Abstract
Background
Infective endocarditis (IE) remains a severe disease with a high mortality rate. Therefore, guidelines encourage the setup of a multidisciplinary group in reference centers. The present study evaluated the impact of this “Endocarditis Team” (ET).
Methods
We conducted a monocentric observational study at Strasbourg University Hospital, Strasbourg, France, between 2012 and 2017. The primary end point was in-hospital mortality. Secondary end points were 6-month and 1-year mortality, surgery rate, time to surgical procedure, duration of effective antibiotic therapy, length of in-hospital stay, and sequelae. We also assessed predictors of in-hospital mortality.
Results
We analyzed 391 episodes of IE. In the post-ET period, there was a nonsignificant decrease in in-hospital mortality (20.3% vs 14.7%, respectively; P = .27) and sequelae, along with a significant reduction in time to surgery (16.4 vs 10.3 days, respectively; P = .049), duration of antibiotic therapy (55.2 vs 47.2 days, respectively; P < .001), and length of in-hospital stay (40.6 vs 31.9 days, respectively; P < .01). In a multivariate analysis, the post-ET period was positively associated with survival (odds ratio, 0.45; 95% confidence interval, 0.20–0.96; P = .048).
Conclusions
This multidisciplinary approach exerted a positive impact on the management of IE and should be considered in all hospitals managing IE.
Keywords: infective endocarditis, multidisciplinary management, Endocarditis Team, cardiac surgery, guidelines, valve disease, prognosis
The implementation of an 'Endocarditis Team' is recommended in reference centres for infective endocarditis. This study evaluated its impact and showed improvements on the management of this disease with a decrease in time to surgery and nonsignificant reduction in mortality.
The epidemiology of infective endocarditis (IE) has changed in recent decades [1, 2]. Despite the constant evolution of antibiotic regimens, the prognosis of IE remains poor, with in-hospital mortality ranging from 15% to 22% [3, 4]. Although surgery is required for approximately half of the patients, its indications and timing in certain situations are unclear [5, 6]. Furthermore, diagnosis may be challenging, especially in cases with prosthetic valve endocarditis.
Considering the complexity of this disease and the involvement of various medical and surgical specialties, the guidelines established in 2015 by the American Heart Association (AHA) [7] and the European Society of Cardiology (ESC) [8] recommend the setup of an “Endocarditis Team” (ET) in every reference center. This approach has been implemented for several years in numerous fields (eg, oncology) but remained to be proposed in such a complex disease.
An ET was set up at Strasbourg University Hospital, Strasbourg, France, in December 2016, after the publication of the ESC and AHA guidelines. This ET meets systematically on a weekly basis. It is led by cardiac surgeons and brings together cardiac surgeons, cardiologists, echocardiographers, and infectious disease specialists to discuss the diagnosis and management of all cases of endocarditis managed in this institution. Strasbourg University Hospital is a reference center for this pathology, managing approximately 70 cases per year.
The objective of the study was to determine the impact of this new approach on the management of endocarditis—in terms of mortality—by performing a before-and-after analysis. The secondary objectives were to examine the impact on the management of these patients (surgery rates, time to surgery, duration of antibiotic therapy and in-hospital stay) on their evolution (6-month and 1-year mortality rates, cardiac and neurologic sequelae) and to identify predictors of mortality.
METHODS
Study Design
This descriptive study was divided into 2 periods. The data were prospectively collected from January to December 2017, after establishing an ET that convened on a weekly basis to discuss all definite IE cases. The data were retrospectively compared with those from IE cases recorded in the previous 5 years. Since 2012, all IE data have been entered in a centralized database to improve disease management at Strasbourg University Hospital. This database—termed “Registre des Endocardites Infectieuses” (RENDI)—collects medical and paramedical information and therapeutic decisions for all patients with a final diagnosis of IE. All data from the study period were extracted from this database. To ensure that all IE cases from the retrospective period were included in the study, we investigated all endocarditis diagnoses recorded during that period in the Medicalization Program of the Information Systems (PMSI) register.
All patients diagnosed with definite IE according to the modified Duke criteria were included in this analysis. Patients aged <18 years and those with ventricular assist devices were excluded. Patients with multiple episodes of IE during the study period were eligible for re-enrollment in the study.
The primary end point of the study was to determine the impact of the ET on the rate of all-cause in-hospital mortality. The secondary end point was to evaluate the effect of the ET on 6-month and 1-year mortality, rate of surgery, time to surgery, duration of antibiotic treatment, length of in-hospital stay, and occurrence of cardiac or neurological sequelae. Moreover, we assessed predictors of in-hospital mortality. Significant variables determined in the univariate analysis were included in a multivariate model.
Definitions
The day of definite diagnosis of IE was defined as the first day when “definite diagnosis” according to the modified Duke criteria was met. Time to surgery was defined as the time from the day of definite diagnosis of IE to the day of the surgical procedure. Late surgery (ie, surgery after the end of antibiotic therapy) was excluded from this definition. Length of in-hospital stay was defined as the time from the day of the definite diagnosis of IE to the day of hospital discharge. Cardiac sequelae were defined as the persistence of heart failure symptoms (dyspnea, edema, or jugular vein distension) and/or left ventricular ejection fraction <50% after the end of treatment. Neurological sequelae were defined as new neurological deficits (motor, sensory, or cognitive) after the end of treatment that were not present before the development of IE. In accordance with the Sepsis-3 definition, septic shock denoted the requirement for a vasopressor to maintain a mean arterial pressure of ≥65 mmHg and serum lactate level >2 mmol/L [9].
Statistical Analysis
Statistical analysis included a descriptive part and an inferential part. The descriptive analysis of continuous data was performed by obtaining the location parameters (ie, mean, median, minimum, maximum, first and third quartiles) and the dispersion parameters (ie, variance, standard deviation, range, interquartile range) for each variable. The Gaussian distribution of the data was tested using the Shapiro-Wilk test and quantile–quantile plots. The description of the categorical data was performed by obtaining the numbers and proportions for each category in the sample. Whenever useful, cross-tabulations were produced for each box of the table, which included numbers and proportions by row, column, and relative to the total. The inferential analysis for the categorical data was performed using the χ 2 test or Fisher exact test, depending on the theoretical size of the samples. Continuous data were compared using the Student t test (Gaussian distribution of the variable) or a nonparametric test in the opposite case (Mann-Whitney U test). Significant variables in the univariate analysis were included in the multivariate analysis. A stepwise regression method based on Akaike’s information criterion minimization was used after multiple imputation of variables with missing data. The analyses were performed using R software, version 3.4.3, and other software packages.
Ethical Statements
This study was approved by the Ethics Committee of Strasbourg University Hospital (registration number: FC/dossier 2017–36) and was registered under ClinicalTrials.gov (NCT03429153). According to French legislation (Jardé law, No. 2016–800), the nonopposition of all patients was sought.
RESULTS
Study Population
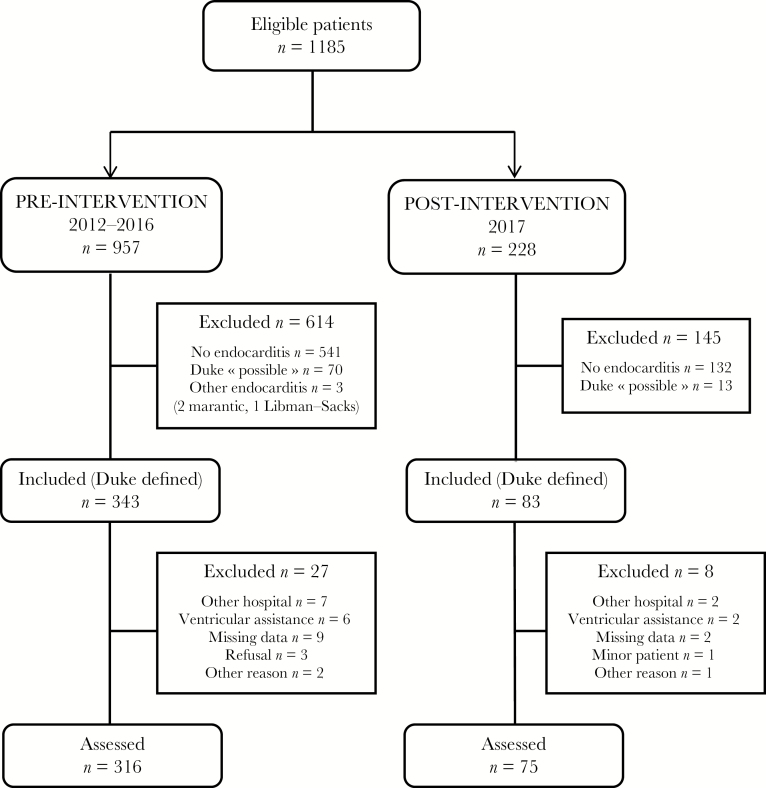
The investigation of the RENDI and PMSI databases yielded 1185 eligible patients, of whom 426 patients fulfilled the Duke criteria of definite IE. A total of 35 patients (including 8 with ventricular assist devices and 9 treated in another hospital) were excluded. A total of 391 episodes of IE (ie, 316 in the pre-ET period and 75 in the post-ET period) occurred in 369 patients (Figure 1).
Figure 1.
Study flowchart.
The characteristics of patients are provided in Tables 1 and 2. The mean age was 65.4 ± 15.7 years, and 70% of patients were male. Most cases exhibited aortic valve involvement (222 patients; 57%), followed by mitral involvement (139 patients; 36%). Development of IE in prosthetic material was reported in 154 patients (39%). A total of 79 patients (20%) and 32 (8%) developed bioprosthetic and mechanical valve IE, respectively. IE after transcatheter aortic valve replacement occurred in only 6 patients (2%). Intracardiac device IE occurred in 43 patients (11%), including 29 patients (7%) without valve involvement, 8 patients (2%) with native valve IE, and 6 patients (2%) with prosthetic valve IE. Staphylococci were the most frequently identified pathogens (38%) (including 115 Staphylococcus aureus patients [29%] and 35 coagulase-negative staphylococci patients [9%]), followed by streptococci (32%) and enterococci (12%). Heart failure was the most common complication of IE, occurring in 213 patients (54%), whereas septic shock was less frequent (67 patients; 17%). Approximately half of the patients exhibited embolism detected through brain imaging (135 patients; 47%). Most of these cases were asymptomatic, with only 57 patients (15%) presenting clinical signs of stroke.
Table 1.
Demographic and Clinical Characteristics and Biological Findings at Admission of the 391 Episodes of IE
| Patient Characteristics | All Patients | Pre-ET (n = 316) | Post-ET (n = 75) | P |
|---|---|---|---|---|
| Female sex | 116 (29.7) | 94 (29.7) | 22 (29.3) | .94 |
| Age, y | 65.4 ± 15.7 | 65.0 ± 15.9 | 67.1 ± 14.6 | .31 |
| Body mass index, kg/m2 | 26.1 ± 5.8 | 26.2 ± 5.7 | 25.7 ± 5.9 | .52 |
| History and risk factors | ||||
| Hypertension | 232 (59.3) | 186 (58.9) | 46 (61.3) | .69 |
| Diabetes mellitus | 110 (28.1) | 84 (26.6) | 26 (34.7) | .16 |
| Current or former smoker | 193 (49.4) | 158 (50.0) | 35 (46.7) | .60 |
| Chronic renal failure | 66 (16.9) | 49 (15.5) | 17 (22.7) | .14 |
| Hemodialysis | 12 (3.1) | 7 (2.2) | 5 (6.7) | .06 |
| Intravenous drug user | 38 (9.7) | 33 (10.4) | 5 (6.7) | .32 |
| Stroke | 37 (9.5) | 30 (9.5) | 7 (9.3) | .97 |
| Immunosuppression | 68 (18.2) | 54 (18.1) | 14 (18.7) | .91 |
| Cancer (incl. hematological malignancy) | 85 (21.7) | 64 (20.3) | 21 (28.0) | .14 |
| Heart disease | 167 (42.7) | 134 (42.4) | 33 (44.0) | .80 |
| Coronary heart disease | 84 (21.5) | 64 (20.3) | 20 (26.7) | .22 |
| Previous IE | 46 (11.8) | 38 (12.0) | 8 (10.7) | .74 |
| Heart surgery | 144 (36.8) | 124 (39.2) | 20 (26.7) | .04 |
| Intracardiac device (PM or ICD) | 71 (18.2) | 59 (18.7) | 12 (16.0) | .59 |
| Clinical findings | ||||
| Fever | 359 (94.0) | 287 (93.5) | 72 (96.0) | .59 |
| Septic shock | 67 (17.1) | 47 (14.9) | 20 (26.7) | .01 |
| Worsening or new heart murmur | 268 (68.5) | 218 (69.0) | 50 (66.7) | .70 |
| Heart failure | 213 (54.5) | 178 (56.3) | 35 (46.7) | .13 |
| Stroke | 57 (14.6) | 50 (15.8) | 7 (9.3) | .18 |
| Biological findings | ||||
| Positive blood culture | 356 (91.0) | 288 (91.1) | 68 (90.7) | .90 |
| Median glomerular filtration rate, mL/min/1.73 m2 | 74 | 72 | 77.5 | .95 |
| Blood leukocyte count, ×109/L | 14.7 ± 8.2 | 14.4 ± 8.2 | 15.8 ± 8.4 | .25 |
| Serum CRP level, mg/L | 169 ± 103 | 167 ± 105 | 176 ± 99 | .55 |
| Serum BNP level, ng/L | 773 ± 831 | 765 ± 839 | 808 ± 800 | .76 |
| Median serum troponin level, µg/L | 0.165 | 0.16 | 0.17 | .71 |
Data are presented as No. (%) or mean ± SD, if not otherwise specified.
Abbreviations: BNP, brain natriuretic peptide; CRP, C-reactive protein; ET, Endocarditis Team; ICD, implantable cardioverter-defibrillator; IE, infective endocarditis; PM, pacemaker.
Table 2.
Infection Characteristics, Microbiological and Echocardiographic Findings, and Embolic Complications of the 391 Episodes of IE
| IE Characteristics | All Patients | Pre-ET (n = 316) | Post-ET (n = 75) | P |
|---|---|---|---|---|
| Valve involved | ||||
| Aortic | 222 (56.8) | 182 (57.6) | 40 (53.3) | .50 |
| Mitral | 139 (35.5) | 112 (35.4) | 27 (36.0) | .93 |
| Tricuspid | 36 (9.2) | 31 (9.8) | 5 (6.7) | .40 |
| Pulmonary | 5 (1.3) | 4 (1.3) | 1 (1.3) | 1 |
| Type of valve | .06 | |||
| Native | 245 (62.7) | 190 (60.1) | 55 (73.3) | |
| Bioprosthetic valve | 79 (20.2) | 72 (22.8) | 7 (9.3) | |
| Mechanical valve | 32 (8.2) | 26 (8.2) | 6 (8.0) | |
| TAVR | 6 (1.5) | 4 (1.3) | 2 (2.7) | |
| Intracardiac device alone | 29 (7.4) | 24 (7.6) | 5 (6.7) | |
| Health care–associated nosocomial IE | 46 (11.8) | 34 (10.8) | 12 (16.0) | .19 |
| Causative microorganisms | .42 | |||
| Staphylococcus aureus | 115 (29.4) | 89 (28.2) | 26 (34.7) | |
| Coagulase-negative staphylococci | 35 (9.0) | 32 (10.1) | 3 (4.0) | |
| Streptococci (incl. pneumococcus) | 126 (32.2) | 105 (33.2) | 21 (28.0) | |
| Enterococci | 46 (11.8) | 35 (11.1) | 11 (14.7) | |
| Gram-negative bacilli | 17 (4.3) | 12 (3.8) | 5 (6.7) | |
| Others | 22 (5.6) | 18 (5.7) | 4 (5.3) | |
| ≥2 microorganisms | 17 (4.3) | 13 (4.1) | 4 (5.3) | |
| Unknown | 13 (3.3) | 12 (3.8) | 1 (1.3) | |
| Echocardiography findings | ||||
| LVEF, % | 58 ± 13.3 | 58 ± 13.4 | 59 ± 12.9 | .36 |
| Aortic location | 193 (49.4) | 158 (50.0) | 35 (46.7) | .60 |
| Mitral location | 130 (33.2) | 106 (33.5) | 24 (32.0) | .80 |
| Right-sided | 40 (10.2) | 31 (9.8) | 9 (12.0) | .57 |
| Maximum length of vegetation, mm | 14.6 | 14.8 | 13.5 | .10 |
| Severe/important regurgitation | 162 (41.4) | 132 (41.8) | 30 (40.0) | .78 |
| Intracardiac device IE | 43 (11.0) | 36 (11.4) | 7 (9.3) | .61 |
| Intracardiac abscess | 84 (21.5) | 72 (22.8) | 12 (16.0) | .20 |
| Intracardiac fistula | 22 (5.6) | 19 (6.0) | 3 (4.0) | .78 |
| Systemic embolism | ||||
| Cerebral embolism | 135 (46.7) | 115 (49.1) | 20 (36.4) | .09 |
| Cerebral ischemia | 125 (43.7) | 106 (45.7) | 19 (35.2) | .37 |
| Cerebral hemorrhage (incl. microbleed) | 58 (20.5) | 47 (20.6) | 11 (20.0) | .97 |
| Cerebral abscess | 10 (2.6) | 9 (2.8) | 1 (1.3) | .69 |
| Other noncerebral embolism | 166 (42.5) | 134 (42.4) | 32 (42.7) | .97 |
| Total embolism | 228 (58.3) | 187 (59.2) | 41 (54.7) | .48 |
| Spondylodiscitis | 33 (8.4) | 30 (9.5) | 3 (4.0) | .12 |
Data are presented as No. (%) or mean ± SD.
Abbreviations: IE, infective endocarditis; LVEF, left ventricular ejection fraction; TAVR, transcatheter aortic valve replacement.
Comparison of the 2 study groups in Tables 1 and 2 shows that a history of heart surgery was more prevalent before the setup of the ET (39% vs 27%, respectively; P = .04). In the pre-ET period, bioprosthetic valve infections were more frequent (23% vs 9%, respectively) and native valve infections were less frequent (60% vs 73%, respectively), but these differences were not significant (P = .06). Moreover, there were significantly fewer septic shocks in the pre-ET period (15% vs 27%, respectively; P = .01). All other parameters did not differ significantly between the 2 study periods.
Outcomes
In-hospital mortality decreased from 20.3% in the pre-ET period (n = 64) to 14.7% in the post-ET period (n = 11). However, the difference was not statistically significant (odds ratio [OR], 0.68; 95% confidence interval [CI], 0.30–1.39; P = .27) (Table 3). This nonsignificant decrease was also observed for 6-month (21.2% vs 16.0%, respectively; OR, 0.71; 95% CI, 0.33–1.42; P = .31) and 1-year mortality (23.4% vs 16.0%, respectively; OR, 0.62; 95% CI, 0.29–1.25; P = .16). The rates of surgery were similar between the 2 periods (47.8% vs 45.3%, respectively; OR, 0.91; 95% CI, 0.53–1.55; P = .70). However, the time to surgical procedure was significantly reduced in the post-ET period (from 16.4 to 10.3 days, respectively; P = .049). Moreover, the mean duration of antibiotic therapy (55.2 vs 47.2 days, respectively; P < .001) and length of in-hospital stay (40.6 vs 31.9 days, respectively; P < .01) were shorter during the post-ET period. Furthermore, cardiac (17.7% vs 9.8%, respectively; P = .14) and neurological (10.4% vs 3.3%, respectively; P = .09) sequelae were less frequent during the post-ET period. However, the differences were not statistically significant.
Table 3.
Management and Outcome of Patients
| All Patients | Pre-ET (n = 316) | Post-ET (n = 75) | P | OR (95% CI) | |
|---|---|---|---|---|---|
| Cardiac surgery | 185 (47.3) | 151 (47.8) | 34 (45.3) | .70 | 0.91 (0.53–1.55) |
| Late surgery | 14 (3.6) | 12 (3.8) | 2 (2.7) | 1 | 0.69 (0.07–3.22) |
| Duration of antibiotic therapy, d | 53.7 ± 21.1 | 55.2 ± 22.5 | 47.2 ± 11.9 | <.001 | |
| Time to surgery, d | 15.4 ± 14.2 | 16.4 ± 15.0 | 10.3 ± 7.5 | .049 | |
| Length of in-hospital stay, d | 38.9 ± 21.8 | 40.6 ± 22.0 | 31.9 ± 19.5 | <.01 | |
| In-hospital mortality | 75 (19.2) | 64 (20.3) | 11 (14.7) | .27 | 0.68 (0.30–1.39) |
| Cardiac sequelaea | 50 (16.1) | 44 (17.7) | 6 (9.8) | .14 | 0.51 (0.17–1.29) |
| Neurological sequelaea | 28 (9.0) | 26 (10.4) | 2 (3.3) | .09 | 0.30 (0.03–1.25) |
| 6-mo mortality | 79 (20.2) | 67 (21.2) | 12 (16.0) | .31 | 0.71 (0.33–1.42) |
| 1-y mortality | 86 (22.0) | 74 (23.4) | 12 (16.0) | .16 | 0.62 (0.29–1.25) |
Data are presented as No. (%) or mean ± SD.
Abbreviations: CI, confidence interval; ET, Endocarditis Team; ICD, implantable cardioverter-defibrillator; OR, odds ratio; PM, pacemaker.
an = 310.
In the multivariate analysis, independent predictors of in-hospital mortality were age (OR, 1.03 per year; 95% CI, 1.01–1.06; P < .01), septic shock (OR, 4.13; 95% CI, 2.10–8.19; P < .0001), and heart failure (OR, 2.05; 95% CI, 1.14–3.77; P < .05) (Table 4). Surgical treatment was independently associated with lower in-hospital mortality (OR, 0.50; 95% CI, 0.27–0.89; P < .05), as was the post-ET period (OR, 0.45; 95% CI, 0.20–0.96; P < .05).
Table 4.
Independent Predictors of In-Hospital Mortality
| Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| Age (per year) | 1.03 (1.01–1.06) | <.01 |
| Staphylococcus aureus | 1.69 (0.91–3.08) | .09 |
| Septic shock | 4.13 (2.10–8.19) | <.0001 |
| Heart failure | 2.05 (1.14–3.77) | .018 |
| Systemic embolism | 1.57 (0.88–2.85) | .13 |
| Surgical treatment | 0.50 (0.27–0.89) | .02 |
| Post-ET period | 0.45 (0.20–0.96) | .048 |
Abbreviations: CI, confidence interval; ET, Endocarditis Team; OR, odds ratio.
Discussion
Our study revealed major changes in patient management and significant improvements after the establishment of an ET, namely reductions in the time to surgical procedure, duration of antibiotic treatment, and length of in-hospital stay. We also found nonsignificant reductions in in-hospital, 6-month, and 1-year mortality rates after the setup of the ET. Nevertheless, by investigating factors associated with in-hospital mortality, we found that the ET and surgery were independent factors linked to survival. In 2009, Botelho-Nevers et al. demonstrated an important reduction in IE mortality using a management-based approach [10]. However, the extremely low mortality rate observed in that study may be attributed to the exclusion of certain microorganisms and the particularly high rate of surgery. In 2013, Chirillo et al. reported similar results; however, their study only involved native valve IE [11]. More recently, another study showed a survival benefit in medically treated patients after the setup of an ET [12]. Our study emphasizes the positive impact of the ET, demonstrating its beneficial effect on survival.
Despite recent epidemiological changes, the death rates of IE have remained stable over time. In most studies, in-hospital mortality approaches 20% and 5-year mortality reaches 40% [13]. Several investigations have demonstrated an improved prognosis in operated patients when carefully selected [13–16]. In addition, the difficulties in determining all patient characteristics in a short period of time may explain the observed conflicting results, particularly with regard to IE caused by Staphylococcus aureus [17]. The timing of surgery may also influence mortality in IE [18]. Our study confirms that surgery and the ET are factors associated with good outcome in IE. Thus, bringing together all the specialists involved in this pathology on a regular basis offers the best chance to promptly and accurately determine patients eligible for surgery and facilitate earlier surgical procedures. Half of the patients in this study underwent surgical valve replacement—a finding consistent with data in the literature [5]—and the rates of surgery were comparable between the 2 groups. However, we observed a significant decrease in the time to surgery, reduced by 6 days in the post-ET period. According to the 2015 ESC guidelines, surgery indicated for the management of IE should be performed within 2 weeks from the time of diagnosis and initiation of antibiotic therapy [8]. Therefore, the observed reduction in the time to surgery is a major improvement, related to the responsiveness of the coordinated multidisciplinary approach, assuming regular and frequent meetings, which are time-consuming and probably difficult to implement if the number of endocarditis cases to be discussed is not sufficient.
The reduction of neurological embolic events has recently been proposed as a critical parameter regarding the decision to perform surgery on a patient with IE. Notably, early surgery may prevent cerebral embolism in selected patients, particularly within the first 2 weeks of antibiotic treatment [19, 20]. Moreover, surgery is correlated with improved survival through reduction of heart failure and prevention of embolic sequelae [21]. In our study, we observed a nonsignificant decrease in the frequency of cardiac and neurological complications during the post-ET period.
Furthermore, we observed significant reductions in the length of antibiotic therapy and duration of hospitalization, which decreased by 8 days on average and may be linked to the early surgical management of patients.
Our study is characterized by several strengths. This is the first analysis with a prospective part investigating the impact of the ET on surgical delay. Considering the rarity of the disease and strict selection criteria (ie, exclusion of possible IE), the number of included patients is substantial. Therefore, the characteristics of patients are consistent with the epidemiology of IE observed in most large studies in this setting [3, 4].
Our study also has limitations. The observational design does not allow us to determine a causal link between the establishment of the ET and the observed differences. Although our hospital is a referral center for a large geographical area in France, this was a single-center study. A multicenter study, though introducing a center-to-center variability approach, would have added strength. Therefore, the present results must be generalized with caution. In addition, our study was not statistically powered to highlight small differences, which may explain why the primary outcome was not significant. The post-ET period lasted only 1 year. We cannot be sure that the improvements in care we observed were not due to a cognitive bias (halo effect), as the post-ET period was not long. Moreover, the physicians involved in the management of IE before the ET were mostly the same individuals involved in the ET. This overlap might minimize the differences between the 2 periods. Finally, the shorter time to surgery in the post-ET period may be explained by a higher rate of septic shock in this group, by a higher frequency of S. aureus IE after the ET, or by a decrease in cerebral embolism that may delay urgent surgery.
In conclusion, the multidisciplinary ET exerted a positive effect on the management of IE. In this observational study, we noted a significant reduction in surgical delay, length of in-hospital stay, and antibiotic therapy after the setup of the ET. We also noted a nonsignificant decrease in mortality rate during hospitalization and at 6 months and 1 year. Finally, the ET and surgery were independently associated with survival. Currently, the prognosis of IE remains poor despite documented therapeutic progress. However, the establishment of an ET—which can be further optimized—provides additional leverage to improve the management of this disease. Further prospective studies are warranted to evaluate and promote this multidisciplinary approach.
Acknowledgments
The authors would like to thank Enago (www.enago.com) for the English language review.
Financial support. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Y.R., X.A., J.P.M., and Y.H. designed the study. Y.R., X.A., Y.H., A.M., N.L., N.D., T.H.M., and J.P.M. collected the data. Y.R. and X.A. analyzed the data. Y.R. and F.L. performed the statistical analysis. Y.R. and X.A. wrote the manuscript. E.P.H. analyzed the echocardiographical data. P.R. analyzed the microbiological data. All authors reviewed, revised, and approved the final report.
References
- 1. Slipczuk L, Codolosa JN, Davila CD, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013; 8:e82665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA 2018; 320:72–83. [DOI] [PubMed] [Google Scholar]
- 3. Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012; 54:1230–9. [DOI] [PubMed] [Google Scholar]
- 4. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iung B, Doco-Lecompte T, Chocron S, et al. Cardiac surgery during the acute phase of infective endocarditis: discrepancies between European Society of Cardiology guidelines and practices. Eur Heart J 2016; 37:840–8. [DOI] [PubMed] [Google Scholar]
- 6. Yanagawa B, Pettersson GB, Habib G, et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation 2016; 134:1280–92. [DOI] [PubMed] [Google Scholar]
- 7. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 8. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128. [DOI] [PubMed] [Google Scholar]
- 9. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med 2009; 169:1290–8. [DOI] [PubMed] [Google Scholar]
- 11. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol 2013; 112:1171–6. [DOI] [PubMed] [Google Scholar]
- 12. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart 2017; 4:e000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bannay A, Hoen B, Duval X, et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: do differences in methodological approaches explain previous conflicting results? Eur Heart J 2011; 32:2003–15. [DOI] [PubMed] [Google Scholar]
- 14. Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366:2466–73. [DOI] [PubMed] [Google Scholar]
- 15. Aksoy O, Sexton DJ, Wang A, et al. Early surgery in patients with infective endocarditis: a propensity score analysis. Clin Infect Dis 2007; 44:364–72. [DOI] [PubMed] [Google Scholar]
- 16. Thuny F, Grisoli D, Collart F, Habib G, Raoult D. et al. Management of infective endocarditis: challenges and perspectives. Lancet 2012; 379:965–75. [DOI] [PubMed] [Google Scholar]
- 17. Chirouze C, Alla F, Fowler VG Jr, et al. Impact of early valve surgery on outcome of Staphylococcus aureus prosthetic valve infective endocarditis: analysis in the International Collaboration of Endocarditis-Prospective Cohort Study. Clin Infect Dis 2015; 60:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thuny F, Beurtheret S, Mancini J, et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J 2011; 32:2027–33. [DOI] [PubMed] [Google Scholar]
- 19. Yoshioka D, Sakaguchi T, Yamauchi T, et al. Impact of early surgical treatment on postoperative neurologic outcome for active infective endocarditis complicated by cerebral infarction. Ann Thorac Surg 2012; 94:489–95; discussion 496. [DOI] [PubMed] [Google Scholar]
- 20. García-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditisclinical perspective: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127:2272–84. [DOI] [PubMed] [Google Scholar]
- 21. Chu VH, Park LP, Athan E, et al. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation 2015; 131:131–40. [DOI] [PubMed] [Google Scholar]