Aβ deposition in the basal ganglia is common in autosomal dominant Alzheimer’s disease. Vöglein et al. report an increased severity of motor symptoms in autosomal dominant versus sporadic disease in advanced disease stages. Motor symptoms are more severe in post-codon 200 presenilin 1 mutation carriers and correlate with basal ganglia Aβ.
Keywords: Alzheimer’s disease, motor symptoms, amyloid-β, genetics, Unified Parkinson Disease Rating Scale
Abstract
Owing to an early and marked deposition of amyloid-β in the basal ganglia, autosomal dominant Alzheimer’s disease could distinctly involve motor symptoms. Therefore, we aimed to assess the prevalence and characteristics of motor signs in autosomal dominant Alzheimer’s disease. Baseline Unified Parkinson Disease Rating Scale part three scores (UPDRS-III) from 433 participants of the Dominantly Inherited Alzheimer’s Network observational study were analysed. Motor symptoms were scrutinized with respect to associations with mutation carrier status, mutation site within PSEN1, basal ganglia amyloid-β as measured by Pittsburgh compound B PET, estimated years to symptom onset and Clinical Dementia Rating Scale-Sum of Boxes. Motor findings in mutation carriers were compared to patients with sporadic Alzheimer’s disease using data of the National Alzheimer’s Coordination Center. Mutation carriers showed motor findings at a higher frequency (28.4% versus 12.8%; P < 0.001) and severity (mean UPDRS-III scores 2.0 versus 0.4; P < 0.001) compared to non-carriers. Eleven of the 27 UPDRS-III items were statistically more frequently affected in mutation carriers after adjustment for multiple comparisons. Ten of these 11 items were subscale components of bradykinesia. In cognitively asymptomatic mutation carriers, dysdiadochokinesia was more frequent compared to non-carriers (right hand: 3.8% versus 0%; adjusted P = 0.023; left: 4.4% versus 0.6%; adjusted P = 0.031). In this cohort, the positive predictive value for mutation carrier status in cognitively asymptomatic participants (50% a priori risk) of dysdiadochokinesia was 100% for the right and 87.5% for the left side. Mutation carriers with motor findings more frequently were basal ganglia amyloid-β positive (84% versus 63.3%; P = 0.006) and showed more basal ganglia amyloid-β deposition (Pittsburgh compound B-standardized uptake value ratio 2.472 versus 1.928; P = 0.002) than those without. Frequency and severity of motor findings were greater in post-codon 200 PSEN1 mutations (36%; mean UPDRS-III score 3.03) compared to mutations pre-codon 200 PSEN1 (19.3%, P = 0.022; 0.91, P = 0.013). In mutation carriers, motor symptom severity was significantly positively correlated with basal ganglia amyloid-β deposition, Clinical Dementia Rating scores and estimated years to symptom onset. Mutation carriers with a Clinical Dementia Rating global score of 2 exhibited more pronounced motor symptoms than sporadic Alzheimer’s disease patients with the same Clinical Dementia Rating global score (mean UPDRS-III scores 20.71 versus 5.96; P < 0.001). With a prevalence of approximately 30% and increasing severity with progression of dementia, motor symptoms are proven as a clinically relevant finding in autosomal dominant Alzheimer’s disease, in particular in advanced dementia stages, that correlates with deposition of amyloid-β in the basal ganglia. In a very small per cent of cognitively asymptomatic members of families with autosomal dominant Alzheimer’s disease, dysdiadochokinesia may increase the chance of an individual’s status as mutation carrier.
Introduction
Autosomal dominant Alzheimer’s disease (ADAD) is a monogenic neurodegenerative disease caused by pathogenic sequence variants in one of the three genes, PSEN1, PSEN2 or the gene encoding the amyloid precursor protein (Bateman et al., 2011). Compared to sporadic Alzheimer’s disease, the average age of clinical onset is earlier, at a mean of 45 years (Ryman et al., 2014; Masters et al., 2015). Because of its predictable course, ADAD serves as a model to explore Alzheimer’s disease pathophysiology (Schindler and Fagan, 2015). Studies in ADAD have led to crucial insights into the temporal sequence of pathological events that result in the clinical manifestation of Alzheimer’s disease (Bateman et al., 2012; Preische et al., 2019).
Beyond its typical cognitive manifestation, a subset of patients with ADAD display non-cognitive features such as parkinsonism, ataxia, or spasticity (Tang et al., 2016). In single cases, an association of motor findings in ADAD with the presence of amyloid-β plaques in the basal ganglia at autopsy has been reported, conceivably indicating a possible pathomechanism (Takao et al., 2002). In sporadic Alzheimer’s disease, motor dysfunction is present in a substantial portion of patients and increases with cognitive impairment (Portet et al., 2009). Motor impairment has been reported in early disease stages and may even precede cognitive decline in a small subset of patients (Albers et al., 2015).
Different mutation sites within the PSEN1 gene, i.e. a location before or after codon 200, were reported to impact clinical course, neurological and neuropsychological manifestations, neuropathological features, and the extent of MRI white matter hyperintensities in ADAD (Mann et al., 2001; Ryan and Rossor, 2010; Ryan et al., 2015; Ringman et al., 2016; Shea et al., 2016; Tang et al., 2016).
ADAD mutation carriers exhibit an increased burden of amyloid-β in the basal ganglia earlier than 10 years before expected symptom onset (Bateman et al., 2012). Therefore, we hypothesized that motor findings may play a significant role in ADAD. In particular with respect to the cognitively asymptomatic disease stage, currently there are few comprehensive clinical data on motor function in ADAD and potential neuropathological correlations. In addition, the interaction between specific mutation effects and motor function is also unknown. We used data from the Dominantly Inherited Alzheimer Network (DIAN) observational study (Morris et al., 2012) to fill this gap.
Materials and methods
Participants
To assess motor findings in ADAD we used data from the DIAN observational study gathered at 15 sites in the USA, Australia, UK, Germany and Argentina between January 2009 and December 2015. Four hundred and thirty-three participants, including 261 ADAD mutation carriers (PSEN1, PSEN2 and the gene encoding the amyloid precursor protein, APP) and 172 non-carriers were identified, the latter serving as a control group. In the DIAN observational study, examiners are blinded to the mutation status of the participants. Baseline visit data of all participants were used. Clinical and demographic data were collected using the Uniform Data Set version 2 from the National Alzheimer’s Coordinating Center (NACC) (Morris et al., 2006). The dataset analysed included comprehensive clinical, demographic, genetic, and imaging data.
To analyse motor findings in sporadic Alzheimer’s disease we used data from the NACC, gathered using the Uniform Data Set (Morris et al., 2006) between September 2005 and March 2015 at 36 Alzheimer’s disease centres. NACC data have been described in detail before (Beekly et al., 2004, 2007; Morris et al., 2006; Weintraub et al., 2009).
The protocol for the DIAN observational study has received approval by the institutional review boards of all participating sites. The DIAN observational study is performed in accordance with the Declaration of Helsinki and written informed consent was obtained from each participant. Research utilizing the NACC database was approved by the Institutional Review Board of the University of Washington. Informed consent from individuals that are part of the NACC dataset was obtained at the respective Alzheimer’s disease centres.
Motor assessment
The motor examination in part three of the Unified Parkinson Disease Rating Scale (UPDRS-III) (Fahn and Elton, 1987), being a part of Uniform Data Set version 2 from the NACC, was used. UPDRS-III comprises 14 items and its scale ranges from 0 to 108, where greater numbers indicate increasing impairment. UPDRS-III scores were assessed by trained clinicians at all participating sites of the DIAN observational study. All UPDRS-III raters were blinded to the mutation status of the participants. There was no blinding of UPDRS-III raters regarding the cognitive state of the participants.
For comparison of frequency of motor findings, mutation carriers and non-carriers were each divided into two groups: one with normal UPDRS-III results (0) and the other with suspicious values (>0), both for total scores as well as for each item separately. The positive predictive value, sensitivity and specificity regarding mutation carrier status of impaired rapid alternating hand movements in cognitively asymptomatic participants [defined by a Clinical Dementia Rating (CDR) global score of 0] were calculated. Mean UPDRS-III scores were compared between mutation carriers and non-carriers. In mutation carriers, we investigated correlations between UPDRS-III score and estimated years to symptom onset and CDR-Sum of Boxes (CDR-SB), respectively. CDR-SB is a global clinical cognitive assessment with a scale from 0 to 18 (none to severe impairment) (Morris et al., 1997). Stratified by global CDR scores, frequencies of UPDRS-III scores >0 and mean UPDRS-III scores were compared between cognitively symptomatic ADAD mutation carriers from the DIAN observational study and patients with a clinical diagnosis of Alzheimer’s disease from the NACC. Participants from the NACC with an indicated ADAD mutation in their family or an ADAD mutation found post-mortem examination were excluded from analyses. Individuals with a CDR global score = 3 were not analysed because of a very small number (n = 4) in the ADAD group from the DIAN cohort. Further, cognitively normal controls from the DIAN cohort (non-carrier with a CDR global score = 0) were compared to cognitively normal controls from the NACC cohort (individuals with a CDR global score = 0 that were additionally rated cognitively normal at baseline and all occurring follow-up visits).
Estimated years to symptom onset
Estimated years to symptom onset were calculated from the age of a participant at the time of the baseline visit minus his/her expected age of onset. Expected age of onset was determined using the mean onset of a respective mutation (deriving from combined data of the DIAN and prior publications) (Ryman et al., 2014) or, if unavailable, the age of onset of the participants’ affected family member. In symptomatic participants, the actual time of symptom onset was taken as the expected age of onset.
Amyloid-β imaging
Amyloid-β imaging was conducted after a bolus injection of about 15 mCi of Pittsburgh compound B (11C-PiB). Dynamic imaging acquisition began either at injection for 70 min or 40 min post-injection for 30 min. The data acquired between 40 to 70 min were used for further analysis. Each participant’s PiB-PET data underwent motion correction and were registered to his or her MRI using established procedures (Eisenstein et al., 2012). The standardized uptake value ratio (SUVR) was calculated with the cerebellum serving as the reference for each region of interest (defined by FreeSurfer) (Benzinger et al., 2013). The mean of the SUVRs of the caudate nucleus, of putamen, pallidum and the nucleus accumbens was calculated for each participant to obtain a mean basal ganglia SUVR. Amyloid-β positivity was defined as PiB-SUVR > 1.3 (Dominantly Inherited Alzheimer Network Imaging Core Methods and Definitions; version 1.1; 5 August 2015). The rates of amyloid-β positivity and the means of basal ganglia SUVRs were compared among mutation carriers (with and without motor findings, respectively). Correlation of UPDRS-III scores and basal ganglia SUVRs were analysed. PiB-PET data at baseline visits were available from 200 participants and had been acquired at the time of clinical assessment. PSEN1 and PSEN2 mutation carriers with dysdiadochokinesia were compared to those without dysdiadochokinesia regarding PiB SUVRs in the cerebellar cortex. Brainstem was used as the reference region.
Genetic analyses
To determine the presence or absence of an ADAD mutation and for characterization of apolipoprotein E (APOE) genotypes the respective exons were amplified by polymerase chain reaction, followed by Sanger sequencing (Bateman et al., 2012). Distributions of ADAD mutation types (PSEN1, PSEN2 or APP) and APOE genotypes were compared between mutation carriers with and without motor findings. PSEN1 mutations post-codon 200 were compared to those pre-codon 200 with respect to frequency and degree of motor findings, respectively. Four intronic PSEN1 mutations were excluded from the latter analysis because mutations in introns were not part of the first description of a clustering relative to PSEN1 codon 200 with respect to phenotypic features (Mann et al., 2001) and their effects on the protein structure substantially differ from and are less predictable than in exonic mutations (Vaz-Drago et al., 2017).
Statistical analysis
For statistical analysis the Statistical Package for the Social Sciences (IBM SPSS Statistics, Version 24) was used. Baseline clinical and demographic characteristics were analysed using Student’s t-tests and Fisher’s exact tests. To compare frequencies of motor findings, amyloid-β positivity, and distributions of genetic variants between groups, Fisher’s exact tests or Pearson’s chi-square tests were used. Benjamini-Hochberg procedure was performed to adjust for multiple testing with respect to 27 UPDRS-III subscale components. The positive predictive value, sensitivity and specificity were calculated using a 2D contingency table. For group comparisons with respect to mean UPDRS-III scores and basal ganglia PiB SUVRs Student’s t-tests or Mann-Whitney U-tests were performed. Distribution patterns were analysed with the Kolmogorov-Smirnov test. For correlation analyses, Spearman’s rank correlation coefficient was calculated and tested for statistical significance. P-values <0.05 were considered statistically significant. All tests were performed two-sided.
Data availability
The data that support the findings of this study are openly available from the Dominantly Inherited Alzheimer Network (DIAN) at https://dian.wustl.edu/our-research/observational-study/dian-observational-study-investigator-resources/data-request-form/ and the National Alzheimer’s Coordinating Center (NACC) at https://www.alz.washington.edu/NONMEMBER/QUERY/datareqnew.html.
Results
Participants
The dataset consisted of comprehensive data from 433 members of 107 ADAD families, with 261 (60.3%) carrying a mutation in PSEN1, PSEN2 or APP or a duplication of APP, respectively. One hundred and seventy-two individuals did not carry an ADAD mutation. One hundred and fifty-nine mutation carriers (60.9%) were cognitively asymptomatic (global CDR score = 0). Baseline clinical and demographic data are provided in Table 1.
Table 1.
Comparison of population characteristics between ADAD mutation carriers and non-carriers
| Mutation carriers (n = 261) | Non-carriers (n = 172) | Total (n = 433) | P-value | |
|---|---|---|---|---|
| Mean age, years | 39.3 | 39.6 | 39.4 | 0.789 |
| Females, n (%) | 146 (56) | 102 (59) | 248 (57) | 0.551 |
| Mean years of education | 14.1 | 14.6 | 14.3 | 0.145 |
| Mean EAO, years | 47.2 | N/A | N/A | N/A |
| Mean EYO | −7.9 | N/A | N/A | N/A |
| Mean global CDR score | 0.32 | 0.04 | 0.21 | <0.001 |
| Mean CDR-SB score | 1.55 | 0.07 | 0.96 | <0.001 |
| Participants with UPDRS-III score > 0, n (%) | 74 (28.4%) | 22 (12.8%) | 96 (22.2%) | <0.001 |
Bold indicates P-values <0.05. EAO = expected age of onset; EYO = estimated years to symptom onset; N/A = not applicable.
Additionally, the dataset included data from 1120 patients with a clinical diagnosis of sporadic Alzheimer’s disease, and 8185 cognitively normal controls from the NACC dataset (Table 4).
Table 4.
Comparison of motor symptoms between cognitively symptomatic mutation carriers for ADAD and patients with sporadic Alzheimer’s disease, stratified for CDR global scores, and between non-carriers controls from the DIAN cohort and controls from the NACC cohort
| CDR global score = 0.5 | |||
|---|---|---|---|
| ADAD (n = 65) | sAD (n = 1869) | P-value | |
| Mean UPDRS-III score | 2.15 | 2.32 | 0.76 |
| Participants with motor findings, n (%) | 28 (43.1) | 805 (43.1) | 1 |
| Mean age, years | 43.88 | 72.35 | <0.001 |
| CDR global score = 1 | |||
|---|---|---|---|
| ADAD (n = 26) | sAD (n = 947) | P-value | |
| Mean UPDRS-III score | 5.38 | 3.86 | 0.27 |
| Participants with motor findings, n (%) | 16 (61.5) | 488 (51.5) | 0.31 |
| Mean age, years | 46.96 | 72.19 | <0.001 |
| CDR global score = 2 | |||
|---|---|---|---|
| ADAD (n = 7) | sAD (n = 209) | P-value | |
| Mean UPDRS-III score | 20.71 | 5.96 | <0.001 |
| Participants with motor findings, n (%) | 5 (71.4) | 130 (62.2) | 0.71 |
| Mean age, years | 52.14 | 73.88 | <0.001 |
| Non-carrier controls (DIAN-OBS) (n = 159) | Controls (NACC) (n = 8185) | P-value | |
|---|---|---|---|
| Mean UPDRS-III score | 0.33 | 1.49 | <0.001 |
| Participants with motor findings, n (%) | 16 (10.1) | 2217 (27.1) | <0.001 |
| Mean age, years | 39.04 | 69.32 | <0.001 |
Controls from the DIAN cohort are non-carrier with a CDR global score = 0. Controls from the NACC cohort are individuals with a CDR global score = 0 that were additionally rated cognitively normal at baseline and all occurring follow-up visits.
sAD = sporadic Alzheimer’s Disease; DIAN-OBS = DIAN Observational Study.
Motor assessment
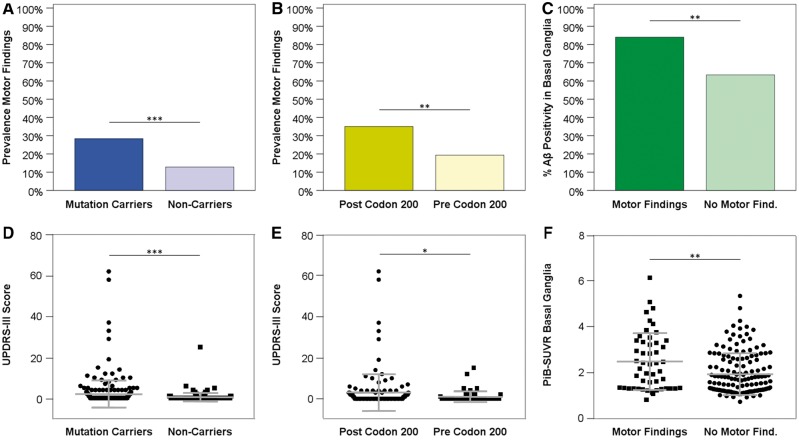
Motor findings, as illustrated in Fig. 1A, were present at a significantly higher frequency in mutation carriers (28.4% versus 12.8%; P < 0.001; with 74/261 mutation carriers and 22/172 non-carriers affected). Comparing each of the 27 UPDRS-III items between the carrier and non-carrier groups, we found 13 items statistically more frequently abnormal in mutation carriers of which seven remained statistically significantly different after correction for multiple testing. Scores >0 on assessing rigidity of the right lower extremity (7.3% versus 1.7%; P = 0.030), right and left hand finger taps (6.9% versus 0%; P < 0.001; 6.5% versus 1.2%; P = 0.025, respectively), right and left hand movements (5.7% versus 0%; P = 0.004; 6.1% versus 0.6%; P = 0.016, respectively), right and left hand rapid alternating movements (7.7% versus 0%; P < 0.001; 9.6% versus 0.6%; P < 0.001, respectively), right and left leg agility (4.6% versus 0%; P = 0.013; 5.0% versus 0.6%; P = 0.030), gait (4.2% versus 0%; P = 0.016), as well as posture stability (6.1% versus 1.2%; P = 0.030) (given P-values are adjusted for multiple comparisons) occurred significantly more often in mutation carriers as compared to non-carriers (Table 2). No UPDRS-III item was scored >0 more frequently in non-carriers than in carriers.
Figure 1.
Different aspects of motor findings in ADAD. Prevalence and degree of motor findings, as assessed by UPDRS-III, in ADAD mutation carriers compared to non-carriers (A and D) and in PSEN1 post-codon 200 mutation carriers compared to PSEN1 pre-codon 200 (B and E). Percentage of amyloid-β-positive basal ganglia, defined by a PiB-SUVR > 1.3, and mean PiB-SUVRs in the basal ganglia in mutations carriers with motor findings compared to those without (C and F). In D–F single data points are shown. Bars indicate medians and interquartile intervals. P-values: *P < 0.05; **P < 0.01; ***P < 0.001. Aβ = amyloid-β.
Table 2.
Prevalence of abnormality in each UPDRS-III item (i.e. item score > 0) in mutation carriers and non-carriers
| UPDRS-III items | Mutation carriers, % (n = 261) | Non-carriers, % (n = 172) | P-value |
|---|---|---|---|
| Speech | 4.2 | 1.2 | 0.129 |
| Facial expression | 5.4 | 1.7 | 0.128 |
| Tremor at rest | |||
| Face, lips, chin | 0.8 | 0.6 | 1 |
| Right hand | 0.8 | 0 | 0.585 |
| Left hand | 0.8 | 0 | 0.585 |
| Right foot | 0.4 | 0 | 1 |
| Left foot | 0 | 0 | 1 |
| Action or postural tremor of hands | |||
| Right hand | 7.3 | 2.9 | 0.101 |
| Left hand | 8.0 | 2.9 | 0.077 |
| Rigidity | |||
| Neck | 2.3 | 0 | 0.129 |
| Right upper extremity | 8.8 | 4.7 | 0.181 |
| Left upper extremity | 8.4 | 5.2 | 0.312 |
| Right lower extremity | 7.3 | 1.7 | 0.030 |
| Left lower extremity | 6.1 | 1.7 | 0.070 |
| Finger taps | |||
| Right hand | 6.9 | 0 | <0.001 |
| Left hand | 6.5 | 1.2 | 0.025 |
| Hand movements | |||
| Right hand | 5.7 | 0 | 0.004 |
| Left hand | 6.1 | 0.6 | 0.016 |
| Rapid alternating movements of hands | |||
| Right hand | 7.7 | 0 | <0.001 |
| Left hand | 9.6 | 0 | <0.001 |
| Leg agility | |||
| Right leg | 4.6 | 0 | 0.013 |
| Left leg | 5.0 | 0.6 | 0.030 |
| Arising from chair | 1.5 | 0 | 0.209 |
| Posture | 2.3 | 0.6 | 0.312 |
| Gait | 4.2 | 0 | 0.016 |
| Posture stability | 6.1 | 1.2 | 0.030 |
| Body bradykinesia and hypokinesia | 3.8 | 0.6 | 0.101 |
All P-values are derived from Fisher’s exact tests and are adjusted for 27 comparisons with Benjamini-Hochberg procedure. Bold indicates P-values <0.05.
Impaired rapid alternating hand movements (dysdiadochokinesia) occurred more often in cognitively asymptomatic mutation carriers (right: 6/159, 3.8%; left: 7/159, 4.4%) than in non-carriers (right: 0/172; 0%; left: 1/172, 0.6%) (adjusted P = 0.023 and 0.031, respectively). In cognitively asymptomatic mutation carriers with a value >0 in rapid alternating hand movements, they were scored ‘2’ (moderately impaired; definite and early fatiguing; may have occasional arrests in movement) or ‘1’ (mild slowing and/or reduction in amplitude) (Fahn and Elton, 1987), whereas the one non-carrier with a value >0 in this item was scored ‘1’ with respect to the left side. The positive predictive value of dysdiadochokinesia for presence of a pathogenic mutation in cognitively asymptomatic first-degree relatives of individuals with symptomatic ADAD was 100% for the right and 87.5% for the left side. While specificity was high (right: 100%; left: 99.4%), sensitivity was low (right: 3.8%; left: 4.4%). For both sides, the negative predictive value was 52.9%.
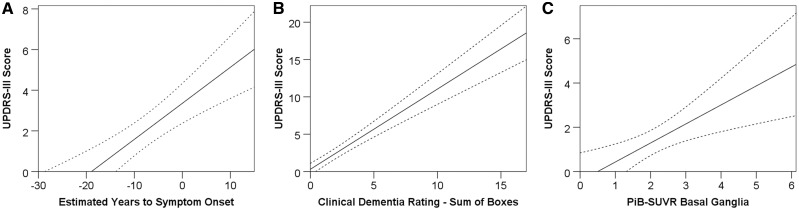
Overall motor findings were more pronounced in mutation carriers (mean UPDRS-III score 2.0) than in non-carriers (mean UPDRS-III score 0.4) (P < 0.001) (Fig. 1D). The extent of motor findings (UPDRS-III scores) in mutation carriers was positively correlated both with disease duration (rs = 0.409; P < 0.001), as estimated via estimated years to symptom onset (Fig. 2A), and with cognitive decline (rs = 0.420; P < 0.001) as assessed with CDR-SB (Fig. 2B). Frequencies of abnormal UPDRS-III values increased with global CDR scores (0: 14.5%; 0.5: 43.1%; ≥1: 62.2%) and with estimated years to symptom onset (−30 to −20: 2.8%; −20 to −10: 18.3%; −10 to 0: 26.1%; 0 to 10: 52.6%; 10 to 2: 75.0%) in mutation carriers.
Figure 2.
Correlations between UPDRS-III score estimated years to symptom onset, CDR-SB and PiB-SUVR. (A) Estimated years to symptom onset (rs = 0.409; P < 0.001), (B) CDR-SB (rs = 0.420; P < 0.001) and (C) the basal ganglia PiB-SUVR (rs = 0.233; P = 0.001) in ADAD mutation carriers. Dashed lines represent 95% confidence intervals.
Cognitively symptomatic ADAD mutation carriers with a CDR global score of 2 showed more pronounced motor symptoms than patients with sporadic Alzheimer’s disease with the same CDR global score (mean UPDRS-III scores 20.71 versus 5.96; P < 0.001). Frequencies of abnormal UPDRS-III scores were 71.4% for ADAD mutations carriers and 62.2% for sporadic Alzheimer’s disease patients in the CDR global score = 2 group (P = 0.71). Frequencies of abnormal UPDRS-III scores and mean UPDRS-III scores were 43.1% versus 43.1% (P = 1) and 2.15 versus 2.32 (P = 0.76) in the group with global CDR scores of 0.5, and 61.5 versus 51.5 (P = 0.31) and 5.38 versus 3.86 (P = 0.27) in the group with global CDR scores of 1 (Table 4).
Cognitively normal controls from the NACC database were significantly older, and a higher percentage of individuals showed abnormal UPDRS-III scores as well as had higher mean UPDRS-III scores compared to cognitively normal non-carrier controls from the DIAN cohort (69.32 years versus 39.04 years, P < 0.001; 27.1% versus 10.1%, P < 0.001; 1.49 versus 0.33, P < 0.001) (Table 4).
Amyloid-β imaging
Eighty-four per cent of the mutation carriers with motor findings that had undergone PiB-PET were amyloid-β positive in the basal ganglia (42 of 50), in contrast to 63.3% (95/150) of mutation carriers without motor findings (P = 0.006) (Fig. 1C). Mean basal ganglia PiB-SUVR was significantly higher in carriers with motor findings as opposed to those without (2.472 and 1.928, respectively, P = 0.002) (Fig. 1F). Overall motor dysfunction as assessed by UPDRS-III scores was positively correlated with basal ganglia amyloid-β burden (rs = 0.233; P = 0.001) (Fig. 2C).
All analyses that included basal ganglia amyloid burden measured by PiB-PET were repeated using the brainstem as the reference region. All results were consistent with the results of the analyses that used the cerebellar reference. Details are shown in Supplementary Table 1.
There was no statistically significant difference between PSEN1 and PSEN2 mutation carriers with dysdiachokinesia (n = 15) and those without (n = 154) regarding cerebellar cortex PiB-SUVRs (0.59 versus 0.56; P = 0.23).
Genetic analyses
Among the 261 mutation carriers, 197 carried PSEN1 (75.5%), 20 PSEN2 (7.7%) and 44 mutations or duplications in or of APP (16.9%). No significant differences regarding the distribution of the three affected ADAD genes between mutation carriers with and without motor findings were found (P = 0.259). Neither did distribution of APOE genotypes differ between the groups (P = 0.554). Carriers of PSEN1 mutations that were localized after codon 200 more commonly showed motor findings that were also more pronounced (36%; mean UPDRS-III score 3.03) (Fig. 1B and E) in comparison to participants with PSEN1 mutations before codon 200 (19.3%, P = 0.022; mean UPDRS-III score 0.91, P = 0.013) (Table 3).
Table 3.
Extent of motor symptoms in mutation carriers of ADAD, analysed separately regarding affected gene (i.e. PSEN1, PSEN2 or APP), mutation site within PSEN1, and APOE genotype
| ADAD mutation | P-value | |||
|---|---|---|---|---|
| PSEN1 | PSEN2 | APP | ||
| Participants with motor findings, n (%) | 61 (31) | 4 (20) | 9 (20.5) | 0.259 |
| Total participant number, n | 197 | 20 | 44 | N/A |
| Mutation site | P-value | ||
|---|---|---|---|
| PSEN1 post-codon 200 | PSEN1 pre-codon 200 | ||
| Participants with motor findings, n (%) | 49 (36) | 11 (19.3) | 0.022 |
| Different mutations in participants with motor findings, n | 19 | 10 | N/A |
| Total participant number, n | 136 | 57 | N/A |
| Mean UPDRS-III score | 3.03 | 0.91 | 0.013 |
| Mean EYO | −5.9 | −8.7 | 0.090 |
| APOE genotype | P-value | ||||||
|---|---|---|---|---|---|---|---|
| e2e2 | e2e3 | e2e4 | e3e3 | e3e4 | e4/e4 | ||
| Participants with motor findings, n (%) | 0 (0) | 5 (19.2) | 2 (28.6) | 47 (29.9) | 16 (26.7) | 4 (50) | 0.554 |
| Total participant number, n | 2 | 26 | 7 | 157 | 60 | 8 | N/A |
Percentages in brackets refer to affected gene, mutation site or APOE genotype, respectively. The APOE genotype was not available in one mutation carrier. Bold indicates P-values <0.05. EYO = estimated years to symptom onset; N/A = not applicable.
Discussion
In the DIAN observational study, motor signs were found to be present in ∼30% of ADAD mutation carriers, with their severity increasing as the disease progresses (Figs 1A and 2A). Motor function was abnormal in nearly a fifth of mutation carriers between estimated years to symptom onset −20 and −10, and in more than half of those between estimated years to symptom onset 0 and 10. As reflected by the mean age of mutation carriers of around 39 years, the study subjects were young in comparison to cohorts with sporadic Alzheimer’s disease. Hence, this population is more unlikely to have relevant comorbidities that might contribute to the occurrence of motor findings. Our analysis therefore may indicate that early motor findings, before the onset of cognitive symptoms, could be a distinct feature of ADAD in a very small subset of individuals. The early occurrence of motor symptoms in this small subgroup could possibly relate to the early basal ganglia pattern of amyloid-β in ADAD that is not typically seen in sporadic Alzheimer’s disease (Bateman et al., 2012; Benzinger et al., 2013; Villemagne et al., 2013; McDade et al., 2014; Fleisher et al., 2015). Motor signs in ADAD can be assessed and scored using the UPDRS, which has great strengths in reliability and validity (Goetz et al., 2003), because of precisely defined subscale components (Fahn and Elton, 1987). Hereby even slight differences in UPDRS scores are distinguishable for trained clinicians.
UPDRS-III allows measurement of a range of distinct motor phenotypes. Compared to non-carriers, ADAD mutation carriers showed motor abnormalities in 41% (11/27) of the UPDRS-III items. Interestingly, the majority (91%) of the abnormalities were found in subscale components that focus on the detection of bradykinesia, not of tremor or rigidity (Table 2). This suggests that motor symptoms in ADAD primarily manifest with a bradykinetic profile.
With an UPDRS-III score of 2 on average, motor symptoms were rather mildly pronounced in ADAD mutations carriers. This is also reflected by only one mutation carrier with motor findings who was treated with levodopa at the time of his baseline visit. However, 61% of the studied mutation carriers were cognitively asymptomatic, with a mean estimated years to symptom onset of approximately −8.
Our suggestion of motor symptoms as a distinct feature of ADAD is consistent with associations between the presence, respectively the amount of fibrillar amyloid-β in the basal ganglia and the manifestation of motor findings in mutation carriers (Figs 1C, F and 2C). This association of ADAD pathology with motor symptoms, which can be caused by basal ganglia dysfunction (Nelson and Kreitzer, 2014), accords with the concept that the anatomical distribution of pathology determines the clinical phenotype (Weintraub and Mesulam, 2009).
The significant increase of the prevalence of motor signs reaching almost 20% between estimated years to symptom onset −20 and −10, compared to a proportion of ∼3% between estimated years to symptom onset −30 and −20, also complies with a potential association between amyloid-β pathology and motor symptoms in ADAD, as it coincides with the proposed starting point of amyloid-β accumulation in the timeline of ADAD (Bateman et al., 2012). However, motor symptoms were solely more pronounced in ADAD than in sporadic Alzheimer’s disease at the stage of moderately severe dementia, and not at earlier stages.
Other conditions with different neuroanatomical substrates such as cerebellar pathologies, corticospinal dysfunction or cognitive dysfunction, i.e. apraxia, may influence motor function as measured by UPDRS-III. Therefore, the results of our study do not warrant a link of motor dysfunction specifically to amyloid-β in the basal ganglia. Regarding cerebellar amyloid-β deposition, no difference between PSEN1 and PSEN2 mutation carriers with and without dysdiadochokinesia was found.
Potential basic premises for the association of subcortical amyloid-β with basal ganglia symptoms include a directly induced neuronal dysfunction, as well as a mediation of regional neurodegeneration through tau pathology (Nelson et al., 2012; Shinohara et al., 2014). Further, a potential impact of Lewy body pathology, which is frequently present in ADAD (Lippa et al., 1998; Leverenz et al., 2006; Cairns et al., 2015; Ringman et al., 2016), on the manifestation of motor symptoms has to be considered (Chung et al., 2015). To investigate the conceivable influence of these and other non-amyloid-β pathologies on motor function in ADAD tau imaging and clinicopathological correlation studies are required in the future.
In the context of the various current and ongoing observational and treatment trials, in particular those with a focus on very early Alzheimer’s disease stages (Bateman et al., 2012, 2017) as well as in terms of clinical diagnosis and care of Alzheimer’s disease, early and easy to assess clinical signs could become important for the identification of individuals in initial disease stages. Dysdiadochokinesia appears to be such an indicator and can be rapidly evaluated in clinical routine settings. In distinction from seizures, which we have also shown to be an early feature of ADAD in a subset of individuals and a predictor of mutation status in persons at risk for ADAD (Vöglein et al., 2019), dysdiadochokinesia is independent from the individual’s history but is assessed in a standardized manner, also to be re-evaluated as deemed necessary. However, given that only a small percentage (<5%) manifest this symptom, its general utility is clearly limited.
In our investigation of effects of mutation position in PSEN1, we concur with Mann et al. (2001) who first described a mutation clustering within the gene in relation to distinct neuropathological findings in the frontal cortex and cerebellum of PSEN1 mutation carriers. The first cluster, comprising mutations that affect codons 1 to 200, was associated with an amyloid plaque profile similar to sporadic Alzheimer’s disease. The second mutation cluster, after PSEN1 codon 200, was associated with severe cerebral amyloid angiopathy (Mann et al., 2001). This finding was subsequently corroborated (Ryan et al., 2015; Ringman et al., 2016). More extensive cerebral amyloid angiopathy could contribute to the greater extent of motor findings that we found in PSEN1 post-codon 200 mutation carriers. This is of particular interest in the light of a marginally higher burden of cerebellar amyloid angiopathy in PSEN1 post-codon 200 mutation carriers compared to pre-codon 200 mutations (Ryan et al., 2015). Findings of an increased amount of MRI white matter hyperintensities, more severe neurofibrillary pathology and an increased likelihood for ischaemic, haemorrhagic, or vascular pathology in PSEN1 post-codon 200 mutation carriers (Ryan et al., 2015; Ringman et al., 2016) might also account for the more pronounced motor signs that we found in this subpopulation.
Regarding clinical manifestation, PSEN1 mutations after codon 200 were reported to be more frequently associated with spasticity, spastic paraparesis and visuospatial impairment, whereas mutations before codon 200 more frequently with seizures and myoclonus (Shea et al., 2016; Tang et al., 2016). Broadening the clinical characterization of PSEN1 mutation carriers and adding to the evidence that their exact mutation site influences the clinical phenotype, we found motor symptoms more common and even more severe with PSEN1 mutations after codon 200 (Fig. 1B and E). There have been interpretations regarding the impact of the mutation site in PSEN1 with respect to codon 200 on neuropathological and clinical manifestations of ADAD (Mann et al., 2001; Ryan and Rossor, 2010). However, the underlying mechanisms remain unclear and deserve further study.
Our results indicate that ADAD patients with a CDR global score of 2 show more pronounced motor findings than sporadic Alzheimer’s disease patients with the same CDR global score. Prevalence and degree of motor symptoms did not differ between ADAD and sporadic Alzheimer’s disease patients with global CDR scores of 0.5 and 1, respectively. This indicates that progressing dementia is the most significant factor that leads to more severe motor symptoms. Additionally, these findings might be in accordance with the delay of up to 20 years between deposition of amyloid-β and manifestation of symptoms that is already known for cortical amyloid deposition and cognitive impairment in ADAD and sporadic Alzheimer’s disease (Mintun et al., 2006; Bateman et al., 2012). In ADAD, accumulation of amyloid-β in the basal ganglia is more pronounced at early disease stages than in sporadic Alzheimer’s disease (Bateman et al., 2012). Therefore, subsequent motor symptoms may occur at the stage of moderately severe dementia in ADAD, while patients with sporadic Alzheimer’s disease may manifest motor symptoms at the stage of severe dementia, if at all in their lifetime. Hence, the findings of this study would be in accordance with a common, while yet unknown, mechanism of substantially delayed functional impairment by amyloid-β in cortex and basal ganglia. Of note, a limitation could be that clinical assessment could be more challenging at the stage of severe dementia.
Cognitively symptomatic mutation carriers from the DIAN observational study, on average ∼ 47 years old, were equally affected by motor symptoms (at CDR global score 0.5 and 1) or worse (at CDR global score 2) compared to patients with sporadic Alzheimer’s disease from the NACC database who were on average ∼72 years old, while normal controls from the NACC database (mean age 70 years) exhibited more pronounced motor symptoms than non-carriers from the DIAN cohort (mean age 40 years). This could be explained in two different ways. First, symptomatic mutations carriers develop more pronounced motor symptoms if age is factored out. Second, because motor symptoms are usually rare in healthy controls who are at an age similar to the mean age of mutation carriers studied here, motor symptoms could be recognized as an irregular symptom of ADAD at a young age. Therefore, an alternative interpretation may be that it could be the early age of manifestation but not the early phase of ADAD that is associated with the increase notion of motor symptoms.
Motor symptoms affect a relevant proportion of ADAD mutation carriers (Table 1) as well as of patients with sporadic Alzheimer’s disease and worsen along with progression of cognitive impairment in Alzheimer’s disease. In particular, ADAD and Alzheimer’s disease patients at the stage of moderately severe dementia are affected by motor symptoms (Fig. 2 and Table 4) (Albers et al., 2015). Identification of motor dysfunction is relevant for clinical care and for patient and family/caregiver interaction, as it is associated with disability (Murray et al., 2004) and predictive of Alzheimer’s disease mortality (Bennett et al., 1998; Zhou et al., 2010).
In summary, our study describes motor symptoms in ADAD that are associated with disease stage and cognitive symptoms, particularly affecting patients in advanced dementia stages. In a very small percentage of cognitively asymptomatic individuals, motor signs can predict mutation carrier status. Further, the prevalence of motor findings is increased in PSEN1 mutations after codon 200.
Motor assessment is therefore proposed as an integral component in the clinical work-up of individuals from ADAD families. Evaluation of motor function should be considered to be comprehensively included in current and future observational and therapeutic trials of ADAD.
Supplementary Material
Acknowledgements
This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications. We acknowledge the altruism of the participants and their families and contributions of the DIAN research and support staff at each of the participating sites for their contributions to this study. The authors thank Ingrid Ricard (Institute for Medical Informatics, Biometry & Epidemiology, Ludwig-Maximilians-Universität München, Munich, Germany) for statistical consultation.
Glossary
Abbreviations
- ADAD
autosomal dominant Alzheimer’s disease
- CDR
Clinical Dementia Rating Scale
- CDR-SB
Clinical Dementia Rating Scale – Sum of Boxes
- DIAN
Dominantly Inherited Alzheimer Network
- NACC
National Alzheimer’s Coordination Center
- SUVR
standardized uptake value ratio
- UPDRS-III
Unified Parkinson Disease Rating Scale part three
Funding
This project was supported by The Dominantly Inherited Alzheimer’s Network (DIAN, UF1 AG032438) funded by the National Institute on Aging (NIA), the German Center for Neurodegenerative Diseases (DZNE), the NIHR Queen Square Dementia Biomedical Research Centre and the MRC Dementias Platform UK (MR/L023784/1 and MR/009076/1), and AMED under Grant Number JP17dk0207036 and JP17kk0205009. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) within the framework of the Munich Cluster for Systems Neurology (EXC 1010 SyNergy), the European Research Council under the European Union’s Seventh Framework Program (FP7/2007–2013)/ERC Grant Agreement No. 321366-Amyloid, the general legacy of Mrs. Ammer, the MetLife award, and the Cure Alzheimer’s fund. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). G.H was funded by the Deutsche Forschungsgemeinschaft (DFG, HO2402/18–1, Munich Cluster for Systems Neurology SyNergy), the German Federal Ministry of Education and Research (BMBF, 01EK1605A HitTau), the NOMIS foundation (FTLD project).
Competing interests
A.G. served on SAB at Denali Therapeutics from 2015–2018. She has also served as a consultant for Biogen, Eisai, Pfizer, GSK, AbbVie and Cognition Therapeutics. C.H. collaborates with Denali and received a speaker honorarium of Roche and Novartis. J.L. reports consulting fees from Aesku, speakers fees from Bayer Vital, speakers fees from Willi Gross Foundation, consulting fees from Axon Neuroscience, consulting fees from Ionis Pharmaceuticals, non-financial support from Abbvie, outside the submitted work. All other authors report no competing interests.
References
- Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement 2015; 11: 70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther 2011; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM et al. The DIAN-TU next generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement 2017; 13: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012; 367: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord 2007; 21: 249–58. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME et al. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004; 18: 270–7. [PubMed] [Google Scholar]
- Bennett DA, Beckett LA, Wilson RS, Murray AM, Evans DA. Parkinsonian signs and mortality from Alzheimer’s disease. Lancet 1998; 351: 1631. [DOI] [PubMed] [Google Scholar]
- Benzinger TL, Blazey T, Jack CR Jr, Koeppe RA, Su Y, Xiong C et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci USA 2013; 110: E4502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, Perrin RJ, Franklin EE, Carter D, Vincent B, Xie M et al. Neuropathologic assessment of participants in two multi-center longitudinal observational studies: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). Neuropathology 2015; 35: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EJ, Babulal GM, Monsell SE, Cairns NJ, Roe CM, Morris JC. Clinical features of Alzheimer disease with and without lewy bodies. JAMA Neurol 2015; 72: 789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Koller JM, Piccirillo M, Kim A, Antenor-Dorsey JA, Videen TO et al. Characterization of extrastriatal D2 in vivo specific binding of [(1)(8)F](N-methyl)benperidol using PET. Synapse 2012; 66: 770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton R. Unified Parkinson’s disease rating scale. Florham Park, NJ: Macmillan Healthcare Information, 1987. [Google Scholar]
- Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gutierrez Gomez M, Langois CM et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol 2015; 72: 316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Goetz CG, Goetz CG, Goetz CG. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003; 18: 738–50. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Fishel MA, Peskind ER, Montine TJ, Nochlin D, Steinbart E et al. Lewy body pathology in familial Alzheimer disease: evidence for disease- and mutation-specific pathologic phenotype. Arch Neurol 2006; 63: 370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 1998; 153: 1365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Pickering-Brown SM, Takeuchi A, Iwatsubo T. Amyloid angiopathy and variability in amyloid beta deposition is determined by mutation position in presenilin-1-linked Alzheimer’s disease. Am J Pathol 2001; 158: 2165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers 2015; 1: 15056. [DOI] [PubMed] [Google Scholar]
- McDade E, Kim A, James J, Sheu LK, Kuan DC-H, Minhas D et al. Cerebral perfusion alterations and cerebral amyloid in autosomal dominant Alzheimer disease. Neurology 2014; 83: 710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006; 67: 446–52. [DOI] [PubMed] [Google Scholar]
- Morris JC, Aisen PS, Bateman RJ, Benzinger TL, Cairns NJ, Fagan AM et al. Developing an international network for Alzheimer research: the Dominantly Inherited Alzheimer Network. Clin Investig 2012; 2: 975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology 1997; 48: 1508–10. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006; 20: 210–6. [DOI] [PubMed] [Google Scholar]
- Murray AM, Bennett DA, Mendes de Leon CF, Beckett LA, Evans DA. A longitudinal study of parkinsonism and disability in a community population of older people. J Gerontol A Biol Sci Med Sci 2004; 59: 864–70. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012; 71: 362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Kreitzer AC. Reassessing models of Basal Ganglia function and dysfunction. Annu Rev Neurosci 2014; 37: 117–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portet F, Scarmeas N, Cosentino S, Helzner EP, Stern Y. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol 2009; 66: 1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med 2019; 25: 277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Monsell S, Ng DW, Zhou Y, Nguyen A, Coppola G et al. Neuropathology of autosomal dominant Alzheimer disease in the National Alzheimer Coordinating Center Database. J Neuropathol Exp Neurol 2016; 75: 284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NS, Biessels GJ, Kim L, Nicholas JM, Barber PA, Walsh P et al. Genetic determinants of white matter hyperintensities and amyloid angiopathy in familial Alzheimer’s disease. Neurobiol Aging 2015; 36: 3140–51. [DOI] [PubMed] [Google Scholar]
- Ryan NS, Rossor MN. Correlating familial Alzheimer’s disease gene mutations with clinical phenotype. Biomark Med 2010; 4: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology 2014; 83: 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SE, Fagan AM. Autosomal dominant Alzheimer disease: a unique resource to study CSF biomarker changes in preclinical AD. Front Neurol 2015; 6: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea YF, Chu LW, Chan AO, Ha J, Li Y, Song YQ. A systematic review of familial Alzheimer’s disease: differences in presentation of clinical features among three mutated genes and potential ethnic differences. J Formos Med Assoc 2016; 115: 67–75. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Fujioka S, Murray ME, Wojtas A, Baker M, Rovelet-Lecrux A et al. Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer’s disease. Brain 2014; 137 (Pt 5): 1533–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao M, Ghetti B, Hayakawa I, Ikeda E, Fukuuchi Y, Miravalle L et al. A novel mutation (G217D) in the Presenilin 1 gene (PSEN1) in a Japanese family: presenile dementia and parkinsonism are associated with cotton wool plaques in the cortex and striatum. Acta Neuropathol 2002; 104: 155–70. [DOI] [PubMed] [Google Scholar]
- Tang M, Ryman DC, McDade E, Jasielec MS, Buckles VD, Cairns NJ et al. Neurological manifestations of autosomal dominant familial Alzheimer’s disease: a comparison of the published literature with the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS). Lancet Neurol 2016; 15: 1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz-Drago R, Custodio N, Carmo-Fonseca M. Deep intronic mutations and human disease. Human Genet 2017; 136: 1093–111. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 2013; 12: 357–67. [DOI] [PubMed] [Google Scholar]
- Vöglein J, Noachtar S, McDade E, Quaid KA, Salloway S, Ghetti B et al. Seizures as an early symptom of autosomal dominant Alzheimer’s disease. Neurobiol Aging 2019; 76: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Mesulam M. With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain 2009; 132 (Pt 11): 2906–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the Neuropsychological Test Battery. Alzheimer Dis Assoc Disord 2009; 23: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Duan L, Sun F, Yan B, Ren S. Association between mild parkinsonian signs and mortality in an elderly male cohort in China. J Clin Neurosci 2010; 17: 173–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available from the Dominantly Inherited Alzheimer Network (DIAN) at https://dian.wustl.edu/our-research/observational-study/dian-observational-study-investigator-resources/data-request-form/ and the National Alzheimer’s Coordinating Center (NACC) at https://www.alz.washington.edu/NONMEMBER/QUERY/datareqnew.html.