Abstract
Gilt progeny (GP) often have restricted growth performance and health status in comparison to sow progeny (SP) from birth, with the underlying mechanisms responsible for this yet to be fully understood. The present study aimed to compare differences in growth and development between GP and SP in the first 24 h after birth and in the periweaning period. Two cohorts of pigs including 36 GP and 37 SP were euthanized at 1 of 4 time points: a birth cohort (at birth before suckling, 0 h; and 24 h after birth, 24 h; n = 33) and a weaning cohort (at approximately 29 d of age; “pre-weaning,” PrW; and 24 h after weaning; “post-weaning,” PoW; n = 40). Pigs were individually weighed at 0 h, 24 h, PrW, and PoW up until the point of euthanasia, at which time the weights of selected tissues and organs were recorded and analyzed relative to BW. The length of the small intestine (SI), femur, and body were also measured, and a serum sample was collected and analyzed for IgG concentration. Samples of jejunal and ileal mucosa were collected and analyzed for total protein and specific activity of lactase. Euthanized GP were lighter (P < 0.01) than SP at all time points. At all time points, the ratios of quadriceps weight to femur length, BW to body length, spleen to BW (all P < 0.05), and SI weight to length (P < 0.10) were lower in GP than in SP. There was no difference (P ≥ 0.05) in stomach or heart to BW ratios between GP and SP in either cohort. The brain to liver weight ratio was greater (P = 0.044) in GP than in SP in the birth cohort, and the brain to BW ratio was greater (P < 0.01) in GP in both the birth and weaning cohorts. The liver to BW ratio was similar (P = 0.35) at birth but greater (P = 0.014) in GP around weaning. Total mucosal protein content in the jejunum and ileum was lower (P = 0.007) in GP at 24 h compared with SP, and specific activity of lactase was greater (P = 0.022) in GP in the birth cohort, whereas there were no differences in the weaning cohort (P ≥ 0.10). Gilt progeny had lower (P < 0.001) serum IgG concentration compared with SP at 24 h, but there was no difference (P ≥ 0.10) in the weaning cohort. Collectively, these findings suggest that the early development of GP may be delayed compared with SP and that a number of the anatomical differences between GP and SP that exist after birth are also present at weaning.
Keywords: gilt progeny, lactation, parity, pig, sow progeny, weaning
Introduction
On average, gilt progeny (GP) are born and wean lighter than sow progeny (SP; Carney-Hinkle et al., 2013; Craig et al., 2017a, b), which persists as slower growth to slaughter (Craig et al., 2017b). Light-for-age pigs are less likely to ingest colostrum and acquire sufficient maternal immunity for survival (Rooke and Bland, 2002). This reduction in live weight in GP, along with compromised immune status of these animals (Klobasa et al., 1986; Carney-Hinkle et al., 2013), are thought to be the major contributors to higher mortality rates seen in GP before and after weaning (Miller et al., 2012b; Craig et al., 2017b). Reduction in the acquisition of maternal immunity in GP may be due to the lower immunity of the comparatively naive primiparous dam (Klobasa et al., 1985). Alternatively, if primiparous sows have lower colostrum and milk yields, GP may have lower intakes (King, 2000; Quesnel, 2011), and (or) GP may have reduced gastrointestinal tract (GIT) function resulting in limitation of their ability to absorb nutrients from colostrum and milk (Cottrell et al., 2017). To investigate possible mechanisms for these differences, this study examined anatomical and GIT functional development of GP and SP in the first 24 h after birth and during the periweaning period. It is in these critical stages where colostrum and milk ingestion is vital for survival (Rooke and Bland, 2002) and where a number of stressors are known to affect physiological development of the piglet (Pluske et al., 1997; Dunshea, 2003; Baxter et al., 2008). It was hypothesized that, in addition to having slower rates of growth, GP would have reduced anatomical development of several organs and delays in early functional development of the GIT compared with SP around birth and weaning.
MATERIALS AND METHODS
All experimental procedures were approved by both the Rivalea (Australia) Animal Care and Ethics Committee (protocol number 16P014) and the Murdoch University Animal Ethics Committee (protocol number N2847/16) in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council, 2013).
Experimental Design
The experiment was conducted under commercial conditions at a piggery in Corowa, NSW (Rivalea Australia Pty Ltd, Australia). Thirty-six entire male and 37 female piglets born to 41 Large White × Landrace F1 sows (Primegro genetics, NSW, Australia) were selected for the experiment. This consisted of 36 piglets born to 16 primiparous (parity 1) sows and 37 born to 25 multiparous sows (parities 3 and 4), with a minimum of 1 and a maximum of 4 piglets sampled from each dam. In the birth cohort, total piglets born per pregnancy were 15.0 ± 2.1 and 12.8 ± 1.8 (mean ± SD) for primiparous and multiparous sows, respectively, and total piglets born alive were 13.9 ± 2.3 and 12.4 ± 1.6 for primiparous and multiparous sows, respectively. In the weaning cohort, these figures were 12.9 ± 3.7, 13.0 ± 3.3, 12.0 ± 3.4, and 11.8 ± 2.7 piglets, respectively. Piglets were euthanized, tissue samples were collected, and morphological traits of various organs were measured (see Piglet Euthanasia and Gross Anatomical Measurements section) either at birth (0 h), 24 h after birth (24 h), before weaning (“pre-weaning,” at approximately 29 d of age; PrW), or 24 h after weaning (“post-weaning,” PoW). Live weights of all pigs were measured at 0 h, 24 h, PrW, and PoW for each pig up until the time of euthanasia. Two separate cohorts of piglets were used for the experiment, including a birth cohort (0 and 24 h) and a weaning cohort (PrW and PoW). The experiment was performed over an intensive 2-wk period, with piglets in the weaning cohort born in March and piglets in the birth cohort born in April, such that piglets from both groups were euthanized in April, with both dam parity treatments and sexes represented within each cohort.
For the birth cohort of progeny (0 and 24 h), dams were selected at parturition based on parity and the time of piglet birth was recorded. For the 0-h time point, piglets were opportunistically selected immediately after birth (within 1 h, before suckling), their birth weight (BWT) recorded, and immediately transported to an on-farm facility for euthanasia and dissection. For the 24-h time point, piglets were selected immediately after birth (within 1 h, before suckling), individually numbered with a marker, and BWT and time of day recorded before being returned to their farrowing pen to suckle. Exactly 24 h later, piglets were weighed again (24WT) and transported for euthanasia and dissection. Piglets were selected based on their representation of an average BWT piglet in that litter.
Within the weaning cohort of progeny (PrW and PoW), sows were selected for the experiment before farrowing based on parity. At farrowing, up to 6 pigs per litter of average weight and conformation were weighed (BWT) and individually tagged. These pigs were then weighed again at 24 h after birth (24WT) and left to suckle their birth dams throughout lactation. Piglets allocated for euthanasia at the PrW and PoW time points were then selected from these tagged piglets around the time of weaning (28.6 ± 0.1 d; mean ± SE), with PrW piglets weighed and euthanized before weaning (28.7 ± 0.2 d) and PoW piglets weighed and weaned (28.6 ± 0.2 d) from their dams, then weighed again and euthanized exactly 24 h after weaning. The number of piglets in each cohort was balanced between dam parity treatment (GP or SP), time point (0 h, 24 h, PrW, or PoW), and sex, with 5 pigs per group allocated where possible depending on the timing of natural farrowings and availability of dissection facilities (Table 1). Piglets were all euthanized over a period of 11 d, with sampling time randomly based on availability of facilities and farrowing times, as timing of parturition was natural and not induced in experimental gilts and sows.
Table 1.
Number of animals (N) euthanized and number of observations for each parameter (n) within each dam parity (sow progeny, SP; or gilt progeny, GP), sex (male, M; or female, F), and time point
| n | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dam parity | SP | GP | ||||||||||||||
| Sex | M | F | M | F | ||||||||||||
| Timepoint1 | 0 h | 24 h | PrW | PoW | 0 h | 24 h | PrW | PoW | 0 h | 24 h | PrW | PoW | 0 h | 24 h | PrW | PoW |
| Total N | 4 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 2 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
| Parameter | ||||||||||||||||
| Piglet liveweight | ||||||||||||||||
| BW at 0 h | 4 | 4 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 4 | 5 | 5 | 4 | 4 | 5 | 5 |
| BW at 24 h | — | 5 | 5 | 5 | — | 3 | 5 | 5 | — | 5 | 5 | 5 | — | 5 | 5 | 5 |
| 24-h BW gain | — | 4 | 5 | 5 | — | 3 | 5 | 5 | — | 4 | 5 | 5 | — | 4 | 5 | 5 |
| BW at PrW | — | — | 5 | 5 | — | — | 5 | 5 | — | — | 5 | 5 | — | — | 5 | 5 |
| BW at PoW | — | — | — | 5 | — | — | — | 5 | — | — | — | 5 | — | — | — | 5 |
| Musculoskeletal | ||||||||||||||||
| Quadriceps weight (QD) | 4 | 5 | 4 | 4 | 5 | 3 | 5 | 5 | 2 | 4 | 5 | 4 | 4 | 5 | 5 | 4 |
| Femur length (FEM) | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 5 | 5 | 4 | 4 | 5 | 5 | 5 |
| QD:FEM | 4 | 5 | 4 | 4 | 5 | 3 | 5 | 5 | 2 | 4 | 5 | 4 | 4 | 5 | 5 | 4 |
| Body length (BL) | — | — | 5 | 5 | — | — | 5 | 5 | — | — | 5 | 5 | — | — | 5 | 5 |
| BW:BL | — | — | 5 | 5 | — | — | 5 | 5 | — | — | 5 | 5 | — | — | 5 | 5 |
| Gastrointestinal tract | ||||||||||||||||
| Stomach weight (STOM) | 3 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 1 | 4 | 5 | 5 | 4 | 5 | 5 | 5 |
| STOM:BW | 3 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 1 | 4 | 5 | 5 | 4 | 5 | 5 | 5 |
| SI weight (SIWT)2 | 4 | 5 | 5 | 5 | 3 | 3 | 5 | 4 | 2 | 5 | 4 | 5 | 4 | 5 | 5 | 4 |
| SI length (SIL) | 4 | 5 | 5 | 5 | 4 | 3 | 4 | 4 | 2 | 5 | 4 | 5 | 4 | 5 | 5 | 5 |
| SIWT:SIL | 4 | 5 | 5 | 5 | 3 | 3 | 4 | 4 | 2 | 5 | 4 | 5 | 4 | 5 | 5 | 4 |
| Liver weight (LIV) | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 5 | 5 | 5 | 3 | 5 | 5 | 5 |
| LIV:BW | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
| Total protein per g SI mucosa3 | 8 | 10 | 10 | 10 | 10 | 8 | 10 | 10 | 4 | 7 | 9 | 10 | 8 | 10 | 10 | 8 |
| Specific lactase activity in SI3 | 8 | 10 | 10 | 10 | 10 | 8 | 10 | 10 | 4 | 7 | 9 | 10 | 8 | 10 | 10 | 8 |
| Other | ||||||||||||||||
| Brain weight (BR) | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 5 | 4 | 5 | 4 | 5 | 5 | 5 |
| BR:LIV | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 5 | 4 | 5 | 3 | 5 | 5 | 5 |
| BR:BW | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 5 | 4 | 5 | 3 | 5 | 5 | 5 |
| Heart weight (HRT) | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
| HRT:BW | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 2 | 5 | 4 | 5 | 4 | 5 | 5 | 5 |
| Spleen weight (SPL) | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 4 | 2 | 4 | 5 | 5 | 4 | 5 | 5 | 5 |
| SPL:BW | 4 | 5 | 5 | 5 | 5 | 3 | 5 | 4 | 2 | 4 | 5 | 5 | 4 | 5 | 5 | 5 |
| Serum IgG concentration | 4 | 5 | 5 | 5 | 5 | 4 | 4 | 5 | 2 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
1Pigs were euthanized at 4 different time points within a birth cohort (at birth before suckling colostrum, 0 h; and at 24 h after birth, 24 h) and a weaning cohort (pre-weaning, approximately 29 d of age, PrW; and 24 h post-weaning, PoW).
2SI = small intestine (jejunum and ileum).
3Two samples (jejunum and ileum) per animal.
Animal Management
Sows were moved into the farrowing house approximately 8 d before their expected farrow date and housed in individual farrowing crates. Sows within the birth cohort farrowed over 3 farrowing sheds, whereas sows within the weaning cohort were all located within the 1 shed. Each farrowing crate was fitted with drinker nipples for both the sows and piglets, and a creep area fitted with a heat lamp. Piglets were tail docked and given 200 mg of Fe (Gleptosil; Champion Alstoe, ON, Canada) and 2 mL of a mycoplasma vaccine (RespiSure One; Zoetis, NSW, Australia) via i.m. injection 2 d after birth. All sows were fed a common gestation [averaging 12.9 MJ DE/kg; 12.8% CP; 0.5% standardized ileal digestible (SID) Lys; as-fed basis] and lactation (14.8 MJ DE/kg; 16.9% CP; 0.9% SID Lys) diet throughout the experiment. Piglets were not provided access to creep feed and were vaccinated against porcine circovirus type 2 (Ingelvac CircoFLEX; Boehringer Ingelheim, NSW, Australia) approximately 1 wk before weaning. Although minimal cross-fostering was practiced soon after parturition to standardize litters to 10 to 12 piglets, fostered piglets were not included in the experiment and experimental piglets were left to suckle their birth dam for the entirety of the suckling period up until weaning. Piglets euthanized at the PoW time point were weaned and housed until euthanasia in individual metabolism pens (0.5 m × 0.85 m) each fitted with a drinker nipple and a heat lamp shared between every 2 pens. These pigs were given ad libitum access to a commercial starter diet (averaging 14.5 MJ DE/kg; 17.4% CP; 1.3% SID Lys) during the 24 h post-weaning period.
Piglet Euthanasia and Gross Anatomical Measurements
Pigs were initially sedated with an intraperitoneal (i.p.) injection of 17 mg/kg of BW of xylazine hydrochloride (Ilium Xylazil-100; Troy Laboratories Pty Ltd, NSW, Australia) before being administered an anesthetic dose of 0.1 mL/kg i.p. sodium pentobarbitone (Lethabarb; Virbac, NSW, Australia) and finally a lethal dose of 0.5 mL/kg intra-cardiac sodium pentobarbitone. Two separate doses of sodium pentobarbitone were administered to retrieve a blood sample via jugular venepuncture from each piglet before full euthanasia, and a cardiac blood sample was collected after euthanasia. All euthanasia procedures were carried out by a veterinarian. Blood samples were collected into vacuum tubes containing EDTA (BD Vacutainer, Macquarie Park NSW, Australia). Samples were then refrigerated and left to clot for a minimum of 2 h and then centrifuged at 3,000 × g for 10 min at 4 °C to obtain serum. Serum samples were then transferred to a separate tube and stored at −80 °C until further analysis. A midline incision was made using a scalpel from the sternum to the pubis, and the GIT, its accessory organs, and selected muscles were gently removed. Absolute weights of major organs were recorded using a digital weighing scale, after being emptied of their contents and flushed with cold saline. Weights of the quadriceps muscle, stomach, small intestine (SI; excluding duodenum), liver, brain, heart, and spleen were recorded. The brain was separated from the lower brain stem/upper spinal cord at the foramen magnum and absolute brain weight included the cerebellum, olfactory bulbs, and the majority of the lower brain stem. The length of the SI (excluding the duodenum) and femur bone (excluding cartilage) were also measured. The jejunum and ileum of the SI from the duodenojejunal flexure to the ileocecal junction was removed and rinsed with cold 0.01 M PBS (pH 7.2). Approximately 5-cm sections of proximal jejunum and distal ileum from each terminal 15 cm of SI were collected and the mucosal layer scraped off using a ruler. Mucosa samples were flash-frozen in liquid nitrogen and stored at −80 °C until further analysis.
Biological Assays
Cardiac serum samples were analyzed for the birth cohort and jugular serum samples were analyzed for the weaning cohort. As cardiac serum samples taken from the birth cohort showed a high amount of hemolysis, they were treated just before laboratory analysis to remove hemoglobin using a commercial product (hemoglobind; Biotech Support Group, Monmouth Junction, NJ). As per the manufacturer’s instructions, 250 µL of hemoglobind was added to 250 µL of hemolyzed serum, vortexed for 30 s before mixing via inversion for 10 min, and then centrifuged at 6,000 × g for 2 min at 4 °C. Purified serum was then aspirated and assessed in duplicate for IgG concentration along with the jugular serum samples from the weaning cohort using a commercial ELISA kit (Bethyl Laboratories, Montgomery, TX). The intra-assay CV for the ELISA was 12.1% and the interassay CV was 9.1%.
Scrapings of mucosa from the proximal jejunum and the distal ileum were also assayed for specific activity of lactase. Approximately 0.1 g of each frozen mucosal scraping was suspended in 1.5 mL of PBS and homogenized in a benchtop homogenizer (FastPrep-24, MP Biomedicals, Seven Hills, NSW, Australia). Tubes were then centrifuged at 21,000 × g for 20 min at 4 °C, and homogenates aspirated and refrozen at −20 °C until analysis. The specific activity of lactase was measured by the liberation of glucose from lactose in the methods previously described by Dudley et al. (1992, 1994). Homogenates were diluted 1:10 with PBS and 600 mM lactose solution containing 0.13 mM p-hydroxymercuribenzoate (Sigma–Aldrich, St Louis, MO) was added. After 60 min the reaction was stopped by the addition of 200 µL each of 1.8% Ba(OH)2 and 2% ZnSO4 and the amount of glucose liberated was measured using the Infinity Glucose Oxidase Liquid Stable Reagent (Thermo Fisher, Waltham, MA). For the final glucose assay, the intra-assay CV was 1.5%, and the interassay CV was 7.4%. The assay was performed in triplicate for each homogenized sample. Total protein was determined for each homogenate, with the sample further diluted 1:10 in PBS using the Pierce BCA assay kit (Thermo Fisher, Waltham, MA). The intra- and interassay CVs for this assay were 1.9% and 7.6%, respectively. Specific lactase activity was normalized to protein and expressed as micromole of glucose liberated per minute per gram of protein.
Statistical Analysis
Data were analyzed as a linear mixed model using the MIXED procedure of SPSS (IBM SPSS Version 21.0; IBM, Armonk, NY) with the individual piglet as the experimental unit and dam parity group (GP vs. SP), time point (0 h vs. 24 h in the birth cohort; PrW vs. PoW in the weaning cohort), and piglet sex as fixed factors. Data from the birth and weaning cohorts were analyzed separately such that each model was a 2 × 2 × 2 factorial comparison, except in the case of measures at 24 h (birth cohort) and at PoW (weaning cohort), where a dam parity group × sex factorial comparison was carried out. Sex and any of its interactions were removed from the model if their effect was not significant (P < 0.05) and not of primary interest to the hypothesis, using the backwards elimination procedure (Field, 2013). Specific activity of lactase and total mucosal protein were also analyzed with intestinal section (jejunum vs. ileum) as an additional fixed factor, such that a 2 × 2 × 2 × 2 factorial arrangement was employed. All absolute tissue and organ weights were analyzed, as well as the relative weights of the stomach (STM:BW), liver (LIV:BW), brain (BR:BW), heart (HRT:BW), and spleen (SPL:BW), expressed relative to piglet BW. In addition, quadriceps weight and femur length were expressed as a ratio (QD:FEM, g/cm), along with SI weight to length ratio (SIWT:SIL, g/cm), brain to liver weight ratio (BR:LIV, g/g), and BW to body length ratio (BW:BL, g/cm; weaning cohort only).
Random effects (e.g., farrowing shed, litter, and day of euthanasia) did not have a significant effect (P ≥ 0.05) on any parameter studied and were therefore not included in the overall model. Between-treatment comparisons from significant interaction effects were carried out using simple effects analysis in SPSS syntax using the COMPARE function. The number of individual pig observations (n) for each trait after editing for outliers is represented in Table 1. Extreme outliers were removed if they were greater than 2 SD from the group mean and were deemed to be aberrant data. A P-value of <0.05 was considered significant, and a P-value of <0.10 was considered a trend. Estimates are reported herein as least square mean (LSM) ± SEM.
RESULTS
The 3-way interaction of dam parity group × time point × sex was not significant (P ≥ 0.05) for any parameter in either cohort and was therefore excluded from all models. Sex and its interactions were only significant for SPL and SPL:BW in the weaning cohort (dam parity group × sex interaction; P = 0.036 and P = 0.038, respectively) and for SIL and STOM:BW in the weaning cohort (main effect of sex; P = 0.045 and P = 0.049, respectively), and were otherwise removed from all other models. Only the time point × section interaction for specific lactase activity in the weaning cohort contributed to the model (P < 0.05), and therefore, all other interactions involving SI section were removed from the analysis of total mucosal protein and specific lactase activity in the SI. The dam parity × time point interaction was only significant (P < 0.05) if stated herein but was left in the model regardless of whether it made a significant contribution or not.
Piglet Liveweight and 24-h BW Gain
In the birth cohort, BW was significantly lower in GP compared with SP at 0 h (1.20 ± 0.07 vs. 1.57 ± 0.06 kg, respectively; P < 0.001) and at 24 h (1.32 ± 0.07 vs. 1.70 ± 0.08 kg, respectively; P = 0.004). In the weaning cohort, BW was not significantly different (P = 0.12) between GP and SP at 0 h (1.41 ± 0.06 vs. 1.55 ± 0.06 kg; respectively), but GP tended (P = 0.099) to be lighter than SP at 24 h (1.51 ± 0.06 vs. 1.66 ± 0.06 kg, respectively), and pigs selected for the PrW time point were lighter than piglets selected for the PoW time point at 0 h (1.39 ± 0.06 vs. 1.56 ± 0.06 kg, respectively; P = 0.060) and at 24 h (1.48 ± 0.06 vs. 1.70 ± 0.06 kg, respectively; P = 0.019). Gilt progeny were lighter than SP at PrW (7.54 ± 0.30 vs. 8.97 ± 0.30 kg, respectively; P = 0.002) and PoW (6.95 ± 0.41 vs. 8.88 ± 0.41 kg, respectively; P = 0.004). Average BW gain in the first 24 h was not different (P ≥ 0.10) between GP and SP in the birth cohort (115 ± 16 vs. 135 ± 17 g, respectively) or the weaning cohort (101 ± 18 vs. 115 ± 18 g, respectively). Pigs selected for the PrW time point tended (P = 0.057) to gain less weight in the first 24 h than pigs selected for the PoW time point (83 ± 18 vs. 133 ± 18 g, respectively).
Musculoskeletal System
Gilt progeny had a heavier (P = 0.007; Table 2) quadriceps muscle than SP in the birth cohort and in the weaning cohort (P = 0.006; Table 3). Femur length was not different between GP and SP in either the birth (P = 0.29; Table 2) or the weaning (P = 0.82; Table 3) cohorts. Gilt progeny had a lower QD:FEM than SP both in the birth cohort (0.83 ± 0.04 vs. 1.00 ± 0.03 g/cm, respectively; P = 0.002) and the weaning cohort (3.69 ± 0.19 vs. 4.48 ± 0.19 g/cm, respectively; P = 0.005). Body length was greater (P = 0.005) in SP than in GP in the weaning cohort and increased (P = 0.023) between PrW and PoW (Table 3). Values for BW:BL were greater (P = 0.010) in SP (0.141 ± 0.004 g/cm) than in GP (0.126 ± 0.004 g/cm) and decreased (P = 0.037) from PrW to PoW (data not shown).
Table 2.
Least square means (± SE) from the linear mixed model analysis of absolute tissue and organ weights and other absolute organ dissection parameters for the birth cohort
| Factor1 | ||||||
|---|---|---|---|---|---|---|
| SP | GP | P-value2 | ||||
| Trait3 | 0 h | 24 h | 0 h | 24 h | D | T |
| Musculoskeletal | ||||||
| Quadriceps, g | 3.9 ± 0.2 | 4.2 ± 0.3 | 3.3 ± 0.3 | 3.3 ± 0.2 | *** | ns |
| Femur length, cm | 4.0 ± 0.1 | 4.0 ± 0.1 | 3.9 ± 0.1 | 4.0 ± 0.1 | ns | ns |
| Gastrointestinal tract | ||||||
| Stomach, g | 7.6 ± 0.5 | 9.6 ± 0.5 | 5.5 ± 0.6 | 7.8 ± 0.5 | *** | *** |
| SI weight, g | 41.8 ± 6.1 | 77.5 ± 5.7 | 40.3 ± 6.6 | 58.8 ± 5.1 | ns | *** |
| SI length, m | 3.62 ± 0.20 | 4.50 ± 0.20 | 3.59 ± 0.23 | 4.09 ± 0.18 | ns | *** |
| Liver, g | 50.3 ± 4.5 | 51.6 ± 4.7 | 45.9 ± 6.0 | 44.0 ± 4.2 | ns | ns |
| Other | ||||||
| Brain, g | 31.7 ± 0.8 | 32.0 ± 0.9 | 32.6 ± 1.0 | 31.5 ± 0.8 | ns | ns |
| Heart, g | 10.4 ± 0.7 | 13.7 ± 0.7 | 8.9 ± 0.8 | 9.8 ± 0.6 | *** | *** |
| Spleen, g | 1.9 ± 0.2 | 2.8 ± 0.2 | 1.3 ± 0.2 | 1.9 ± 0.2 | *** | *** |
1Analyzed as a 2 × 2 factorial treatment design (dam parity × time point; including the interaction term); sow progeny (SP) vs. gilt progeny (GP); at birth (0 h) vs. 24 h post-partum (24 h).
2 P-values for the main effects of dam parity (D) and time point (T) within the birth cohort; ***P < 0.01; **P < 0.05; *P < 0.10; and ns = not significant (P ≥ 0.10). The dam parity × time point interaction was not significant (P ≥ 0.05) for any model.
3SI = small intestine (jejunum and ileum).
Table 3.
Least square means (± SE) from the linear mixed model analysis of absolute tissue and organ weights and other absolute organ dissection parameters for the weaning cohort
| Factor1 | |||||||
|---|---|---|---|---|---|---|---|
| SP | GP | P-value2 | |||||
| Trait3 | PrW | PoW | PrW | PoW | D | T | D × T |
| Musculoskeletal | |||||||
| Quadriceps, g | 30.9 ± 1.9 | 29.7 ± 1.9 | 26.0 ± 1.8 | 23.5 ± 2.0 | *** | ns | ns |
| Femur, cm | 6.8 ± 0.1 | 6.8 ± 0.2 | 6.7 ± 0.1 | 6.8 ± 0.1 | ns | ns | ns |
| Body length, cm | 59.6 ± 1.1 | 63.9 ± 1.1 | 58.1 ± 1.1 | 58.9 ± 1.1 | *** | ** | ns |
| Gastrointestinal tract | |||||||
| Stomach, g | 50.9 ± 2.4 | 52.4 ± 2.4 | 48.1 ± 2.4 | 45.2 ± 2.4 | ** | ns | ns |
| SI weight, g | 281.7 ± 14.3 | 244.8 ± 15.1 | 248.9 ± 15.3 | 213.1 ± 15.1 | ** | ** | ns |
| SI length, m | 9.43 ± 0.30 | 9.51 ± 0.30 | 9.01 ± 0.30 | 9.59 ± 0.28 | ns | ns | ns |
| Liver, g | 223.9 ± 11.4 | 223.7 ± 11.4 | 215.6 ± 11.4 | 204.9 ± 11.4 | ns | ns | ns |
| Other | |||||||
| Brain, g | 47.7 ± 1.3 | 52.8 ± 1.3 | 50.6 ± 1.4 | 52.5 ± 1.3 | ns | ** | ns |
| Heart, g | 43.7 ± 2.4 | 50.6 ± 2.4 | 44.2 ± 2.4 | 39.9 ± 2.4 | ** | ns | ** |
| Spleen, g | 41.6 ± 2.3 | 47.5 ± 2.4 | 32.9 ± 2.3 | 28.0 ± 2.3 | *** | ns | ** |
1Analyzed as a 2 × 2 × 2 factorial treatment design [dam parity × time point × sex; including interaction terms where significant (P < 0.05)]; sow progeny (SP) vs. gilt progeny (GP); pre-weaning (PrW) vs. post-weaning (PoW); male vs. female.
2 P-values for the main effects of dam parity (D), time point (T), and their interaction (D × T) within the weaning cohort; ***P < 0.01; **P < 0.05; *P < 0.10; and ns = not significant (P ≥ 0.10).
3SI = small intestine (jejunum and ileum).
Gastrointestinal Tract
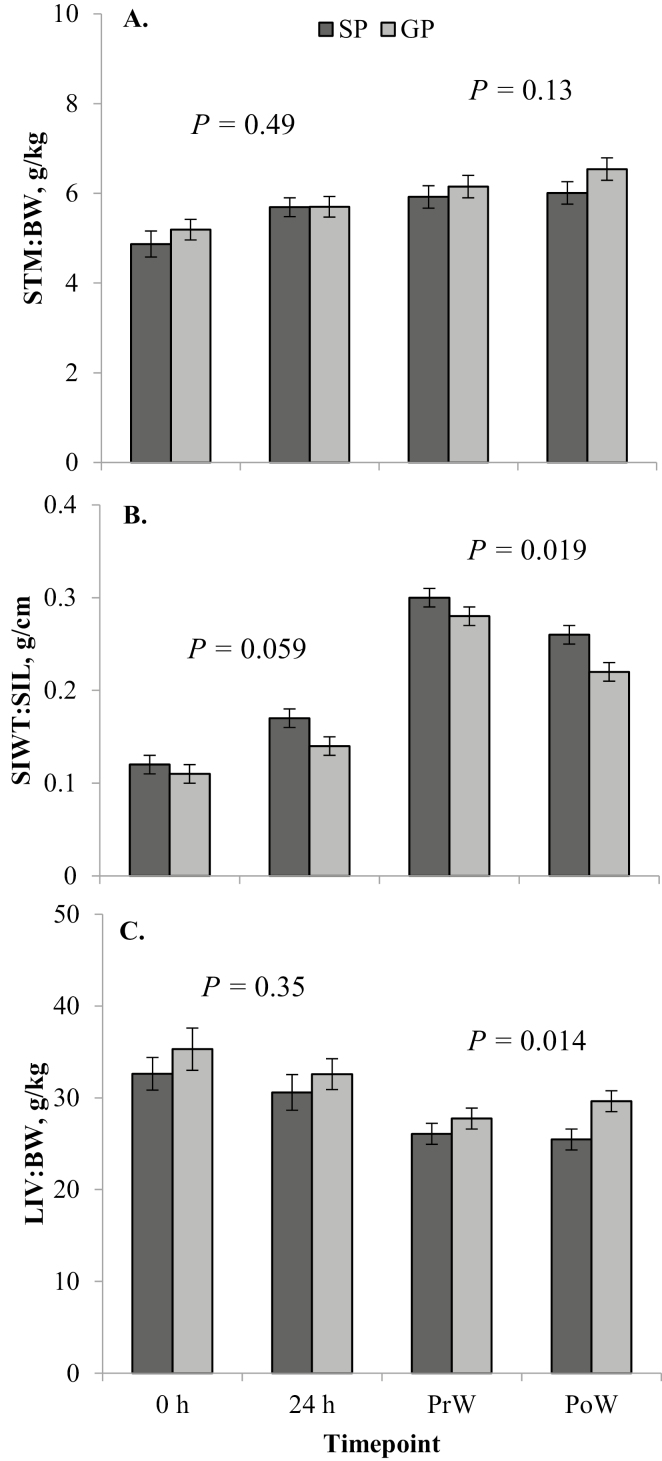
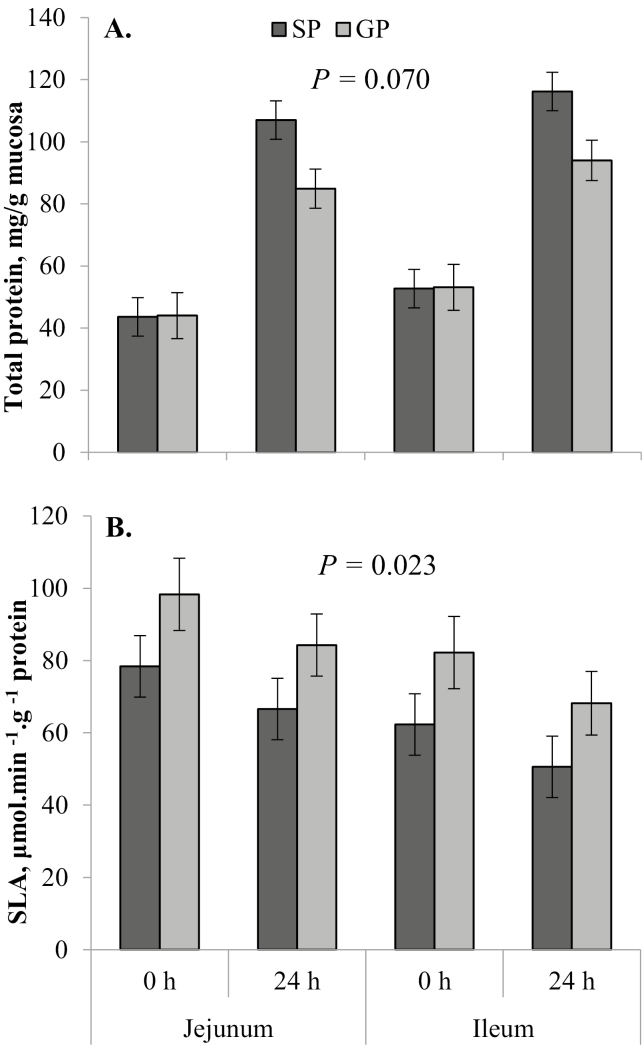
In the birth cohort, GP had lighter (P = 0.001) absolute stomach weights than SP (Table 2). However, there was no difference (P = 0.49) in STM:BW between GP and SP (Fig. 1A). Both absolute stomach weight (P < 0.001; Table 2) and STM:BW (P = 0.010; Fig. 1A) increased from 0 to 24 h. The absolute SI weight of GP was similar (P = 0.10) to that of SP, and absolute SI weight increased substantially (P < 0.001) from 0 to 24 h in both GP and SP (Table 2). There was no difference (P = 0.28) in SI length between GP and SP in the birth cohort (Table 2), which increased (P = 0.002) from 0 to 24 h. Weight of the SI relative to the length (SIWT:SIL) tended to be greater (P = 0.059) in SP compared with GP (Fig. 1B) and increased (P < 0.001) from 0 to 24 h. There was no difference between GP and SP in terms of absolute liver weight (P = 0.23; Table 2) or LIV:BW (P = 0.35; Fig. 1C) in the birth cohort. There was no effect (P ≥ 0.05) of time point on absolute or relative (LIV:BW) liver weight. There tended (P = 0.060) to be an interactive effect of dam parity × time point on total protein per gram of mucosa in the SI in the birth cohort, being no different (P = 0.96) between GP and SP at 0 h and lower (P = 0.007) in GP than in SP at 24 h (Fig. 2A). Gilt progeny had greater (P = 0.023) specific activity of lactase in the SI compared with SP, which was similar at 0 and 24 h (P = 0.11) and lower (P = 0.047) in the ileum compared with the jejunum (Fig. 2B).
Figure 1.
Least square means (± SE) from the linear mixed model analysis of relative gastrointestinal tract (GIT) growth parameters for each dam parity treatment (gilt progeny, GP; sow progeny, SP) at each time point (birth, 0 h; 24 h after birth, 24 h; weaning, at approximately 29 d of age; PrW; and 24 h after weaning, PoW), including stomach weight relative to body weight (STM:BW; A); weight of the small intestine relative to its length (SIWT:SIL; B); and liver weight relative to body weight (LIV:BW; C). P-values given are for the main effect of dam parity within that cohort (birth, 0 and 24 h; weaning, PrW and PoW).
Figure 2.
Least square means (± SE) from the linear mixed model analysis of total mucosal protein (A) and specific lactase activity (SLA) per gram of protein (B) in the proximal jejunum and distal ileum at each birth cohort time point (birth, 0 h; and 24 h after birth, 24 h). P-value given is the main effect of dam parity.
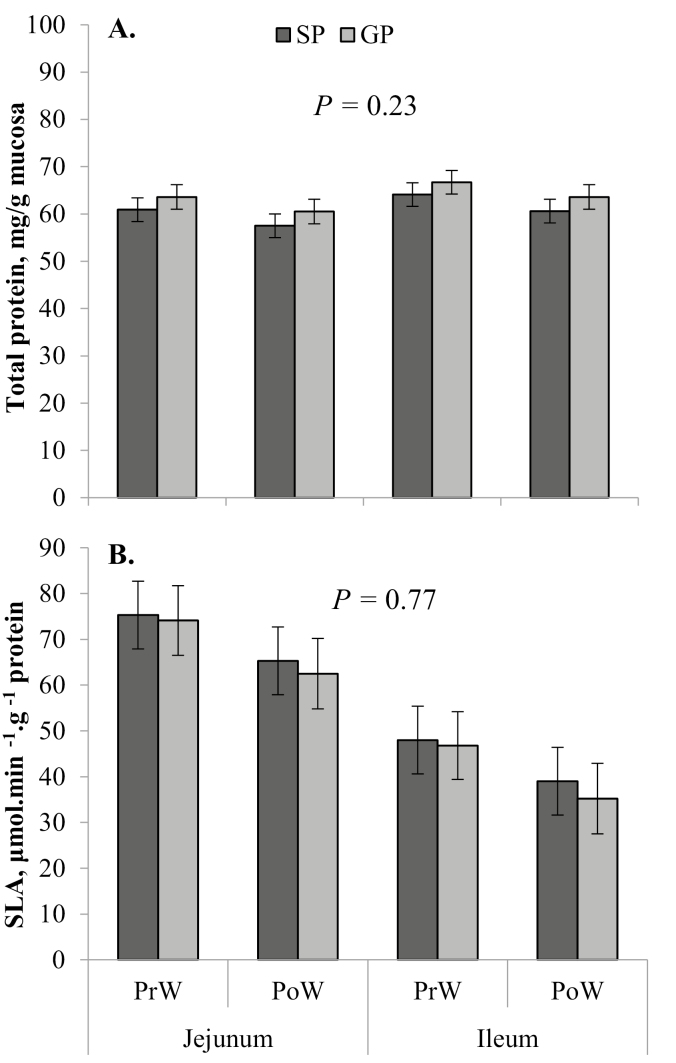
In the weaning cohort, GP had lower (P = 0.045) absolute stomach weights than SP (Table 3). However, there was no difference (P = 0.13) in STM:BW between GP and SP (Fig. 1A). Both absolute stomach weight and STM:BW did not change (P ≥ 0.05) from PrW to PoW. In the weaning cohort, male pigs had a greater (P = 0.049) STM:BW than females (6.4 ± 0.2 vs. 5.9 ± 0.2 g/kg, respectively). Gilt progeny had lighter (P = 0.038) absolute SI weights in the weaning cohort compared with that of SP (Table 3). Absolute SI weight decreased (P = 0.020) after weaning (Table 3). There was no difference (P = 0.57) in SI length between GP and SP in the weaning cohort and no change (P = 0.26) from PrW to PoW (Table 3). Weight of the SI relative to the length (SIWT:SIL) was greater (P = 0.019) in SP compared with GP and decreased (P < 0.001) from PrW to PoW (Fig. 1B). Males had a greater SI length (P = 0.045) than females in the weaning cohort (9.7 ± 0.2 vs. 9.1 ± 0.2 m, respectively). There was no difference (P = 0.24) between GP and SP in terms of absolute liver weight in the weaning cohort (Table 3). However, GP had a greater (P = 0.014) LIV:BW than SP (Fig. 1C). There was no effect (P ≥ 0.05) of time point on absolute liver weight (Table 2) or LIV:BW (Fig. 1C). There was no significant effect of dam parity (P = 0.23), time point (P = 0.16), or section (P = 0.18) on total protein per gram of mucosa in the SI in the weaning cohort (Fig. 3A). Specific activity of lactase was not different (P = 0.77) between GP and SP or between weaning cohort time points (P = 0.11), but it was significantly higher (P < 0.001) in the jejunum than in the ileum (Fig. 3B).
Figure 3.
Least square means (± SE) from the linear mixed model analysis of total mucosal protein (A) and specific lactase activity (SLA) per gram of protein (B) in the proximal jejunum and distal ileum at each weaning cohort time point (weaning, at approximately 29 d of age; PrW; and 24 h after weaning, PoW). P-value given is the main effect of dam parity.
Other Organs
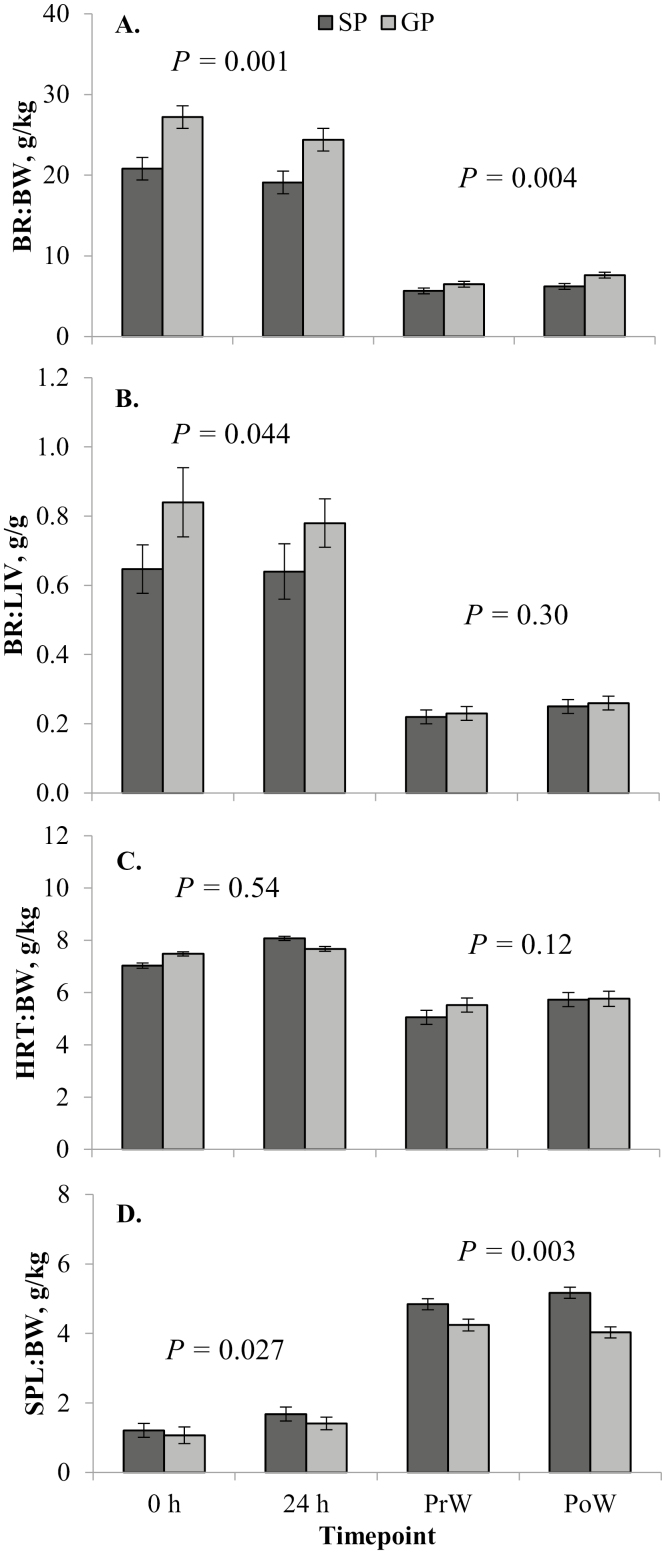
There was no difference (P = 0.80) in absolute brain weight between GP and SP in the birth cohort (Table 2), although BR:BW was greater (P = 0.001) for GP compared with SP (Fig. 4A). The BR:LIV ratio was greater (P = 0.044) for GP than it was for SP (Fig. 4B). There was no change (P ≥ 0.05) in absolute brain weight (Table 2), BR:BW (Fig. 4A), or BR:LIV (Fig. 4B) from 0 to 24 h. There was a trend (P = 0.095) for a dam parity × time point interaction for absolute heart weight in the birth cohort, as there was a greater increase from 0 to 24 h for SP (P = 0.002) than there was for GP (P = 0.42; Table 2), with most of the difference between GP and SP present at 24 h (P < 0.001), and no difference (P = 0.18) at 0 h between the 2 groups (Table 2). The same was true for HRT:BW, except the interaction was significant (P = 0.003) in this model (Fig. 4C). There was a greater difference in HRT:BW at 24 h between GP and SP (P = 0.007) than there was at 0 h (P = 0.092), and there was a greater increase in HRT:BW from 0 to 24 h in SP (P < 0.001) compared with GP (P = 0.54). Sow progeny had greater absolute spleen weights (P < 0.001; Table 2) and SPL:BW (P = 0.027; Fig. 4D) than GP in the birth cohort. Both absolute spleen weight (Table 2) and SPL:BW (Fig. 4D) increased (P < 0.001) from 0 to 24 h.
Figure 4.
Least square means (± SE) from the linear mixed model analysis of relative organ dissection parameters for each dam parity treatment (gilt progeny, GP; sow progeny, SP) at each time point (birth, 0 h; 24 h after birth, 24 h; weaning, at approximately 29 d of age; PrW; and 24 h after weaning, PoW), including brain weight relative to BW (BR:BW; A); brain to liver weight ratio (BR:LIV; B); heart (HRT:BW; C); and spleen weight relative to BW (SPL:BW; D). P-values given are for the main effect of dam parity within that cohort (birth, 0 and 24 h; weaning, PrW and PoW).
In the weaning cohort, there was no difference (P = 0.33) in absolute brain weight between GP and SP (Table 3), while BR:BW ratio was greater (P = 0.004) for GP compared with SP (Fig. 4A). Both absolute brain weight (P = 0.012; Table 3) and BR:BW (P = 0.027; Fig. 4A) increased from PrW to PoW. There was no difference (P = 0.30) in BR:LIV between GP and SP, which tended (P = 0.054) to increase from PrW to PoW (Fig. 4B). The dam parity × time point interaction was significant (P = 0.027) for absolute heart weight, which was similar between PrW and PoW for GP (P = 0.22), and increased between PrW and PoW for SP (P = 0.052; Table 2). The difference in absolute heart weight between GP and SP was seen mostly at PoW (P = 0.004) rather than at PrW (P = 0.88; Table 2). Gilt progeny had similar HRT:BW at PrW and PoW (P = 0.12; Fig. 4C), whereas HRT:BW increased from PrW to PoW in SP (P = 0.008; Fig. 4C).
The dam parity × sex interaction was significant for both absolute spleen weight (P = 0.036) and SPL:BW (P = 0.038), with the majority of the difference in GP and SP occurring in males for absolute spleen weight (27.6 ± 2.3 vs. 46.7 ± 2.3 g, respectively; P < 0.001) and SPL:BW (4.0 ± 0.3 vs. 5.5 ± 0.3 g/kg, respectively; P = 0.001). Within the females, absolute spleen weight was lower (P = 0.009) in GP compared with SP (33.2 ± 2.3 vs. 42.3 ± 2.4 g, respectively), but SPL:BW was similar (4.3 ± 0.3 vs. 4.6 ± 0.3 g/kg, respectively; P = 0.48). In SP, females had a lower (P = 0.034) SPL:BW than males, but this difference was not shown in absolute spleen weight (P = 0.19), and there was no difference (P ≥ 0.05) in either parameter between sexes of GP. The dam parity × time point interaction was significant (P = 0.024) for absolute spleen weight, with SP having higher (P = 0.010) weights than GP at PrW, and this difference was even greater (P < 0.001) at PoW (Table 3). Absolute spleen weight did not change from PrW to PoW in GP (P = 0.13), but increased slightly in SP (P = 0.082; Table 3). There was no effect (P = 0.80) of time point on SPL:BW (Fig. 4D).
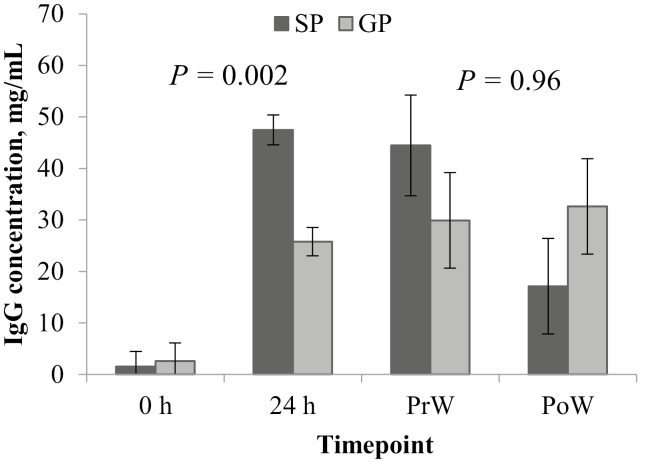
Serum IgG
In the birth cohort, the dam parity × time point interaction influenced (P = 0.001) the piglet serum IgG concentration (Fig. 5), with no difference (P = 0.83) between GP and SP at 0 h, but significantly lower (P < 0.001) in GP at 24 h. Both GP and SP serum IgG concentrations increased (P < 0.001) from 0 to 24 h. In the weaning cohort, there was no difference (P = 0.98) in serum IgG concentration between GP and SP or between PrW and PoW (P = 0.20), and the dam parity × time point interaction was not significant (P = 0.12; Fig. 5).
Figure 5.
Least square means (± SE) from the linear mixed model analysis of piglet serum immunoglobulin G (IgG) concentration at each time point (birth, 0 h; 24 h after birth, 24 h; weaning, at approximately 29 d of age; PrW; and 24 h after weaning, PoW). P-values given are for the main effect of dam parity within that cohort (birth, 0 and 24 h; weaning, PrW and PoW).
Discussion
Gilt progeny are generally born lighter and suffer inferior performance to SP throughout the production cycle, with increased mortality and reduced growth rates (Holyoake, 2006; Miller et al., 2012b; Craig et al., 2017b). The findings from this study allow a further understanding of the underlying mechanisms behind differences between GP and SP, which are generally believed to be due to factors such as inferior colostrum and milk production, intake and composition (Inoue et al., 1980; Klobasa and Butler, 1987; King, 2000), as well as differences in the ability to digest and (or) absorb specific components of colostrum and (or) milk in the GIT (Cottrell et al., 2017). The results of the current experiment confirm that GP are born and weaned lighter than SP and may suggest that they suffer poorer acquisition of maternal immunity and delays in development of the GIT and skeletal muscle in utero and soon after birth, and around weaning. Unfortunately, due to constraints of time, resources, commercial pig production flows, and due to the fact that euthanasia was the end point of sampling for the current experiment, the sample size was limited and confined to 2 completely separate cohorts of animals; hence, results should be interpreted with this in mind.
In early gestation, development of the nervous system and skeleton is prioritized (McMeekan, 1940; Hammond, 1944). Discrepancies were found in the present study between GP and SP in weights of organs of body systems developed later in gestation, such as those of the GIT and of skeletal muscle, reflected in the lower absolute and relative weights of these tissues at birth (e.g., SI, stomach, spleen, and quadriceps muscle). In particular, GP having greater BR:LIV and BR:BW ratios compared with SP at birth may be as a result of development of the nervous system earlier in gestation and (or) because of fetal mechanisms in place to protect the development of the brain relative to some other tissues such as the skeletal muscle and organs of the GIT (i.e., the liver). This was supported by lower QD weight (both absolute and relative to femur length) in GP compared with SP at all time points in the present study. Femur length was highly conserved between animals at every age studied, which is in agreement with Liu et al. (1999), and was not different between SP and GP, suggesting skeletal growth was comparable between the 2 groups. Da Silva et al. (2013) found absolute semitendinosus muscle weight and area was lower in GP at birth compared with progeny born to multiparous sows of parities 3 and 4, and related this to the energy demands of GP being unmet during late gestation.
These asymmetrical growth patterns are often seen during intrauterine growth restriction, often observed in “runt” piglets from large litters that are born light as a result of uterine crowding (Bauer et al., 1998; Town et al., 2004). Because fat deposition in utero is part of the last fetal growth phase (Hammond, 1944), fetal growth restriction in GP may result in lesser fat stores at birth for thermoregulation, which are already inadequate in newborn pigs (Herpin et al., 2002). This would also be impacted by a greater surface area to volume ratio in GP, which is suggested by their lower BWT compared with SP, and may increase heat loss and compromise their thermoregulatory ability. In the present study, GP had a lower BW:BL ratio in the weaning cohort, calculated in a similar way to body mass index, which has been shown to be a good predictor of pre- and post-weaning performance in newborn piglets (Douglas et al., 2016; Huting et al., 2018). If uterine space is restricted in late gestation in gilts, focused management strategies in mid-to-late gestation to increase the BWT of GP may be in vain. In the experimental herd in the present study, primiparous sows are given a lower feed allowance than multiparous sows during gestation based on recommended gestation requirements (NRC, 2012), and it must be kept in mind that this may have impacted the development of GP compared with that of SP. However, when additional nutrients are supplied to primiparous sows, they generally direct these toward their own growth rather than that of the fetus (Clowes et al., 1998; Pluske et al., 1998; Zak et al., 1998).
Previous findings (Edwards et al., 2013), along with the present study, show that GP have a lower weight per unit length of SI in comparison to SP. In the present study, the SI grew rapidly in terms of weight, length, and weight per unit length in the first 24 h after birth, as has been shown previously in response to intake of colostrum (Widdowson et al., 1976; Burrin et al., 1992; Xu, 1996). This probably occurs due to an increase in intestinal cell number and size, as well as uptake of IgG and other macromolecules into enterocytes (Werhahn et al., 1981). Furthermore, in our previous experiment, we have shown that colostrum composition between primiparous and multiparous sows is largely similar in terms of IgG sand macronutrient concentrations (Craig et al., 2019). Data from the present study, therefore, points toward GP being restricted in their ability to obtain and digest colostrum in the first 24 h of life. The lower SIW:SIL and total jejunal and ileal protein per gram of mucosa in GP compared with SP may indicate that there is a reduction in the uptake of these proteins from colostrum possibly due to compromised development of the GIT, and ultimately affecting the capacity to absorb nutrients and components for the development of immunity.
Lower relative spleen weights in GP in both the birth and weaning cohort may suggest that their capacity to synthesize and store blood cells is compromised. Consequently, this may affect their ability to resolve infection around these challenge periods. Gilt progeny have been reported to suffer higher rates of mortality than SP, especially around weaning (Holyoake, 2006; Edwards et al., 2013; Craig et al., 2017b), thought due to impaired acquisition of maternal immunity from colostrum. This is supported by the finding that GP had lower serum IgG concentrations than SP at 24 h in the present study. However, other studies investigating the difference between IgG levels in colostrum and milk of gilts and sows and in the serum of their progeny are conflicting (Klobasa and Butler, 1987; Cabrera et al., 2012; Miller et al., 2012a; Kielland et al., 2015) in comparison to our study (Craig et al., 2019). Differences in splenic growth and poorer acquisition of IgG in these early periods between GP and SP may favor the hypothesis that GP are less able to mount an immune response to infection, such as to a lipopolysaccharide endotoxic challenge, but this requires further investigation. Furthermore, it may be speculated that greater relative liver weights in GP may be a sign of an increase in absorption of endotoxin due to compromised intestinal development causing a more permeable epithelium in these pigs (Cottrell et al., 2017), but again this requires further elucidation.
The fact that a number of these differences were also seen, and often exaggerated, in the weaning cohort (e.g., BR:BW, QD:FEM, BW:BL, SIW:SIL, LIV:BW, etc.), may be a further indication that GP do not exhibit compensatory organ growth before weaning and suffer a cumulative long-term disadvantage from being born lighter than SP and immunocompromised early in life. This is unsurprising given the relative impacts of lighter BWT of GP on their weaning weight and later performance (Craig et al., 2017b), but further implies that GP may be unable to optimally convert nutrients to carcass growth later in the grower-finisher period. A lower milk lactose concentration at day 21 of lactation in gilts compared with multiparous sows (Craig et al., 2019) may indicate that gilts cannot maintain the metabolic demands of late lactation and that their milk production is inadequate for effective growth of their progeny to weaning. This concurs with the finding that a number of vital organs remained underdeveloped in GP in comparison to SP in the weaning cohort. This further highlights the need for energy and nutrient supplementation, for example through creep feed, milk replacers and (or) energy supplements, for GP closer to weaning.
Absolute spleen and heart weights showed opposing trends in the first 24 h after weaning between parity groups, decreasing in GP after weaning, whereas heart and spleen weights increased in SP after weaning. This may suggest that GP are relatively unable to adapt to weaning stress and may reflect poorer cardiovascular health and (or) a reduction in red blood cell production in these animals. There was no creep feed supplied in the present study, and it would be of interest to determine whether these negative weaning stressors may be negated by exposure to creep feed before weaning. Further research in this area is warranted.
Higher specific activity of lactase in the brush border membrane of GP compared with SP at 24 h in the present study was surprising, and it is unclear from these findings what the cause or implications of this may be, and due to the small sample size in our study, this phenomenon needs to be confirmed with future studies. These findings are in contrast with that of Huygelen et al. (2015), who found no difference in enzyme activities at birth between low and normal birth weight piglets. Regardless, upregulation of the activity of lactase at birth may not result in improvements in glucose metabolism in GP unless glucose uptake by the enterocyte is simultaneously upregulated (Buddington, 2002). It seems from differences in SI weight to length ratios that the intestinal cells of GP are growing at a slower rate to that of SP in the first 24 h of life, and we, therefore, may speculate that brush border glucose metabolism is less efficient in these animals. No differences in specific lactase activity were found between GP and SP in the weaning cohort, where the efficiency of milk carbohydrate digestion may be more imperative in determining the piglets’ propensity for growth (Le Huërou-Luron, 2002), especially given that levels of lactose in milk from gilts were found to be lower than in milk from multiparous sows by this stage (Craig et al., 2019). Specific activities of disaccharidases alone are often thought inadequate as indicators of digestive capacity (Shields et al., 1980; Kelly et al., 1991), and it was unfortunate that whole mucosal weights or histological sections were unable to be taken in the present study. Another possible speculation is that greater specific lactase activity of GP in the birth cohort may be indicative of a longer, finger-like villus in the SI of GP compared with SP, as the majority of lactase activity is observed at the villous apex (Tsuboi et al., 1981, 1985). Further histological studies are required in to investigate early development of the GIT barrier structure and function in GP compared with SP.
In conclusion, the findings from this study suggest that the development of a number of organs in late gestation—in particular those of the GIT and the skeletal muscle—may be compromised in GP in comparison to SP, culminating in differences at birth and persisting until weaning. To identify the best way to manage GP to maximize their performance, it is important to further investigate these physiological differences between GP and SP in future studies. Although it may be important to focus management strategies toward improving fetal development of GP in late gestation, the success of such strategies depends on the gilts’ capacity to supply uterine space, and direct additional nutrients toward the placenta to ensure improvements in the growth of her progeny in utero. Management strategies targeted toward the growth of GP in lactation, with consideration for their poorer GIT development and acquisition of maternal immunity (in comparison to SP), may, therefore, be more successful.
Footnotes
The authors gratefully acknowledge the research teams from Rivalea (Australia) Pty. Ltd. (Corowa, Australia), the University of Melbourne (Parkville, Australia), and Murdoch University (Murdoch, Australia) for their assistance, particularly K. O’Halloran, L. Fothergill, M. Ringuet, and Dr. D. Turpin. Funding was supplied by Australian Pork Limited (APL; Canberra, Australia; project number 2014/461), and J.R.C. was supported by an Australian Postgraduate Award (PhD) from Murdoch University.
LITERATURE CITED
- Bauer R., Walter B., Hoppe A., Gaser E., Lampe V., Kauf E., and Zwiener U.. 1998. Body weight distribution and organ size in newborn swine (sus scrofa domestica) – A study describing an animal model for asymmetrical intrauterine growth retardation. Exp. Toxicol. Pathol. 50:59–65. doi: 10.1016/S0940-2993(98)80071-7 [DOI] [PubMed] [Google Scholar]
- Baxter E. M., Jarvis S., D’Eath R. B., Ross D. W., Robson S. K., Farish M., Nevison I. M., Lawrence A. B., and Edwards S. A.. 2008. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 69:773–783. doi: 10.1016/j.theriogenology.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Buddington R. K. 2002. Intestinal absorption of nutrients during early development of vertebrates: Patterns of appearance and change. In: Zabielski R., Gregory P. C., Weström B., and Salek E., editors, Biology of growing animals. Chapter 18. Vol. 1 Elsevier, Amsterdam, The Netherlands: p. 539–562. [Google Scholar]
- Burrin D. G., Shulman R. J., Reeds P. J., Davis T. A., and Gravitt K. R.. 1992. Porcine colostrum and milk stimulate visceral organ and skeletal muscle protein synthesis in neonatal piglets. J. Nutr. 122:1205–1213. doi: 10.1093/jn/122.6.1205 [DOI] [PubMed] [Google Scholar]
- Cabrera R. A., Lin X., Campbell J. M., Moeser A. J., and Odle J.. 2012. Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J. Anim. Sci. Biotechnol. 3:42. doi: 10.1186/2049-1891-3-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney-Hinkle E. E., Tran H., Bundy J. W., Moreno R., Miller P. S., and Burkey T. E.. 2013. Effect of dam parity on litter performance, transfer of passive immunity, and progeny microbial ecology. J. Anim. Sci. 91:2885–2893. doi: 10.2527/jas.2011-4874 [DOI] [PubMed] [Google Scholar]
- Clowes E. J., Williams I. H., Baracos V. E., Pluske J. R., Cegielski A. C., Zak L. J., and Aherne F. X.. 1998. Feeding lactating primiparous sows to establish three divergent metabolic states: II. Effect on nitrogen partitioning and skeletal muscle composition. J. Anim. Sci. 76:1154–1164. doi: 10.2527/1998.7641154x [DOI] [PubMed] [Google Scholar]
- Cottrell J. J., Craig J., Wijesiriwardana U. A., Fothergill L., Ringuet M. T., O’Hallorhan K., Turpin D. L., Munoz L. M., Collins C. L., Furness J. B., Dunshea F. R., and Pluske J.. 2017. The gastrointestinal tract of piglets from first parity sows develops more slowly and is more permeable than piglets from later parity sows. FASEB J. 31:792. [Google Scholar]
- Craig J. R., Collins C. L., Athorn R. Z., Dunshea F. R., and Pluske J. R.. 2017a. Investigating the reproductive performance of gilt progeny entering the breeding herd. J. Swine Health Prod. 25:230–237. [Google Scholar]
- Craig J. R., Collins C. L., Bunter K. L., Cottrell J. J., Dunshea F. R., and Pluske J. R.. 2017b. Poorer lifetime growth performance of gilt progeny compared with sow progeny is largely due to weight differences at birth and reduced growth in the preweaning period, and is not improved by progeny segregation after weaning. J. Anim. Sci. 95:4904–4916. doi: 10.2527/jas2017.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. R., Dunshea F. R., Cottrell J. J., Wijesiriwardana U. A., and Pluske J. R.. 2019. Primiparous and multiparous sows have largely similar colostrum and milk composition profiles throughout lactation. Animals. 9:35. doi: 10.3390/ani9020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva A., Dalto D., Lozano A., De Oliveira E., Gavioli D., De Oliveira J., Romero N., and Da Silva C.. 2013. Differences in muscle characteristics of piglets related to the sow parity. Can. J. Anim. Sci. 93:471–475. doi: 10.4141/CJAS2013-049 [DOI] [Google Scholar]
- Douglas S. L., Edwards S. A., and Kyriazakis I.. 2016. Are all piglets born lightweight alike? Morphological measurements as predictors of postnatal performance. J. Anim. Sci. 94:3510–3518. doi: 10.2527/jas.2015-0142 [DOI] [PubMed] [Google Scholar]
- Dudley M. A., Jahoor F., Burrin D. G., and Reeds P. J.. 1994. Brush-border disaccharidase synthesis in infant pigs measured in vivo with [2H3]leucine. Am. J. Physiol. 267(6 Pt 1):G1128–G1134. doi: 10.1152/ajpgi.1994.267.6.G1128 [DOI] [PubMed] [Google Scholar]
- Dudley M. A., Shulman R. J., Reeds P. J., Rosenberger J. N., Putman M., Johnston P. K., Perkinson J. S., and Nichols B. L.. 1992. Developmental changes in lactase-phlorizin hydrolase precursor isoforms in the rat. J. Pediatr. Gastroenterol. Nutr. 15:260–269. [DOI] [PubMed] [Google Scholar]
- Dunshea F. R. 2003. Metabolic and endocrine changes around weaning. In: Pluske J. R., Le Dividich J., and Verstegen M. V. A. editors, Weaning the pig, concepts and consequences. Wageningen Press, University of Wageningen, Wageningen, The Netherlands: p. 61–80. [Google Scholar]
- Edwards M. V., Campbell R. G., Chapman T., Brouwers H., Pierzynowski S. G., Weström B. R., Prykhod’ko O., Gabor L., and Choct M.. 2013. Spray-dried porcine plasma and yeast derived protein meal influence the adaption to weaning of primiparous and multiparous sow progeny in different ways. Anim. Prod. Sci. 53:75–86. doi: 10.1071/AN12151 [DOI] [Google Scholar]
- Field A. 2013. Regression. In: M. Carmichael (ed.) Discovering statistics using IBM SPSS Statistics. Chapter 8. 4th ed. Sage Publ., Thousand Oaks, CA: p. 293–356. [Google Scholar]
- Hammond J. 1944. Physiological factors affecting birth weight. Proc. Nutr. Soc. 2:14. [Google Scholar]
- Herpin P., Damon M., and Le Dividich J.. 2002. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 78:25–45. doi: 10.1016/S0301-6226(02)00183-5 [DOI] [Google Scholar]
- Holyoake P. K. 2006. Dam parity affects the performance of nursery pigs. In: 19th Int. Pig Vet. Soc. Cong., Copenhagen, Denmark p. 149. [Google Scholar]
- Huting A. M. S., Sakkas P., Wellock I., Almond K., and Kyriazakis I.. 2018. Once small always small? To what extent morphometric characteristics and post-weaning starter regime affect pig lifetime growth performance. Porcine Health Manag. 4:21. doi: 10.1186/s40813-018-0098-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygelen V., De Vos M., Prims S., Vergauwen H., Fransen E., Casteleyn C., Van Cruchten S., and Van Ginneken C.. 2015. Birth weight has no influence on the morphology, digestive capacity and motility of the small intestine in suckling pigs. Livest. Sci. 182:129–136. doi: 10.1016/j.livsci.2015.11.003 [DOI] [Google Scholar]
- Inoue T., Kitano K., and Inoue K.. 1980. Possible factors influencing the immunoglobulin G concentration in swine colostrum. Am. J. Vet. Res. 41:1134–1136. [PubMed] [Google Scholar]
- Kelly D., Smyth J. A., and McCracken K. J.. 1991. Digestive development of the early-weaned pig. 2. Effect of level of food intake on digestive enzyme activity during the immediate post-weaning period. Br. J. Nutr. 65:181–188. doi: 10.1079/BJN19910078 [DOI] [PubMed] [Google Scholar]
- Kielland C., Rootwelt V., Reksen O., and Framstad T.. 2015. The association between immunoglobulin G in sow colostrum and piglet plasma. J. Anim. Sci. 93:4453–4462. doi: 10.2527/jas.2014-8713 [DOI] [PubMed] [Google Scholar]
- King R. H. 2000. Factors that influence milk production in well-fed sows. J. Anim. Sci. 78:19–25. doi: 10.2527/2000.78suppl_319x [DOI] [PubMed] [Google Scholar]
- Klobasa F., and Butler J. E.. 1987. Absolute and relative concentrations of immunoglobulins G, M, and A, and albumin in the lacteal secretion of sows of different lactation numbers. Am. J. Vet. Res. 48:176–182. [PubMed] [Google Scholar]
- Klobasa F., Butler J. E., Werhahn E., and Habe F.. 1986. Maternal-neonatal immunoregulation in swine. II. Influence of multiparity on de novo immunoglobulin synthesis by piglets. Vet. Immunol. Immunopathol. 11:149–159. doi: 10.1016/0165-2427(86)90094-2 [DOI] [PubMed] [Google Scholar]
- Klobasa F., Habe F., Werhahn E., and Butler J. E.. 1985. The influence of age and breed on the concentrations of serum IgG, IgA and IgM in sows throughout the reproductive cycle. Vet. Immunol. Immunopathol. 10:355–366. doi: 10.1016/0165-2427(85)90024-8 [DOI] [PubMed] [Google Scholar]
- Le Huërou-Luron I. 2002. Production and gene expression of brush border disaccharidases and peptidases during development in pigs and calves. In: Zabielski R., Gregory P. C., Weström B., and Salek E., editors, Biology of growing animals. Chapter 16. Vol. 1 Elsevier, Amsterdam, The Netherlands: p. 491–513. [Google Scholar]
- Liu M. F., He P., Aherne F. X., and Berg R. T.. 1999. Postnatal limb bone growth in relation to live weight in pigs from birth to 84 days of age. J. Anim. Sci. 77:1693–1701. doi: 10.2527/1999.7771693x [DOI] [PubMed] [Google Scholar]
- McMeekan C. P. 1940. Growth and development in the pig, with special reference to carcass quality characters. J. Agric. Sci. 30:276–343. doi: 10.1017/S0021859600048024 [DOI] [Google Scholar]
- Miller Y. J., Collins A. M., Emery D., Begg D. J., Smits R. J., and Holyoake P. K.. 2012a. Piglet performance and immunity is determined by the parity of both the birth dam and the rearing dam. Anim. Prod. Sci. 53:46–51. doi: 10.1071/AN12063 [DOI] [Google Scholar]
- Miller Y. J., Collins A. M., Smits R. J., Thomson P. C., and Holyoake P. K.. 2012b. Providing supplemental milk to piglets preweaning improves the growth but not survival of gilt progeny compared with sow progeny. J. Anim. Sci. 90:5078–5085. doi: 10.2527/jas.2011-4272 [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council 2013. Australian code for the care and use of animals for scientific purposes. 8th ed. National Health and Medical Research Council, Canberra, ACT, Australia. [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Pluske J. R., Williams I. H., Zak L. J., Clowes E. J., Cegielski A. C., and Aherne F. X.. 1998. Feeding lactating primiparous sows to establish three divergent metabolic states: III. Milk production and pig growth. J. Anim. Sci. 76:1165–1171. doi: 10.2527/1998.7641165x [DOI] [PubMed] [Google Scholar]
- Quesnel H. 2011. Colostrum production by sows: Variability of colostrum yield and immunoglobulin G concentrations. Animal 5:1546–1553. doi: 10.1017/S175173111100070X. [DOI] [PubMed] [Google Scholar]
- Rooke J. A., and Bland I. M.. 2002. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 78:13–23. doi: 10.1016/S0301-6226(02)00182-3 [DOI] [Google Scholar]
- Shields R. G. Jr., Ekstrom K. E., and Mahan D. C.. 1980. Effect of weaning age and feeding method on digestive enzyme development in swine from birth to ten weeks. J. Anim. Sci. 50:257–265. doi: 10.2527/jas1980.502257x [DOI] [PubMed] [Google Scholar]
- Town S. C., Putman C. T., Turchinsky N. J., Dixon W. T., and Foxcroft G. R.. 2004. Number of conceptuses in utero affects porcine fetal muscle development. Reproduction 128:443–454. doi: 10.1530/rep.1.00069 [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Kwong L. K., D’Harlingue A. E., Stevenson D. K., Kerner J. A. Jr., and Sunshine P.. 1985. The nature of maturational decline of intestinal lactase activity. Biochim. Biophys. Acta 840:69–78. doi: 10.1016/0304-4165(85)90163-1 [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Kwong L. K., Neu J., and Sunshine P.. 1981. A proposed mechanism of normal intestinal lactase decline in the postweaned mammal. Biochem. Biophys. Res. Commun. 101:645–652. doi: 10.1016/0006-291x(81)91307-3 [DOI] [PubMed] [Google Scholar]
- Werhahn E., Klobasa F., and Butler J. E.. 1981. Investigation of some factors which influence the absorption of IgG by the neonatal piglet. Vet. Immunol. Immunopathol. 2:35–51. doi: 10.1016/0165-2427(81)90037-4 [DOI] [PubMed] [Google Scholar]
- Widdowson E. M., Colombo V. E., and Artavanis C. A.. 1976. Changes in the organs of pigs in response to feeding for the first 24 h after birth. II. The digestive tract. Biol. Neonate 28:272–281. doi: 10.1159/000240828 [DOI] [PubMed] [Google Scholar]
- Xu R. J. 1996. Development of the newborn GI tract and its relation to colostrum/milk intake: A review. Reprod. Fertil. Dev. 8:35–48. doi: 10.1071/RD9960035 [DOI] [PubMed] [Google Scholar]
- Zak L. J., Williams I. H., Foxcroft G. R., Pluske J. R., Cegielski A. C., Clowes E. J., and Aherne F. X.. 1998. Feeding lactating primiparous sows to establish three divergent metabolic states: I. Associated endocrine changes and postweaning reproductive performance. J. Anim. Sci. 76:1145–1153. doi: 10.2527/1998.7641145x [DOI] [PubMed] [Google Scholar]