ABSTRACT
Background
Exercise is recommended for weight management, yet exercise produces less weight loss than expected, which is called weight compensation. The mechanisms for weight compensation are unclear.
Objective
The aim of this study was to identify the mechanisms responsible for compensation.
Methods
In a randomized controlled trial conducted at an academic research center, adults (n = 198) with overweight or obesity were randomized for 24 wk to a no-exercise control group or 1 of 2 supervised exercise groups: 8 kcal/kg of body weight/wk (KKW) or 20 KKW. Outcome assessment occurred at weeks 0 and 24. Energy intake, activity, and resting metabolic rate (RMR) were measured with doubly labeled water (DLW; with and without adjustments for change in RMR), armband accelerometers, and indirect calorimetry, respectively. Appetite and compensatory health beliefs were measured by self-report.
Results
A per-protocol analysis included 171 participants (72.5% women; mean ± SD baseline body mass index: 31.5 ± 4.7 kg/m2). Significant (P < 0.01) compensation occurred in the 8 KKW (mean: 1.5 kg; 95% CI: 0.9, 2.2 kg) and 20 KKW (mean: 2.7 kg; 95% CI: 2.0, 3.5 kg) groups, and compensation differed significantly between the exercise groups (P = 0.01). Energy intake by adjusted DLW increased significantly (P < 0.05) in the 8 KKW (mean: 90.7 kcal/d; 95% CI: 35.1, 146.4 kcal/d) and 20 KKW (mean: 123.6 kcal/d; 95% CI: 64.5, 182.7 kcal/d) groups compared with control (mean: −2.3 kcal/d; 95% CI: −58.0, 53.5 kcal/d). Results were similar without DLW adjustment. RMR and physical activity (excluding structured exercise) did not differentially change among the 3 groups. Participants with higher compared with lower compensation reported increased appetite ratings and beliefs that healthy behaviors can compensate for unhealthy behaviors. Furthermore, they increased craving for sweet foods, increased sleep disturbance, and had worsening bodily pain.
Conclusions
Compensation resulted from increased energy intake and concomitant increases in appetite, which can be treated with dietary or pharmacological interventions. Compensation was not due to activity or metabolic changes. This trial was registered at clinicaltrials.gov as NCT01264406.
Keywords: exercise, compensation, weight loss, food intake, physical activity, Food Craving Inventory, SF-36, compensatory health beliefs
Introduction
Excess body weight is associated with increased risk for adverse health conditions (1), and ∼70% of Americans are overweight or obese (2). Exercise is commonly recommended for weight management, yet in long-term (≥6 mo) but not short-term studies, actual weight loss consistently is only ∼30–40% of expected based on measured energy expenditure (3–5). The discrepancy between the amount of weight loss predicted from exercise-associated energy expenditure and observed weight loss is called compensation (6).
Compensation has been a focus of research for many years. Blundell and colleagues (6) reported that ∼30% of exercise-induced increases in energy expenditure are compensated for, and they concluded that compensation could be due to poor adherence to the exercise protocols or other behavioral or physiological factors. A single group trial highlighted the large amount of individual variation in weight loss from exercise, although compensation was not detected when data from the entire sample were analyzed (7). Once compensators were identified and compared to noncompensators, however, increased appetite and food intake appeared to be a significant source of compensation (7). Due to the dynamic nature of energy balance and the frequency with which compensation is detected, particularly at higher doses of exercise, recent efforts have focused on identifying and quantifying the exact mechanisms responsible for compensation.
Compensation could be due to reduced metabolic rate, decreased spontaneous activity, and/or increased energy intake. Previous studies examining compensation could be improved upon by designing and powering studies to specifically identify the source or sources of compensation, relying on measures of energy intake that are sufficiently sensitive to detect small changes over time, and determining if physical activity levels change outside of structured exercise (4, 5).
The Examination of Mechanisms of Exercise-Induced Weight Compensation (E-MECHANIC) study was designed to identify mechanisms of compensation by examining the effect over 6 mo of 2 doses of exercise training on energy intake; resting metabolism; non-exercise physical activity; and constructs such as eating attitudes, eating behaviors, and health beliefs. Understanding the mechanisms of exercise-induced weight compensation is critical to refining clinical weight loss guidelines and informing clinicians how to counsel patients who exercise with the expectation of losing weight.
Methods
Participants and design
The study methods are described in detail elsewhere (8). Briefly, healthy overweight or obese [BMI (in kg/m2): ≥25 to ≤45], sedentary (not exercising >20 min on ≥3 d/wk) men and women (N = 198) were recruited between November 2010 and August 2014 (Figure 1). The study was a 6-mo randomized controlled trial with 3 groups: 1) a no-exercise control group, 2) a group that was prescribed exercise that reflected recommendations for general health [8 kcal/kg of body weight/wk (8 KKW) or ∼700 kcal/wk], and 3) a group that was prescribed a higher exercise dose that is recommended for weight loss and weight loss maintenance (20 KKW or ∼1760 kcal/wk) (9). The 8 KKW group completed their full exercise dose from the outset, whereas the 20 KKW group ramped up their exercise dose from 8 KKW during week 1 to 14 KKW during week 2 and 20 KKW during week 3. Exercise intensity was self-selected between 65% and 85% peak oxygen uptake (VO2peak), which participants have been found to tolerate over the length of the exercise sessions. All exercise sessions were monitored/supervised. Exercise sessions varied in length to meet each participant's energy expenditure goal (10). In addition to energy expenditure rate being measured with a metabolic cart at baseline and week 24, measurements were collected at weeks 2, 4, 6, 8, 12, 16, and 20 to adjust the daily exercise time to account for changes in metabolic or biomechanical efficiency.
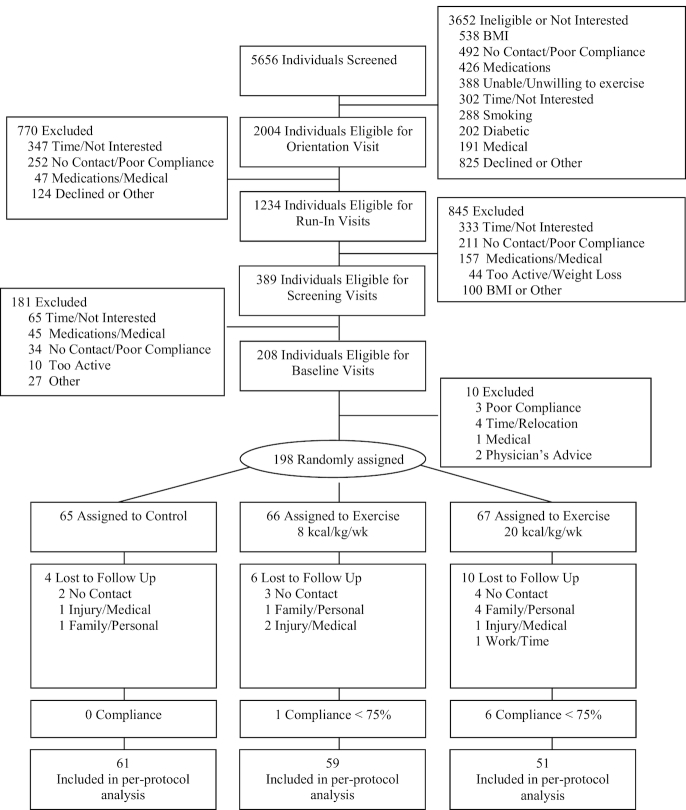
FIGURE 1.
CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials.
Recruitment and data collection occurred from November 2010 to March 2015. A 1:1:1 randomization ratio was created by the biostatistician, and randomization was concealed in a sealed envelope until opened with the participant by the study manager or a staff member responsible for exercise training. Sex was stratified to promote an equal number of men and women among the groups. The assessment team and investigators were blind to participant assignment. Participants and staff prescribing and supervising the exercise sessions were not blind.
Based on previous research demonstrating that compensation most frequently occurs at higher doses of exercise (4), it was hypothesized that energy intake, measured with doubly labeled water (DLW) and laboratory-based food intake tests, would increase significantly in the 20 KKW group but not in the 8 KKW or control groups. Similarly, it was hypothesized that compensation or the difference between observed and predicted weight loss would be significantly larger in the 20 KKW group.
Ethics
The Pennington Biomedical Institutional Review Board approved the protocol, and all participants provided written informed consent. A data and safety monitoring board supervised the study.
Outcomes variables
Weight compensation
The primary outcome variables were weight compensation and energy intake measured with the 2 methods described herein. Compensation is the difference between the amount of weight loss predicted from exercise-associated energy expenditure and observed weight loss from baseline to follow-up (actual – predicted weight change). Predicted weight loss was calculated using 2 methods. The primary measure utilized a validated dynamic energy balance model that overcomes the limitations of the conventional 7700 kcal/kg of body weight and accounts for the effects of changing body mass on components of energy balance over time (11–13). To be comparable to previous studies, a second analysis was conducted assuming 1 kg of body weight = 7700 kcal, although this method overestimates weight loss (11–13).
Energy intake
Energy intake, measured with DLW and food intake tests, was the primary outcome variable (all other outcomes were secondary). DLW data were collected over 2 wk at baseline and 2 wk at week 24. The DLW period at baseline occurred before participants in the 2 exercise groups began exercising. During the DLW period at week 24, participants in the exercise groups exercised at their prescribed dose. DLW measures total daily energy expenditure, which equals total daily energy intake during energy balance or weight stability (14, 15). All 3 groups were weight stable at months 0 and 6; specifically, in all 3 groups, weight changed by ≤0.15 kg over each of the 2 wk of DLW measurements at baseline and follow-up based on a regression of the 3 clinic weights obtained during the DLW periods. Change in energy intake by DLW was calculated with and without adjusting for change in resting metabolic rate, as described in the Supplemental Methods. Adjusting DLW did not meaningfully affect the results. Adjusted values are reported in the text and both values reported in the tables.
At baseline and week 24, validated laboratory-based food intake tests were conducted at lunch and dinner following a standard breakfast consisting of a 190-kcal nutrition bar, which was consumed between 0700 and 0800. Participants returned to the center between 1100 and 1200 to complete their test lunch, which consisted of ad libitum sandwiches, potato chips, cookies, water, and choice of an artificially sweetened soda or tea and sugar-sweetened soda or tea. Participants returned to the center 5.5 h after the start of their lunch to complete their dinner meal, which consisted of a previously described buffet meal (16). Food intake testing occurred at least 24 h after the last exercise session. Food intake at lunch and dinner was quantified by covertly weighing food provision and waste.
Resting metabolic rate and respiratory quotient
Resting metabolic rate (RMR) and respiratory quotient (RQ; VCO2/VO2) were measured with indirect calorimetry over 30 min after a 12-h overnight fast with Max II metabolic carts (AEI Technologies) at baseline and week 24. RMR decreases with weight loss; therefore, RMR values were adjusted using 2 methods. First, RMR values were adjusted for change in body composition—that is, lean mass measured with dual-energy X-ray absorptiometry (the statistical models also adjusted for age and sex). Second, to obtain a measure of metabolic adaptation or reduced metabolic rate that could limit weight loss, regression analysis was used to adjust RMR values for age, sex, and body mass (fat mass and fat-free mass), which results in a participant's residual RMR value at baseline and week 24. To assess change in RMR, the residual at week 24 was subtracted from the baseline residual following the procedures of Galgani and Santos (17).
Physical activity
SenseWear armbands (BodyMedia) measured the number of steps taken per day, minutes per day spent in activities of different intensities, and minutes per day spent in physical activity (defined as time ≥3 metabolic equivalents). Measurements spanned 24 h/d, except during activities involving water, over 2 wk at baseline and 1 wk at weeks 4 and 24. Armbands detect and record wear time, and only full days of data were included in the analyses. A full day of data required that the device be worn 95% of the time, which equates to 22 h and 48 min. Armbands provide valid measures of free-living total daily energy expenditure (18) and time performing activity (19).
Participants in the exercise groups wore the armband during exercise sessions, and data were tracked to allow data collected during the exercise sessions to be removed. This results in the exercise groups having fewer minutes per day of wear time; therefore, these data were also expressed by minute to evaluate if activity changed over time differently between the groups.
Weight and body composition
Baseline and follow-up weights were the average from 3 fasting weights collected over 14 d separated by 1 wk (e.g., days 0, 7, and 14). Body composition was measured with dual-energy X-ray absorptiometry (iDXA, encore software version 13.60; GE Healthcare) at baseline and week 24.
Questionnaires
Visual analog scales (VAS) assessed subjective ratings of appetite before and after the test meals (20). Retrospective VAS assessed average ratings of appetite during the previous week (21). Additional questionnaires included the Multifactorial Assessment of Eating Disorders Symptoms (22), Eating Inventory (23), Food Preference Questionnaire (24), Food Craving Inventory (25), Yale Food Addiction Scale (26), Activity Temperament Questionnaire (27, 28), Medical Outcomes Study Short Form 36 (SF-36) (29), Pittsburgh Sleep Quality Index (30), Compensatory Health Beliefs Scale (31), and Body Morph Assessment (32).
Cardiorespiratory fitness
Cardiorespiratory fitness testing occurred following medical clearance using a standardized graded exercise testing protocol on a treadmill (Trackmaster 425), unless orthopedic constraints required use of a bicycle ergometer (Excalibur Sport Cycle Ergometer; Lode, BV). Testing began at 2.4 miles per hour and a 0% grade, equal to 2.8 metabolic equivalents. Every two minutes, intensity increased by altering speed (0.2 miles per hour increments), grade (increments of two percentage points), or both and the test continued until individual maximal exertion. Measurements were collected during testing with a TrueMax 2400 metabolic measurement cart (Parvo Medics). Data collected during the test included maximal heart rate, respiratory exchange ratio, VO2, pulmonary ventilation, ventilatory equivalents for oxygen (pulmonary ventilation/VO2), ventilatory equivalents for carbon dioxide (pulmonary ventilation/CO2), end-tidal partial pressure of oxygen, and end-tidal partial pressure of carbon dioxide. Ratings of perceived exertion were obtained using the Borg scale (33)
Metabolic syndrome
Metabolic syndrome is marked by 3 or more of the following 5 symptoms: 1) waist circumference >40 inches (>101 cm) for men or >35 inches (>89 cm) for women, 2) triglycerides ≥150 mg/dL, 3) HDL <40 mg/dL for men or <50 mg/dL for women, 4) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, and 5) fasting glucose ≥100 mg/dL. These measurements were collected following standardized clinic procedures, and participants were categorized at baseline as having or not having the metabolic syndrome.
Data analysis
Power analyses are detailed elsewhere (8). The calculations assumed 2-tailed tests, α = 0.05, and randomizing 198 individuals equally across groups with 10% attrition resulting in ∼60 individuals per group (171 participants were included in the per-protocol analysis, which reflected the original power analyses). For estimated compensation of 2 kg, statistical power was 90%.
E-MECHANIC was designed to identify mechanisms responsible for weight compensation; therefore, per-protocol analyses were utilized that included participants with follow-up data and ≥75% adherence to their exercise prescription. This resulted in virtually identical exercise adherence between the exercise groups, and the study results did not differ when the 7 participants who failed to meet the per-protocol cutoff were included in the analysis (see the Supplemental Methods and Supplemental Table 1). Differences in outcomes among groups were tested by ANCOVAs or nonparametric Kruskal–Wallis (if 3 groups were compared) or Wilcoxon (if 2 groups were compared) tests, with adjustment for sex and age (additional analyses were conducted that included race, and the results did not differ meaningfully; therefore, the models without race are reported). Post hoc comparisons were Tukey–Kramer adjusted (ANCOVAs), or Dwass, Steel, Critchlow-Fligner multiple comparison analysis was used. Results are presented as adjusted least-squares means with 95% CIs or SDs. Between-group differences for categorical variables were examined using chi-square tests. Subgroup analyses examined differences in baseline and change variables between compensators and noncompensators in the exercise groups.
Due to the high prevalence of compensation, participants in the exercise groups were classified as compensators or noncompensators based on a median split (compensators > −58.5% and noncompensators ≤ −58.5%) of percentage compensation using data from the 2 exercise groups (percentage compensation = actual – predicted weight loss/predicted weight loss). These procedures relied on predicted weight loss from the energy balance model. Percentage rather than absolute compensation was utilized because the latter was biased toward the higher exercise group. Change on the outcome variables was examined by compensation status. All P values are 2-sided, α = 0.05, and analyses were conducted with SAS software (version 9.3; SAS Institute).
Results
Descriptive characteristics and exercise adherence
Of 198 randomized participants, 178 completed follow-up testing (89.9%) (Figure 1). There were 7 participants in the exercise groups with <75% adherence, resulting in 171 (86.4%) in the analysis (adherence = achieved exercise energy expenditure/prescribed exercise energy expenditure). Table 1 includes the sample characteristics, Table 2 includes achieved exercise energy expenditure from the exercise sessions, and exercise-training data are summarized in Supplemental Table 2. The 8 and 20 KKW groups achieved ∼600 and ∼1350 metabolic equivalents per week, respectively, and 100.8% and 98.2% of prescribed exercise-associated energy expenditure, respectively.
TABLE 1.
Descriptive baseline characteristics of the 171 participants included in the per-protocol analysis1
| Characteristic | All (n = 171) | Control (n = 61) | 8 KKW (n = 59) | 20 KKW (n = 51) |
|---|---|---|---|---|
| Women, n (%) | 124 (72.5) | 45 (74) | 43 (73) | 36 (71) |
| Ethnicity, n (%) | ||||
| Caucasian | 114 (66.7) | 38 (62.3) | 39 (66.1) | 37 (72.6) |
| African American | 53 (31) | 21 (34.4) | 20 (33.9) | 12 (23.5) |
| Hispanic/other | 4 (2.3) | 2 (3.3) | 0 (0) | 2 (3.9) |
| Age, n (y) | 171 (48.9 ± 11.4) | 61 (49.5 ± 10.8) | 59 (48.3 ± 11.2) | 51 (48.7 ± 12.4) |
| Weight, n (kg) | 171 (88.6 ± 15.4) | 61 (90.1 ± 15.4) | 59 (88.7 ± 15.8) | 51 (86.5 ± 15.1) |
| BMI, n (kg/m2) | 171 (31.5 ± 4.7) | 61 (32.3 ± 4.8) | 59 (31.4 ± 4.6) | 51 (30.6 ± 4.4) |
| Percentage fat, n | 170 (42.1 ± 7.2) | 61 (43.0 ± 7.6) | 59 (41.5 ± 6.6) | 50 (41.6 ± 7.3) |
| Fat mass, n (kg) | 170 (37.5 ± 10.0) | 61 (38.9 ± 10.3) | 59 (37.0 ± 9.7) | 50 (36.3 ± 10.0) |
| Lean mass, n (kg) | 170 (48.2 ± 10.1) | 61 (48.2 ± 10.3) | 59 (48.8 ± 10.0) | 50 (47.4 ± 10.0) |
| VO2, n (L/min) | 171 (2.1 ± 0.6) | 61 (2.1 ± 0.6) | 59 (2.1 ± 0.5) | 51 (2.0 ± 0.5) |
| VO2, n (mL·kg-1·min-1) | 171 (23.6 ± 5.3) | 61 (23.1 ± 5.3) | 59 (23.9 ± 5.5) | 51 (23.7 ± 5.1) |
| Systolic blood pressure, n (mm Hg) | 171 (120.8 ± 10.6) | 61 (121.7 ± 11.1) | 59 (119.6 ± 10.6) | 51 (121.0 ± 9.9) |
| Diastolic blood pressure, n (mm Hg) | 171 (77.4 ± 7.4) | 61 (78.6 ± 7.8) | 59 (77.2 ± 6.1) | 51 (76.8 ± 8.4) |
| Steps/d, n | 167 (610 8 ± 2250) | 59 (5958 ± 2246) | 57 (6458 ± 2565) | 51 (5892 ± 1834) |
| Active, n (min/d) | 167 (59.8 ± 44.4) | 59 (59.2 ± 41.4) | 57 (62.8 ± 48.9) | 51 (57.3 ± 43.2) |
| Moderate, n (min/d) | 167 (58.6 ± 43.2) | 59 (57.1 ± 39.5) | 57 (61.8 ± 47.9) | 51 (56.7 ± 42.3) |
| Vigorous, n (min/d) | 167 (1.0 ± 5.3) | 59 (1.6 ± 8.1) | 57 (1.0 ± 3.7) | 51 (0.5 ± 1.0) |
| Very vigorous, n (min/d) | 167 (0.2 ± 1.9) | 59 (0.5 ± 3.2) | 57 (0.0 ± 0.0) | 51 (0.1 ± 0.6) |
| RMR, not adjusted, n (kcal/d) | 170 (1526.1 ± 316.4) | 61 (1516.1 ± 353.9) | 58 (1530.7 ± 258.1) | 51 (1532.8 ± 334.7) |
| RQ, n | 170 (0.82 ± 0.05) | 61 (0.81 ± 0.05) | 58 (0.82 ± 0.05) | 51 (0.82 ± 0.04) |
| Energy intake (DLW), n (kcal/d) | 171 (2479.1 ± 455.9) | 61 (2437.5 ± 447.4) | 59 (2537.3 ± 435.9) | 51 (2461.5 ± 489.4) |
| Intake (buffet), n (kcal at lunch and dinner combined) | 171 (1733.5 ± 573.6) | 61 (1626.3 ± 607.7) | 59 (1814.4 ± 485.6) | 51 (1768.1 ± 615.7) |
Values in parentheses are percentages or means ± SD. Chi-square tests were used to test for differences among the groups on categorical variables, and ANOVA was used to test for differences among the 3 groups on baseline values of continuous variables. The control, 8 KKW, and 20 KKW groups did not differ significantly on any baseline measures listed in the table. DLW, doubly labeled water; RMR, resting metabolic rate by indirect calorimetry (ventilated hood); RQ, respiratory quotient; VO2, peak oxygen uptake.
TABLE 2.
Predicted weight loss, compensation, change from week 0 to week 24 by group on outcome variables, and mean energy expenditure during exercise training for the 2 exercise groups1
| Variable | Control | 8 KKW | 20 KKW | Group P value |
|---|---|---|---|---|
| Predicted weight loss2 (kg) | 0.0 (−0.1, 0.1) | −1.9 (−2.1, −1.7) | −4.3 (−4.5, −4.1) | |
| Actual weight change (kg) | −0.2a (−1.0, 0.6) | −0.4a,b (−1.2, 0.4) | −1.6b (−2.4, −0.8) | 0.02* |
| Compensation (kg) | 0.0a (−0.5, 0.5) | 1.5b (0.9, 2.2) | 2.7c (2.0, 3.5) | 0.01* |
| Energy intake, adjusted DLW (kcal/d) | −2.3a (−58.0, 53.5) | 90.7b (35.1, 146.4) | 123.6b (64.5, 182.7) | <0.01* |
| Energy intake, DLW (kcal/d) | −24.7a (−82.2, 32.9) | 71.1b (13.6, 128.6) | 90.5b (29.5, 151.6) | <0.01* |
| Change in energy intake, adjusted DLW (%) | 0.0a (−2.2, 2.3) | 3.9b (1.7, 6.2) | 5.5b (3.1, 7.9) | <0.01* |
| Change in energy intake, DLW (%) | −0.8a (−3.1, 1.5) | 3.2b (0.9, 5.6) | 4.2b (1.8, 6.7) | <0.01* |
| Energy intake—buffet (kcal at lunch and dinner combined) | −88.1 (−197.4, 21.2) | −106.2 (−216.0, 3.6) | −72.0 (−183.9, 40.0) | 0.90 |
| Percentage body fat | 0.1a (−0.4, 0.6) | −0.05a,b (−0.6, 0.5) | −0.8b (−1.4, −0.3) | 0.04* |
| Body fat (kg) | 0.1a (−0.6, 0.8) | −0.2a,b (−0.9, 0.5) | −1.4b,† (−2.1, −0.6) | <0.01* |
| Lean body mass (kg) | −0.4 (−0.8, −0.1) | −0.3 (−0.6, 0.1) | −0.1 (−0.5, 0.2) | 0.51 |
| VO2 (L/min) | −0.11a (−0.16, −0.05) | 0.11b (−0.05, 0.16) | 0.28c (0.21, 0.34) | <0.01 |
| VO2 (mL·kg-1·min-1) | −1.5a (−2.4, −0.6) | 0.6b (−0.3, 1.5) | 3.1c (2.2, 4.1) | <0.01 |
| Systolic blood pressure (mm Hg) | −1.9 (−4.0, 0.2) | −1.1 (−3.2, 1.1) | −0.3 (−2.5, 2.0) | 0.56 |
| Diastolic blood pressure (mm Hg) | −0.8 (−2.3, 0.8) | −0.2 (−1.8, 1.4) | 1.4 (−0.3, 3.1) | 0.14 |
| Total activity (min/d) | −7.2 (−16.0, 1.7) | −8.5 (−17.3, 0.3) | 2.7 (−6.5, 11.8) | 0.16 |
| Moderate (min/d) | −5.7 (−14.1, 2.6) | −8.2 (−16.5, 0.0) | 2.1 (−6.6, 10.7) | 0.19 |
| Vigorous (min/d) | 0.2 (−0.2, 0.5) | 0.0 (−0.3, 0.4) | 0.4 (0.1, 0.8) | 0.21 |
| Very vigorous3 (min/d) | −0.5 (−1.1, 0.1) | 0.0 (−0.5, 0.6) | −0.0 (−0.6, 0.5) | 0.39 |
| RMR-adjusted (kcal/d) | 16.4 (−44.9, 77.8) | 57.0 (−5.7, 119.6) | 17.6 (−48.7, 83.9) | 0.57 |
| RMR-Galgani (kcal/d) | 14.3 (−46.8, 75.5) | 56.7 (−5.9, 119.4) | 19.3 (−46.9, 85.5) | 0.56 |
| RQ | 0.000 (−0.015, 0.015) | −0.000 (−0.016, 0.015) | −0.017 (−0.033, −0.001) | 0.20 |
| FPQ, high fat/high carb | −0.14a,b (−0.53, 0.26) | 0.20a (−0.21, 0.61) | −0.53b (−0.97, −0.10) | 0.04* |
| VAS, after dinner, desire3 | 4.47a (0.58, 8.37) | 3.41a,b (−0.56, 7.37) | −2.75b (−6.99, 1.48) | 0.02* |
| SF-36, social functioning3 | −4.99a,b (−9.83, −0.15) | −1.75a (−6.73, 3.24) | −5.80b (−11.06, −0.53) | 0.046* |
| Exercise EE prescribed (kcal) | — | 16,973 (15,606, 18,340) | 39,690 (38,220, 41,161) | <0.01* |
| Exercise EE achieved (kcal) | — | 17,114 (15,708, 18,520) | 38,956 (37,444, 40,468) | <0.01* |
Values are least-squares means (95% CIs) (n = 171; n = 61, 59, and 51 participants in the control, 8 KKW, and 20 KKW groups, respectively). Post hoc comparisons were Tukey–Kramer adjusted (ANCOVAs), or Dwass, Steel, Critchlow-Fligner multiple comparison analysis was used. Within rows, means with different superscript letters differ significantly, and significant P values (P < 0.05) are indicated with a *. †The P value comparing the 8 KKW and 20 KKW groups was 0.06. The control group had no exercise intervention; therefore, compensation was held at 0. Results are only shown for questionnaires that had a significant group by time interaction; Supplemental Table 4 includes all questionnaire results. All values are adjusted for age and sex except for exercise EE. RMR-adjusted values represent change in RMR adjusted for sex and age, as well as fat-free mass. RMR-Galgani is a measure of metabolic adaptation where regression analysis was used to adjust RMR values for age, sex, and body mass (fat mass and fat-free mass), which results in a participant's residual RMR values at baseline and week 24. Change in RMR or metabolic adaptation is then calculated by subtracting the residual at week 24 from the baseline residual. DLW, doubly labeled water; EE, energy expenditure; FPQ, food-preference questionnaires; RMR, resting metabolic rate by indirect calorimetry (ventilated hood); RQ, respiratory quotient; SF-36, Medical Outcomes Study Short Form 36; VAS, visual analog scale.
Weight loss was predicted from an energy balance model. Differences in outcomes among groups were tested by ANCOVAs or nonparametric Kruskal–Wallis tests, with adjustment for sex and age.
Nonparametric Kruskal–Wallis test.
Weight change and weight compensation
The percentage of individuals who lost weight was 52.5%, 57.6%, and 76.5% in the control, 8 KKW, and 20 KKW groups, respectively, with the 20 KKW group significantly different compared with the control (P = 0.05) (Figure 2). In the 8 and 20 KKW groups, 76.3% and 90.2% of participants compensated, respectively, with no significant difference between groups (P = 0.09). Compensation status did not differ by sex [χ2 (1, N = 110) = 0.40; P = 0.52] or race [χ2 (1, N = 110) = 0.17; P = 0.68].
FIGURE 2.
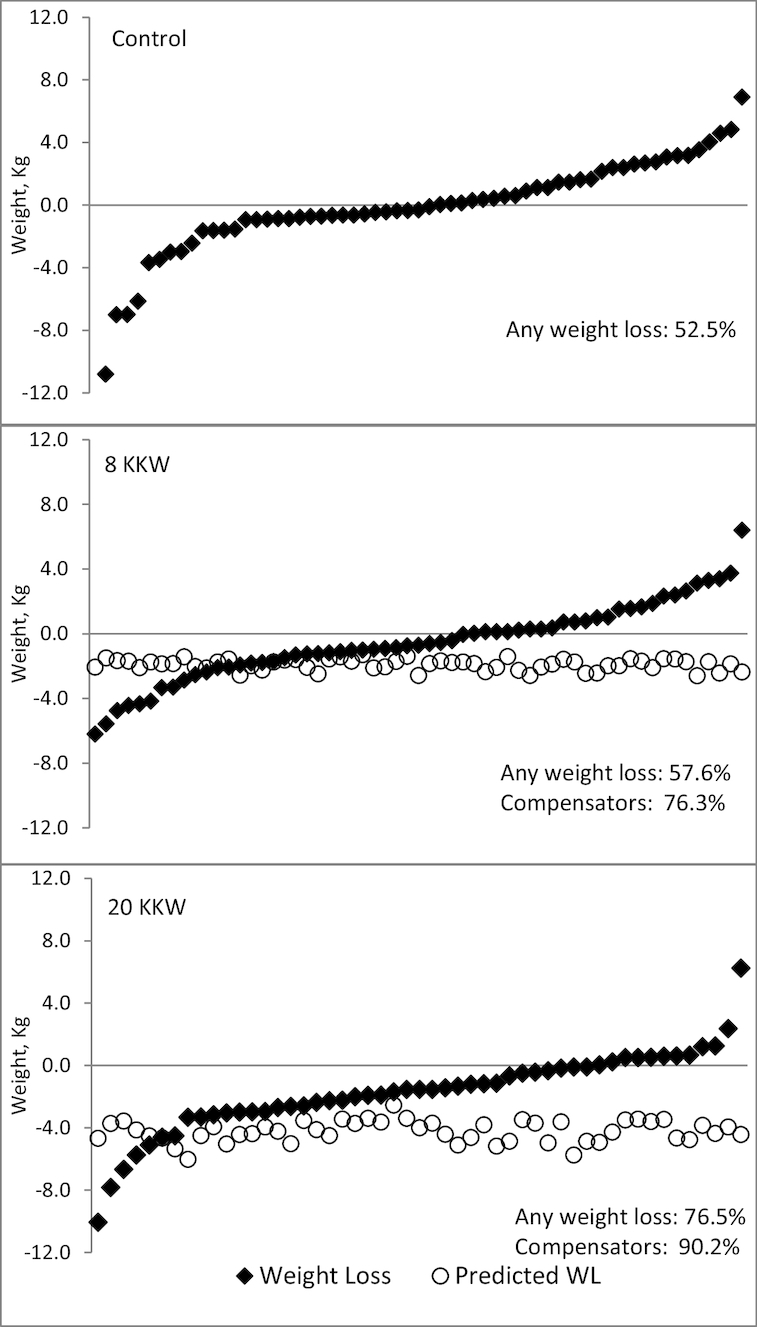
Actual weight change (diamonds) and predicted weight loss (WL) from the energy balance model (circles) for participants in the control group (n = 61; top), 8 KKW group (n = 59; middle), and 20 KKW group (n = 51; bottom).
Table 2 shows weight change, predicted weight change from the energy balance model, and compensation across the 3 groups. Mean weight change in the control (−0.2 kg) was less than that in the 20 KKW group (−1.6 kg, P = 0.02), but neither group differed from the 8 KKW group (−0.4 kg). Significant (P < 0.01) compensation occurred in the 8 KKW (1.5 kg) and 20 KKW (2.7 kg) groups, and the amount of compensation was significantly different between the exercise groups (P = 0.01). Using 7700 kcal/kg of body weight to derive compensation produced similar results, with significant differences between the 8 KKW (1.9 kg; 95% CI: 1.1, 2.7 kg) and the 20 KKW (3.5 kg; 95% CI: 2.7, 4.4 kg; P = 0.01) groups. The 20 KKW group lost more body fat compared with the control group (Table 2), and change in lean mass did not differ among groups.
Energy intake
Change in energy intake measured by adjusted DLW differed significantly (P < 0.01) between the control (−2.3 kcal/d) and both the 8 KKW (90.7 kcal/d) and the 20 KKW (123.6 kcal/d) groups (Table 2). Individual-level energy intake change for participants in the 8 and 20 KKW groups is depicted in Supplemental Figure 1. Change in energy intake did not differ between the 8 and 20 KKW groups (P = 0.69). Percentage change in DLW measured energy intake was significantly lower (P < 0.01) in the control (0.0%) compared with both the 8 KKW (3.9%) and the 20 KKW (5.5%) groups (Table 2). Food intake data from the lunch and dinner test meals were combined. Change in food intake measured during the test meals did not differ (P = 0.90) between the control (−88.1 kcal), 8 KKW (−106.2 kcal), and 20 KKW (−72.0 kcal) groups (Table 2).
Physical activity, RMR, and RQ
Baseline physical activity, RMR, and RQ data are shown in Table 1, and change data are shown in Table 2. No measures of activity, RMR, or resting RQ differed by group from baseline to week 24 (Table 2). Change in activity energy expenditure, total energy expenditure, and steps did not differ among the groups when activity was expressed per minute to account for different accelerometry wear times in the exercise groups at week 24 (all P values >0.34; data not shown).
Questionnaires
Baseline questionnaire data by group are provided in Supplemental Table 3.
The 8 KKW group had increased preference for high-fat/high-carbohydrate foods (e.g., French fries) compared with the 20 KKW group (P = 0.04) (Table 2). The control group had increased desire to consume food measured with the VAS after dinner compared with the 20 KKW group (P = 0.02). The 20 KKW group had a larger reduction in social functioning compared with the 8 KKW group (P = 0.046). Table 2 and Supplemental Table 4 include all comparisons.
Compensators compared with noncompensators (exercise groups only)
Baseline questionnaire data by compensator group are provided in Supplemental Table 5. Compensators did not differ from noncompensators in age, weight, BMI (P values > 0.45), or the proportion of men (30.9%) and women (69.1%) (P = 0.52). Compensators and noncompensators had virtually identical adherence (mean ± SD: 99.9 ± 6.2% and 99.5 ± 6.4%, respectively; P = 0.76). At baseline, a greater (P = 0.02) proportion of compensators (31%) met criteria for metabolic syndrome compared with noncompensators (11%).
Change on outcomes by compensator status is provided in Table 3. Also, Supplemental Table 6 includes results for all questionnaire and other analyses by compensator status. As expected, compensators lost significantly less body mass compared with noncompensators (P < 0.01). Compensators had smaller increases in cardiorespiratory fitness corrected for body mass (P = 0.046) but larger reductions in fasting glucose concentrations (P = 0.04). Compensators had increased adjusted RMR (P = 0.05), RMR adjusted using the method described by Galgani and Santos (17) (P = 0.04), energy intake by adjusted DLW (P = 0.03), compensatory health beliefs (P = 0.01), cravings for sweets (P = 0.01), and hunger (P = 0.03) and prospective food consumption (P = 0.03) from retrospective VAS. Compensators had reduced preference for low-fat/high-carbohydrate foods (e.g., wild rice; P = 0.01) and smaller reductions in disinhibition (P = 0.02) measured with the Eating Inventory. Compensators had worsening sleep disturbance based on the Pittsburgh Sleep Quality Index (P = 0.02). In addition, on the SF-36, they had worsening bodily pain (P = 0.02) and physical component summary (P = 0.01) scores but improved role emotional, which reflects a decrease in role limitations due to emotional problems (P = 0.03), and mental component summary (0.84 compared with −2.50, P = 0.01) scores.
TABLE 3.
Change on the outcome variables for compensators and noncompensators1
| Variable | Noncompensators | Compensators | P value |
|---|---|---|---|
| Weight (kg) | −2.69 (−3.23, −2.16) | 0.78 (−0.25, 1.30) | <0.01* |
| BMI (kg/m2) | −0.73 (−0.98, −0.47) | 0.46 (0.20, 0.72) | <0.01* |
| Percentage body fat | −0.99 (−1.47, −0.52) | 0.20 (−0.27, 0.67) | <0.01* |
| Fat mass (kg) | −1.90 (−2.50, −1.30) | 0.49 (−0.10, 1.07) | <0.01* |
| Lean mass (kg) | −0.59 (−0.93, −0.24) | 0.17 (−0.17, 0.51) | <0.01* |
| VO2 (mL·kg-1·min-1) | 2.73 (1.65, 3.81) | 1.27 (0.22, 2.33) | 0.046* |
| Glucose (mg/dL) | 0.21 (−1.60, 2.03) | −2.33 (−4.10, −0.56) | 0.04* |
| Very vigorous2 (min/d) | −0.01 (−0.11, 0.09) | −0.10 (−0.20, 0.01) | 0.03* |
| RMR-adjusted (kcal/d) | −0.3 (−63.5, 63.0) | 85.4 (23.7, 147.1) | 0.05* |
| RMR-Galgani (kcal/d) | 0.5 (−60.7, 61.7) | 84.8 (24.6, 144.9) | 0.04* |
| Energy intake, adjusted DLW (kcal/d) | 60.4 (0.8, 120.1) | 149.3 (91.1, 207.6) | 0.03* |
| Energy intake, DLW (kcal/day) | 14.26 (−44.94, 73.47) | 144.36 (86.56, 202.16) | <0.01* |
| Compensatory health beliefs | −2.14 (−4.13, −0.15) | 1.25 (−0.68, 3.17) | 0.01* |
| Eating Inventory, disinhibition | −1.27 (−1.93, −0.61) | −0.28 (−0.92, 0.36) | 0.02* |
| FCI, sweets | −0.17 (−0.44, 0.11) | 0.31 (0.05, 0.57) | 0.01* |
| FPQ, high carbs | 0.26 (−0.18, 0.70) | −0.37 (−0.79, 0.06) | 0.03* |
| FPQ, low fat | 0.25 (−0.16, 0.66) | −0.40 (−0.79, 0.00) | 0.02* |
| FPQ, low fat/high carb | 0.40 (−0.05, 0.85) | −0.39 (−0.83, 0.05) | 0.01* |
| PSQI, sleep disturbance2 | −0.13 (−0.28, 0.03) | 0.12 (−0.03, 0.27) | 0.02* |
| SF-36, bodily pain | 1.30 (−3.72, 6.31) | −6.61 (−11.46, −1.76) | 0.02* |
| SF-36, role emotional2 | −5.18 (−10.97, 0.61) | 2.36 (−3.23, 7.96) | 0.03* |
| SF-36, physical component summary | 1.88 (0.16, 3.59) | −1.20 (−2.86, 0.46) | 0.01* |
| SF-36, mental component summary | −2.50 (−4.34, −0.67) | 0.84 (−0.93, 2.62) | 0.01* |
| VAS, retrospective, hunger | −2.31 (−7.42, 2.81) | 5.11 (0.13, 10.09) | 0.03* |
| VAS, retrospective, PCF | −3.82 (−8.15, 0.52) | 2.34 (−1.88, 6.56) | 0.03* |
Values are least-squares means (95% CIs) (n = 110; 55 compensators and 55 noncompensators). Differences in outcomes among groups were tested by ANCOVAs or nonparametric Wilcoxon tests, with adjustment for sex and age. Post hoc comparisons were Tukey–Kramer adjusted (ANCOVAs), or Dwass, Steel, Critchlow-Fligner multiple comparison analysis was used. Results are only shown for questionnaires that had a significant group by time interaction; Supplemental Table 6 includes all questionnaire results. *Significant P values (P < 0.05). RMR-adjusted values represent change in RMR adjusted for sex and age, as well as change in fat-free mass. RMR-Galgani is a measure of metabolic adaptation where regression analysis was used to adjust RMR values for age, sex, and body mass (fat mass and fat-free mass), which results in a participant's residual RMR values at baseline and week 24. Change in RMR or metabolic adaptation is then calculated by subtracting the residual at week 24 from the baseline residual. DLW, doubly labeled water; FCI, Food Craving Inventory; FPQ, food-preference questionnaire; PCF, prospective food consumption; PSQI, Pittsburg Sleep Quality Index; RMR, resting metabolic rate; SF-36, Medical Outcomes Study Short Form 36; VAS, visual analog scales.
Wilcoxon test.
Discussion
In this large randomized controlled trial with 2 doses of exercise, energy intake assessed with DLW increased significantly and as hypothesized in the 20 KKW group, as well as in the 8 KKW group, which was unexpected. The hypotheses were based on previous research demonstrating that compensation most frequently occurs at higher doses of exercise (4), although the results of this study indicate that compensation also occurs at lower doses of exercise. This discrepancy may be due to differences in the study samples since the current study recruited men and women aged 18–65 y, whereas the Church et al. (4) study recruited postmenopausal women. Change in energy intake measured with the less sensitive test meals did not differ among the groups, which also was unexpected. Significant weight compensation was detected in the 8 and 20 KKW groups. Compensation in the 8 KKW group was not predicted, although it was predicted that compensation would be significantly higher in the 20 KKW group compared with the 8 KKW group, as observed.
Although most participants in the 8 KKW (76.3%) and 20 KKW (90.2%) groups compensated, there was great heterogeneity in weight loss and compensation with both exercise doses. Large individual variability in weight loss in response to exercise has been observed in other studies, including a 12-wk single-dose exercise study with 35 participants (7). Almost half (42.4%) and 23.5% of participants in the 8 and 20 KKW groups, respectively, did not lose any weight or gained weight. These percentages are much higher than the 13% of participants who did the same in the 12-wk exercise study by King et al. (7), thus suggesting that longer durations of exercise increase compensatory responses. This conclusion is further supported by Church et al. (4), who found that compensation, assessed on a weekly basis, did not occur until approximately week 12. Very few participants in the exercise groups lost more weight than expected. Participants in the 8 and 20 KKW groups lost only 36.2% and 40.8% of predicted weight loss, respectively, which is similar to previous studies (34), including ours, although previously we had not always found compensation at lower exercise doses (4, 5). Body fat loss was greater in the 20 KKW group compared to control, and change in lean mass did not differ among groups. Compensation appears to be a response to long-term exercise training because short-term studies (e.g., 13 wk) show smaller compensation only at higher doses (35) and acute studies (e.g., 2 d) show no compensation (36). Weight change in the control group was minimal (−0.2 kg), and participants in all groups were not instructed to change their diet.
To our knowledge, this is one of the first studies to prospectively attempt to identify the mechanisms of compensation by assessing changes in energy intake, metabolism, and self-report measures of appetite and other constructs. The study found that compensation appears to be primarily the result of increased energy intake and not changes in metabolism/energy expenditure, RQ, or non-exercise physical activity. Energy intake by DLW increased by 90.7 and 123.6 kcal/d (3.9% and 5.5%) in the 8 and 20 KKW groups, respectively. Increases of this magnitude are difficult to detect over the short term, and energy intake is known to vary widely from day to day (37, 38), indicating that long-term measures of energy intake are optimal. This may have contributed to the failure to detect increased energy intake with test meals and is consistent with the results of King et al. (7), who found that energy intake measured with test meals did not increase among the entire sample of exercisers (energy intake and hunger did increase among compensators in their study, however). Another interpretation is that increased energy intake likely occurs outside of meals (e.g., from snacking or drinking calorie-containing beverages between meals). Finally, it can be difficult for people to detect changes in daily energy intake of this size (∼100 kcal), which could contribute to unawareness of compensation and hence the inability to address it.
Changes in RQ, RMR, and non-exercise physical activity were similar by group, the latter of which is consistent with results from other studies (39, 40). A review concluded that exercise does not result in decreased non-exercise activity or energy expenditure (41). At the exercise doses achieved in this study, people do not appear to become more sedentary outside of structured exercise after they begin to exercise, although it is possible that activities of daily living limit the ability to detect such an effect.
More participants in the exercise groups classified as compensators met the criteria for metabolic syndrome compared with noncompensators (31% compared with 11%, respectively), suggesting that the compensatory increase in energy intake might be related to the predisposition to diabetes and insulin insensitivity. Also, compensators had a smaller response to exercise in terms of improved cardiorespiratory fitness compared with noncompensators, but they had larger reductions in glucose levels, although the difference was small (2.5 mg/dL). Compensators had an increase in adjusted RMR, indicating that their bodies were expending more energy relative to body mass after exercise training, which could be a physiological response to increased energy intake, or they had longer lasting excess post-exercise oxygen consumption (42). Increased RMR, adjusted using the methods described by Galgani and Santos (17), was also observed in the compensators compared with the noncompensators. Nonetheless, the Galgani values were also higher at baseline in the compensators than in the noncompensators (Supplemental Table 5), whereas baseline RMR did not differ by compensator status when adjusted only for fat-free mass (data not shown). Thus, compensators could have higher relative RMR regardless of exercise levels, and adjustments to RMR beyond fat-free mass are needed to detect such differences.
Supporting the conclusion that compensation is due to increased energy intake, compensators had an ∼90 kcal/d larger increase in energy intake and increased cravings for sweets, hunger, prospective food consumption, and compensatory health beliefs (and a smaller reduction in disinhibition). The results suggest that compensators might 1) differ in important ways prior to exercise that contribute to a differential response, 2) not experience some physiological (e.g., increased cardiorespiratory fitness) benefits of exercise that lead to exercise being more difficult/aversive, 3) have different psychological responses to exercise (e.g., increased compensatory beliefs and smaller reductions in disinhibition) that affect other health behaviors, and 4) experience an increased drive to eat. Furthermore, the results indicate that compensators, who one could argue are most in need of exercise based on the higher prevalence of the metabolic syndrome, are most likely to fail to fully realize the benefits of exercise and possibly discontinue exercise due to the perceived lack of benefit or aversive aspects of exercise.
The results suggest that clinicians should include a concomitant lifestyle, dietary, or possibly pharmacological intervention when prescribing exercise if weight loss is the goal. Further research is needed to determine what interventions are most effective at reducing compensation and improving the beneficial response to exercise, and many of the constructs that were different between compensators and noncompensators (e.g., change in disinhibition and cravings) can be targeted by various interventions.
Study strengths include being the first prospective randomized controlled trial powered to identify the mechanisms by which exercise fails to produce the expected level of weight loss. Also, energy intake, energy expenditure, and activity were measured with gold standard techniques, and questionnaires assessed constructs associated with health behaviors and health-related quality of life. Finally, exercise adherence was excellent, and the dose of exercise achieved was carefully quantified. Weaknesses include lack of data regarding what foods participants ate and when, as well as the fact that the majority of the sample was composed of women, although the randomization procedures considered sex.
In conclusion, in this 6-mo randomized controlled exercise trial, participants in the exercise groups compensated and lost less weight than expected. Compensation was found to primarily be the result of increased energy intake and not changes in metabolism/energy expenditure or non-exercise physical activity. Participants who compensated were more likely to meet criteria for metabolic syndrome, responded differently to exercise, and reported increased appetite and compensatory health beliefs. These findings provide insight into how to reduce compensation during long-term exercise.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—TSC and WDJ: had full access to all of the study data and take responsibility for the integrity of the data and the accuracy of the data analyses; CKM and TSC: conception and design, data collection and analysis, writing of the manuscript, and final approval of the manuscript; CAM, JWA, CPE, DMT, JCR, NMJ, CT-L, DSH, and MH: reviewed, edited, and approved the manuscript; all authors: contributed to the final version of the manuscript and were responsible for conducting the trial, analyzing and interpreting data, and preparing the results for publication; and all authors: read and approved the final manuscript. Medical monitoring was completed by Timothy S. Church and Daniel S. Hsia. CPE is a scientific consultant for Catapult Health, Naturally Slim, and ACAP Health. All other authors report no conflicts of interest related to this study.
Notes
Research reported in this article was supported by the NIH via the National Heart, Lung, and Blood Institute, with the Multiple Principal Investigators being CKM and TSC (R01 HL102166); NORC Center grant P30 DK072476, titled “Nutritional Programming: Environmental and Molecular Interactions,” sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute of General Medical Sciences, which funds the Louisiana Clinical and Translational Science Center (U54 GM104940); and NIH grant F32 HL123242.
The sponsor had no role in the design, data collection, data analysis, interpretation of results, or preparation of the results for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplemental Methods, Supplemental Tables 1–6, and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: DLW, doubly labeled water; E-MECHANIC, Examination of Mechanisms of Exercise-Induced Weight Compensation; RMR, resting metabolic rate; RQ, respiratory quotient; SF-36, Medical Outcomes Study Short Form 36; VAS, visual analog scales; VO2, peak oxygen uptake.
References
- 1. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF et al.. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States 2011–2012. JAMA. 2014;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ross R, Janssen I. Physical activity, total and regional obesity: dose–response considerations. Med Sci Sports Exerc. 2001;33(6 Suppl):S521–9. [DOI] [PubMed] [Google Scholar]
- 4. Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Church TS, Earnest CP, Thompson AM, Priest EL, Rodarte RQ, Saunders T, Ross R, Blair SN. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports Exerc. 2010;42(4):708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite?. Proc Nutr Soc. 2003;62(3):651–61. [DOI] [PubMed] [Google Scholar]
- 7. King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes (2005). 2008;32(1):177–84. [DOI] [PubMed] [Google Scholar]
- 8. Myers CA, Johnson WD, Earnest CP, Rood JC, Tudor-Locke C, Johannsen NM, Cocreham S, Harris M, Church TS, Martin CK. Examination of Mechanisms (E-MECHANIC) of Exercise-Induced Weight Compensation: Study protocol for a randomized controlled trial. Trials. 2014;15:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Institute of Medicine. Adequacy of Evidence for Physical Activity Guidelines Development: Workshop Summary. Washington (DC): National Academies Press; 2007. [Google Scholar]
- 10. American College of Sports Medicine. American College of Sports Medicine's Guidelines for Exercise Testing and Prescription, 9th ed.;Philadelphia (PA): Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 11. Thomas DM, Martin CK, Lettieri S, Bredlau C, Kaiser K, Church T, Bouchard C, Heymsfield SB. Can a weight loss of one pound a week be achieved with a 3500-kcal deficit? Commentary on a commonly accepted rule. Int J Obes. 2013;37(12):1611–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, Martin CK, Silva AM, Vossen M, Westerterp K et al.. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev. 2012;13(10):835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heymsfield SB, Thomas D, Nguyen AM, Peng JZ, Martin C, Shen W, Strauss B, Bosy-Westphal A, Muller MJ. Voluntary weight loss: systematic review of early phase body composition changes. Obes Rev. 2011;12:e348–61. [DOI] [PubMed] [Google Scholar]
- 14. Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(Suppl 3):895S–920S. [DOI] [PubMed] [Google Scholar]
- 15. Schoeller DA. How accurate is self-reported dietary energy intake?. Nutr Rev. 1990;48(10):373–9. [DOI] [PubMed] [Google Scholar]
- 16. Martin CK, Coulon SM, Markward N, Greenway FL, Anton SD. Association between energy intake and viewing television, distractibility, and memory for advertisements. Am J Clin Nutr. 2009;89(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galgani JE, Santos JL. Insights about weight loss-induced metabolic adaptation. Obesity. 2016;24(2):277–8. [DOI] [PubMed] [Google Scholar]
- 18. St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85(3):742–9. [DOI] [PubMed] [Google Scholar]
- 19. Welk GJ, McClain JJ, Eisenmann JC, Wickel EE. Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity. 2007;15(4):918–28. [DOI] [PubMed] [Google Scholar]
- 20. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 21. Womble LG, Wadden TA, Chandler JM, Martin AR. Agreement between weekly vs. daily assessment of appetite. Appetite. 2003;40(2):131–5. [DOI] [PubMed] [Google Scholar]
- 22. Anderson DA, Williamson DA, Duchmann EG, Gleaves DH, Barbin JM. Development and validation of a multifactorial treatment outcome measure for eating disorders. Assessment. 1999;6(1):7–20. [DOI] [PubMed] [Google Scholar]
- 23. Stunkard AJ, Messick S. Eating Inventory Manual (The Psychological Corporation). San Antonio (TX): Harcourt Brace; 1988. [Google Scholar]
- 24. Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav. 1998;63(5):919–28. [DOI] [PubMed] [Google Scholar]
- 25. White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10(2):107–14. [DOI] [PubMed] [Google Scholar]
- 26. Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52(2):430–6. [DOI] [PubMed] [Google Scholar]
- 27. Anderson SE, Bandini LG, Dietz WH, Must A. Relationship between temperament, nonresting energy expenditure, body composition, and physical activity in girls. Int J Obes Relat Metab Disord. 2004;28(2):300–6. [DOI] [PubMed] [Google Scholar]
- 28. Thomas A, Chess S. Temperament and Development. New York: Brunner/Mazel; 1977. [Google Scholar]
- 29. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 30. Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychom Res. 1998;45(1):5–13. [DOI] [PubMed] [Google Scholar]
- 31. Knauper B, Rabiau M, Cohen O, Patriciu N. Compensatory health beliefs: scale development and psychometric properties. Psychol Health. 2004;19(5):607–24. [Google Scholar]
- 32. Stewart TM, Williamson DA, Allen HR, Han H. The Body Morph Assessment Version 2.0 (BMA 2.0): psychometrics and interesting findings. ObesityResearch 2005;13(Program Abstract Suppl):A129. [Google Scholar]
- 33. Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med. 1982;3(3):153–8. [DOI] [PubMed] [Google Scholar]
- 34. Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, Heelan K, Hise M, Fennessey PV, Sonko B et al.. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163(11):1343–50. [DOI] [PubMed] [Google Scholar]
- 35. Rosenkilde M, Auerbach P, Reichkendler MH, Ploug T, Stallknecht BM, Sjodin A. Body fat loss and compensatory mechanisms in response to different doses of aerobic exercise—a randomized controlled trial in overweight sedentary males. Am J Physiol Regul Integr Comp Physiol. 2012;303(6):R571–9. [DOI] [PubMed] [Google Scholar]
- 36. Douglas JA, King JA, McFarlane E, Baker L, Bradley C, Crouch N, Hill D, Stensel DJ. Appetite, appetite hormone and energy intake responses to two consecutive days of aerobic exercise in healthy young men. Appetite. 2015;92:57–65. [DOI] [PubMed] [Google Scholar]
- 37. Apolzan JW, Bray GA, Hamilton MT, Zderic TW, Champagne CM, Shepard D, Martin CK. Short-term overeating results in incomplete energy intake compensation regardless of energy density or macronutrient composition. Obesity. 2014;22(1):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bray GA, Flatt JP, Volaufova J, Delany JP, Champagne CM. Corrective responses in human food intake identified from an analysis of 7-d food-intake records. Am J Clin Nutr. 2008;88(6):1504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hollowell RP, Willis LH, Slentz CA, Topping JD, Bhakpar M, Kraus WE. Effects of exercise training amount on physical activity energy expenditure. Med Sci Sports Exerc. 2009;41(8):1640–4. [DOI] [PubMed] [Google Scholar]
- 40. Rangan VV, Willis LH, Slentz CA, Bateman LA, Shields AT, Houmard JA, Kraus WE. Effects of an 8-month exercise training program on off-exercise physical activity. Med Sci Sports Exerc. 2011;43(9):1744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Washburn RA, Lambourne K, Szabo AN, Herrmann SD, Honas JJ, Donnelly JE. Does increased prescribed exercise alter non-exercise physical activity/energy expenditure in healthy adults? A systematic review. Clinical Obesity. 2014;4(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc. 2003;62(3):621–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.