Abstract
Surgical resection represents the only potentially curative therapy for patients with pancreatic adenocarcinoma (PDAC), an aggressive malignancy with a very limited 5-year survival rate. However, even after complete tumor resection, many patients are still facing an unfavorable prognosis underlining the need for better preoperative stratification algorithms. Here, we explored the role of the secreted glycoprotein soluble urokinase plasminogen activator receptor (suPAR) as a novel circulating biomarker for patients undergoing resection of PDAC. Serum levels of suPAR were measured by enzyme-linked immunosorbent assay (ELISA) in an exploratory as well as a validation cohort comprising a total of 127 PDAC patients and 75 healthy controls. Correlating with a cytoplasmic immunohistochemical expression of uPAR in PDAC tumor cells, serum levels of suPAR were significantly elevated in PDAC patients compared to healthy controls and patient with PDAC precursor lesions. Importantly, patients with high preoperative suPAR levels above a calculated cutoff value of 5.956 ng/ml showed a significantly reduced overall survival after tumor resection. The prognostic role of suPAR was further corroborated by uni- and multivariate Cox-regression analyses including parameters of systemic inflammation, liver and kidney function as well as clinico-pathological patients’ characteristics. Moreover, high baseline suPAR levels identified those patients particularly susceptible to acute kidney injury and surgical complications after surgery. In conclusion, our data suggest that circulating suPAR represents a novel prognostic marker in PDAC patients undergoing tumor resection that might be a useful addition to existing preoperative stratification algorithms for identifying patients that particularly benefit from extended tumor resection.
We show that elevated levels of circulating suPAR in patients with resectable pancreatic adenocarcinoma are associated with a significantly impaired overall survival as well as increased rates of postoperative kidney failure and other surgical complications after resection.
Introduction
Pancreatic adenocarcinoma (PDAC) is a gastrointestinal cancer with a particularly devastating prognosis. Global incidence rates range from 2.4 to 8.6 cases per 100 000 population and are highest in developed countries and among men (1). Mortality rates have only slightly decreased over the last years and still nearly equal incidence rates. Therefore, PDAC represents the fourth most common cause of cancer-related death worldwide (2,3). The overall 5-year survival rate for all stages of PDAC is still below 10% (4), and surgical resection has remained the only potentially curative therapeutic approach but is often not feasible due to advanced stage of disease at time of diagnosis (5). Moreover, even after curatively intended resection of PDAC the prognosis of most patients remains poor with a 5-year survival rate between 18 and 50% (6–8). Currently available stratification tools assessing the postoperative outcome of patients undergoing tumor resection are not well established and are primarily based on imaging techniques and the patients’ clinical performance status, whereas aspects of the individual tumor biology only play a minor role (9,10). Thus, there is a vital need for novel stratification strategies that help to better understand which patients represent the ideal candidates for curative PDAC resection.
The soluble urokinase plasminogen activator receptor (suPAR), a secreted circulating glycoprotein ranging from 20 to 50 kDa, was recently described as a promising biomarker in various clinical conditions (11,12). Circulating suPAR mainly originates from cleavage of the membrane plasminogen activator receptor (uPAR), which is expressed on the cell surface of epithelial and immune cells, regulating cell migration and adhesion processes (13,14). Elevated suPAR serum levels have been described in different clinical conditions including systemic inflammation (15), kidney disease (16) and cancer (17–19). However, the potential role of circulating suPAR in patients with PDAC has remained obscure.
Here, we aimed at evaluating circulating suPAR levels as a novel biomarker in the context of pancreatic cancer in patients undergoing curatively intended tumor resection at our tertiary referral hospital.
Patients and Methods
Study design and patient characteristics
We designed this observational cohort study to evaluate serum levels of suPAR as a biomarker in patients undergoing resection of pancreatic adenocarcinoma (PDAC). Patients with histologically confirmed pancreatic cancer who were admitted to the Department of Visceral and Transplantation Surgery at the University Hospital RWTH Aachen for surgical resection were prospectively recruited in two cohorts between 2011 and 2016 and enrolled into this study (exploratory cohort: 23 patients (enrolled between 2011 and 2012), confirmatory cohort: 104 patients (enrolled between 2012 and 2016), see Table 1 and Supplementary Table 1, available at Carcinogenesis Online, for detailed patient characteristics). Serum samples were collected prior to surgery and 6–7 days after tumor resection. The occurrence of acute kidney injury (AKI) I was defined according to the current KDIGO criteria (20). As control populations we analyzed a total of 75 healthy, cancer-free blood donors with normal values for blood counts, C-reactive protein, kidney and liver function. Moreover, we included a small cohort of patients with pancreatic intraepithelial neoplasia (PanIN) lesions (n = 9). The study protocol was approved by the local ethics committee and conducted in accordance with the ethical standards laid down in the Declaration of Helsinki (Ethics committee of the University Hospital Aachen, RWTH University, Aachen, Germany, EK 206/09). Written informed consent was obtained from the patients.
Table 1.
Characteristics of study population
| Exploratory cohort | Confirmatory cohort | |
|---|---|---|
| PDAC patients | 23 | 104 |
| Sex (%) | ||
| Male–female | 50.0–50.0 | 64.4–35.6 |
| Age (years, median and range) | 64.0 (44–83) | 68.5 (42–84) |
| BMI (kg/m2, median and range) | 25.03 (16.40–33.30) | 24.58 (17.26–43.21) |
| PDAC characteristics (%) | ||
| T1–T2–T3–T4 | 0–0–88.9–11.4 | 4.4–4.4–86.7–4.4 |
| N0–N1 | 38.9–61.1 | 28.9–71.1 |
| M0–M1 | 85.7–14.3 | 82.1–17.8 |
| G2–G3 | 55.6–44.4 | 50.6–49.4 |
| R0–R1 | 67.9–32.1 | |
| Clinical performance status (%) | ||
| ECOG 0–1–2–3 | 41.7–45.8–12.5 | 56.2–29.2–9.0–5.6 |
| Postoperative AKI (%) | ||
| Yes–No | 77.8–22.2 | 88.3–11.7 |
| Deceased during follow-up (%) | ||
| Yes–No | 87.5–12.5 | 71.2–28.8 |
AST, aspartate transaminase; BMI, body mass index; CRP, C-reactive protein; ECOG PS, ‘Eastern Cooperative Oncology Group’ performance status; LDH, lactate dehydrogenase.
Immunohistochemistry
Pseudonymized human pancreatic tissue samples were provided by the tissue bank of the National Center for Tumor Diseases (NCT) in Heidelberg in accordance with the regulations of the tissue bank and after approval by the ethics committee of the medical faculty Heidelberg (project ID: 2804). Haematoxylin and eosin (H&E) stainings were performed on formalin-fixed, paraffin-embedded sections and evaluated by experienced pathologists (F.B. and T.L.). Each 10 samples with non-neoplastic pancreatic tissue, n = 5; chronic pancreatitis, n = 5, PanIN (PanIN1: n = 4, PanIN2: n = 3, PanIN3: n = 3), and pancreatic ductal adenocarcinoma were randomly selected and used for further analyses. Immunohistochemistry was performed using an automated staining system (Techmateä 500+, Dako/Agilent Pathology Solution, Santa Clara, CA). Dako Target Retrieval Solution (pH9, Dako/Agilent Pathology Solution) was used for antigen retrieval. The following antibody was used: uPAR (Thermo Fisher Scientific, Waltham, MA, R-4, 1:2000). Staining was assessed qualitatively and semiquantitatively using the immunoreactive score (IRS) as described previously (21).
Measurement of serum suPAR levels
Serum levels of suPAR were measured using a commercial enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Nr. A001, suPARnostic, ViroGates, Birkerød, Denmark). Standard laboratory parameters were measured in the laboratory center for blood analysis at University Hospital RWTH Aachen.
Statistical analysis
Statistical analyses were performed as recently described (22,23). Serum data are given as median and range. Kolmogorov-Smirnov- and Shapiro-Wilk-Test were used to test for normal distribution. Non-parametric data were compared using the Mann-Whitney-U-Test and the Kruskal-Wallis-Test for multiple comparisons. Box plot graphics show a statistical summary of the median, quartiles and ranges. Receiver operating characteristic (ROC) curves were generated by plotting sensitivity against 1-specificity. The optimal cutoff values for ROC curves were established using the Youden-Index (YI = sensitivity + specificity − 1). The predictive value of suPAR with respect to AKI was tested in a binary logistic regression model. The odds ratio and the 95% confidence interval are displayed. Kaplan-Meier curves were plotted to display the impact on survival. The Log-rank test was used to test for differences between subgroups in Kaplan-Meier curve analysis. The optimal cutoff value for the identification of patients with an impaired overall survival (OS) was established using a recently published biometric software, which fits Cox proportional hazard models to the dichotomized survival status (dead versus alive) and the survival variable (survival time). The optimal cutoff is then defined as the point with the most significant (log-rank test) split (24). The prognostic value of variables was further tested by uni- and multivariate Cox-regression analysis. Parameters with a P-value of < 0.250 in univariate testing were included into multivariate testing. The hazard ratio and the 95% confidence interval are displayed. All statistical analyses were performed with SPSS 23 (SPSS, Chicago, IL) (15). A P-value of <0.05 was considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Tissue expression of uPAR and circulating levels of suPAR in pancreatic cancer—an exploratory analysis
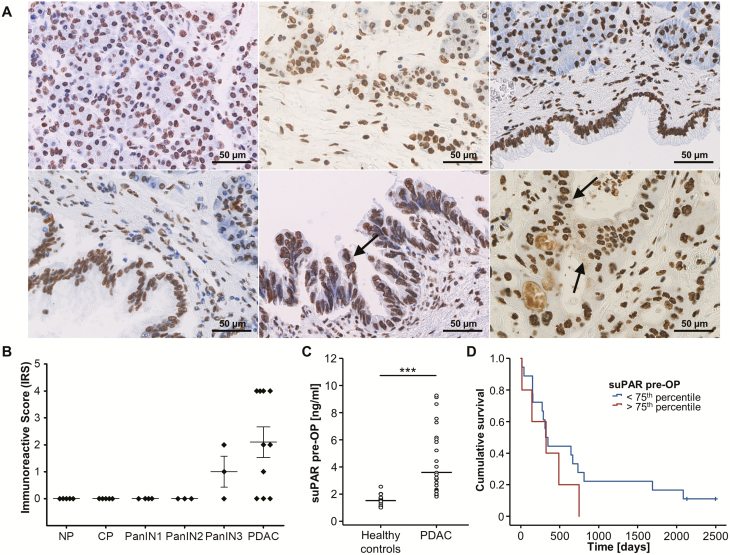
We first analyzed tissue expression levels of uPAR in a small set of pancreas resection samples including PDAC (n = 10), PanIN with different grades of dysplasia (n = 10), chronic pancreatitis (CP, n = 5) and non-neoplastic pancreatic tissue (NP, n = 5). Interestingly, immunohistochemical stainings revealed cytoplasmic uPAR expression in PDAC and PanIN3 lesions (Figure 1A, lower middle and lower right panel, black arrows), whereas NP, CP and PanIN 1/2 samples only displayed an unspecific nuclear staining that was observed in all samples analyzed an may be related to antigen retrieval (Figure 1A and B). Next, we compared circulating levels of suPAR in a small exploratory cohort of PDAC patients before tumor resection (n = 23) as well as healthy controls (n = 10). Interestingly, this analysis revealed a highly significant elevation of circulating suPAR levels in PDAC patients compared to healthy controls (Figure 1B). Moreover, we explored if the elevation of suPAR serum levels might also be associated with the patients’ prognosis after tumor resection. Interestingly, Kaplan-Meier curve analysis showed a strong trend towards a reduced OS in patients with high suPAR levels (e.g. above the 75th percentile) compared to patients with circulating suPAR levels below this cutoff value (Figure 1C). Importantly, none of the patients with high suPAR serum levels reached long-term survival, prompting us to perform an analysis in a larger cohort of PDAC patients undergoing tumor resection at our center.
Figure 1.
uPAR expression and circulating levels of suPAR in pancreatic cancer—exploratory analyses. (A) Representative uPAR expression as detected by immunohistochemistry in normal pancreas (NP, upper left panel), chronic pancreatitis (CP, upper middle panel), PanIN1 (upper right panel), PanIN2 (lower left panel), PanIN3 (lower middle panel), and PDAC (lower right panel, all 400-fold magnification). Only PDAC and PanIN3 cells revealed small uPAR-positive intracytoplasmic speckles (black arrow) while a likely unspecific nuclear staining was present in all samples analyzed. Noteworthy, PDAC cells seem to secrete uPAR as indicated by positive staining of intraductular mucoid material in PDAC glands only. (B) Quantification of cytoplasmic uPAR expression according to the immunoreactive score (IRS) in NP, CP, PanIN, and PDAC samples. (C) Circulating levels of suPAR are significantly elevated in the exploratory cohort of PDAC patients (n = 23) when compared to healthy controls (n = 10). (D) Kaplan-Meier curve analysis shows a trend towards a reduced overall survival in patients with high suPAR levels (above the 75th percentile).
Serum levels of suPAR are elevated in patients with pancreatic adenocarcinoma
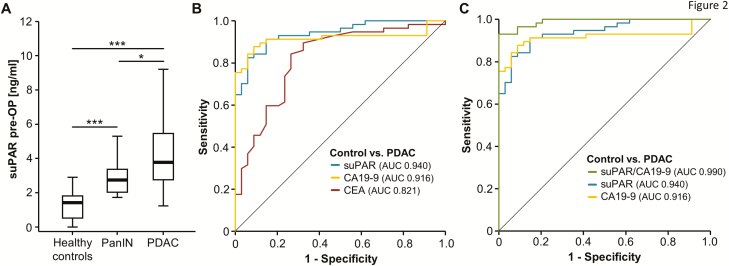
Based on the promising data on elevated serum suPAR levels in the exploratory cohort of PDAC patients, we next extended our analyses to a larger cohort of 104 PDAC patients (not including patients from the previous analysis) and compared them to a group of 60 healthy control samples (patients’ characteristics are summarized in Table 1).
In line with our previous results, PDAC patients displayed significantly higher serum suPAR levels compared to healthy control samples (Figure 2A). Moreover, we evaluated suPAR serum levels in patients with PanIN lesions (n = 9) as an example for pancreatic cancer precursor lesions. Interestingly, these patients had significantly elevated suPAR levels when compared to healthy controls but significantly lower serum levels compared to pancreatic cancer patients (Figure 2A). ROC curve analysis revealed an AUC value of 0.948 for the discrimination between PDAC patients and healthy controls, which was superior to the diagnostic power of established tumor markers such as CA19-9 (AUC 0.917) and CEA (AUC 0.820) (Figure 2B). By applying the Youden-index method, an optimal cutoff value of 2.18 ng/ml was established. At this cutoff value, suPAR serum levels showed a diagnostic sensitivity and specificity of 87.2 and 91.8%, respectively. Importantly, the combination of circulating suPAR and CA19-9 levels even further improved the diagnostic power with a sensitivity of 88.5% and specificity of 98% for the discrimination between PDAC patients and healthy controls (Figure 2C).
Figure 2.
suPAR serum levels are elevated in PDAC patients. (A) PDAC patients display significantly elevated serum suPAR levels compared to healthy control samples and patients with pancreatic intraepithelial neoplasia (PanIN) lesions. (B) Baseline suPAR levels show an AUC value of 0.940 regarding the discrimination of PDAC patients and healthy controls. (C) The combination of circulating suPAR and CA19-9 levels further improved the diagnostic potential.
Next, we analyzed if preoperative suPAR serum levels correlated with disease-specific characteristics such as the tumor staging or grading. However, we observed no significant alteration of suPAR serum concentrations when comparing PDAC patients with different T-stages (Supplementary Figure 1A, available at Carcinogenesis Online), nodal negative versus nodal positive cases (Supplementary Figure 1B, available at Carcinogenesis Online) as well as non-metastasized versus those metastasized patients that were still eligible for surgical tumor resection (Supplementary Figure 1C, available at Carcinogenesis Online). Moreover, patients with moderately differentiated tumors (G2) had similar preoperative suPAR levels compared to poorly differentiated (G3) patients (Supplementary Figure 1D, available at Carcinogenesis Online). Finally, suPAR serum levels were unaltered between patients with R0 or R1 resection status (Supplementary Figure 1E, available at Carcinogenesis Online) as well as between male and female patients (Supplementary Figure 1F, available at Carcinogenesis Online).
Elevated preoperative suPAR serum levels are associated with a reduced OS after resection of pancreatic cancer
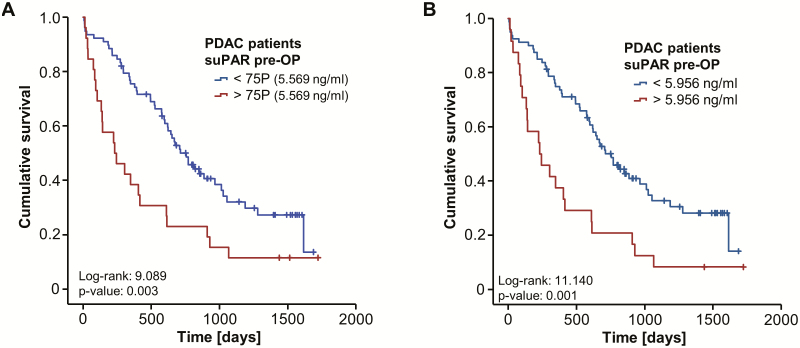
To validate our hypothesis on a potential impact of high initial circulating suPAR levels on the patients’ prognosis after PDAC tumor resection, we next subdivided our cohort of patients into two subgroups according to the respective preoperative suPAR concentration. We first chose the 75th percentile (5.569 ng/ml) as an arbitrary cutoff value to identify patients with particularly high levels of suPAR. Interestingly, Kaplan-Meier curve analysis revealed a significantly impaired long-term survival for patients with serum suPAR concentrations above this cutoff value (Figure 3A). Based on this finding, we next calculated an optimal prognostic cutoff value by fitting Cox proportional hazard models to the survival status and the survival time and testing for the respective suPAR level with the most significant log-rank test as recently described (see Patients and Methods for further specifications) (24). When applying this specific cutoff value of 5.956 ng/ml, Kaplan-Meier curve analysis showed a strikingly reduced OS of 231 days for PDAC patients with initial serum suPAR levels above the cutoff value compared to 756 days for patients with low initial suPAR levels below the cutoff (Figure 3B).
Figure 3.
Elevated levels of circulating suPAR are associated with a reduced overall survival after resection of pancreatic cancer. (A) Kaplan-Meier curve analysis reveals a significantly impaired survival for patients with high baseline serum suPAR concentrations (>50th percentile). (B) PDAC patients with initial serum suPAR level above the calculated ideal cutoff value (5.956 ng/ml) show a strikingly reduced OS of 231 days compared to 756 days for patients with suPAR serum levels below the cutoff.
To further corroborate the prognostic role of circulating suPAR in the clinical context of PDAC resection, we subsequently performed univariate Cox-regression analysis. In this analysis, preoperative suPAR serum levels above the ideal cutoff value turned out as a prognostic factor for OS following PDAC resection (hazard ratio 2.304 [1.392–3.813], P = 0.001). In uni- and subsequent multivariate analyses, in which markers of systemic inflammation (leucocyte count and CRP), liver and kidney function (AST, bilirubin and creatinine) as well as clinic-pathological parameters (T-stage, age, BMI and ECOG PS) were included, circulating levels of suPAR remained an independent prognostic factor for OS (hazard ratio 2.533 [1.185–5.418], P = 0.017; Table 2).
Table 2.
Uni- and multivariate Cox-regression analyses for the prediction of overall survival
| Univariate Cox-regression | Multivariate Cox-regression | |||
|---|---|---|---|---|
| Parameter | P-value | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) |
| suPAR (>5.956 ng/ml) | 0.001 | 2.304 (1.392–3.813) | 0.017 | 2.533 (1.185–5.418) |
| Leukocyte count | 0.926 | 0.997 (0.930–1.068) | ||
| CRP | 0.033 | 1.006 (1.000–1.011) | 0.180 | 1.005 (0.998–1.013) |
| Platelets | 0.751 | 1.000 (0.997–1.002) | ||
| AST | 0.178 | 1.002 (0.999–1.005) | 0.088 | 1.003 (0.999–1.007) |
| Bilirubin | 0.098 | 1.051 (0.991–1.116) | 0.107 | 0.916 (0.823–1.019) |
| ALP | 0.873 | 1.000 (0.999–1.001) | ||
| LDH | 0.480 | 0.992 (0.972–1.013) | ||
| Creatinine | 0.223 | 1.706 (0.772–4.031) | 0.557 | 1.325 (0.518–3.393) |
| BMI | 0.927 | 0.998 (0.948–1.050) | ||
| ECOG PS | 0.221 | 1.192 (0.899–1.581) | 0.251 | 0.797 (0.540–1.175) |
| Age | 0.059 | 1.024 (0.999–1.050) | 0.198 | 1.022 (0.989–1.055) |
| Sex | 0.663 | 0.889 (0.547–1.443) | ||
| T-stage | 0.271 | 1.318 (0.807–2.153) |
ALP, alkaline phosphatase; AST, aspartate transaminase; BMI, body mass index; CRP, C-reactive protein; ECOG PS, ‘Eastern Cooperative Oncology Group’ performance status; LDH, lactate dehydrogenase.
Postoperative suPAR serum levels are unsuitable for the prediction of OS
We next analyzed if postoperative suPAR serum concentration might also reflect patients’ outcome after PDAC resection. Postoperative suPAR serum levels were available for 52 of all resected PDAC patients, but were not significantly altered compared to the respective preoperative levels (Supplementary Figure 2A, available at Carcinogenesis Online). Moreover, postoperative suPAR serum concentrations were unaltered between patients with different TNM stages, tumor grading (G2 versus G3) and R0/R1 resection status (Supplementary Figure 2B–E, available at Carcinogenesis Online). In contrast to preoperative suPAR serum levels, Kaplan-Meier curve analysis did not reveal an impaired prognosis for patients with high postoperative serum levels (above the 50th percentile; Supplementary Figure 3A, available at Carcinogenesis Online). In addition, the individual longitudinal kinetics of suPAR serum levels before and after surgery (∆ pre/post-OP) did not provide further information regarding the patients’ prognosis after tumor resection (Supplementary Figure 3B, available at Carcinogenesis Online).
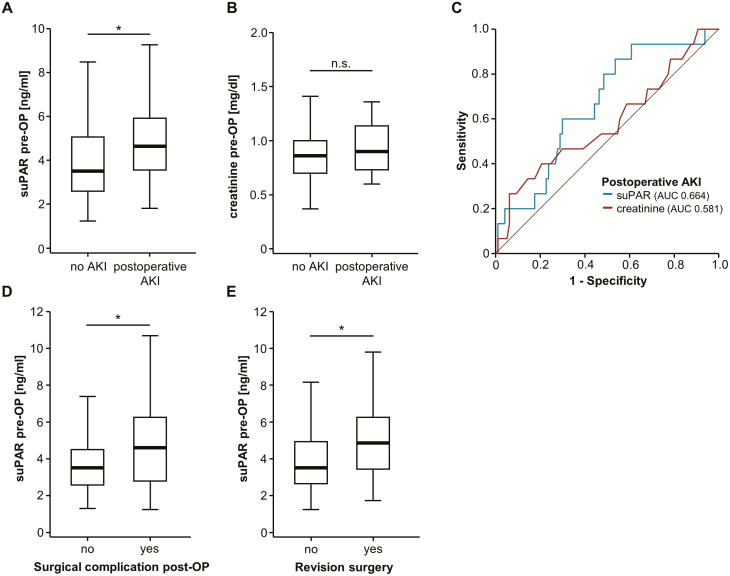
Preoperative levels of suPAR predict AKI and surgical complications after surgical resection of PDAC
Based on our data on a prognostic role of suPAR serum levels in the context of PDAC resection, we finally analyzed if suPAR serum levels might correlate with specific clinical postoperative characteristics. Of note, SuPAR was suggested as a predictor of chronic kidney disease and was also described as a predictor of AKI after major cardiac surgery (16,25). In our cohort of patients, we observed 16 cases of postoperative stage I AKI according to KDIGO criteria (20). Importantly, we observed a striking elevation of preoperative suPAR levels in those patients developing postoperative AKI I compared to those patients with normal renal function after pancreatic surgery (Figure 4A). In contrast, preoperative creatinine levels (the clinically most established marker of acute and chronic renal failure (26)) were unaltered between AKI and non-AKI patients (Figure 4B). Compared to preoperative creatinine levels (AUC 0.581), preoperative suPAR serum concentrations displayed a superior AUC value of 0.664 for the discrimination between AKI and non-AKI patients in ROC curve analysis (Figure 4C). Applying the Youden-Index method, we established an optimal cutoff value of 3.35 ng/ml, at which suPAR serum concentrations had a sensitivity of 86.7% with a specificity of 46.4% for the prediction of postoperative AKI. In line, binary logistic regression analysis revealed preoperative suPAR serum levels above our ideal cutoff as a predictor of postoperative AKI (odds ratio 5.625 [1.204–26.271], P = 0.028). However, the number of events was too small to perform sufficient multivariate analysis. Finally, we evaluated if preoperative suPAR levels might also be indicative for surgical complications after tumor resection. A total of 38 patients presented with postoperative complications including intraabdominal or gastrointestinal bleeding, abscess formation, anastomotic leak, sepsis or pulmonary embolism. Of these, 22 required revision surgery. Interestingly, initial levels of circulating suPAR were significantly elevated in patients with postoperative complications compared to patients with an uncomplicated clinical course as well as in patients that need surgical revision (Figure 4D and E).
Figure 4.
Preoperative levels of suPAR predict risk for AKI and postoperative complications after surgical resection. (A) Preoperative suPAR serum levels are significantly elevated in patients who develop postoperative AKI I compared to non-AKI patients. (B) Preoperative creatinine levels are unaltered between AKI and non-AKI patients. (C) In comparison to initial creatinine levels (AUC 0.581), preoperative suPAR serum concentrations have a superior AUC value of 0.664 for the discrimination between AKI and non-AKI patients. (D) Patients who presented with postoperative surgical complications have significantly higher preoperative levels of circulating suPAR. (E) Initial suPAR concentrations are higher in patients who require revision surgery after initial tumor resection.
Discussion
Pancreatic cancer represents a gastrointestinal malignancy with a particularly poor prognosis (2). While surgical resection represents the only potentially curative therapeutic option, many successfully resected patients are still facing a limited prognosis due to disease recurrence and postoperative complications (5). However, the knowledge on potential biomarkers for the identification of these patients is still limited. To date, CA19-9 represents the most widely used biomarker in the context of PDAC but its diagnostic and prognostic power is limited, and serum levels of CA19-9 are mainly measured for monitoring of chemotherapy and postoperative surveillance (27). Here, we examined serum levels of suPAR in two independent cohorts of PDAC patients undergoing extended tumor resection and demonstrate for the first time that suPAR serum levels are elevated in PDAC patients. While it is important to notice that suPAR serum levels are elevated in a variety of clinical conditions including systemic inflammation and other tumor entities (15,17,18) and therefore do not represent a disease-specific marker for pancreatic cancer, measurements of suPAR might be implicated into diagnostic algorithms for pancreatic cancer patients. Our data further suggest that preoperative serum levels of suPAR can unravel important information on the patient’s individual postoperative course. As such, PDAC patients with high preoperative suPAR levels above our ideal cutoff value of 5.956 ng/ml had a strongly impaired outcome showing a median OS of just 231 days. In contrast, patients with initial suPAR concentrations below the cutoff value showed a significantly better long-term survival with a median OS of 756 days (Figure 3). This finding was further confirmed by uni- and multivariate Cox-regression analyses including markers of systemic inflammation, renal- and liver function as well as clinical parameters such as the tumor stage and the patients’ age, BMI or ECOG performance status (Table 2).
Circulating suPAR originates from shedding of the membrane-bound plasminogen activator receptor (uPAR), which is expressed on a variety of cell types including epithelial and immune cells (28). In the context of PDAC, several studies described an overexpression of uPAR in tissue samples of PDAC with a predominant overexpression in malignant tumor cells (29,30). In line, immunohistochemistry confirmed cytoplasmic uPAR expression in PDAC tumor cells and PanIN3 lesions, but not in normal pancreas, chronic pancreatitis, and low-grade PanIN lesions (Figure 1). However, only very little is known on circulating suPAR in the context of PDAC. Since experimental data described a strong correlation between circulating suPAR and the tumor volume (31), it is conceivable to conclude that the elevation of suPAR, which we observed in our cohort of patients, is at least partly related to an increased expression of uPAR in the PDAC tumor tissue. Moreover, increased shedding of uPAR has been described as a surrogate for an increased immune activation within in tumor and might also occur in PDAC patients (32). However, the underlying pathophysiological mechanisms linking a strong uPAR and/or suPAR expression with an impaired patients’ prognosis is not fully understood to date. Several studies suggested that uPAR might act as a promoter of cancer cell survival, proliferation and metastases (33). As such, uPAR was shown to subvert ligand-regulated EGFR signaling, providing cancer cells with a proliferative phenotype (34). In PDAC cell lines, high uPAR expression exerts a strong pro-malignant effect via the p-ERK pathway and strongly contributes to PDAC progression by a positive uPAR/ERK feedback loop (35). uPAR expression also promotes the differentiation of pro-tumorigenic M2 macrophage differentiation via overexpression of TGF-β and IL-4, a strong driver of cancer progression in the tumor microenvironment (36). Moreover, recent data provided evidence that suPAR down-regulates PTEN in endothelial cells to support angiogenesis, a hallmark in cancer progression, via activation of the PI3K/Akt pathway (37). Nevertheless, the exact pathophysiological correlation between elevated levels of suPAR and an impaired prognosis of PDAC patients in our cohort remains not fully understood, underlining the need for further molecular studies ideally including e.g. uPAR knockout mice in the context of PDAC (38). Of note, we observed no significant change of circulating suPAR levels 6 to 7 days after tumor resection and the individual kinetics of circulating suPAR before and after surgery had no impact on the patients’ survival. It is thus unlikely that suPAR serum levels 1 week after surgery reflect successful resection of pancreatic cancer but are rather affected by postoperative systemic inflammation. In line, we have shown a strong correlation between postoperative suPAR serum levels and the patients’ leukocyte count or CRP levels (Supplementary Figure 3, available at Carcinogenesis Online). Moreover, it is conceivable that circulating suPAR did not exclusively origin from uPAR-expressing tumor cells but also from other cell types such as inflammatory or immune cells. However, further clinical studies including later time points of serum analyses after tumor resection are warranted to fully elucidate the postoperative course of circulating suPAR.
Interestingly, our study further revealed a potential role of preoperative suPAR levels as a predictor of postoperative AKI following pancreatic tumor resection. While postoperative AKI is a well-known complication after extensive abdominal surgery, which is associated with poor short- and long-term outcomes (39,40), the preoperative identification of patients who are particularly susceptible to AKI has remained challenging. Serum creatinine levels are the most established biomarker for the evaluation of renal function but its relevance for the prediction of postoperative AKI is restricted (41). In the context of pancreatic resection, preoperative severe chronic kidney disease (CKD grade IV/V) has been described as a negative prognostic factor (42), but also asymptomatic renal dysfunction which often goes along with unaltered creatinine levels represents a significant risk factor for morbidity after pancreatoduodenectomy (43). Moreover, elevated preoperative suPAR levels were associated with postoperative surgical complications and the necessity of revision surgery after initial tumor resection. Serum levels of suPAR might thus not only be a valuable tool in the clinical decision making whether a PDAC patient should undergo extensive surgical resection or not, but could also identify patients who are particularly susceptible to postoperative AKI or surgery-related complications and therefore require specific perioperative attention. Although the exact pathophysiological association between an impaired postoperative renal function and elevated preoperative suPAR levels is not fully understood, it was shown that circulating suPAR binds and activates ß3 integrins, which anchor podocytes to the underlying glomerular basement membrane, leading to significant podocyte dysfunction and proteinuria (44). Our findings are further in line with previous data suggesting a predictive role of circulating suPAR for the occurrence of AKI after major cardiac surgery (25). Moreover, suPAR serum levels have been shown to independently correlate with a decline of the glomerular filtration rate in a large cohort of patients after cardiac catheterization (16).
This study was limited by a few points. First of all, the two patient cohorts were defined by their time of inclusion but were still operated at the same center. Importantly, the single-center study design ensured to have similar criteria for assessing eligibility for a surgical approach to PDCA in both cohorts. Second, our study only gave information on the prognostic role of suPAR in the context of pancreatic tumor resection. However, it remains unknown if patients with high preoperative suPAR levels and poor postoperative survival would have benefitted similarly or even to a greater extent from a different treatment modality such as systemic chemotherapy. In line, suPAR and/or other biomarkers could be helpful to identify patients who might specifically benefit from a more aggressive therapeutic approach including highly active neoadjuvant and adjuvant chemotherapy regimens (e.g. ESPAC-4, PRODIGE24 (8,45)). In this context, the implication of prognostic biomarkers such as suPAR in the preoperative stratification process especially for borderline resectable patients (46) represents a promising novel approach. However, to corroborate the clinical relevance of circulating suPAR in the context of PDAC, further larger and prospective clinical trials including different treatment modalities for PDAC patients are warranted, which we hope to have stimulated with our study.
Funding
Work in the lab of T.L. was funded from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program through the ERC Consolidator Grant PhaseControl (Grant Agreement n° 771083). The lab of T.L. was further supported by the German Cancer Aid (Deutsche Krebshilfe 110043 and a Mildred-Scheel-Professorship), the German-Research-Foundation (SFB-TRR57/P06 and LU 1360/3-1), the IZKF (interdisciplinary center of clinical research) Aachen and a grant from the medical faculty of the RWTH Aachen.
Author contributions
T.L., F.T., S.H.L., C.R., and U.P.N. designed the study; U.P.N., M.B., P.H.A., N.P., and G.W. recruited and operated on patients; S.H.L. and N.P. performed experiments; S.H.L. performed statistical analysis and generated figures and tables; T.L., F.B., and T.R. performed IHC analysis; C.T., F.T., J.N.K., P.P., and A.K. provided intellectual input; S.H.L. and T.L. drafted the manuscript; all authors approved the article.
Supplementary Material
Acknowledgements
The suPAR ELISA kits were kindly provided by Virogates (Denmark). We thank Veronika Geissler for excellent technical assistance. The pancreatic tissue samples were provided by the tissue bank of the National Center for Tumor Diseases (NCT, Heidelberg, Germany) in accordance with the regulations of the tissue bank and the approval of the ethics committee of the medical faculty Heidelberg.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AKI
acute kidney injury
- OS
overall survival
- PanIN
pancreatic intraepithelial neoplasia
- ROC
receiver operating characteristic
- suPAR
soluble urokinase plasminogen activator receptor
References
- 1. Ferlay J., et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer, 136, E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Hariharan D., et al. (2008) Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford)., 10, 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torre L.A., et al. (2015) Global cancer statistics, 2012. CA. Cancer J. Clin., 65, 87–108. [DOI] [PubMed] [Google Scholar]
- 4. American Cancer Society. (2015) Cancer Facts & Figures 2015. Atlanta: American Cancer Society. [Google Scholar]
- 5. Ryan D.P., et al. (2014) Pancreatic adenocarcinoma. N. Engl. J. Med., 371, 2140–2141. [DOI] [PubMed] [Google Scholar]
- 6. Lovecek M., et al. (2016) Long-term survival after resections for pancreatic ductal adenocarcinoma. Single centre study. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub., 160, 280–286. [DOI] [PubMed] [Google Scholar]
- 7. Yamashita Y., et al. (2016) Surgical results of pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a multi-institutional retrospective study of 174 patients. Anticancer Res., 36, 2407–2412. [PubMed] [Google Scholar]
- 8. Conroy T., et al. (2018) FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med., 379, 2395–2406. [DOI] [PubMed] [Google Scholar]
- 9. Balzano G., et al. (2017) A preoperative score to predict early death after pancreatic cancer resection. Dig. Liver Dis., 49, 1050–1056. [DOI] [PubMed] [Google Scholar]
- 10. Popp F.C., et al. (2017) Protocol of the PANCALYZE trial: a multicenter, prospective study investigating the tumor biomarkers CXCR4, SMAD4, SOX9 and IFIT3 in patients with resected pancreatic adenocarcinoma to predict the pattern of recurrence of the disease. BMC Cancer, 17, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Backes Y., et al. (2012) Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med., 38, 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eugen-Olsen J., et al. (2015) suPAR: The unspecific marker for disease presence, severity and prognosis. Int. J. Antimicrob. Agents, 46 (suppl 1), S33–S34. [DOI] [PubMed] [Google Scholar]
- 13. Thunø M., et al. (2009) suPAR: the molecular crystal ball. Dis. Markers, 27, 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montuori N., et al. (2005) Soluble and cleaved forms of the urokinase-receptor: degradation products or active molecules? Thromb. Haemost., 93, 192–198. [DOI] [PubMed] [Google Scholar]
- 15. Koch A., et al. (2011) Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit. Care, 15, R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayek S.S., et al. (2015) Soluble urokinase receptor and chronic kidney disease. N. Engl. J. Med., 373, 1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chounta A., et al. (2015) Serum soluble urokinase plasminogen activator receptor as a screening test for the early diagnosis of hepatocellular carcinoma. Liver Int., 35, 601–607. [DOI] [PubMed] [Google Scholar]
- 18. Fidan E., et al. (2013) Diagnostic and prognostic significance of CA IX and suPAR in gastric cancer. Med. Oncol., 30, 540. [DOI] [PubMed] [Google Scholar]
- 19. Loosen S.H., et al. (2018) Serum levels of soluble urokinase plasminogen activator receptor (suPAR) predict outcome after resection of colorectal liver metastases. Oncotarget, 9, 27027–27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khwaja A. (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract., 120, c179–c184. [DOI] [PubMed] [Google Scholar]
- 21. Schlaeger C., et al. (2008) Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology, 47, 511–520. [DOI] [PubMed] [Google Scholar]
- 22. Loosen S.H., et al. (2017) Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J. Hepatol., 67, 749–757. [DOI] [PubMed] [Google Scholar]
- 23. Loosen S., et al. (2018) Circulating levels of osteopontin predict patients’ outcome after resection of colorectal liver metastases. J. Clin. Med., 7, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Budczies J., et al. (2012) Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One, 7, e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mossanen J., et al. (2017) Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int. J. Mol. Sci., 18, 1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown J.R., et al. ; Northern New England Cardiovascular Disease Study Group. (2006) Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation, 114(1 Suppl), I409–I413. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y., et al. (2015) Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int. J. Clin. Exp. Med., 8, 11683–11691. [PMC free article] [PubMed] [Google Scholar]
- 28. Genua M., et al. (2015) The urokinase plasminogen activator receptor (uPAR) controls macrophage phagocytosis in intestinal inflammation. Gut, 64, 589–600. [DOI] [PubMed] [Google Scholar]
- 29. de Geus S.W., et al. (2017) Prognostic impact of urokinase plasminogen activator receptor expression in pancreatic cancer: malignant versus stromal cells. Biomark. Insights, 12, 1177271917715443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harvey S.R., et al. (2003) Evaluation of urinary plasminogen activator, its receptor, matrix metalloproteinase-9, and von Willebrand factor in pancreatic cancer. Clin. Cancer Res., 9, 4935–4943. [PubMed] [Google Scholar]
- 31. Holst-Hansen C., et al. (1999) Soluble urokinase receptor released from human carcinoma cells: a plasma parameter for xenograft tumour studies. Br. J. Cancer, 81, 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koch A., et al. (2014) Clinical relevance and cellular source of elevated soluble urokinase plasminogen activator receptor (suPAR) in acute liver failure. Liver Int., 34, 1330–1339. [DOI] [PubMed] [Google Scholar]
- 33. Huber M.C., et al. (2016) uPAR enhances malignant potential of triple-negative breast cancer by directly interacting with uPA and IGF1R. BMC Cancer, 16, 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu D., et al. (2002) EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell, 1, 445–457. [DOI] [PubMed] [Google Scholar]
- 35. Xue A., et al. (2009) Suppression of urokinase plasminogen activator receptor inhibits proliferation and migration of pancreatic adenocarcinoma cells via regulation of ERK/p38 signaling. Int. J. Biochem. Cell Biol., 41, 1731–1738. [DOI] [PubMed] [Google Scholar]
- 36. Hu J., et al. (2014) uPAR induces expression of transforming growth factor β and interleukin-4 in cancer cells to promote tumor-permissive conditioning of macrophages. Am. J. Pathol., 184, 3384–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Unseld M., et al. (2015) PTEN expression in endothelial cells is down-regulated by uPAR to promote angiogenesis. Thromb. Haemost., 114, 379–389. [DOI] [PubMed] [Google Scholar]
- 38. Connolly B.M., et al. (2010) Selective abrogation of the uPA-uPAR interaction in vivo reveals a novel role in suppression of fibrin-associated inflammation. Blood, 116, 1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. An Y., et al. (2018) Risk factors for and the prevention of acute kidney injury after abdominal surgery. Surg. Today, 48, 573–583. [DOI] [PubMed] [Google Scholar]
- 40. Gameiro J., et al. (2018) Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann. Intensive Care, 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Geus H.R., et al. (2012) Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin. Kidney J., 5, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Squires M.H., III, et al. (2014) Effect of preoperative renal insufficiency on postoperative outcomes after pancreatic resection: a single institution experience of 1,061 consecutive patients. J. Am. Coll. Surg., 218, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagai M., et al. (2015) Impact of preoperative asymptomatic renal dysfunction on clinical course after pancreatoduodenectomy. J. Hepatobiliary. Pancreat. Sci., 22, 810–818. [DOI] [PubMed] [Google Scholar]
- 44. Wei C., et al. (2011) Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med., 17, 952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neoptolemos J.P., et al. ; European Study Group for Pancreatic Cancer. (2017) Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet, 389, 1011–1024. [DOI] [PubMed] [Google Scholar]
- 46. Sabater L., et al. (2018) Borderline resectable pancreatic cancer. Challenges and controversies. Cancer Treat. Rev., 68, 124–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.