ABSTRACT
Background
Infertility is an important public health problem with few known modifiable risk factors. Dietary factors including folic acid have been associated with improved fertility, but the association between iron and fertility is understudied. One study among US nurses found a 40% lower risk of ovulatory infertility with higher intake of nonheme iron and iron supplements.
Objectives
The aim of this study was to determine the extent to which iron intake from diet and supplements reported on structured questionnaires is associated with fecundability.
Methods
We conducted parallel analyses that used data from 2 prospective cohort studies of pregnancy planners from Denmark (Snart Foraeldre; n = 1693) and North America (PRESTO; n = 2969) during 2013–2018. Follow-up comprised menstrual cycles at risk until pregnancy or censoring for fertility treatment, stopped trying to conceive, withdrawal, loss to follow-up, or 12 cycles of attempt. We used proportional probabilities regression models to estimate fecundability ratios (FRs) and 95% CIs, adjusting for confounders.
Results
We found little association between dietary heme iron intake and fecundability in either cohort. The FR for nonheme iron intake (≥11 mg/d compared with <9 mg/day) was 1.11 for Snart Foraeldre participants (95% CI: 0.92, 1.34) and 1.01 for PRESTO participants (95% CI: 0.89, 1.14). The FR for iron-containing supplements was 1.01 in Snart Foraeldre (95% CI: 0.90, 1.13) and 1.19 in PRESTO (95% CI: 1.03, 1.38). In PRESTO, but not Snart Foraeldre, stronger positive associations were found for nonheme iron intake and iron supplement use among women with heavy menses or short menstrual cycles.
Conclusions
Overall, dietary intake of iron was not consistently associated with fecundability, although there was some evidence for a positive association among women with risk factors for iron deficiency. We also found a small positive association between supplemental iron intake and fecundability among North American, but not Danish, pregnancy planners.
Keywords: dietary iron, iron supplements, fecundability, cohort study, epidemiology, fertility, preconception
Introduction
Infertility, defined as trying to conceive for ≥12 mo, is a common public health problem with few confirmed risk factors. Approximately 10–15% of couples experience infertility and, of these, 25% experience ovulatory infertility (1). Fertility treatment often results in psychological stress and a large economic burden for couples (1, 2), underscoring the importance of identifying modifiable risk factors associated with infertility.
Iron, a micronutrient for which the major sources include meat, seafood, fortified cereals, legumes, and spinach, is an important component of hemoglobin, cytochromes, and myoglobin (3). Absorption of iron in its 2 forms, heme (derived primarily from animal sources) and nonheme (derived primarily from vegetable sources), is regulated in the gastrointestinal tract. Many multivitamin-mineral supplements, including prenatal “vitamins,” are an important source of nonheme iron, although some supplements also contain heme iron. In the United States and Canada, but not in Denmark, many foods such as cereals and flour are fortified with nonheme iron. Extremes of serum iron concentration are associated with disrupted glucose and androgen metabolism (3–6) and impaired immunologic function (7, 8). These biologically important systems can affect fertility.
A prospective cohort study of US nurses reported a lower risk of ovulatory infertility among women who used iron supplements (RR = 0.60; 95% CI: 0.39, 0.92) (9) compared with nonusers. The same study (9) also found inverse associations of nonheme and total iron consumption with infertility. In the present report, we examine the associations of iron intake from foods and use of iron-containing supplements with fecundability in 2 prospective cohort studies of pregnancy planners.
Methods
Study populations
The Snart Foraeldre (“Soon Parents”) study is an internet-based prospective cohort study of female pregnancy planners and their male partners in Denmark. Snart Foraeldre was designed as an expansion of the Snart Gravid (“Soon Pregnant”) study, described elsewhere (10, 11). Recruitment for Snart Foraeldre began in 2011, with placement of advertisements on Danish health-related websites and social media. Enrollment and primary data collection were conducted via a self-administered questionnaire. Beginning in January 2013, female participants were invited to complete a comprehensive FFQ designed for and validated in the Snart Foraeldre cohort (12).
Women eligible for the Snart Foraeldre study are aged 18–45 y, residents of Denmark, planning a pregnancy, in a stable relationship with a male partner, and not receiving fertility treatment. From 3986 potentially eligible women, we excluded 72 whose last menstrual period (LMP) was >6 mo before study entry and 30 who had missing or implausible LMP information. We further limited our analyses to women who had been trying to conceive for ≤6 cycles at study entry. Of these 3094 women, 1740 completed the FFQ after it was introduced into the study data collection, with an 83% completion rate among women who were presented with this questionnaire. Based on responses to the FFQ, we further excluded 24 women with implausible total energy intake (<600 or >3800 kcal/d) and 23 who had >12 missing food items on the questionnaire, for a final analytic sample of 1693 women.
Pregnancy Study Online (PRESTO) (13) is an internet-based preconception cohort study conducted in the United States and Canada. It is modeled on Snart Foraeldre. Recruitment began in 2013 and eligible women are aged 21–45 y, planning a pregnancy, not receiving fertility treatment, and in a stable relationship with a male partner. As in Snart Foraeldre, PRESTO participants are invited to complete a baseline questionnaire. Ten days after enrollment, they are asked to complete the National Cancer Institute's Diet History Questionnaire II (DHQII) (14), an internet-based FFQ. For the current analysis there were 5734 eligible women who completed the baseline questionnaire. We excluded 72 women whose baseline LMP was >6 mo before study entry and 14 women with missing or implausible LMP data. Of the 4595 women who had been trying to conceive for ≤6 cycles at study entry, 3027 (68%) completed the FFQ. Based on responses to the FFQ, we further excluded 58 women with implausible total energy intake (<600 or >3800 kcal/d), for a final analytic sample of 2969 women.
We excluded women who had been trying to conceive for >6 cycles at study entry from our analyses due to concerns that women may change some behaviors (especially those frequently associated with lower fertility such as smoking, caffeine intake, and vigorous physical activity) with increasing attempt time. As expected, there were some differences in baseline characteristics between women trying to conceive for ≤6 mo compared with >6 mo at study entry. Most women in both cohorts (68%) had been trying to conceive for <3 cycles at study entry (median number of cycles trying at entry = 1.00 in Snart Foraeldre and PRESTO). The per-cycle probability of conception shows similar patterns across the 2 cohorts (15), with declining fecundability over time as the more fertile couples are removed from the population.
Baseline questionnaires for Snart Foraeldre and PRESTO include information on demographics, reproductive and medical history, and lifestyle and behavioral factors, including use of dietary supplements. To determine pregnancy status, self-administered online follow-up questionnaires are completed every 8 wk for 12 mo or until a reported conception.
This study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Boston Medical Center Institutional Review Board and the Danish Data Protection Agency. Informed consent was obtained online from all subjects.
Assessment of iron intake
We estimated total, heme, and nonheme dietary iron intake based on the nutrient composition of all food items in the 2 FFQs. Total dietary iron intake, calculated from responses to the Danish FFQ and the DHQII, was validated against a 4-d food record in Denmark and repeated 24-h dietary recalls in the United States (deattenuated Pearson correlation coefficients 0.58 and 0.59, respectively) (12, 14). Before data analysis, we adjusted nutrient intakes for energy intake with the use of the nutrient residual method (16).
We asked participants in both cohorts if they took multivitamin supplements or other single-nutrient supplements, including vitamin C or iron. In Snart Foraeldre, participants reported the brand of multivitamin they took, which we were able to classify as iron-containing or not. In PRESTO, participants reported their use of “multivitamins or prenatal vitamins” as a single-line item. The questionnaire did not specify the brand of multivitamin, but did elicit data on whether the vitamin supplement contained minerals; thus, we assumed that all “multivitamins or prenatal vitamins containing minerals” contained iron.
Assessment of Time to Pregnancy
We estimated time to pregnancy (TTP) through the use of data from the baseline and follow-up questionnaires. Women who reported regular menstrual cycles (defined as “being able to predict from one menstrual period to the next about when the next menstrual period would start”) were asked to report their usual menstrual cycle length. For women with irregular cycles, we estimated cycle length based on date of LMP reported at baseline and on prospectively reported LMP dates during follow-up. We estimated TTP, rounded to the nearest whole cycle, with the use of the following formula: [(cycles of pregnancy attempt at study entry/cycle length) + [(LMP date from most recent follow-up questionnaire – date of baseline questionnaire)/cycle length] + 1] (17).
Assessment of covariates
On the baseline questionnaire, women reported their age, education, height, weight, physical activity, smoking, alcohol consumption, use of oral contraceptives as last method of birth control, doing something to improve chances of conception (e.g., timing intercourse during the fertile window), parity, use of vitamin C supplements, and use of multivitamins. Dietary vitamin C intake and energy intake were computed from the respective FFQs. We calculated BMI as weight (kg) divided by height squared (m2). In Snart Foraeldre, total metabolic equivalents (METs) per week were calculated through the use of the International Physical Activity Questionnaire short-form by summing the MET-hours from walking, moderate physical activity, and vigorous physical activity (h/wk × 3.3 METs, 4 METs, and 8 METs, respectively) (18). In PRESTO, total MET-hours per week were calculated by multiplying the average number of hours per week spent participating in various activities by metabolic equivalents estimated from the Compendium of Physical Activities (19).
The confounders considered for adjustment in each cohort were identical except for race/ethnicity (ascertained in PRESTO only) and education, which was categorized differently in the 2 studies. In Snart Foraeldre, education was reported as years of vocational training (none, semiskilled/basic training, <3 y, 3–4 y, and >4 y). In PRESTO, education was reported as overall years of schooling and was categorized as less than a college/university degree, graduation from a 4-y college/university, and any graduate schooling.
Data analysis
We performed identical parallel analyses across the 2 cohorts with the use of the methods described below. For ease of comparison, the same categories for dietary intake of total, heme, and nonheme iron were used in each cohort analysis, based on the underlying distribution of iron intake in both cohorts combined. The categories for total iron intake were <10, 10–11.9, and ≥12 mg/d. Daily heme iron intake was categorized as <0.5, 0.5–0.9, and ≥1 mg/d, and daily nonheme iron intake was categorized as <9, 9–10.9, and ≥11 mg/d. In addition, we examined the shape and magnitude of the associations of fecundability with heme and nonheme iron as continuous variables by fitting restricted cubic splines (20). Restricted cubic splines fit a curvilinear relation between a continuous independent variable (iron intake) and a dependent variable (fecundability). The range of the independent variable is split into subintervals defined by “knots” or boundary points at which the separate curves for each subinterval meet to produce an overall smooth curve that is locally well adapted to the data.
We assessed fecundability in relation to iron-containing dietary supplement use as follows. First, we examined any use of an iron-containing supplement (multivitamin or iron-only supplement) compared with nonuse. We then divided supplement users into multivitamin users and iron-only supplement users.
Women contributed menstrual cycles at risk to the analysis from study entry until a reported pregnancy or a censoring event, whichever came first. Censoring events included initiation of fertility treatment, no longer trying to conceive, withdrawal, loss to follow-up, or 12 menstrual cycles of pregnancy attempt. To account for variation in attempt time at study entry (range: 0–6 cycles) and to minimize bias due to left truncation, we only analyzed observed cycles at risk. We used proportional probabilities regression models (21, 22) to estimate fecundability ratios (FRs), i.e., the cycle-specific probability of conception comparing exposed with unexposed women, with 95% CIs.
Potential confounders were selected based on a literature review and assessment of a causal graph. We included potential risk factors for subfertility that were associated with dietary iron intake or iron metabolism. Final models were adjusted for age (<25, 25–29, 30–34, and ≥35 y), education (≤12, 13–15, 16, and >16 years), race/ethnicity in PRESTO (white non-Hispanic compared with Hispanic or nonwhite), BMI (<25, 25–29, 30–34, and ≥35), physical activity (<10, 10–19, 20–39, and ≥40 MET-h/wk), alcohol consumption (0, 1–6, 7–13, and ≥14 drinks/wk), use of oral contraceptives as last method of contraception, doing something to improve chances of conception, parity (parous compared with nulliparous), cycle length and regularity (irregular cycles, regular cycles of <26 d, regular cycles of 26–30 d, and regular cycles of >30 d), use of individual iron and vitamin C supplements, daily multivitamin use, dietary vitamin C (continuous), and energy intake (continuous). We also mutually adjusted heme and nonheme iron intake. Because of their potential effects on iron absorption, we also examined consumption of caffeine (23), tea (24), dietary fiber (25, 26), and dietary calcium (27) as potential confounders. These did not substantially change the observed estimates and we did not include them in the regression models. We also controlled for total fruit and vegetable intake, with little effect on the estimates.
In secondary analyses, we evaluated the extent to which relations between iron intake and TTP varied by age (<30 y compared with ≥30 y), BMI (<25 compared with ≥25), attempt time at study entry (<3 cycles compared with 3–6 cycles), or parity (nulliparous compared with parous). We also conducted stratified analyses among women with and without heavy menstrual blood loss [defined as heavy/moderately heavy bleeding or short menstrual cycles (<25 d)]. Finally, we analyzed dietary iron and fecundability separately among users and nonusers of iron supplements.
We used multiple imputation to impute missing covariate and outcome data (28). Covariate missingness in Snart Foraeldre ranged from 0% (age, dietary vitamin C intake, and energy intake) to 2.7% for menstrual cycle regularity. In PRESTO, covariate missingness ranged from 0% [age, education, dietary vitamin C intake, supplement use (vitamin C, iron, and multivitamins) and energy intake] to 0.8% for last method of contraception. Outcome information was imputed for the 5.8% of participants from Snart Foraeldre and the 2.1% of participants from PRESTO who did not complete any follow-up questionnaires, due to concerns about potential selection bias from excluding this group. These women were assigned the minimum amount of follow-up time (1 cycle) and pregnancy status (yes, no) was multiply imputed for that cycle. Removal of these women from the analysis had little effect on the estimates. We used PROC MI to create 5 imputed datasets with >100 variables in the imputation models. We combined coefficients and standard errors across the imputed datasets with the use of PROC MIANALYZE. All analyses were performed with SAS version 9.4 statistical software (SAS Institute).
Results
In the Snart Foraeldre study, mean ± SD energy-adjusted iron intake from dietary sources was 10.4 ± 1.4 mg/d for total iron, 9.7 ± 1.3 mg/d for nonheme iron, and 0.7 ± 0.3 mg/d for heme iron. In the PRESTO study, mean energy-adjusted iron intake was 12.1 ± 3.0 mg/d for total iron, 11.5 ± 3.1 mg/d for nonheme iron, and 0.6 ± 0.4 mg/d for heme iron.
In both cohorts, after adjustment for age, dietary heme iron intake was positively associated with BMI, parity, and current smoking, and inversely associated with education (Table 1). Nonheme iron intake was positively associated with education and physical activity, and inversely associated with BMI in the 2 cohorts.
TABLE 1.
Baseline characteristics of women planning a pregnancy in the Snart Foraeldre and PRESTO cohorts by categories of dietary iron intake1
| Snart Foraeldre (n = 1693) | PRESTO (n = 2969) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary heme iron, mg/d | Dietary nonheme iron, mg/d | Dietary heme iron, mg/d | Dietary nonheme iron, mg/d | |||||||||
| <0.5 | 0.5–0.9 | ≥1 | <9 | 9–10.9 | ≥11 | <0.5 | 0.5–0.9 | ≥1 | <9 | 9–10.9 | ≥11 | |
| Number of participants | 395 | 1019 | 279 | 767 | 432 | 494 | 1433 | 1126 | 410 | 984 | 573 | 1412 |
| Age, y | 29.1 ± 4.5 | 28.5 ± 4.3 | 28.1 ± 4.4 | 28.2 ± 4.3 | 28.5 ± 4.2 | 29.2 ± 4.5 | 30.5 ± 4.1 | 30.0 ± 3.7 | 29.9 ± 4.2 | 29.9 ± 4.1 | 30.2 ± 3.7 | 30.5 ± 3.9 |
| BMI, kg/m2 | 23.1 ± 4.2 | 24.3 ± 5.1 | 25.1 ± 5.3 | 24.4 ± 5.1 | 23.9 ± 4.9 | 23.8 ± 4.6 | 25.3 ± 5.9 | 26.9 ± 6.7 | 29.4 ± 7.8 | 27.0 ± 6.5 | 26.6 ± 6.8 | 26.2 ± 6.7 |
| Non-Hispanic white | — | — | — | — | — | — | 87.3 | 87.9 | 82.9 | 87.1 | 87.7 | 86.2 |
| Education <college degree | 18.3 | 22.6 | 29.8 | 27.8 | 19.0 | 18.6 | 15.0 | 17.4 | 27.6 | 22.1 | 15.0 | 15.6 |
| Parous | 28.6 | 35.9 | 37.1 | 32.7 | 34.5 | 36.4 | 24.4 | 26.1 | 34.6 | 24.3 | 28.0 | 27.5 |
| Multivitamin use in last 12 mo | 60.2 | 61.5 | 58.7 | 59.5 | 60.7 | 61.9 | 90.2 | 89.3 | 85.5 | 88.6 | 88.6 | 89.9 |
| Iron-only supplement use | 5.2 | 6.5 | 3.6 | 4.7 | 7.7 | 5.6 | 11.5 | 9.7 | 9.8 | 9.2 | 10.2 | 11.7 |
| Vitamin C–only supplement use | 4.1 | 4.0 | 3.6 | 3.6 | 4.0 | 4.8 | 17.0 | 20.8 | 19.6 | 16.3 | 18.0 | 20.6 |
| Fruit intake, servings/d | 1.7 ± 1.1 | 1.7 ± 1.0 | 1.9 ± 1.2 | 1.3 ± 0.8 | 1.8 ± 1.0 | 2.3 ± 1.2 | 1.2 ± 0.9 | 1.2 ± 0.9 | 1.3 ± 1.0 | 0.8 ± 0.7 | 1.1 ± 0.8 | 1.5 ± 1.0 |
| Vegetable intake (servings/day) | 5.6 ± 3.8 | 5.8 ± 4.1 | 6.5 ± 4.1 | 4.2 ± 2.7 | 6.1 ± 3.9 | 8.3 ± 4.6 | 1.4 ± 0.8 | 1.7 ± 0.8 | 2.1 ± 1.0 | 1.1 ± 0.5 | 1.5 ± 0.6 | 2.0 ± 0.9 |
| Heme iron intake, mg/d | 0.4 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.3 | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.4 ± 0.2 | 0.7 ± 0.3 | 1.0 ± 0.4 | 0.7 ± 0.4 | 0.6 ± 0.3 | 0.5 ± 0.3 |
| Nonheme iron intake, mg/d | 9.7 ± 1.4 | 9.6 ± 1.3 | 9.8 ± 1.2 | 9.1 ± 1.2 | 9.9 ± 1.2 | 10.4 ± 1.2 | 12.0 ± 3.3 | 11.2 ± 2.9 | 10.6 ± 2.3 | 9.7 ± 1.9 | 10.9 ± 2.1 | 13.0 ± 3.3 |
| Alcohol intake, g/wk | 2.5 ± 3.7 | 2.5 ± 3.5 | 2.9 ± 3.5 | 2.4 ± 3.0 | 2.7 ± 4.3 | 2.7 ± 3.5 | 3.4 ± 4.6 | 3.4 ± 4.1 | 3.2 ± 4.0 | 3.4 ± 4.5 | 3.6 ± 4.2 | 3.3 ± 4.4 |
| Current cigarette smoking | 9.3 | 9.6 | 15.5 | 12.9 | 7.8 | 9.8 | 5.4 | 8.5 | 11.5 | 8.4 | 9.0 | 6.0 |
| Physical activity, MET-hr/w | 64.8 ± 85.1 | 62.0 ± 70.4 | 72.1 ± 90.7 | 60.4 ± 76.0 | 67.0 ± 80.1 | 67.8 ± 75.4 | 38.6 ± 26.7 | 35.9 ± 24.5 | 31.9 ± 23.5 | 33.2 ± 24.8 | 36.4 ± 25.9 | 39.1 ± 25.8 |
| Regular menstrual cycles | 67.7 | 69.6 | 71.7 | 68.0 | 72.5 | 68.7 | 53.8 | 55.0 | 54.3 | 56.0 | 55.0 | 53.0 |
| Cycle length, d | 30.1 ± 4.6 | 30.6 ± 7.0 | 30.5 ± 7.4 | 30.7 ± 7.5 | 30.3 ± 5.5 | 30.3 ± 5.5 | 30.0 ± 4.7 | 29.8 ± 4.3 | 30.4 ± 8.9 | 30.0 ± 4.7 | 29.8 ± 4.7 | 30.0 ± 6.1 |
| Moderate or heavy menstrual flow | 10.0 | 14.1 | 20.7 | 11.2 | 15.3 | 17.5 | 21.3 | 21.8 | 30.0 | 20.8 | 23.9 | 24.0 |
| OCs as last contraceptive method | 41.9 | 44.4 | 41.7 | 46.7 | 37.8 | 43.9 | 26.5 | 28.1 | 24.3 | 27.5 | 25.6 | 26.8 |
| Doing something to improve chances of conception | 72.0 | 75.2 | 71.3 | 73.6 | 73.2 | 74.8 | 76.5 | 74.1 | 71.3 | 76.9 | 71.5 | 74.9 |
Characteristics are presented as percentages or means ± SD within levels of iron consumption, standardized to the age distribution of the cohorts at baseline. MET, metabolic equivalent; OC, oral contraceptive.
In the Snart Foraeldre study, consuming the highest amount of total dietary iron (≥12 mg/d) was not meaningfully associated with fecundability, compared with consuming <10 mg/d (FR: 0.98; 95% CI: 0.81, 1.20) (Table 2). Consuming between 10 and 11.9 mg/d of total dietary iron also was not materially associated with fecundability (FR: 1.11; 95% CI: 0.98, 1.24). In the PRESTO study, FRs for 10–11.9 and ≥12 mg/d were 0.98 (95% CI: 0.87, 1.10) and 1.06 (95% CI: 0.94, 1.19), respectively, compared with <10 mg/d.
TABLE 2.
Association of dietary iron and fecundability among women planning a pregnancy in the Snart Foraeldre and PRESTO cohorts1
| Pregnancies, n | Cycles at risk, n | Age adjusted FR (95% CI) | Multivariable2 FR (95% CI) | Mutually adjusted multivariable3 FR (95% CI) | |
|---|---|---|---|---|---|
| Snart Foraeldre, n = 1693 | |||||
| Total iron, mg/d | |||||
| <10 | 419 | 2348 | 1.00 (Ref.) | 1.00 (Ref.) | — |
| 10–11.9 | 606 | 3088 | 1.08 (0.97, 1.21) | 1.10 (0.98, 1.24) | — |
| ≥12 | 114 | 730 | 0.92 (0.76, 1.11) | 0.98 (0.81, 1.20) | — |
| Heme iron, mg/d | |||||
| <0.5 | 205 | 1125 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| 0.5–0.9 | 737 | 4025 | 1.02 (0.89, 1.17) | 0.99 (0.86, 1.14) | 0.99 (0.86, 1.14) |
| ≥1.0 | 197 | 1016 | 1.05 (0.88, 1.25) | 1.01 (0.85, 1.22) | 1.00 (0.83, 1.20) |
| Nonheme iron, mg/d | |||||
| <9 | 319 | 1801 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| 9–10.9 | 668 | 3495 | 1.07 (0.95, 1.21) | 1.12 (0.99, 1.27) | 1.12 (0.99, 1.27) |
| ≥11 | 152 | 870 | 1.02 (0.85, 1.22) | 1.11 (0.92, 1.34) | 1.11 (0.92, 1.34) |
| PRESTO, n = 2969 | |||||
| Total iron, mg/d | |||||
| <10 | 377 | 2832 | 1.00 (Ref.) | 1.00 (Ref.) | — |
| 10–11.9 | 608 | 4310 | 1.06 (0.94, 1.19) | 0.98 (0.87, 1.10) | — |
| ≥12 | 831 | 5258 | 1.16 (1.04, 1.30) | 1.06 (0.94, 1.19) | — |
| Heme iron, mg/d | |||||
| <0.5 | 776 | 5262 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| 0.5–0.9 | 837 | 5543 | 1.00 (0.91, 1.09) | 1.03 (0.94, 1.12) | 1.03 (0.94, 1.13) |
| ≥1.0 | 203 | 1595 | 0.87 (0.76, 1.01) | 1.01 (0.87, 1.17) | 1.01 (0.87, 1.18) |
| Nonheme iron, mg/d | |||||
| <9 | 293 | 2168 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| 9–10.9 | 573 | 4116 | 1.03 (0.91, 1.18) | 0.96 (0.84, 1.09) | 0.96 (0.84, 1.09) |
| ≥11 | 950 | 6116 | 1.14 (1.01, 1.29) | 1.01 (0.89, 1.14) | 1.01 (0.89, 1.15) |
FR, fecundability ratio.
Adjusted for age, vocational training/education, BMI, physical activity, smoking, alcohol consumption, use of oral contraceptives, parity, cycle regularity and length, doing something to improve chances of conception, use of iron and vitamin C supplements, multivitamin use, dietary vitamin C (continuous), and total energy (continuous). The PRESTO models are also adjusted for race/ethnicity
Also adjusted for dietary heme or nonheme iron intake, respectively.
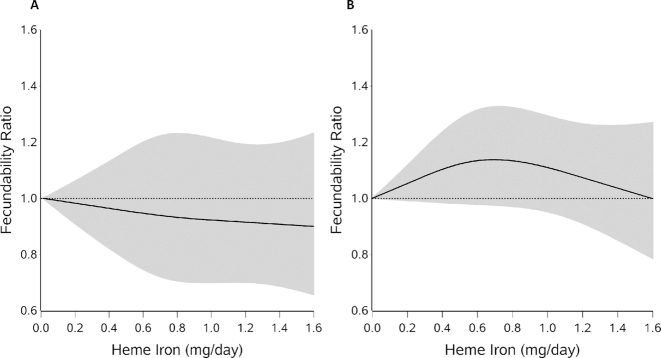
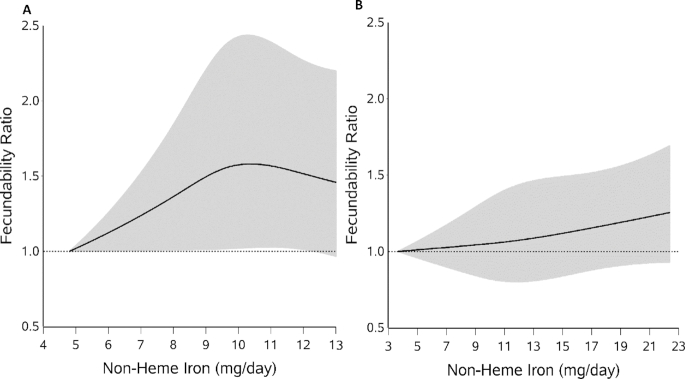
We found little association between heme iron intake and fecundability in either cohort. Multivariable adjusted FRs ranged from 0.99 to 1.03, and results from models that mutually adjusted for heme and nonheme iron did not vary substantially (Table 2). Figure 1A, B displays the associations between heme iron and fecundability in the Snart Foraeldre and PRESTO cohorts through the use of restricted cubic splines. The spline curves showed slightly different patterns for the 2 cohorts, but the curves remained close to the null, indicating little overall association, consistent with findings from the categoric analyses. For nonheme iron (Table 2), the categoric analyses indicated slight increases in fecundability >9 mg/d in the Snart Foraeldre cohort, but little association in the PRESTO cohort. However, the spline curves for both cohorts were similar, showing slight increases in the fecundability curve with increasing intake of nonheme iron (Figure 2A, B). Adjustment for fruit and vegetable consumption did not change the estimates (data not shown).
FIGURE 1.
Dietary heme iron intake and fecundability among women planning a pregnancy in the Snart Foraeldre (A) and PRESTO (B) studies, fitted with the use of restricted cubic splines. The reference level is the lowest value in each cohort (0.01 for both). There are 3 knots located at the 10th, 50th, and 95th percentiles of the distribution. The line indicates the FR and the shaded area indicates the 95% CI. The spline is trimmed at the 99th percentile. Splines are adjusted for age, vocational training/education, BMI, physical activity, smoking, alcohol consumption, use of oral contraceptives, parity, cycle regularity and length, doing something to improve chances of conception, use of iron and vitamin C supplements, multivitamin use, dietary vitamin C (continuous), and total energy (continuous). The PRESTO models are also adjusted for race/ethnicity. FR, fecundability ratio.
FIGURE 2.
Dietary nonheme iron intake and fecundability among women planning a pregnancy in the Snart Foraeldre (A) and PRESTO (B) studies, fitted with the use of restricted cubic splines. The reference level is the lowest value in each cohort (4.81 for Snart Foraeldre and 3.67 for PRESTO). There are 3 knots located at the 10th, 50th, and 95th percentiles of the distribution. The line indicates the FR and the shaded area indicates the 95% CI. The spline is trimmed at the 99th percentile. Splines are adjusted for age, vocational training/education, BMI, physical activity, smoking, alcohol consumption, use of oral contraceptives, parity, cycle regularity and length, doing something to improve chances of conception, use of iron and vitamin C supplements, multivitamin use, dietary vitamin C (continuous), and total energy (continuous). The PRESTO models are also adjusted for race/ethnicity. FR, fecundability ratio.
In both cohorts, the relation between fecundability and total, heme, or nonheme iron intake was similar across strata of maternal age, BMI, and attempt time at study entry. Results for dietary iron intake were also broadly consistent, although imprecise, among women who used and did not use iron-containing supplements (Supplemental Table 1).
Among parous women in both cohorts, nonheme iron intake was associated with slightly increased fecundability (overall FR for ≥11 mg/d compared with <9 mg/d: 1.32; 95% CI: 0.98, 1.79 in Snart Foraeldre, and 1.17; 95% CI: 0.91, 1.50 in PRESTO), but little association was observed among nulliparous women (FR ≥11 mg/d compared with <9 mg/d: 0.97; 95% CI: 0.76, 1.23 in Snart Foraeldre, and FR: 0.97; 95% CI: 1.84, 1.12 in PRESTO) (Table 3). In the PRESTO cohort, but not Snart Foraeldre, nonheme iron intake was positively associated with fecundability among women with heavy menses or short cycles, or a combination of both (for nonheme iron intake of 9–10.9 mg/d and ≥11 mg/d compared with <9 mg/d, FRs were 1.31; 95% CI: 0.97, 1.78 and 1.54; 95% CI: 1.16, 2.04, respectively) compared with women without heavy menses/short cycles (FR: 0.87; 95% CI: 0.75, 1.01 and FR: 0.89; 95% CI: 0.77, 1.02) (Table 4).
TABLE 3.
Association of dietary iron and fecundability among women planning a pregnancy in the Snart Foraeldre and PRESTO cohorts, stratified by parity1
| Parous | Nulliparous | |||||
|---|---|---|---|---|---|---|
| Pregnancies, n | Cycles at risk, n | Adjusted2 FR (95% CI) | Pregnancies, n | Cycles at risk, n | Adjusted2 FR (95% CI) | |
| Snart Foraeldre, n = 1693 | ||||||
| Total iron, mg/d | ||||||
| <10 | 185 | 751 | 1.00 (Ref.) | 234 | 1597 | 1.00 (Ref.) |
| 10–11.9 | 219 | 932 | 1.03 (0.86, 1.24) | 387 | 2156 | 1.18 (1.01, 1.39) |
| ≥12 | 43 | 187 | 1.07 (0.78, 1.46) | 71 | 543 | 0.92 (0.71, 1.20) |
| Heme iron, mg/d | ||||||
| <0.5 | 77 | 333 | 1.00 (Ref.) | 128 | 792 | 1.00 (Ref.) |
| 0.5–0.9 | 290 | 1217 | 0.97 (0.77, 1.23) | 447 | 2808 | 1.00 (0.83, 1.20) |
| ≥1.0 | 80 | 320 | 0.83 (0.62, 1.12) | 117 | 696 | 1.08 (0.85, 1.37) |
| Nonheme iron, mg/d | ||||||
| <9 | 136 | 590 | 1.00 (Ref.) | 183 | 1211 | 1.00 (Ref.) |
| 9–10.9 | 256 | 1063 | 1.18 (0.96, 1.43) | 412 | 2432 | 1.11 (0.94, 1.32) |
| ≥11 | 55 | 217 | 1.32 (0.98, 1.79) | 97 | 653 | 0.97 (0.76, 1.23) |
| PRESTO, n = 2969 | ||||||
| Total iron, mg/d | ||||||
| <10 | 105 | 695 | 1.00 (Ref.) | 272 | 2137 | 1.00 (Ref.) |
| 10–11.9 | 185 | 1065 | 1.00 (0.80, 1.26) | 423 | 3245 | 0.96 (0.83, 1.11) |
| ≥12 | 252 | 1273 | 1.07 (0.86, 1.33) | 579 | 3985 | 1.03 (0.90, 1.19) |
| Heme iron, mg/d | ||||||
| <0.5 | 220 | 1219 | 1.00 (Ref.) | 556 | 4043 | 1.00 (Ref.) |
| 0.5–0.9 | 260 | 1386 | 0.98 (0.83, 1.15) | 577 | 4157 | 1.05 (0.94, 1.17) |
| ≥1.0 | 62 | 428 | 1.02 (0.77, 1.34) | 141 | 1167 | 1.01 (0.84, 1.20) |
| Nonheme iron, mg/d | ||||||
| <9 | 73 | 542 | 1.00 (Ref.) | 220 | 1626 | 1.00 (Ref.) |
| 9–10.9 | 187 | 1020 | 1.21 (0.93, 1.58) | 386 | 3096 | 0.86 (0.74, 1.01) |
| ≥11 | 282 | 1471 | 1.17 (0.91, 1.50) | 668 | 4645 | 0.97 (0.84, 1.12) |
FR, fecundability ratio.
Adjusted for age, vocational training/education, BMI, physical activity, smoking, alcohol consumption, use of oral contraceptives, parity, cycle regularity and length, doing something to improve chances of conception, use of iron and vitamin C supplements, multivitamin use, dietary vitamin C (continuous), and total energy (continuous). The PRESTO models are also adjusted for race/ethnicity. Heme and non-heme iron models are mutually-adjusted for each other.
TABLE 4.
Association of dietary iron and fecundability among women planning a pregnancy in the Snart Foraeldre and PRESTO cohorts, stratified by heavy menses/short cycles1
| Heavy menses or short cycles | Not heavy menses or short cycles | |||||
|---|---|---|---|---|---|---|
| Pregnancies, n | Cycles at risk, n | Adjusted2 FR (95% CI) | Pregnancies, n | Cycles at risk, n | Adjusted2 FR (95% CI) | |
| Snart Foraeldre, n = 1693 | ||||||
| Total iron, mg/d | ||||||
| <10 | 111 | 546 | 1.00 (Ref.) | 312 | 1806 | 1.00 (Ref.) |
| 10–11.9 | 170 | 806 | 1.10 (0.88, 1.37) | 443 | 2289 | 1.11 (0.97, 1.28) |
| ≥12 | 36 | 216 | 0.96 (0.65, 1.41) | 79 | 515 | 0.99 (0.78, 1.27) |
| Heme iron, mg/d | ||||||
| <0.5 | 49 | 259 | 1.00 (Ref.) | 160 | 870 | 1.00 (Ref.) |
| 0.5–0.9 | 209 | 1015 | 0.99 (0.74, 1.33) | 535 | 3017 | 0.98 (0.84, 1.16) |
| ≥1.0 | 59 | 294 | 0.92 (0.64, 1.31) | 139 | 723 | 1.05 (0.85, 1.31) |
| Nonheme iron, mg/d | ||||||
| <9 | 82 | 371 | 1.00 (Ref.) | 241 | 1434 | 1.00 (Ref.) |
| 9–10.9 | 192 | 922 | 1.03 (0.80, 1.33) | 483 | 2580 | 1.15 (0.99, 1.34) |
| ≥11 | 43 | 275 | 0.89 (0.58, 1.35) | 110 | 596 | 1.20 (0.96, 1.51) |
| PRESTO, n = 2969 | ||||||
| Total iron, mg/d | ||||||
| <10 | 77 | 853 | 1.00 (Ref.) | 300 | 1979 | 1.00 (Ref.) |
| 10–11.9 | 133 | 1109 | 1.24 (0.95, 1.63) | 475 | 3201 | 0.94 (0.82, 1.07) |
| ≥12 | 219 | 1396 | 1.57 (1.22, 2.03) | 612 | 3862 | 0.96 (0.85, 1.10) |
| Heme iron, mg/d | ||||||
| <0.5 | 181 | 1387 | 1.00 (Ref.) | 595 | 3875 | 1.00 (Ref.) |
| 0.5–0.9 | 203 | 1457 | 0.97 (0.80, 1.18) | 634 | 4086 | 1.03 (0.93, 1.14) |
| ≥1.0 | 45 | 514 | 0.76 (0.55, 1.05) | 158 | 1081 | 1.12 (0.95, 1.33) |
| Nonheme iron, mg/d | ||||||
| <9 | 58 | 670 | 1.00 (Ref.) | 235 | 1498 | 1.00 (Ref.) |
| 9–10.9 | 135 | 1114 | 1.31 (0.97, 1.78) | 438 | 3002 | 0.87 (0.75, 1.01) |
| ≥11 | 236 | 1574 | 1.54 (1.16, 2.04) | 714 | 4542 | 0.89 (0.77, 1.02) |
FR, fecundability ratio.
Adjusted for age, vocational training/education, BMI, physical activity, smoking, alcohol consumption, use of oral contraceptives, parity, cycle regularity and length, doing something to improve chances of conception, use of iron and vitamin C supplements, multivitamin use, dietary vitamin C (continuous), and total energy (continuous). The PRESTO models are also adjusted for race/ethnicity. Heme and non-heme iron models are mutually-adjusted for each other.
In the Snart Foraeldre cohort, baseline use of multivitamins containing iron or iron-only supplements during the last 12 mo was 61%, compared with 89% in the PRESTO cohort. Overall prevalence was driven chiefly by use of multivitamins with minerals. Compared with nonusers of iron-containing supplements, there was little association between use of any iron-containing supplements and fecundability in Snart Foraeldre (FR: 1.01; 95% CI: 0.90, 1.13), but slightly increased fecundability in PRESTO (FR: 1.19; 95% CI: 1.03, 1.38). Similar results were found for iron-only supplements (Snart Foraeldre, FR: 1.05; 95% CI: 0.84, 1.32; PRESTO, FR: 1.20; 95% CI: 0.99, 1.44) (Table 5). We found stronger associations in PRESTO, but not Snart Foraeldre, for multivitamin/mineral use and iron-only supplement use among women with heavy menses or short menstrual cycles, or a combination of both (FR: 1.57; 95% CI: 1.16, 2.12 and FR: 1.62; 95% CI: 1.10, 2.37 in PRESTO) than among women without heavy menses/short cycles (FR: 1.09; 95% CI: 0.93, 1.29 and FR: 1.09; 95% CI: 0.88, 1.36 in PRESTO) (Tables 6 and 7). Results for iron-only supplements stratified by maternal age, BMI, and cycles of attempt time at study entry were broadly consistent across strata in both cohorts (Supplemental Table 2).
TABLE 5.
Supplementary iron intake and fecundability among women planning a pregnancy in the Snart Foraeldre and PRESTO cohorts1
| Pregnancies, n | Cycles at risk, n | Age-adjusted FR (95% CI) | Multivariable model2 FR (95% CI) | |
|---|---|---|---|---|
| Snart Foraeldre, n = 1693 | ||||
| Iron-containing supplements | ||||
| Nonuser | 453 | 2494 | 1.00 (Ref.) | 1.00 (Ref.) |
| Any iron-containing supplement user | 686 | 3672 | 1.06 (0.95, 1.18) | 1.01 (0.90, 1.13) |
| Supplement type | ||||
| Nonuser | 453 | 2494 | 1.00 (Ref.) | 1.00 (Ref.) |
| Multivitamin user | 616 | 3346 | 1.05 (0.94, 1.17) | 1.01 (0.90, 1.13) |
| Iron-only supplement user | 70 | 326 | 1.15 (0.92, 1.44) | 1.05 (0.84, 1.32) |
| PRESTO, n = 2969 | ||||
| Iron-containing supplements | ||||
| Nonuser | 185 | 1559 | 1.00 (Ref.) | 1.00 (Ref.) |
| Any iron-containing supplement user | 1631 | 10,841 | 1.25 (1.08, 1.43) | 1.19 (1.03, 1.38) |
| Supplement type | ||||
| Nonuser | 185 | 1559 | 1.00 (Ref.) | 1.00 (Ref.) |
| Multivitamin user | 1436 | 9509 | 1.25 (1.08, 1.44) | 1.19 (1.03, 1.38) |
| Iron-only supplement user | 195 | 1332 | 1.23 (1.02, 1.48) | 1.20 (0.99, 1.44) |
FR, fecundability ratio
Adjusted for age, vocational training/education, BMI, physical activity, smoking, alcohol consumption, use of oral contraceptives, cycle regularity and length, doing something to improve chances of conception, use of vitamin C supplements, and dietary vitamin C (continuous). The PRESTO models are also adjusted for race/ethnicity.
TABLE 6.
Supplementary iron intake and fecundability among women planning a pregnancy in the Snart Foraeldre and PRESTO cohorts, stratified by parity1
| Parous | Nulliparous | |||||
|---|---|---|---|---|---|---|
| Pregnancies, n | Cycles at risk, n | Adjusted2 FR (95% CI) | Pregnancies, n | Cycles at risk, n | Adjusted2 FR (95% CI) | |
| Snart Foraeldre, n = 1693 | ||||||
| Nonuser | 168 | 686 | 1.00 (Ref.) | 285 | 1808 | 1.00 (Ref.) |
| Any iron-containing supplement user | 279 | 1184 | 1.02 (0.86, 1.21) | 407 | 2488 | 1.02 (0.88, 1.17) |
| Supplement type | ||||||
| Nonuser | 168 | 686 | 1.00 (Ref.) | 285 | 1808 | 1.00 (Ref.) |
| Multivitamin user | 249 | 1070 | 1.02 (0.86, 1.22) | 367 | 2276 | 1.01 (0.87, 1.16) |
| Iron-only supplement user | 30 | 114 | 0.99 (0.69, 1.40) | 40 | 212 | 1.12 (0.83, 1.52) |
| PRESTO, n = 2969 | ||||||
| Iron-containing supplements | ||||||
| Nonuser | 61 | 439 | 1.00 (Ref.) | 124 | 1120 | 1.00 (Ref.) |
| Any iron-containing supplement user | 481 | 2594 | 1.20 (0.94, 1.55) | 1150 | 8247 | 1.18 (0.99, 1.40) |
| Supplement type | ||||||
| Nonuser | 61 | 439 | 1.00 (Ref.) | 124 | 1120 | 1.00 (Ref.) |
| Multivitamin user | 420 | 2207 | 1.22 (0.95, 1.57) | 1016 | 7302 | 1.17 (0.98, 1.39) |
| Iron-only supplement user | 61 | 387 | 1.11 (0.80, 1.55) | 134 | 945 | 1.24 (0.99, 1.56) |
| Single-iron supplement user | 141 | 875 | 1.21 (0.98, 1.50) | 54 | 457 | 1.21 (0.82, 1.79) |
FR, fecundability ratio.
Adjusted for age, vocational training/education, BMI, physical activity, smoking, alcohol consumption, use of oral contraceptives, parity, cycle regularity and length, doing something to improve chances of conception, use of iron and vitamin C supplements, multivitamin use, dietary vitamin C (continuous), and total energy (continuous). The PRESTO models are also adjusted for race/ethnicity. Heme and nonheme iron models are mutually adjusted for each other.
TABLE 7.
Supplementary iron intake and fecundability among women planning a pregnancy in the Snart Foraeldre and PRESTO cohorts, stratified by heavy menses/short cycles1
| Heavy menses or short cycles | Not heavy menses or short cycles | |||||
|---|---|---|---|---|---|---|
| Pregnancies, n | Cycles at risk, n | Adjusted2 FR (95% CI) | Pregnancies, n | Cycles at risk, n | Adjusted2 FR (95% CI) | |
| Snart Foraeldre, n = 1693 | ||||||
| Nonuser | 113 | 551 | 1.00 (Ref.) | 344 | 1947 | 1.00 (Ref.) |
| Any iron-containing supplement user | 204 | 1017 | 1.09 (0.87, 1.36) | 490 | 2663 | 1.01 (0.89, 1.15) |
| Supplement type | ||||||
| Nonuser | 113 | 551 | 1.00 (Ref.) | 344 | 1947 | 1.00 (Ref.) |
| Multivitamin user | 182 | 930 | 1.10 (0.88, 1.39) | 441 | 2423 | 1.01 (0.89, 1.14) |
| Iron-only supplement user | 22 | 87 | 1.01 (0.67, 1.52) | 49 | 240 | 1.06 (0.80, 1.40) |
| PRESTO, n = 2969 | ||||||
| Iron-containing supplements | ||||||
| Nonuser | 46 | 512 | 1.00 (Ref.) | 139 | 1047 | 1.00 (Ref.) |
| Any iron-containing supplement user | 383 | 2846 | 1.58 (1.17, 2.13) | 1248 | 7995 | 1.09 (0.93, 1.29) |
| Supplement type | ||||||
| Nonuser | 46 | 512 | 1.00 (Ref.) | 139 | 1047 | 1.00 (Ref.) |
| Multivitamin user | 328 | 2416 | 1.57 (1.16, 2.12) | 1108 | 7093 | 1.09 (0.93, 1.29) |
| Iron-only supplement user | 55 | 430 | 1.62 (1.10, 2.37) | 140 | 902 | 1.09 (0.88, 1.36) |
FR, fecundability ratio.
Adjusted for age, vocational training/education, BMI, physical activity, smoking, alcohol consumption, use of oral contraceptives, parity, cycle regularity and length, doing something to improve chances of conception, use of iron and vitamin C supplements, multivitamin use, dietary vitamin C (continuous), and total energy (continuous). The PRESTO models are also adjusted for race/ethnicity. Heme and nonheme iron models are mutually adjusted for each other.
Discussion
We found little association between intake of total dietary iron or heme iron and fecundability. We found some evidence for a positive association between dietary nonheme iron and iron supplement intake and fecundability, particularly among women with a potential iron deficiency (as approximated by heavy menstrual bleeding or short menstrual cycles) and among parous women.
A previous investigation among US female nurses found that iron supplements and dietary intake of nonheme iron were associated with a 40% lower risk of ovulatory infertility (OR: 0.60; 95% CI: 0.39, 0.92) for any iron supplement use compared with nonuse, and an identical value of OR: 0.60 (95% CI: 0.39, 0.92) for quintile 5 compared with quintile 1 intake of nonheme iron (9), findings that are much stronger than in our cohorts. The range of iron intake between our investigation and the US study of nurses may differ considerably and could contribute to the variation in observed results. In addition, we examined all types of subfertility, not just ovulatory subfertility. If we assume that iron only affects 1 type of subfecundity (ovulatory, for example), then our outcome definition would have imperfect specificity. If this difference is nondifferential with respect to exposure, the result of imperfect specificity would be to bias the FR toward the null. Similar to the US study of nurses, we found little association between heme iron intake and fecundability in either cohort (9, 29). We found stronger positive associations between nonheme iron and fecundability among parous compared with nulliparous women in both cohorts. Higher parity and short interpregnancy intervals have been associated with poorer iron status (30). A cross-sectional study of premenopausal women from NHANES found that several biomarkers of iron status (hemoglobin, ferritin, transferrin receptor, and percent transferrin saturation) were lower in parous than in nulliparous women (31). In addition to parity, heavy menstrual blood loss is a potential risk factor for iron deficiency. Women with heavy menstrual bleeding tend to lose about twice as much iron during a menstrual cycle than those with average blood loss (31, 32) and are at greater risk of iron-deficiency anemia (33, 34).
We found improved fecundability with increasing nonheme iron intake among women with heavy menses or short menstrual cycles, or a combination of both, in PRESTO. We also found increased fecundability among iron supplement users in PRESTO, with even stronger results among women with heavy menstrual blood loss.
Iron is absorbed in the gut and is involved in oxygen transport, metabolism, growth, cellular function and support of the immune system (35). Iron homeostasis is tightly regulated; excesses or deficiencies can cause myriad problems. Women with polycystic ovarian syndrome (PCOS) tend to have mild iron overload (36). There is a bidirectional relation between iron and glucose metabolism, and some PCOS patients experience an elevated iron concentration and hyperinsulinemia (5, 37, 38). The metabolic dysregulation of glucose present in PCOS is associated with hyperandrogenism, ovulatory dysfunction, and difficulties conceiving (4). Although the complicated relation between insulin resistance and iron overload is most evident among women with PCOS, these relations also may exist to a lesser degree among women without PCOS.
It is estimated that 5–16% of reproductive-age women in industrialized countries are iron deficient (39). Inadequate iron status, most commonly caused by malabsorption in the gut, heavy menstrual bleeding (40), or a recent full-term pregnancy (41), may also play a role in fertility. For example, celiac disease, a chronic allergic reaction to gluten that causes malabsorption and inflammation of the gut, usually results in low iron status. In women with celiac disease, adoption of a gluten-free diet has been shown to restore fertility by improving nutrient absorption and decreasing inflammation (42, 43).
Although an association between serum iron concentration and improved fertility is biologically plausible, we observed only a small and inconsistent increase in fecundability among women who consumed supplementary iron. Serum iron concentration is tightly controlled by normal body processes. Very low or very high nutrient intakes are needed to produce a meaningful change in serum iron. In addition, many systemic and dietary factors, including intake of vitamin C, polyphenols, and calcium, can modify the absorption, excretion, and bioavailability of iron.
Although recruitment, questionnaires, and general study procedures were nearly identical across the Snart Foraeldre and PRESTO cohorts, the different study populations necessitated separate assessments of dietary intake, which, along with differing diets and food fortification practices, may partially explain the differences in results across the 2 cohorts. The use of 2 different FFQs precluded direct comparison of absolute dietary iron intake between the studies. Although measurement of iron intake was shown to be reasonably valid for both FFQs, questionnaire responses always result in some misclassification, and misclassification from 2 different instruments is unlikely to be equal. Given the prospective study design, exposure misclassification would be nondifferential and would produce bias toward the null for the extreme categories of intake. There is also potential for misclassification of iron supplementation in the PRESTO study. The PRESTO questionnaire did not ask about specific brands of multivitamin use, and we assumed that all multivitamins or prenatal vitamins containing minerals also contained iron. This assumption undoubtedly introduced some misclassification, but the observed effects for use of any iron-containing supplement were similar to the effects related to iron-only supplement use.
Heme iron is more readily absorbed than nonheme iron, and it is therefore somewhat puzzling that we saw slightly increased fecundability for nonheme, but not heme iron intake. Residual confounding by dietary correlates of heme and nonheme iron may partially explain these results. Heme iron is strongly correlated with protein intake from animal sources, which has been associated with lower fecundability (44). Nonheme iron is derived primarily from vegetable sources. Results were similar, however, after we controlled for average daily servings of vegetables and fruits.
The use of TTP as an outcome is a more sensitive measure of subfecundity than the dichotomous clinical measure of infertility (trying to conceive for ≥12 mo without success) (45). Although the studies enrolled volunteers, there is little reason to believe that the physiology of participants would differ from that of persons who did not participate. In an earlier analysis that compared estimates of established perinatal associations (e.g., maternal smoking and low birth weight) between our Danish internet-based preconception cohort with the total population available in the Danish Birth Registry, we obtained similar results from both data sources. These results for known associations support the thesis that the data from the volunteers in our cohorts have reasonable internal and external validity (46).
In summary, we found little evidence that heme iron intake was associated with fecundability. Results for nonheme iron intake and supplement use were inconsistent, with some indication of beneficial effects on fecundability among women with possible iron deficiency.
Supplementary Material
Acknowledgments
We thank Dr Vibeke Knudsen and Ms Tina Christensen for their technical assistance in developing and validating the Snart Foraeldre FFQ, Dr Amy Subar and Mr Ken Bishop from the National Cancer Institute for developing, validating, and providing access to the web-based DHQII, Mr Anders Riis for his preparation of the data for analysis, Mr Michael Bairos for his development of the web-based infrastructure of PRESTO, Dr Ellen Trolle and Tue Christensen of the Danish Technical University for assistance with the web-based FFQ in Denmark, Tanran Wang, MPH, for help with formatting, and Dr Harry McCardle of the University of Aberdeen for his comments on a previous draft of this manuscript.
The authors’ responsibilities were as follows—LAW, EMM, KJR, HTS, and EEH: designed the study; KAH, LAW, EEH, KJR, HTS, AKW, and HTC: conducted the research; KAH and AKW: analyzed the data; KAH, AKW, LAW, EMM, KLT, KJR, EEH, HTC, HTS, and MV: wrote the paper; KAH and EEH: have primary responsibility for all content; and all authors: edited, read and approved the final manuscript.
Notes
Snart Foraeldre is supported by the National Institute of Child Health and Human Development (NICHD) (R21-HD050264, R01-HD060680, and R01-HD086742) and the Danish Medical Research Council (271-07-0338). PRESTO is supported by the NICHD (R21-HD072326 and R01-HD086742). AKW's work was funded by the Boston University Reproductive, Perinatal, and Pediatric Epidemiology training program (NICHD grant T32-HD052458).
Author disclosures: The authors declare no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: DHQII, Diet History Questionnaire II; FR, fecundability ratio; LMP, last menstrual period; MET, metabolic equivalent; PCOS, polycystic ovarian syndrome; TTP, time to pregnancy,
References
- 1. Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA. 2003;290:1767–70. [DOI] [PubMed] [Google Scholar]
- 2. Macaluso M, Wright-Schnapp TJ, Chandra A, Johnson R, Satterwhite CL, Pulver A, Berman SM, Wang RY, Farr SL, Pollack LA. A public health focus on infertility prevention, detection, and management. Fertil Steril. 2010;93:16.e11-10. [DOI] [PubMed] [Google Scholar]
- 3. Hooda J, Shah A, Zhang L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients. 2014;6:1080–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ecklund LC, Usadi RS.. Endocrine and reproductive effects of polycystic ovarian syndrome. Obstet Gynecol Clin North Am. 2015;42:55–65. [DOI] [PubMed] [Google Scholar]
- 5. Kim JW, Kang KM, Yoon TK, Shim SH, Lee WS. Study of circulating hepcidin in association with iron excess, metabolic syndrome, and BMP-6 expression in granulosa cells in women with polycystic ovary syndrome. Fertil Steril. 2014;102:548–54. e542. [DOI] [PubMed] [Google Scholar]
- 6. Martinez-Garcia MA, Luque-Ramirez M, San-Millan JL, Escobar-Morreale HF. Body iron stores and glucose intolerance in premenopausal women: role of hyperandrogenism, insulin resistance, and genomic variants related to inflammation, oxidative stress, and iron metabolism. Diabetes Care. 2009;32:1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhaskaram P. Immunobiology of mild micronutrient deficiencies. Br J Nutr. 2001;85:(Suppl 2):S75–80. [DOI] [PubMed] [Google Scholar]
- 8. Verstraelen H, Delanghe J, Roelens K, Blot S, Claeys G, Temmerman M. Subclinical iron deficiency is a strong predictor of bacterial vaginosis in early pregnancy. BMC Infect Dis. 2005;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Iron intake and risk of ovulatory infertility. Obstet Gynecol. 2006;108:1145–52. [DOI] [PubMed] [Google Scholar]
- 10. Huybrechts KF, Mikkelsen EM, Christensen T, Riis AH, Hatch EE, Wise LA, Sorensen HT, Rothman KJ. A successful implementation of e-epidemiology: the Danish pregnancy planning study ‘Snart-Gravid’. Eur J Epidemiol. 2010;25:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort profile: the Danish web-based pregnancy planning study—‘Snart-Gravid’. Int J Epidemiol. 2009;38:938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knudsen VK, Hatch EE, Cueto H, Tucker KL, Wise L, Christensen T, Mikkelsen EM. Relative validity of a semi-quantitative, web-based FFQ used in the ‘Snart Foraeldre’ cohort—a Danish study of diet and fertility. Public Health Nutr. 2016;19(6):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, Gruschow SM, Horgan CE, Wiley AS, Hahn KA et al.. Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol. 2015;29:360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–99. [DOI] [PubMed] [Google Scholar]
- 15. Wise LA, Wesselink AK, Mikkelsen EM, Cueto H, Hahn KA, Rothman KJ, Tucker KL, Sorensen HT, Hatch EE. Dairy intake and fecundability in 2 preconception cohort studies. Am J Clin Nutr. 2017;105:100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S.; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 17. Wise LA, Mikkelsen EM, Rothman KJ, Riis AH, Sorensen HT, Huybrechts KF, Hatch EE. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol. 2011;174:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF et al.. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 19. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–81. [DOI] [PubMed] [Google Scholar]
- 20. Durrleman S, Simon R.. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 21. Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129:1072–8. [DOI] [PubMed] [Google Scholar]
- 22. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed Philadelphia, (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 23. Zijp IM, Korver O, Tijburg LB. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr. 2000;40:371–98. [DOI] [PubMed] [Google Scholar]
- 24. Nelson M, Poulter J.. Impact of tea drinking on iron status in the UK: a review. J Hum Nutr Diet. 2004;17:43–54. [DOI] [PubMed] [Google Scholar]
- 25. Fernandez R, Phillips SF.. Components of fiber bind iron in vitro. Am J Clin Nutr. 1982;35:100–6. [DOI] [PubMed] [Google Scholar]
- 26. Hallberg L. Wheat fiber, phytates and iron absorption. Scand J Gastroenterol Suppl. 1987;129:73–9. [DOI] [PubMed] [Google Scholar]
- 27. Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461S–7S. [DOI] [PubMed] [Google Scholar]
- 28. Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20:1541–9. [DOI] [PubMed] [Google Scholar]
- 29. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110:1050–8. [DOI] [PubMed] [Google Scholar]
- 30. Milman N, Kirchhoff M, Jorgensen T. Iron status markers, serum ferritin and hemoglobin in 1359 Danish women in relation to menstruation, hormonal contraception, parity, and postmenopausal hormone treatment. Ann Hematol. 1992;65:96–102. [DOI] [PubMed] [Google Scholar]
- 31. Miller EM. Iron status and reproduction in US women: National Health and Nutrition Examination Survey, 1999–2006. PLoS One. 2014;9:e112216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller EM. The reproductive ecology of iron in women. Am J Phys Anthropol. 2016;159:S172–95. [DOI] [PubMed] [Google Scholar]
- 33. Bernardi LA, Ghant MS, Andrade C, Recht H, Marsh EE. The association between subjective assessment of menstrual bleeding and measures of iron deficiency anemia in premenopausal African-American women: a cross-sectional study. BMC Womens Health. 2016;16:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. European Food Safety Authority. Scientific opinion on dietary reference values for iron. EFSA J. 2015;13:4254. [Google Scholar]
- 35. Bendich A. Micronutrients in women's health and immune function. Nutrition. 2001;17:858–67. [DOI] [PubMed] [Google Scholar]
- 36. Escobar-Morreale HF. Iron metabolism and the polycystic ovary syndrome. Trends Endocrinol Metab. 2012;23:509–15. [DOI] [PubMed] [Google Scholar]
- 37. Luque-Ramirez M, Alvarez-Blasco F, Botella-Carretero JI, Sanchon R, San Millan JL, Escobar-Morreale HF. Increased body iron stores of obese women with polycystic ovary syndrome are a consequence of insulin resistance and hyperinsulinism and are not a result of reduced menstrual losses. Diabetes Care. 2007;30:2309–13. [DOI] [PubMed] [Google Scholar]
- 38. Luque-Ramirez M, Alvarez-Blasco F, Alpanes M, Escobar-Morreale HF. Role of decreased circulating hepcidin concentrations in the iron excess of women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:846–52. [DOI] [PubMed] [Google Scholar]
- 39. Beck KL, Conlon CA, Kruger R, Coad J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: a review. Nutrients. 2014;6:3747–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harvey LJ, Armah CN, Dainty JR, Foxall RJ, John Lewis D, Langford NJ, Fairweather-Tait SJ. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br J Nutr. 2005;94:557–64. [DOI] [PubMed] [Google Scholar]
- 41. Bodnar LM, Cogswell ME, McDonald T. Have we forgotten the significance of postpartum iron deficiency? Am J Obstet Gynecol. 2005;193:36–44. [DOI] [PubMed] [Google Scholar]
- 42. Vilppula A, Kaukinen K, Luostarinen L, Krekela I, Patrikainen H, Valve R, Luostarinen M, Laurila K, Maki M, Collin P. Clinical benefit of gluten-free diet in screen-detected older celiac disease patients. BMC Gastroenterol. 2011;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nenna R, Mennini M, Petrarca L, Bonamico M. Immediate effect on fertility of a gluten-free diet in women with untreated coeliac disease. Gut. 2011;60:1023–4. [DOI] [PubMed] [Google Scholar]
- 44. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198:210.e211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–80. [DOI] [PubMed] [Google Scholar]
- 46. Hatch EE, Hahn KA, Wise LA, Mikkelsen EM, Kumar R, Fox MP, Brooks DR, Riis AH, Sorensen HT, Rothman KJ. Evaluation of selection bias in an internet-based study of pregnancy planners. Epidemiology. 2016;27:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.