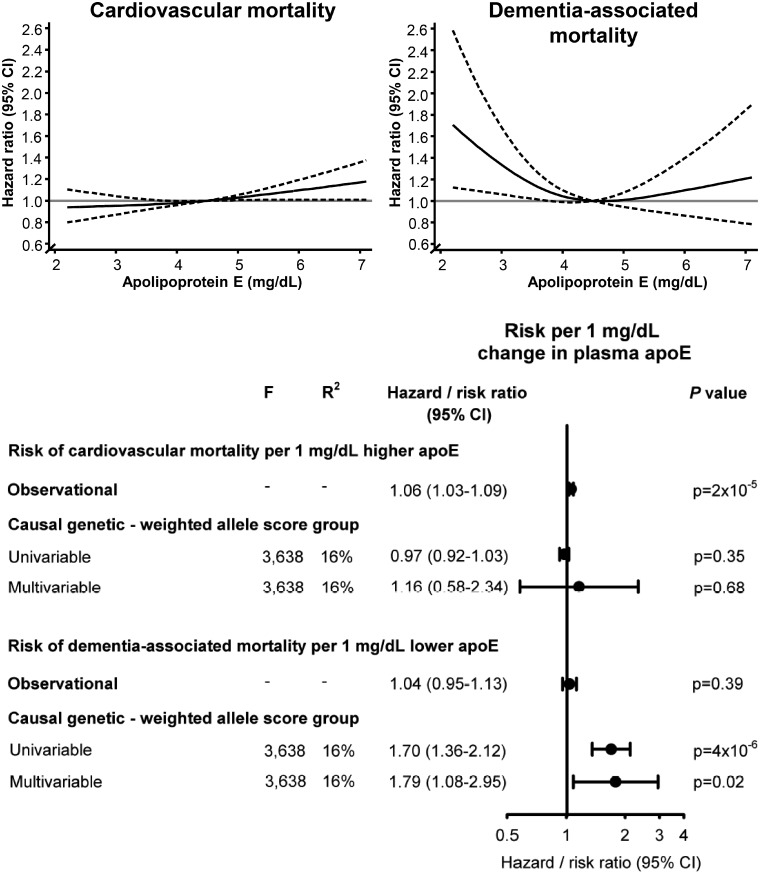
High plasma levels of apoE were associated with increased risk of cardiovascular and cancer mortality in the APOE adjusted model and in ɛ33 carriers only (Figure 4, left and right panels). Conversely, low plasma levels of apoE were associated with increased dementia-associated mortality, in the APOE adjusted model and in ɛ33 carriers only (Figure 4, middle panel).
Figure 4.
Multifactorially adjusted hazard ratios for cause-specific mortality according to plasma levels of apoE in individuals in the general population. Solid lines are hazard ratios, whereas dashed lines indicate 95% confidence intervals derived from restricted cubic spline regression models similar to Figure 3 with respect to reference (4.5 mg/dL) and truncation (2.1–7.1 mg/dL). Cox regression models were multifactorially adjusted similar to Figure 3, with additional adjustment for APOE genotype in upper panels and with analyses restricted to ɛ33 carriers in bottom panels. 95% CI, 95% confidence interval; APOE, APOE ɛ2/ɛ3/ɛ4 genotype; ɛ33, APOE wildtype carriers.
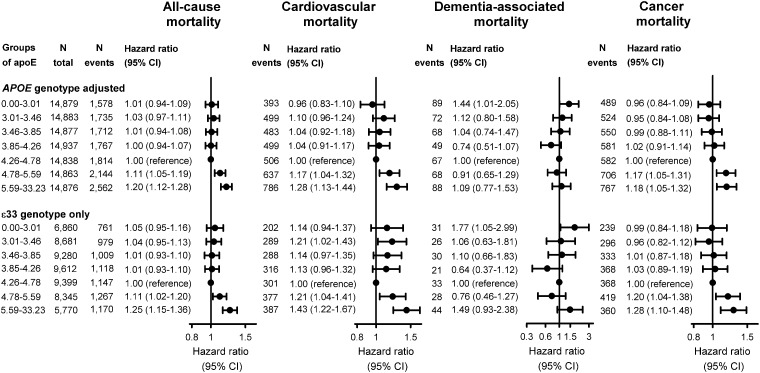
For the highest vs. the fifth septile of apoE, HRs were 1.20 (95% confidence interval 1.12–1.28) for all-cause mortality, 1.28 (1.13–1.44) for cardiovascular mortality, and 1.18 (1.05–1.32) for cancer mortality (Figure 5). Conversely, for the lowest vs. the fifth septile the HR was 1.44 (1.01–2.05) for dementia-associated mortality (Figure 5). Analyses solely in ɛ33 carriers showed similar patterns (Figure 5, lower panels).
Figure 5.
Multifactorially adjusted hazard ratios for all-cause and cause-specific mortality for septiles of apolipoprotein E in individuals in the general population. Cox regression models were multifactorially adjusted for age (time scale), sex, body mass index, smoking, hypertension, diabetes, lipid-lowering therapy, alcohol consumption, physical inactivity, education, postmenopausal status, hormonal replacement therapy, LDL cholesterol, HDL cholesterol, triglycerides (all panels), with further adjustment for APOE genotype (upper panels) and in analyses restricted to ɛ33 carriers (bottom panels). For cause-specific mortality, there are three parallel analyses: when Bonferroni-correction is applied (P = 0.05/3 = 0.02), results for the first septile of apoE for dementia-associated mortality does not adhere to this significance level (P = 0.04) and likewise for the second septile for cardiovascular mortality (P = 0.03). All other results are unaffected. 95% CI, 95% confidence interval; apoE, plasma apolipoprotein E level; APOE, APOE ɛ2/ɛ3/ɛ4 genotype; ɛ33, APOE wildtype carriers.
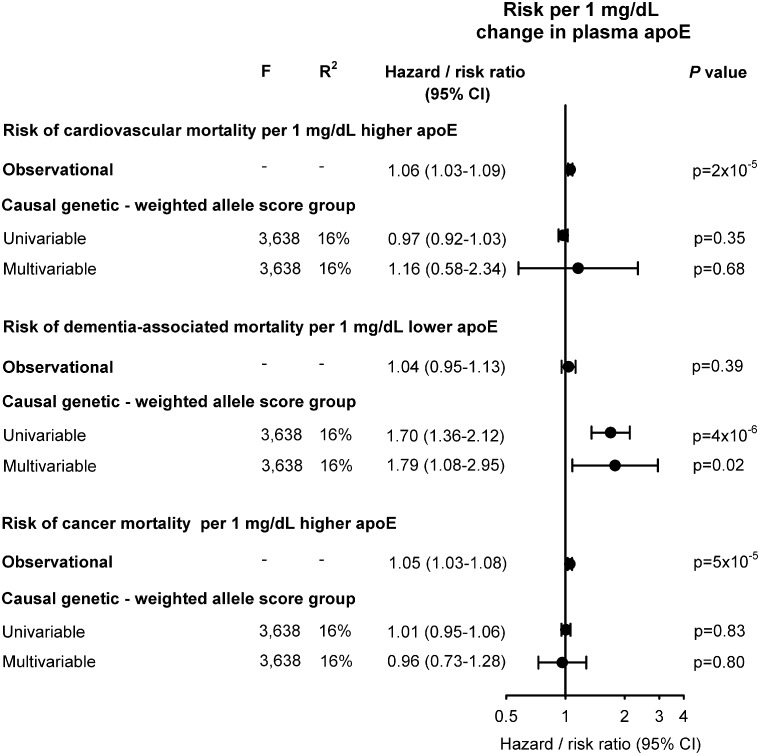
Causal estimates
Estimates from instrumental variable analyses for plasma apoE per se indicated causality for dementia-associated mortality but not for cardiovascular and cancer mortality (Figure 6); because of the observed opposite association observationally between plasma apoE and respectively cardiovascular and dementia-associated mortality, we did not perform instrumental variable analysis for all-cause mortality. Examining genetically determined plasma apoE, a 1 mg/dL increase conferred risk ratios of 0.97 (0.92–1.03) for cardiovascular mortality and 1.01 (0.95–1.06) for cancer mortality, while a 1 mg/dL decrease conferred a risk ratio of 1.70 (1.36–2.12) for dementia-associated mortality for univariable analyses (Figure 6). Plasma levels of apoE and HDL cholesterol decreased, while LDL cholesterol increased across apoE decreasing weighted and simple allele score groups (Supplementary material online, Figure S8).
Figure 6.
Risk of cause-specific mortality for a 1 mg/dL change in observational and causal, genetically determined plasma apolipoprotein E level. The hazard ratio for a 1 mg/dL change in observational plasma apoE was calculated using Cox regression with adjustment for age (time scale), body mass index, smoking, hypertension, diabetes, lipid-lowering therapy, alcohol consumption, physical inactivity, education, postmenopausal status, hormonal replacement therapy, LDL cholesterol, HDL cholesterol, plasma triglycerides, and APOE genotype, whereas the corresponding risk ratios for the genetic change in plasma apoE for the weighted allele score was derived from univariable and multivariable instrumental variable analyses, including LDL cholesterol and triglycerides in multivariable analyses. A total of 74 560 individuals are included in these analyses. apoE, apolipoprotein E; CI, confidence interval; F, strength of the genetic instrument (>10 indicates sufficient statistical strength); P-value, significance of hazard ratios or risk ratios; R2, percent contribution of genetic instrument to the variation in plasma apoE.
Sensitivity analyses
As apoE plays a central part in lipoprotein metabolism and plasma level and isoform specific differences of apoE are associated with levels of lipids and lipoproteins in peripheral lipid metabolism (Figure 2), we also evaluated a multifactorially adjusted model without adjustment for LDL cholesterol, HDL cholesterol, and triglycerides. In these analyses, risk of all-cause mortality increased with both increasing and decreasing levels of apoE relative to the reference value of 4.5 mg/dL, which defined the overall lowest all-cause mortality (Supplementary material online, Figures S9 and S10; compare with Figures 3 and 5). We found results similar to those in Figure 5, when excluding individuals contributing with more than one cause-specific event (Supplementary material online, Figure S11), or when using tertiles, quartiles, quintiles, or deciles instead of septiles (Supplementary material online, Figure S12). We found no interaction between seven groups of plasma apoE and use of two different autoanalyzers on all-cause mortality (P for interaction = 0.80) and estimates before and after the change were similar (Supplementary material online, Figure S13). Sensitivity analyses for risk of narrower cardiovascular mortality endpoints showed similar patterns as for all cardiovascular mortality (compare Supplementary material online, Figure S14 with Figure 1). Results for cause-specific mortality adjusted for multiple hypothesis testing are shown in Supplementary material online, Figure S15. Instrumental variable sensitivity analyses showed no major deviations and did not change conclusions from the main findings in Figure 6 (compare with Supplementary material online, Table S1 and Figure S16).
Discussion
In this study of 105 949 individuals from the white general population, high plasma levels of apoE were associated with high all-cause mortality. For cause-specific mortality, high apoE levels were associated with high cardiovascular mortality, however of a non-causal nature, while low apoE levels were causally associated with high dementia-associated mortality (Take home figure). These findings are novel.
Take home figure.
Epidemiological and causal relationship of plasma apolipoprotein E levels and cardiovascular and dementia-associated mortality in the Copenhagen General Population Study and the Copenhagen City Heart Study. apoE, apolipoprotein E; CI, confidence interval.
That both extreme high and low plasma levels of apoE is associated with cause-specific mortality is biologically plausible and is most likely explained by the fact that low apoE levels are associated with risk of dementia17,23,24 and thus reflected in dementia-associated mortality, whereas high apoE levels are associated with atherogenic lipids and lipoproteins and with risk of ischaemic heart disease25,30,31 and cardiovascular mortality.26,27 A previous meta-analysis comprising ∼9500 individuals found an association for fatal coronary heart disease after adjustment for LDL cholesterol, but showed only tendencies for fatal stroke and non-fatal coronary heart disease.27 In our study, the association between high apoE levels and all-cause and cardiovascular mortality remained after adjustment for lipids, lipoproteins, and APOE genotype, and in analyses restricted to ɛ33 carriers only, most likely reflecting a general correlation between apoE levels and atherogenic triglyceride-rich lipoproteins, independent of APOE genotype. Low apoE levels were associated with dementia-associated mortality in APOE genotype adjusted analyses and in ɛ33 carriers only, more pronounced in men than in women. Since low apoE levels repeatedly have been associated with high risk of dementia, independent of the strong per ɛ4 APOE allele effect,17,23,24 a reflection in dementia-associated mortality makes sense biologically.
Since it is well-established that the APOE ɛ4 allele is associated with increased mortality,32–40 we included analyses of APOE genotype as a positive control and were additionally able to show that the association between apoE levels and cardiovascular mortality, dementia-associated and cancer mortality was independent of the important ɛ4 allele. Our findings of APOE genotype and cause-specific mortality are in agreement with previous findings,33,35,36,40,41 identifying especially ɛ32 as associated with decreased and ɛ43 and ɛ44 as associated with increased mortality. Further, ɛ32 may be protective of dementia-associated mortality, in accordance with the higher apoE levels associated with lower dementia-associated mortality. However, apolipoprotein E is a rather complicated lipid risk factor because quantitative as well as qualitative genotype-determined isoform specific effects each influence levels of lipids and lipoproteins; overall, high levels of apoE are associated with high levels of triglycerides, which we have shown previously.25 ApoE levels are however also influenced by their genotype with the well-established patterns observed in Figure 2. When assessed by genotype, those with the lowest apoE levels (ɛ44) are the ones with the highest risk for both cardiovascular and dementia-associated mortality. This is most likely explained by the fact that genotypes with low apoE in addition are associated with an atherogenic lipid profile, and thus risk of cardiovascular disease and cardiovascular mortality, whereas for dementia and dementia-associated mortality, low apoE in plasma most likely mimic low apoE levels in the brain resulting in decreased β-amyloid clearance.
Mechanisms underlying the association between high apoE levels and increased all-cause and cardiovascular mortality are likely due to the key function of apoE in receptor-mediated endocytosis by binding to triglyceride-rich lipoproteins, and serving as a ligand for the LDL receptor and LDL receptor Related Protein (LRP).9 In mice, overexpression of apoE has been shown to lead to hypertriglyceridaemia, and in vitro increase in apoE expression by hepatoma cells correlates with increased very low-density lipoprotein synthesis and/or secretion irrespective of apoE isoform.42 Hypertriglyceridaemic patients have higher plasma levels of apoE compared with normal control subjects, a finding probably explained by the mechanism that increased levels of apoE stimulate very low-density lipoprotein triglyceride production in the liver and impairs lipoprotein lipase-mediated lipolysis, thus leading to hypertriglyceridaemia.42,43 Also, the well-known propensity of LDL receptor affinity defective ɛ22 carriers to develop type III hyperlipoproteinaemia support this understanding.9 Only <1% of the general population are however ɛ22 carriers and will thus only account for a minor fraction of our overall findings of high plasma apoE and high all-cause and cardiovascular mortality. As triglyceride-rich lipoproteins are atherogenic and causally associated with cardiovascular disease,44,45 and also associated with mortality,46,47 our findings of high apoE levels and high cardiovascular mortality may be mediated by triglyceride-rich lipoproteins via remnant cholesterol content, although our results did not change after adjustment for triglycerides, LDL cholesterol, and HDL cholesterol. It is puzzling that our results do not attenuate after these lipid adjustments, and fuels speculations on a less lipid dependent effect of plasma apoE on cardiovascular mortality, e.g. involvement in thrombosis and bleeding as suggested in a recent experimental study.48 These speculations are however purely theoretical and need further experimental and clinical scrutinization. Further, mechanisms underlying the association between high apoE levels and increased cancer mortality could likely be due to apoE involvement in metastasis and angiogenesis.49–56 The present Mendelian randomization estimates suggest however that the observational findings are not of a causal nature.
Plausible mechanisms underlying the association between low apoE levels and dementia-associated mortality are not explained by peripheral lipid metabolism, but likely by the role of apoE in β-amyloid handling and in neurodegenerative processes.57,58 The ɛ4 allele has detrimental effects on production, deposition, and clearance of amyloid-β, in phosphorylation of tau, in neurotoxicity, mitochondrial dysfunction, and in blood–brain barrier permeability.57,59,60 Also, recently the effect of low apoE levels per se, independent of the ɛ4 allele, was observed to be associated with high risk of dementia in three independent prospective European cohorts,17,23 and further suggested to mark causal pathways in the brain.24 These robust findings of low apoE on dementia risk most likely explain the observed associations with dementia-associated mortality.
As outlined above, apoE is involved in different mechanisms in the periphery and in the central nervous system. Human liver transplantation studies clearly showed that apoE in the peripheral circulation and in the central nervous system originates from two separate compartments.61 Further, previous work suggest a limited blood–brain barrier permeability to circulating lipid-poor apoE,62 suggesting that apoE in blood and brain are regulated independently.61,63 Levels of apoE in human cerebrospinal fluid display a similar genotype-dependent pattern as in plasma,64 and in murine interstitial fluid a similar genotype-dependent pattern as in cerebrospinal fluid was observed.65 Hence, we suggest that plasma levels of apoE mirror levels in cerebrospinal fluid and brain, despite originating from two different compartments.
We further substantiated our findings by causal genetic estimates from instrumental variable analyses, which indicated a causal association between plasma apoE levels and dementia-associated mortality but not with cardiovascular or cancer mortality. We chose to use an instrument consisting of five variants (three promoter variants and two common exonic variants), because this combination of variants reflects the quantitative effects of apoE as well as the qualitative, isoform specific effects. Because APOE variants not only affect plasma levels of apoE but also all other major lipid, lipoprotein, and apolipoprotein classes—a well-established biological fact due to the fundamental role of apoE in lipid metabolism—it is difficult to decipher whether it is plasma levels of apoE per se that is responsible for a potential causal association, or whether the other affected lipid, lipoprotein, or apolipoprotein traits are responsible. This phenomenon is referred to as so-called vertical pleiotropy and may be overcome by applying multivariable instrumental variable analysis, which we did together with a series of other relevant MR sensitivity analyses.66–68 These analyses showed no major deviations and did not change conclusions from the main instrumental variable findings. Mendelian randomization analyses for peripheral effects of apoE should however still be interpreted with caution when pleiotropy is a direct cause of the biology of apoE. In contrast, it seems more straight forward to use the instrument for dementia-associated mortality, because the brain relies almost exclusively on HDL-like apoE-containing lipoprotein particles for its lipid transport and does not have apoB and apoB-containing lipoproteins (LDL and triglyceride-rich lipoproteins). Thus, the lipid and lipoprotein pleiotropic effects in the periphery will most likely not be an issue in the brain. Despite the current limitations, we obtained causal estimates that make biological sense: high plasma levels of apoE are not causally associated with cardiovascular and cancer mortality, whereas low plasma levels of apoE—mimicking brain apoE levels—are suggested to play a causal role in dementia-associated mortality.
Results from the present study may generate therapeutic and clinical considerations. Since both extreme high and low apoE were associated with cause-specific mortality most likely representing two different disease entities, the concern is that a systemic therapeutic increase of apoE—of potential benefit for dementia—could possibly generate harmful hypertriglyceridaemia peripherally due to apoE stimulated very low-density lipoprotein triglyceride production in the liver and apoE inhibited lipoprotein lipase mediated lipolysis as indicated by mechanistic studies.42 Vice versa, a systemic therapeutic decrease of apoE—of potential benefit for ischaemic heart disease due to decreased levels of atherogenic triglyceride-rich lipoproteins—could possibly generate harmful effects in the brain, as supported by experimental evidence in mice,69–72 and indirectly by large human population studies.17,23,24,73 Whether plasma apoE could be a modifiable risk factor for dementia, rather than simply a risk marker, is a crucial question to solve. This would however require that therapeutically modified high plasma apoE level also should be reflected in a high brain apoE level. As brain and periphery are two separate compartments this can only be done by whole body APOE overexpression, which may result in potential detrimental hypertriglyceridaemic effects in plasma.42,43 Alternatively, whole body overexpression of ABCA1 which experimentally increases lipidation of apoE-containing lipoproteins and decreases brain amyloid burden,74 may be a way to increase apoE levels in the brain without potential detrimental hypertriglyceridaemic effects in plasma. Also, small molecule inducers of ABCA1 and apoE that act through indirect activation of the LXR pathway may be a future pharmacological possibility for dementia without the known hepatotoxic side-effects caused by LXR ligands.75 Naturally, these speculations are purely theoretical and can only be confirmed or rebutted in randomized clinical trials. Alternatively, specific therapeutics only targeting brain apoE or liver-derived apoE could be considered.
A major strength of the study is the large prospective general population design with no losses to follow-up: that is, every single individual could be followed to end of follow-up, death, or emigration. Further, due to the large sample size, we were able to perform analyses adjusted for APOE genotype as well as analyses for APOE ɛ33 carriers alone. Finally, by performing APOE adjusted and stratified analyses we were able to differentiate the quantitative effects of apoE levels from the genetically determined isoform specific qualitative effects.
One potential limitation concerns the availability and completeness of the diagnostic information; however, the national Danish Patient Registry includes all hospital visits, as well as out-patient visits. Dementia diagnoses in the Danish registries have high diagnostic validity.24,76 Further, myocardial infarction and cancer as a primary diagnosis or underlying cause of death has been shown to have high diagnostic validity.77,78 Unregistered contributing causes of death would only lead to decreased power of our estimate and bias our results towards the null hypothesis. Given the Danish registration practice it is highly unlikely that an event of death would remain unregistered. Another potential limitation is that blood samples were not drawn in the fasting state, which could add to random measurement error. However, plasma levels of apoE vary only slightly between the fasting and non-fasting state.60,79 Finally, another limitation could be that the generalizability of our study results is limited by the fact that we studied only white individuals. Our results may therefore not necessarily apply to other ethnicities, although we are not aware of data to suggest that our results should not be applicable to all humans.
Conclusion
In conclusion, we found that high plasma levels of apoE were associated with all-cause, cardiovascular, and cancer mortality, however of a non-causal nature, while low apoE levels were causally associated with dementia-associated mortality.
Supplementary Material
Acknowledgements
We thank staff and participants of the CGPS and the CCHS for their important contributions.
Funding
This work was supported by the Independent Research Fund Denmark [10-081618], the Research Council at Rigshospitalet, the Lundbeck Foundation, the Danish Heart Foundation, the Danish Alzheimer Research Fund, M.L. Jørgensen & Gunnar Hansen’s Fund, and the Research Fund at the Capital Region of Denmark, all to [R.F.S].
Conflict of interest: none declared.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP.. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75. [DOI] [PubMed] [Google Scholar]
- 3. Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR.. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med 2017;177:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S.. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 2016;374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmadi-Abhari S, Guzman-Castillo M, Bandosz P, Shipley MJ, Muniz-Terrera G, Singh-Manoux A, Kivimaki M, Steptoe A, Capewell S, O'Flaherty M, Brunner EJ.. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: modelling study. BMJ 2017;358:j2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finegold JA, Asaria P, Francis DP.. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol 2013;168:934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ.. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas PESC Scientific Document Group. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur Heart J 2018;39:508–579. [DOI] [PubMed] [Google Scholar]
- 9. Mahley RW, Rall SC Jr. Type III hyperlipoproteinemia (dysbetalipoproteinemia): the role of apolipoprotein E in normal and abnormal lipoprotein metabolism In Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York, NY: McGraw-Hill; 2001, pp. 2835–2862. [Google Scholar]
- 10. Verghese PB, Castellano JM, Holtzman DM.. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 2011;10:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE.. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 2012;335:1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bennet AM, Di AE, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de FU, Danesh J.. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 2007;298:1300–1311. [DOI] [PubMed] [Google Scholar]
- 13. Sing CF, Davignon J.. Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. Am J Hum Genet 1985;37:268–285. [PMC free article] [PubMed] [Google Scholar]
- 14. Boerwinkle E, Utermann G.. Simultaneous effects of the apolipoprotein E polymorphism on apolipoprotein E, apolipoprotein B, and cholesterol metabolism. Am J Hum Genet 1988;42:104–112. [PMC free article] [PubMed] [Google Scholar]
- 15. Smit M, de KP, Rosseneu M, Bury J, Klasen E, Frants R, Havekes L.. Apolipoprotein E polymorphism in The Netherlands and its effect on plasma lipid and apolipoprotein levels. Hum Genet 1988;80:287–292. [DOI] [PubMed] [Google Scholar]
- 16. Neale MC, de Knijff P, Havekes LM, Boomsma DI.. ApoE polymorphism accounts for only part of the genetic variation in quantitative ApoE levels. Genet Epidemiol 2000;18:331–340. [DOI] [PubMed] [Google Scholar]
- 17. Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R.. Plasma levels of apolipoprotein E and risk of dementia in the general population. Ann Neurol 2015;77:301–311. [DOI] [PubMed] [Google Scholar]
- 18. Davignon J, Gregg RE, Sing CF.. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 1988;8:1–21. [DOI] [PubMed] [Google Scholar]
- 19. Frikke-Schmidt R, Tybjærg-Hansen A, Steffensen R, Jensen G, Nordestgaard BG.. Apolipoprotein E genotype: epsilon32 women are protected while epsilon43 and epsilon44 men are susceptible to ischemic heart disease: the Copenhagen City Heart Study. J Am Coll Cardiol 2000;35:1192–1199. [DOI] [PubMed] [Google Scholar]
- 20. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA.. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 21. Sebastiani P, Solovieff N, Dewan AT, Walsh KM, Puca A, Hartley SW, Melista E, Andersen S, Dworkis DA, Wilk JB, Myers RH, Steinberg MH, Montano M, Baldwin CT, Hoh J, Perls TT.. Genetic signatures of exceptional longevity in humans. PLoS One 2012;7:e29848.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brooks-Wilson AR. Genetics of healthy aging and longevity. Hum Genet 2013;132:1323–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolters FJ, Koudstaal PJ, Hofman A, Duijn CM, Ikram MA.. Serum apolipoprotein E is associated with long-term risk of Alzheimer's disease: the Rotterdam Study. Neurosci Lett 2016;617:139–142. [DOI] [PubMed] [Google Scholar]
- 24. Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R.. Plasma apolipoprotein E levels and risk of dementia: a Mendelian randomization study of 106,562 individuals. Alzheimers Dement 2018;14:71–80. [DOI] [PubMed] [Google Scholar]
- 25. Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R.. Plasma levels of apolipoprotein E and risk of ischemic heart disease in the general population. Atherosclerosis 2016;246:63–70. [DOI] [PubMed] [Google Scholar]
- 26. Mooijaart SP, Berbee JF, van HD, Havekes LM, de Craen AJ, Slagboom PE, Rensen PC, Westendorp RG.. ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med 2006;3:e176.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sofat R, Cooper JA, Kumari M, Casas JP, Mitchell JP, Acharya J, Thom S, Hughes AD, Humphries SE, Hingorani AD.. Circulating apolipoprotein E concentration and cardiovascular disease risk: meta-analysis of results from three studies. PLoS Med 2016;13:e1002146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A.. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 29. Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A.. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524–2532. [DOI] [PubMed] [Google Scholar]
- 30. Corsetti JP, Gansevoort RT, Bakker SJ, Navis G, Sparks CE, Dullaart RP.. Apolipoprotein E predicts incident cardiovascular disease risk in women but not in men with concurrently high levels of high-density lipoprotein cholesterol and C-reactive protein. Metabolism 2012;61:996–1002. [DOI] [PubMed] [Google Scholar]
- 31. Corsetti JP, Bakker SJ, Sparks CE, Dullaart RP.. Apolipoprotein A-II influences apolipoprotein E-linked cardiovascular disease risk in women with high levels of HDL cholesterol and C-reactive protein. PLoS One 2012;7:e39110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindahl-Jacobsen R, Tan Q, Mengel-From J, Christensen K, Nebel A, Christiansen L.. Effects of the APOE epsilon2 allele on mortality and cognitive function in the oldest old. J Gerontol A Biol Sci Med Sci 2013;68:389–394. [DOI] [PubMed] [Google Scholar]
- 33. Rajan KB, Barnes LL, Wilson RS, McAninch EA, Weuve J, Sighoko D, Evans DA.. Racial differences in the association between apolipoprotein E risk alleles and overall and total cardiovascular mortality over 18 years. J Am Geriatr Soc 2017;65:2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosvall L, Rizzuto D, Wang HX, Winblad B, Graff C, Fratiglioni L.. APOE-related mortality: effect of dementia, cardiovascular disease and gender. Neurobiol Aging 2009;30:1545–1551. [DOI] [PubMed] [Google Scholar]
- 35. Beydoun MA, Beydoun HA, Kaufman JS, An Y, Resnick SM, O'Brien R, Ferrucci L, Zonderman AB.. Apolipoprotein E epsilon4 allele interacts with sex and cognitive status to influence all-cause and cause-specific mortality in U.S. older adults. J Am Geriatr Soc 2013;61:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tilvis RS, Strandberg TE, Juva K.. Apolipoprotein E phenotypes, dementia and mortality in a prospective population sample. J Am Geriatr Soc 1998;46:712–715. [DOI] [PubMed] [Google Scholar]
- 37. Jacobsen R, Martinussen T, Christiansen L, Jeune B, Ndersen-Ranberg K, Vaupel JW, Christensen K.. Increased effect of the ApoE gene on survival at advanced age in healthy and long-lived Danes: two nationwide cohort studies. Aging Cell 2010;9:1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ewbank DC. Mortality differences by APOE genotype estimated from demographic synthesis. Genet Epidemiol 2002;22:146–155. [DOI] [PubMed] [Google Scholar]
- 39. Corder EH, Lannfelt L, Viitanen M, Corder LS, Manton KG, Winblad B, Basun H.. Apolipoprotein E genotype determines survival in the oldest old (85 years or older) who have good cognition. Arch Neurol 1996;53:418–422. [DOI] [PubMed] [Google Scholar]
- 40. Hayden KM, Zandi PP, Lyketsos CG, Tschanz JT, Norton MC, Khachaturian AS, Pieper CF, Welsh-Bohmer KA, Breitner JC.. Apolipoprotein E genotype and mortality: findings from the Cache County Study. J Am Geriatr Soc 2005;53:935–942. [DOI] [PubMed] [Google Scholar]
- 41. Pasqualetti G, Seghieri M, Santini E, Rossi C, Vitolo E, Giannini L, Malatesta MG, Calsolaro V, Monzani F, Solini A.. P2X7 receptor and APOE polymorphisms and survival from heart failure: a prospective study in frail patients in a geriatric unit. Aging Dis 2017;8:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang Y, Liu XQ, Rall SC Jr, Taylor JM, von EA, Assmann G, Mahley RW.. Overexpression and accumulation of apolipoprotein E as a cause of hypertriglyceridemia. J Biol Chem 1998;273:26388–26393. [DOI] [PubMed] [Google Scholar]
- 43. Rifai N, Silverman LM.. A simple immunotechnique for the determination of serum concentration of apolipoprotein E. Clin Chim Acta 1987;163:207–213. [DOI] [PubMed] [Google Scholar]
- 44. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res 2016;118:547–563. [DOI] [PubMed] [Google Scholar]
- 45. Nordestgaard BG, Varbo A.. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 46. Varbo A, Freiberg JJ, Nordestgaard BG.. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin Chem 2015;61:533–543. [DOI] [PubMed] [Google Scholar]
- 47. Thomsen M, Varbo A, Tybjærg-Hansen A, Nordestgaard BG.. Low nonfasting triglycerides and reduced all-cause mortality: a Mendelian randomization study. Clin Chem 2014;60:737–746. [DOI] [PubMed] [Google Scholar]
- 48. Baaten C, Meacham S, de Witt SM, Feijge MAH, Adams DJ, Akkerman JN, Cosemans J, Grassi L, Jupe S, Kostadima M, Mattheij NJA, Prins MH, Ramirez-Solis R, Soehnlein O, Swieringa F, Weber C, White JK, Ouwehand WH, Heemskerk J.. A synthesis approach of mouse studies to identify genes and proteins in arterial thrombosis and bleeding. Blood 2018;132:e35–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goodison S, Chang M, Dai Y, Urquidi V, Rosser CJ.. A multi-analyte assay for the non-invasive detection of bladder cancer. PLoS One 2012;7:e47469.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pencheva N, Tran H, Buss C, Huh D, Drobnjak M, Busam K, Tavazoie SF.. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell 2012;151:1068–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boylan KL, Andersen JD, Anderson LB, Higgins L, Skubitz AP.. Quantitative proteomic analysis by iTRAQ(R) for the identification of candidate biomarkers in ovarian cancer serum. Proteome Sci 2010;8:31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oue N, Hamai Y, Mitani Y, Matsumura S, Oshimo Y, Aung PP, Kuraoka K, Nakayama H, Yasui W.. Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res 2004;64:2397–2405. [DOI] [PubMed] [Google Scholar]
- 53. Luo J, Song J, Feng P, Wang Y, Long W, Liu M, Li L.. Elevated serum apolipoprotein E is associated with metastasis and poor prognosis of non-small cell lung cancer. Tumour Biol 2016;37:10715–10721. [DOI] [PubMed] [Google Scholar]
- 54. Chen J, Wu W, Zhen C, Zhou H, Yang R, Chen L, Hu L.. Expression and clinical significance of complement C3, complement C4b1 and apolipoprotein E in pancreatic cancer. Oncol Lett 2013;6:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Su WP, Chen YT, Lai WW, Lin CC, Yan JJ, Su WC.. Apolipoprotein E expression promotes lung adenocarcinoma proliferation and migration and as a potential survival marker in lung cancer. Lung Cancer 2011;71:28–33. [DOI] [PubMed] [Google Scholar]
- 56. Chen YC, Pohl G, Wang TL, Morin PJ, Risberg B, Kristensen GB, Yu A, Davidson B, Shih I.. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res 2005;65:331–337. [PubMed] [Google Scholar]
- 57. Yu JT, Tan L, Hardy J.. Apolipoprotein E in Alzheimer's disease: an update. Annu Rev Neurosci 2014;37:79–100. [DOI] [PubMed] [Google Scholar]
- 58. Wellington CL, Frikke-Schmidt R.. Relation between plasma and brain lipids. Curr Opin Lipidol 2016;27:225–232. [DOI] [PubMed] [Google Scholar]
- 59. Mahley RW, Huang Y.. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron 2012;76:871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rasmussen KL. Plasma levels of apolipoprotein E, APOE genotype and risk of dementia and ischemic heart disease: a review. Atherosclerosis 2016;255:145–144. [DOI] [PubMed] [Google Scholar]
- 61. Linton MF, Gish R, Hubl ST, Butler E, Esquivel C, Bry WI, Boyles JK, Wardell MR, Young SG.. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest 1991;88:270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Martel CL, Mackic JB, Matsubara E, Governale S, Miguel C, Miao W, McComb JG, Frangione B, Ghiso J, Zlokovic BV.. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood-brain barrier transport of circulating Alzheimer's amyloid beta. J Neurochem 2002;69:1995–2004. [DOI] [PubMed] [Google Scholar]
- 63. Wahrle SE, Holtzman DM.. Differential metabolism of ApoE isoforms in plasma and CSF. Exp Neurol 2003;183:4–6. [DOI] [PubMed] [Google Scholar]
- 64. Cruchaga C, Kauwe JS, Nowotny P, Bales K, Pickering EH, Mayo K, Bertelsen S, Hinrichs A, Fagan AM, Holtzman DM, Morris JC, Goate AM.. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer's disease. Hum Mol Genet 2012;21:4558–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ulrich JD, Burchett JM, Restivo JL, Schuler DR, Verghese PB, Mahan TE, Landreth GE, Castellano JM, Jiang H, Cirrito JR, Holtzman DM.. In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol Neurodegener 2013;8:13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burgess S, Thompson SG.. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 2015;181:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bowden J, Davey SG, Burgess S.. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bowden J, Davey SG, Haycock PC, Burgess S.. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL.. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem 2004;279:41197–41207. [DOI] [PubMed] [Google Scholar]
- 70. Koldamova R, Staufenbiel M, Lefterov I.. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem 2005;280:43224–43235. [DOI] [PubMed] [Google Scholar]
- 71. Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM.. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem 2005;280:43236–43242. [DOI] [PubMed] [Google Scholar]
- 72. Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM.. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem 2004;279:40987–40993. [DOI] [PubMed] [Google Scholar]
- 73. Nordestgaard LT, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R.. Loss-of-function mutation in ABCA1 and risk of Alzheimer's disease and cerebrovascular disease. Alzheimers Dement 2015;11:1430–1438. [DOI] [PubMed] [Google Scholar]
- 74. Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, Paul SM, Holtzman DM.. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest 2008;118:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fan J, Zhao RQ, Parro C, Zhao W, Chou HY, Robert J, Deeb TZ, Raynoschek C, Barichievy S, Engkvist O, Maresca M, Hicks R, Meuller J, Moss SJ, Brandon NJ, Wood MW, Kulic I, Wellington CL.. Small molecule inducers of ABCA1 and apoE that act through indirect activation of the LXR pathway. J Lipid Res 2018;59:830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Phung TK, Andersen BB, Hogh P, Kessing LV, Mortensen PB, Waldemar G.. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord 2007;24:220–228. [DOI] [PubMed] [Google Scholar]
- 77. Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M.. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol 2003;56:124–130. [DOI] [PubMed] [Google Scholar]
- 78. Asnaes S. Uncertainty of determining mode and cause of death without autopsy: an autopsy study of medically unattended non-medicolegal deaths. Forensic Sci Int 1980;15:191–196. [DOI] [PubMed] [Google Scholar]
- 79. Annuzzi G, Holmquist L, Carlson LA.. Concentrations of apolipoproteins B, C-I, C-II, C-III, E and lipids in serum and serum lipoproteins of normal subjects during alimentary lipaemia. Scand J Clin Lab Invest 1989;49:73–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.