Abstract
Aims
To evaluate the effectiveness and safety of cryoballoon ablation (CBA) compared with radiofrequency ablation (RFA) for symptomatic paroxysmal or drug-refractory persistent atrial fibrillation (AF).
Methods and results
Prospective cluster cohort study in experienced CBA and RFA centres. Primary endpoint was ‘atrial arrhythmia recurrence’, secondary endpoints were as follows: procedural results, safety, and clinical course. A total of 4189 patients were included: CBA 2329 (55.6%) and RFA 1860 (44.4%). Cryoballoon ablation population was younger, with fewer comorbidities. Procedure time was longer in the RFA group (P = 0.01). Radiation exposure was 2487 (CBA) and 1792 cGycm2 (RFA) (P < 0.001). Follow-up duration was 441 (CBA) and 511 days (RFA) (P < 0.0001). Primary endpoint occurred in 30.7% (CBA) and 39.4% patients (RFA) [adjusted hazard ratio (adjHR) 0.85, 95% confidence interval (CI) 0.70–1.04; P = 0.12). In paroxysmal AF, CBA resulted in a lower risk of recurrence (adjHR 0.80, 95% CI 0.64–0.99; P = 0.047). In persistent AF, the primary outcome was not different between groups. Major adverse cardiovascular and cerebrovascular event rates were 1.0% (CBA) and 2.8% (RFA) (adjHR 0.53, 95% CI 0.26–1.10; P = 0.088). Re-ablations (adjHR 0.46, 95% CI 0.34–0.61; P < 0.0001) and adverse events during follow-up (adjHR 0.64, 95% CI 0.48–0.88; P = 0.005) were less common after CBA. Higher rehospitalization rates with RFA were caused by re-ablations.
Conclusions
The primary endpoint did not differ between CBA and RFA. Cryoballoon ablation was completed rapidly; the radiation exposure was greater. Rehospitalization due to re-ablations and adverse events during follow-up were observed significantly less frequently after CBA than after RFA. Subgroup analysis suggested a lower risk of recurrence after CBA in paroxysmal AF.
Trial Registration
ClinicalTrials.gov (NCT01360008), https://clinicaltrials.gov/ct2/show/NCT01360008.
Keywords: Atrial fibrillation, Catheter ablation, Radiofrequency, Cryoballoon, Paroxysmal, Persistent
What’s new?
This is the first cluster cohort trial with equally sized groups comparing atrial fibrillation (AF) catheter ablation outcomes in clusters of experienced cryoballoon ablation (CBA) and radiofrequency ablation (RFA) centres.
The primary outcome analysis showed no differences in atrial arrhythmia recurrence between the two techniques.
Subgroup analysis suggested a lower risk of atrial recurrence after CBA in paroxysmal AF.
In persistent AF, the risk of atrial arrhythmia recurrence was not different between the techniques.
Cryoballoon ablation procedures were completed more rapidly; however, the radiation exposure measured by dose-area product was higher.
The sample size allowed for analysis of rare but devastating events, such as major adverse cardiovascular and cerebrovascular events (MACCEs), which were rare in both groups with a non-significant trend for increased occurrence of MACCEs after RFA.
Cryoballoon ablation as the initial ablation procedure was associated with a favourable clinical course after discharge; rehospitalization due to re-ablation and adverse events during the follow-up period were less frequently observed.
Introduction
The incidence of atrial fibrillation (AF) increases with age, and demographic developments will lead to a marked increase in the number of patients with symptomatic paroxysmal and persistent AF in the future.1 Catheter ablation is an established treatment option.2 Electrical disconnection of the pulmonary veins (PVs) is the cornerstone of AF ablation and can be achieved by radiofrequency ablation (RFA) or cryoballoon ablation (CBA).2,3 The point-by-point RFA technique is the most commonly used technique. Cryoballoon ablation was demonstrated to be non-inferior to RFA in the treatment of paroxysmal AF in the FIRE AND ICE trial.4 Several secondary endpoints, including procedure time, rates of rehospitalization, cardioversion, and repeat ablation, demonstrated favourable results for the CBA technique.4,5 A meta-analysis demonstrated shorter procedure times and a lower rate of cardiac tamponades in the CBA group.6 Only recently, pooled registry data were published comparing 982 CBA and 3675 RFA procedures and outcomes.7 Cryoballoon ablation was associated with lower re-ablation rates, less antiarrhythmic drug (AAD) use, and better European Heart Rhythm Association (EHRA) scores.7 To date, there is no prospective comparative real-world study with equally large sample sizes of the groups and a cluster cohort design, which allows the evaluation of multiple outcomes.
Methods
Study design
The present study was a prospective multicentre and multinational observational cluster cohort study to compare the effectiveness and safety of either RFA or CBA in the initial ablation procedure in patients with AF.8,9 The cluster design of the study was adopted to compare the characteristics, procedural results, and outcomes of patients treated in experienced CBA centres with those treated in experienced RFA centres.
Before the initiation of a centre, the local principal investigator announced their preferred technique for the initial AF ablation procedure irrespective of the AF type. According to this statement and if the centre could provide experience of at least 50 ablations using the designated technique, the institution was enrolled into the study as either a CBA or an RFA centre. The study team did not verify the statements of the local principal investigators. Only patients treated with the predefined technique of the participating centres were included in the study. The study centres had to report each patient to the coordinating centre in Ludwigshafen, Germany prior to the procedure.
The trial was investigator-initiated, and the steering committee was responsible for the design, execution, and conduct of the study. The local ethics review committees of all the centres approved the study, and informed consent was obtained from all patients. The study complies with the Declaration of Helsinki, and data were collected prospectively. A data and safety monitoring board reviewed the interim results and monitored the safety of the patients. An independent critical event committee was installed to review information on serious adverse events during follow-up. All members of the steering committee approved the statistical analyses and interpretation of data.
Study participants
Forty-two centres in eight countries participated in the study (Supplementary material online, Table S1). Patients with symptomatic paroxysmal or persistent AF (<1-year duration of a single episode) were prospectively enrolled in accordance with the inclusion and exclusion criteria (Supplementary material online, Table S2). All patients who did not fulfil the criteria of the FREEZE Cohort Study were documented in the FREEZEplus Registry, with the same quality of data assessment and follow-up. Therapeutic refractoriness to a Class I or III AAD, not including beta-blockers, was a prerequisite for all types of AF in the initial study protocol. During the course of the study, the international guidelines for AF changed and for patients with symptomatic paroxysmal AF naïve to AAD, first-line catheter ablation became an option.3 With an amendment to the study protocol in 2014, the paroxysmal AF subgroup naïve to AAD from the FREEZEplus Registry was referred to the FREEZE Cohort Study.
Interventions
Prior to the procedure, the recruiting centres assessed baseline characteristics, and all the patients underwent transthoracic and transoesophageal echocardiography. After venous access, transseptal puncture was performed. Pulmonary vein potentials must have been recorded at least before and after complete pulmonary vein isolation (PVI) with a circular mapping catheter. Periprocedural management was performed in accordance with the current practice guidelines.3
The RFA point-by-point and CBA over-the-wire techniques had to strictly follow the internationally accepted techniques described in detail in recent publications3 and in the Supplementary material online, Method Section. PVI was the main procedural endpoint. Additional lesions were allowed as indicated. All approved ablation catheters and all approved 3D-mapping systems were allowed. In CBA, continuous monitoring of the phrenic nerve during ablation of the right superior and inferior PVs by phrenic nerve pacing and palpation of the diaphragmatic movement or visualization, for example with fluoroscopy, to reduce the risk of phrenic nerve palsy (PNP) was a prerequisite.
Study follow-up
All the patients were monitored during the hospital stay for at least 24–48 h post-intervention with telemetry and/or ≥24-h-Holter studies. Clinical visits were performed in accordance with the local standards of care policies.
In cases of symptoms suggestive of PV stenosis or other procedure-related adverse events, each participating centre initiated further diagnostic tests, at its own discretion. Furthermore, the participating centres were asked to organize voluntary Holter recordings (1–7 days of duration) at 3 and 12 months after the index intervention to screen for symptomatic or asymptomatic atrial arrhythmias. Each centre added follow-up information from clinical visits based on the local policies and standards of care.
At least 12 months after the ablation procedure, trained assistant personnel of the ‘Stiftung Institut fuer Herzinfarktforschung’ performed a structured telephone interview with all the patients. To avoid misunderstandings due to linguistic and cultural differences, centres outside of Germany performed the follow-up themselves in accordance with the same electronic case report form questionnaires. An independent critical event committee validated reported critical adverse events during follow-up based on requested medical reports and patient records.
In cases of AF recurrence during follow-up, a second procedure was allowed after the blanking period. The technique of choice for the repeat ablation was according to the physicians’ decision and preference, and the repeat procedure had to be reported to the coordinating study centre. If patients were not available for follow-up, the general practitioner was asked to provide additional contact information, or the civil registry office was involved. Those cases with incomplete follow-up information were analysed as censored observations using the information obtained until last contact.
Objectives and endpoints
The primary objective of the FREEZE cohort study was to evaluate the efficacy of CBA as compared to RFA in a large volume of patients with symptomatic paroxysmal or persistent AF treated in experienced clusters of CBA or RFA centres. The proportion of patients with symptomatic AF or atrial tachycardia lasting ≥30 s during a follow-up of at least 12 months within or more than 3 months after the ablation (blanking period) was statistically compared between the two groups.
The secondary objectives can be summarized as follows: comparison of procedural efficacy and safety results; patient’s radiation exposure; and clinical course after ablation during follow-up, including adverse events such as major adverse cardiovascular and cerebrovascular events (MACCEs), bleeding, syncope, thromboembolism, rehospitalization, repeat ablation, cardioversion (medical or electrical), comparison of symptoms (EHRA score), and quality of life (EQ-5D). Data on quality of life will be addressed in a separate analysis.
Data acquisition, management, and quality control
Detailed information is provided in the Supplementary material online, Appendix.
Statistical analysis
The sample size was determined with the following assumptions: 4000 patients in total were required to show equivalence with respect to the primary efficacy endpoint, with an equivalence level of 5% (test level 5% and power 80%). An intention-to-treat analysis was performed.
Categorical variables are expressed as numbers and percentages and continuous variables as means with standard deviations or as medians with quartiles. In the clustered cohort design, every centre was assigned exclusively to one of the treatment groups; therefore, all differences in patient characteristics were analysed, allowing for random centre effects. For the comparison of continuous variables between the two groups, linear mixed models were used, and for the comparison of binary variables, logistic regression models with normal random effects were used.
As the clinical outcomes would be influenced by several factors, different models were used for the adjustment of confounding baseline parameters (Supplementary material online, Table S3). The multivariable models are described in the Supplementary material online,Table S4. In particular, for binary outcome variables reflecting current status data, the length and type of follow-up were considered in the analysis. The cumulative 1-year MACCE rates were estimated using the Kaplan–Meier method and compared using the log-rank test. Expected adjusted survival curves were calculated for a standard patient using the Breslow method. Reported P-values were calculated using two-tailed tests, and statistical significance was defined as P < 0.05. All analyses were performed using the SAS version 9.3 software (Cary, USA).
Results
Patients
From 2011 to 2016, a total of 5436 patients were prospectively included in the FREEZE Cohort Study (n = 4189, 42 centres) or the FREEZEplus Registry (n = 1247) worldwide (Figure 1 and Supplementary material online,Table S1). In the FREEZE Cohort Study, crossover to the other technique after enrolment (n = 40) occurred rarely in both groups (RFA, n = 26 and CBA, n = 14). Of the total population, 14% (591/4189) with paroxysmal AF naïve to Class I or III AADs were transferred from the FREEZEplus Registry after the protocol amendment in 2014. Of those, 309 (52%) and 282 (48%) patients were enrolled in CBA and RFA centres, respectively. See Supplementary material online, Figure S1 for the course of enrolment in both clusters.
Figure 1.
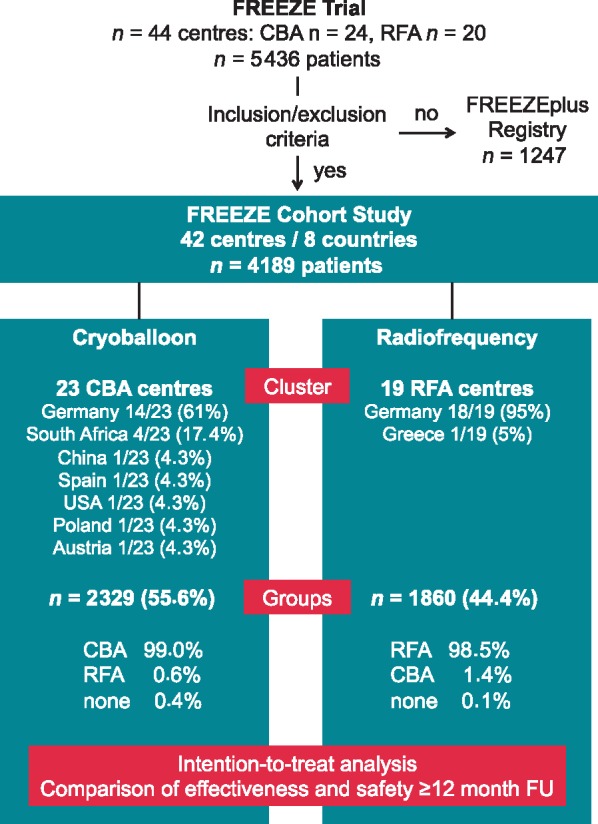
Study population. This flow chart depicts the trial design and provides details on centres, clusters, and groups. Within the cluster cohort trial, 42 experienced centres were enrolled in two clusters: CBA and RFA. Those clusters treated 2329 and 1860 patients with CBA and RFA, respectively. The crossover rate to any other technique was very low in both groups. CBA, cryoballoon ablation; FU, follow-up; RFA, radiofrequency ablation.
In this non-randomized cluster cohort study, important statistically significant differences were found between the groups at baseline (Table 1).
Table 1.
Baseline characteristics
| CBA n = 2329 | RFA n = 1860 | P-valuea | |
|---|---|---|---|
| Year of age | 61.1 ± 10.6 | 63.3 ± 10.6 | 0.008 |
| Female sex category | 36.0% | 36.1% | 0.099 |
| Body mass index (kg/m2) | 26.9 (24.3, 30.4) | 27.2 (24.7, 30.4) | 0.36 |
| Paroxysmal AF | 70.4% | 55.8% | 0.24 |
| EHRA symptom score ≥2 | 90.8% | 97.1% | 0.12 |
| Palpitations | 95.9% | 96.1% | 0.52 |
| LA diameter (mm) | 42.0 (38.0–46.0) | 43.0 (40.0–48.0) | 0.62 |
| LA diameter ≥ 45 mm | 27.2% | 33.1% | 0.62 |
| LVEF (%) | 60.0 (55.0, 62.0) | 55.0 (55.0, 60.0) | 0.92 |
| Inefficacy of AADs, not including beta-blockers | 86.7% | 84.8% | 0.094 |
| Number of cardioversionsb | 1.0 ± 1.4 | 1.2 ± 1.4 | 0.44 |
| Hypertensive heart disease | 24.3% | 16.8% | 0.37 |
| Coronary artery disease | 9.4% | 13.9% | 0.043 |
| Valve disease | 4.9% | 17.4% | 0.030 |
| Cardiomyopathyc | 3.6% | 4.7% | 0.46 |
| Diabetes | 7.8% | 10.5% | 0.080 |
| Renal impairment (GFR <60 mL/min) | 2.9% | 5.9% | 0.007 |
| Serum creatinine (mg/dL) | 0.9 (0.8, 1.0) | 1.0 (0.8, 1.1) | 0.048 |
| CHA2DS2-Vasc Score | 1.8 ± 1.3 | 2.1 ± 1.4 | 0.002 |
| CHA2DS2-Vasc Score ≥2 | 52.5% | 62.6% | <0.0001 |
This table shows the comparison of baseline characteristics in the CBA and RFA groups. Differences were analysed allowing for random centre effects, and the level of statistical significance was set at P < 0.05.
n (%), mean ± SD, or median (IQR).
P-value adjusted for random centre effect.
Electrical or medical cardioversion.
Including tachycardia-induced cardiomyopathy.
AAD, antiarrhythmic drug; AF, atrial fibrillation; CBA, cryoballoon ablation; EHRA, European Heart Rhythm Association; GFR, glomerular filtration rate; LA, left atrial diameter; LVEF, left ventricular ejection fraction; RFA, radiofrequency ablation.
Procedural differences and efficacy results
Prior to the procedure, PV anatomy was determined more frequently in CBA patients (81.2% vs. 38.3%, P < 0.0001). Normal PV anatomy was found in 81.7% and 81.5% (P = 0.88), with a left common ostium in 11.4% and 13.8% (P = 0.09) and right middle PV in 5.6% and 2.7% (P < 0.01) in the CBA and RFA groups, respectively. In the RFA group, most of the patients were treated with open-irrigated radiofrequency (RF) tip catheters (92.4%), and the proportion of contact force-sensing catheters used in this group was unknown. In the CBA group, almost one-fourth of the patients were treated with the first-generation cryoballoon (CB) (24%). The majority of patients was treated with either the second- (72%) or third-generation (4%) CB. The procedural efficacy endpoint ‘all PV isolated at the end of the procedure’ was achieved in 94.7% of patients in the CBA group and 97.4% of patients in the RFA group (P < 0.001, adjusted P = 0.12). In the CBA group, 9089 (98.2%) of 9252 PVs were isolated as compared with 7310 (99.2%) of 7372 PVs in the RFA group (P = 0.17). In the CBA group, focal touch-up applications were used in 2.8% of patients and a second balloon size was used in 10%. After the introduction of the second-generation CB, fewer touch-up applications (4.6% vs. 2.2%, P = 0.03) and a reduced double balloon rate were documented (17.0% vs. 8.7%, P < 0.0001). In the RFA group, significantly more additional ablation lesions beyond PVI were applied as compared to the CBA group (11.3% vs. 19.7%, P < 0.001). The mean total procedure time including PVI and additional ablations (adjusted delta mean CBA vs. RFA −39.5, 95% CI −9.3 to −69.6, P = 0.01) and left atrial (LA) time (adjusted delta mean CBA vs. RFA −35.6 min, 95% CI −12.5 to −58.8, P = 0.002) were significantly shorter in the CBA group than in the RFA group. The radiation exposure measured using dose-area product was significantly higher in the CBA group (Supplementary material online, Table S5).
Procedural complications until discharge
In-hospital MACCE rates (CBA 0.3% vs. RFA 0.4%, P = 0.42) and other major complication rates until discharge (CBA 2.0% vs. RFA 2.0%, P = 0.96) were very low in both groups. Other major complications in the CBA group were PNPs not resolved until discharge (1.1%), groin complications with intervention/surgery (0.5%), relevant pericardial effusion (0.3%), and severe bleeding (0.1%). The following other major complications were documented in the RFA group: severe bleeding (0.6%), groin complications (0.8%), or relevant pericardial effusion (0.5%). The rates of minor complications were not significantly different between the groups after adjustment (CBA 6.3% vs. RFA 9.8%, adjusted P = 0.48). The total complication rates until discharge showed no significant difference between the groups after adjustment. See Table 2 for details.
Table 2.
Procedural complications until discharge
| CBA | RFA | P-valuea | Univariable CBA vs. RFA |
Multivariable CBA vs. RFA |
|||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||||
| MACCE (death, MI, stroke) | 0.3% | 0.4% | 0.42 | n.a. | n.a. | n.a. | n.a. |
| Death | 0.1% = 2 pt | 0.1% = 2 pt | 1.00 | ||||
| 1 tamponade plus stroke, 1 tamponade | 1 tamponade 1 groin bleeding | ||||||
| Myocardial infarction | – | – | |||||
| Stroke | 0.2% | 0.3% | 0.55 | ||||
| Other major complications | 2.0% | 2.0% | 0.96 | 1.01 (0.65–1.57) | 0.96 | 1.33 (0.67–2.66) | 0.41 |
| PNP not resolved until discharge | 1.1% | 0.1% | <0.0001 | ||||
| Pericardial effusion treated by drainage or surgery | 0.3% | 0.5% | 0.35 | ||||
| Severe bleeding | 0.1% | 0.6% | 0.013 | ||||
| TIA | 0.0% | 0.3% | 0.09 | ||||
| Pulmonary embolism | 0.0% | 0.1% | 0.44 | ||||
| Haemato-/pneumothorax | 0.0% | 0.0% | 1.00 | ||||
| Groin complications treated interventionally or by surgery | 0.5% | 0.8% | 0.31 | ||||
| Retroperitoneal haematoma | 0.0% | 0.1% | 0.44 | ||||
| Gastric motility dysfunction | 0.0% | 0.1% | 0.44 | ||||
| Successful resuscitation w/o sequelae | 0.1% | 0.1% | 1.00 | ||||
| Minor complications | 6.3% | 9.8% | <0.0001 | 0.63 (0.50–0.79) | <0.0001 | 0.80 (0.45–1.45) | 0.48 |
| PNP resolved until discharge | 1.6% | 0.1% | <0.0001 | ||||
| Pericardial effusion treated conservatively | 0.6% | 2.6% | <0.0001 | ||||
| Groin complications treated conservatively | 2.5% | 4.3% | 0.002 | ||||
| Moderate bleeding | 0.4% | 0.4% | 1.00 | ||||
| Oesophageal lesion symptomatic or asymptomatic | 0.1% | 0.6% | 0.004 | ||||
| Cardiac complication (e.g. decompensation) | 0.1% | 0.2% | 0.42 | ||||
| Bronchial complication (e.g. haemoptysis, bronchitis) | 0.3% | 0.1% | 0.049 | ||||
| Pericarditis | 0.2% | 0.2% | 0.74 | ||||
| Haematuria | 0.0% | 0.1% | 0.59 | ||||
| Allergic reaction | 0.0% | 0.3% | 0.094 | ||||
| Systemic infection | 0.1% | 0.3% | 0.25 | ||||
| Others | 0.5% | 1.0% | 0.06 | ||||
| Total number of complicationsb | 8.2 | 11.3 | <0.0001 | 0.69 (0.56–0.85) | <0.001 | 0.95 (0.55–1.66) | 0.87 |
The table depicts the event rates of procedural complications, the odds ratios (OR) with 95% confidence intervals (CI), determined by univariable and multivariable analysis. The level of statistical significance was set at P < 0.05. See Supplementary material online, Table S4 for details of the adjustments performed.
n (%), mean ± SD, or median (IQR).
Fisher exact test.
Total number of complications is the sum of MACCEs, major complications, and minor complications.
CBA, cryoballoon ablation; CI, confidence interval; MACCE, major adverse cardiovascular and cerebrovascular event; MI, myocardial infarction; n.a., not applicable; OR, odds ratio; PNP, phrenic nerve palsy; RFA, radiofrequency ablation; TIA, transitory ischaemic attack.
Freedom from arrhythmia until discharge
The incidence rates of ‘atrial tachyarrhythmias until discharge’ showed no significant difference between the groups (9.2% CBA vs. 9.6% RFA, P = 0.71). The rate of AAD treatment at discharge was higher in the RFA group (20.4% vs. 36.1%, P < 0.0001). See Supplementary material online, Tables S5 and S6 for details.
Primary outcome: long-term efficacy results
Complete follow-up was performed in 93.5% of patients in the CBA group and 99.1% in the RFA group (P < 0.001). Arrhythmia diagnostics [electrocardiogram (ECG), Holter, or Event Recorder] were performed in 80.1% and 73.3% of patients in the CBA and RFA groups, respectively (P < 0.0001). Holter-ECG (≥24-h) during follow-up was performed in 64.5% (CBA) and 62.6% (RFA) of patients, P = 0.21. At follow-up, 7.6% of patients in the CBA group and 13.4% in the RFA group were on Class I AADs (P < 0.0001) and 10.1% and 8.3% were on Class III AADs, respectively (P = 0.07).
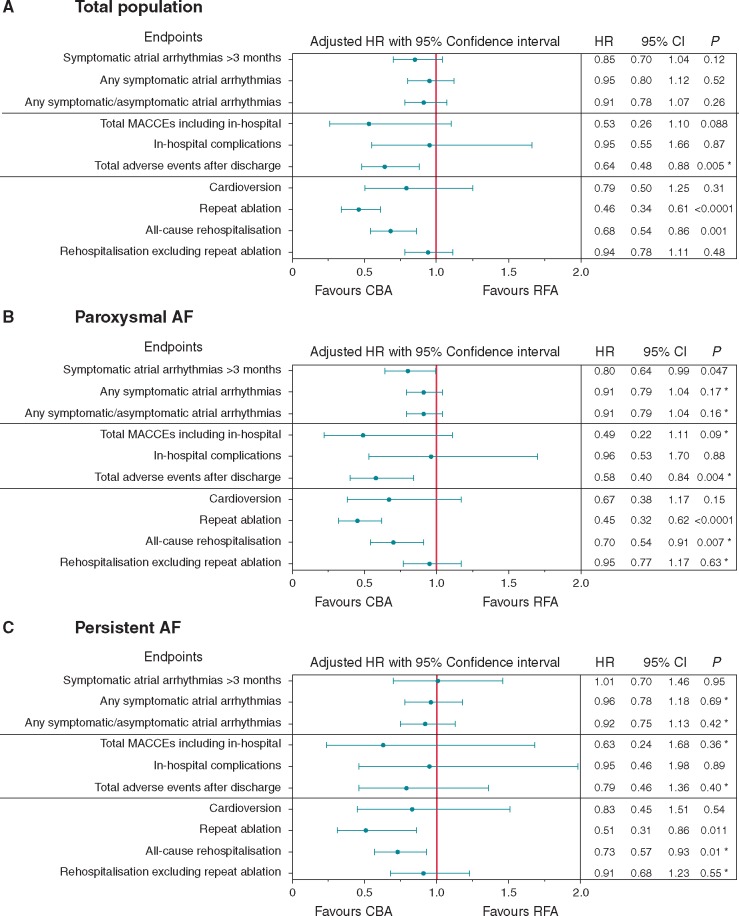
Following a single procedure, on AADs, and after a median follow-up of 441 and 511 days (P < 0.001) in the CBA and RFA groups, respectively, the incidence rates of symptomatic atrial arrhythmia recurrence after the blanking period were 30.7% and 39.4% in the CBA and RFA groups, respectively [adjusted hazard ratio (HR) 0.85, 95% CI 0.70–1.04; P = 0.12]. See Figure2A and Supplementary material online, Table S7 for details. In the subgroup analysis of the patients with PVI only and those with additional ablations beyond PVI, no significant interaction suggesting different outcomes was found (Supplementary material online,Figure S2).
Figure 2.
Comparison of the main adjusted effectiveness and safety outcome parameters of the total population and paroxysmal and persistent AF. The adjusted hazard ratios of the multivariable models with confidence intervals and the P-values for CBA vs. RFA after a single procedure are provided for the major long-term outcome. (A) Depicts the results of the total population; in (B) and (C), the outcomes of the AF-type subgroups are presented. See Supplementary material online, Table S4 for details of the adjustments performed. Level of statistical significance was set at P < 0.05. *‘Centre’ as a random effect was not applicable in the multivariable model. AF, atrial fibrillation; AT, atrial tachycardia; CI, confidence interval; HR, hazard ratio; MACCE, major adverse cardiovascular and cerebrovascular event.
The unadjusted subgroup analysis for the AF type suggested a significant interaction for the primary endpoint favouring CBA for paroxysmal AF, P = 0.039 (Supplementary material online,Figure S2). After adjustment, this result remained statistically significant (adjusted HR 0.80, 95% CI 0.64–0.99; P = 0.047, Figure 2B). In patients with persistent AF, no differences in the primary outcome were observed between CBA and RFA (Figure 2B).
European Heart Rhythm Association symptom score
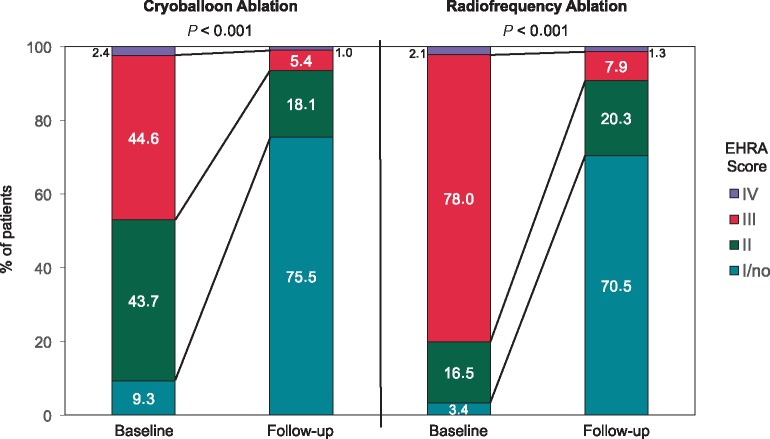
Differences were observed between the groups at baseline. In the RFA group, patients were more symptomatic at baseline as demonstrated by a significantly higher proportion of patients with EHRA scores of 3 or 4 as compared to the CBA group. An improvement in the score of at least one class was observed more frequently in the RFA group than in the CBA group (Figure 3).
Figure 3.
Comparison of the EHRA symptom scores at baseline and follow-up in the CBA and RFA groups. This 100% stacked column chart depicts the differences in the EHRA symptom score distribution at baseline and follow-up in the CBA and RFA groups. Results are displayed as the percentage of patients. In both groups, a significant improvement was observed with P < 0.001. In the RFA group, patients more frequently demonstrated an EHRA score of 3 or 4 at baseline as compared to the CBA group (80.1% vs. 47%, P < 0.001), and an EHRA score of 2 was more often documented in the CBA group. Improvement in the EHRA score of at least one class was observed in 76.3% and 84.9% of the patients in the CBA and RFA groups, respectively (P < 0.0001). More patients in the CBA group demonstrated an EHRA score of 1 (no symptoms) at follow-up (75.5% vs. 70.5%, P < 0.01). CBA, cryoballoon ablation; EHRA, European Heart Rhythm Association; RFA, radiofrequency ablation.
Adverse events after discharge
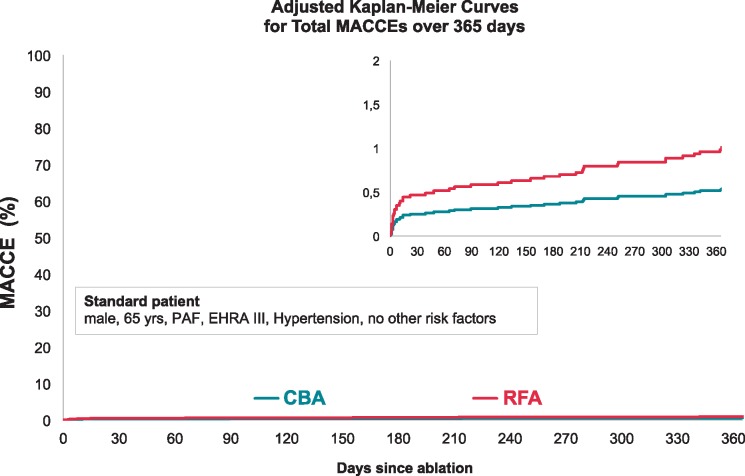
The total MACCE rates, including in- and post-hospital events, were 1.0% in the CBA group and 2.8% in the RFA group (adjusted HR 0.53, 95% CI 0.26–1.10; P = 0.088) (Figure 4).
Figure 4.
Adjusted Kaplan–Meier curve for ‘Total MACCEs’ with CBA vs. RFA. Adjusted Kaplan–Meier curves for total MACCEs were calculated for a standard patient using the Breslow method. After adjustment, a non-significant trend towards a lower MACCE rate in the CBA group (adjusted HR 0.53, 95% CI 0.26–1.10; P = 0.088) was observed. Level of statistical significance was set at P < 0.05. CBA, cryoballoon ablation; CI, confidence interval; EHRA, European Heart Rhythm Association; HR, hazard ratio; MACCE, major adverse cardiovascular and cerebrovascular event; PAF, paroxysmal atrial fibrillation; RFA, radiofrequency ablation.
Significantly fewer other major, minor, and total adverse events occurred in the CBA group than in the RFA group during follow-up after adjustment (Figure 2A, Table 3). Among the AF-type subsets, this result remained statistically significant for the paroxysmal AF patients only (Figure 2 B/C). In this study, no atrial-oesophageal fistula was observed.
Table 3.
Rates of adverse events after discharge
| CBA | RFA | P-value | Univariable CBA vs. RFA |
Multivariable CBA vs. RFA |
|||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||||
| Post-hospital MACCE | 0.6% | 2.2% | <0.001 | n.a. | n.a. | n.a. | n.a. |
| Death | 0.2% | 0.9% | 0.003 | ||||
| Stroke | 0.3% | 0.8% | 0.08 | ||||
| Myocardial infarction | 0.1% | 0.3% | 0.10 | ||||
| Other major adverse events after discharge | 1.3% | 3.2% | <0.001 | 0.44 (0.26–0.72) | <0.001 | 0.47 (0.27–0.84) | 0.010a |
| TIA | 0.1% | 0.1% | 0.42 | ||||
| Atrial-oesophageal fistula | 0.0% | 0.0% | n.a. | ||||
| PNP at follow-up | 0.4% | 0.2% | 0.38 | ||||
| PV stenosisb | 0.5% | 1.5% | 0.004 | ||||
| Severe bleeding | 0.3% | 0.7% | 0.072 | ||||
| Pericardial effusion treated by drainage or surgery | 0.1% | 0.5% | 0.012 | ||||
| Pulmonary and systemic embolism | 0.1% | 0.2% | 0.46 | ||||
| Minor adverse events after discharge | 4.0% | 6.1% | 0.006 | 0.64 (0.47–0.89) | 0.006 | 0.66 (0.46–0.95) | 0.025a |
| Phlebothrombosis | 0.1% | 0.1% | 0.80 | ||||
| Pericardial effusion treated conservatively or treatment indefinite | 0.3% | 0.5% | 0.46 | ||||
| Syncope | 0.6% | 1.3% | 0.028 | ||||
| Moderate bleeding | 0.8% | 1.1% | 0.37 | ||||
| Inguinal problems | 2.3% | 3.3% | 0.079 | ||||
| Total adverse events after discharge | 5.3% | 9.3% | <0.001 | 0.60 (0.47–0.80) | <0.001 | 0.64 (0.48–0.88) | 0.005a |
This table shows the unadjusted rates of those adverse events observed after discharge during follow-up. The HRs were calculated in univariable and multivariable models for adjustments. Binary regression with a complementary log-log-link function was calculated including 21 covariates, follow-up type, and log follow-up time. Given the cumulative reports of events at follow-up, it was not possible to divide the adverse events into those related to the initial ablation procedure or to additional repeat ablation procedures during follow-up. As an example, the higher incidence of PV stenosis observed in the RFA group might be attributed to the initial technique, to the higher rate of repeat procedures in this group, and/or to the fact that the PV stenosis was identified in the second procedure. Level of statistical significance was set at P < 0.05.
Random centre effect not applicable.
In total, 32 cases of PV stenosis (10 CBA vs. 22 RFA) were documented. Of those, 14 (44%) occurred without repeat ablation during FU (rates for CBA 0.48% vs. RFA 0.35%) and 18 (56%) occurred with repeat ablation during FU (rates for CBA 0.05% vs. RFA 1.18%).
CBA, cryoballoon ablation; FU, follow-up; MACCE, major adverse cardiovascular and cerebrovascular event; PNP, phrenic nerve palsy; PV, pulmonary vein; RFA, radiofrequency ablation; TIA, transitory ischaemic attack.
Clinical course after the initial ablation procedure
Significant differences were observed with respect to the clinical course after the index ablation procedure (Figure 2A). Rates of repeat ablations and all-cause re-hospitalizations were significantly higher after RFA than after CBA after adjustment, whereas no statistically significant difference in the cardioversion rate was found between the groups. After excluding rehospitalization events caused by the necessity of repeat ablation from all-cause rehospitalization, there was no longer any difference between the groups. Similar results for ‘repeat ablation’, ‘rehospitalization’, and cardioversion were observed in the subgroup analysis of paroxysmal and persistent AF patients (Figure 2B/C).
Discussion
This is the largest prospective cluster cohort trial comparing two equal-sized groups of patients treated with CBA or RFA. The first observation is that different patient populations were treated at the initial ablation procedure in the centres predefined as CBA or RFA centres. Patients treated in RFA centres were older, with more comorbidity and higher EHRA scores at baseline. Moreover, additional ablations beyond PVI were more frequently performed in the RFA group. At the time of study enrolment, experts were discussing whether additional lesions beyond PVI were necessary in persistent AF.10 The CBA technique is designed to isolate the PV, which is recommended during all AF ablation procedures (Class I, level of evidence A).3 With RFA, additional strategies (e.g. fractionated potential ablation and creation of lines) are possible. This might have led to a selection bias introduced by the differences between the groups at baseline. With respect to the observed differences, an adjustment was performed as described including 21 covariates (see Supplementary material online, Table S4).
This effectiveness study showed no significant difference in the primary endpoint between CBA and RFA. However, more patients in the RFA group were on AAD at the end of follow-up.
In the FIRE AND ICE trial, patients with exclusively paroxysmal AF were included in a randomized controlled study.4 Rates of recurrence and clinical failure of 34.6% and 35.9% in the CBA and RFA groups were observed, respectively (P < 0.001 for non-inferiority).4 Mortsell et al. recently published pooled registry data on CBA and RFA as an initial AF ablation procedure. They observed recurrence rates of 29.8% (CBA) and 31.8% (RFA), P = 0.44.7 In the present cohort trial, the incidence rates of symptomatic atrial arrhythmia recurrence after a blanking period were similar to those reported before, and no statistically significant difference between CBA and RFA was observed. Our study enrolled patients from 2011 to 2016, and one-fourth of the CBA patients were treated with the less effective, first-generation CB.11 The second-generation CB yields a more durable PVI12 and improved outcomes.11 In contrast, contact force-sensing catheters have provided improved outcomes in RF catheter ablation of AF.13 In the present study, the proportion of patients in the RFA group who were treated with contact force-sensing catheters was not known. Therefore, we were not able to compare the second-generation CB with contact force-sensing RF catheters.
Analysis of the subset of patients with paroxysmal AF suggested that CBA might be superior to RFA in terms of atrial arrhythmia recurrence after the blanking period. However, with a P-value of 0.047, the level of significance was barely achieved, and when it was analysed without a blanking period, this observation was no longer significant.
Interestingly, in the subgroup of persistent AF, no difference was observed with respect to the primary outcome. This finding supports the use of CBA and the strategy of a PVI-only approach in the initial ablation procedure for persistent AF.
In the present trial, additional ablations beyond PVI were allowed at the discretion of the treating physician, and a greater number of additional ablations, mostly right atrial flutter and fractionated potential ablation but not LA lines, were documented in the RFA group. We did not find an interaction in the subgroup analysis of patients with or without additional ablations beyond PVI. This finding is in line with the results of the Star AF II Trial10 and with recently published data on prophylactic cavotricuspid isthmus ablation at the time of initial AF ablation, which also demonstrated no benefit as compared to PVI alone.14
Recurrence rates and time-to-event analysis have well-known limitations because they do not provide information on the AF burden and can therefore underestimate the efficacy of catheter ablation.3 Secondary endpoints, e.g., rehospitalization, are reliable if a sporadic arrhythmia like AF is studied. Owing to the large sample size of both groups and the unique cluster cohort design, the analysis of multiple secondary outcome parameters was possible.9
In the present trial, lower re-ablation rates were observed in the CBA group, which may highlight another important advantage of CBA PVI. Only recently, pooled registry results from Europe also demonstrated higher re-ablation rates with RFA as compared to CBA.7 The above-mentioned events are of high economic relevance for health systems15 and expose the patient to additional risks.16 One explanation for the differences in secondary outcomes could be that the CBA procedure is less operator-dependent and more reproducible for creating a durable PVI as compared to RFA.17
The indication of AF ablation in the treatment of AF is mainly based on symptoms. Interestingly, the patients treated by RFA were more symptomatic with higher EHRA scores at baseline, and improvement of at least one EHRA score was observed more frequently in the RFA group. In contrast, a greater number of patients in the CBA group reported no symptoms (EHRA I) at follow-up. These differences must be interpreted with caution. First, the EHRA score is only one puzzle piece in the evaluation of quality of life; second, both groups were not only different in terms of symptoms at baseline, but also comorbidities were more frequently observed in the RFA group. Potentially, those patients with greater comorbidity have more symptomatic AF, e.g., due to concomitant congestive heart failure. Hence, the superior symptom improvement achieved by RFA might be the result of more severe symptoms at baseline.
In the CBA group, PV anatomy was determined more frequently prior to the procedure, suggesting that CBA centres had a tendency to perform patient selection based on imaging findings. Despite this, there were no significant differences in the observed proportion of patients with normal PV and left common ostium anatomy.
In comparison to other studies, this trial provides more detailed information on the radiation exposure beyond the fluoroscopy time. The dose-area product, the more accurate parameter of radiation exposure, was higher in the CBA arm, which may be explained by the use of venography before the CBA to determine the grade of balloon occlusion. Despite the use of 3D-mapping systems in the RFA group (94.3%), the radiation dose was elevated. Most likely, this was a result of the frequent use of LA/PV venography in the RFA group (74%). Radiation exposure should be as low as reasonably achievable, and further continuous efforts to reduce radiation should be prioritized.
The adjusted procedure and LA times were >30 min longer in the RFA group than in the CBA group in our study. This finding is in line with recently published comparative studies.4,7 Shorter procedure times may increase hospital capacity and reduce costs in the treatment of AF.18
However, the total procedure time includes the time taken for PVI and for additional ablations during the procedure. It can be assumed that PVI performed in a point-by-point fashion with RFA, guided by 3D electro-anatomical mapping, and in combination with the higher rate of additional ablations resulted in the longer procedure time in the RFA group. Despite the application of RFA touch-up ablations (2.8%) to isolate the PV and the use of a second CB size (10%), the procedure time remained much shorter in the CBA group. These observations of the CBA group underline that the physicians who perform CBA should be familiar with strategies to close remaining LA-PV gaps in cases of difficult anatomy.
Other studies have reported higher rates of cardiac tamponade with RFA as compared to balloon ablation.6,19,20 Administrative in-hospital data from Germany showed no differences in either the quantity or quality of complications between CBA and RFA.21
In the present study, while the total complication rates until discharge were without significant difference, the types and quality of acute complications were different between the groups: PNP not resolved until discharge was the main contributor to the rate of major complications in CBA. However, the rate of persistent PNP at follow-up was very low (0.4%), in line with administrative safety data on catheter ablation of AF from Germany. In 5608 CBA ablations, PNP was observed in 0.4% of the patients.21 Although PNP is classified as a major complication of CBA, it typically resolves during follow-up and the majority of patients are asymptomatic. In contrast, relevant groin complications and bleeding were the most frequently documented major complications in the RFA group.
The adjusted total MACCE rates demonstrated no statistically significant difference between the two techniques. A non-significant trend for more MACCEs and a significant higher incidence rate of adverse events during follow-up (e.g. relevant pericardial effusions, PV stenosis) in the RFA group, however, warrant serious discussion: the RFA group consisted of a sicker population, for whom more additional lesions beyond PVI were performed in the first procedure and the rate of repeat ablations was higher.
The higher rate of repeat ablation in the RFA group could have resulted in either a higher detection rate or a higher incidence of adverse events. In this context, it should be emphasized that the lower number of adverse events after discharge in the CBA group remained significant in the paroxysmal AF patients only and was not significant in persistent AF. The predictors of adverse events after initial ablation procedures must be identified in further studies.
Despite the well-known limitations of cohort studies, the validity of the real-world experience results of the present study was intended to be high, as many centres in different countries contributed to this prospective effectiveness trial and the large sample size enabled adequate statistical adjustment. A prerequisite for this trial was that each centre had to announce its preferred technique in clinical routine practice for PVI and was then classified as either a CBA or a RFA centre to overcome potential intervention-choice22 and complexity biases.23 The results seem generalizable to clinical settings where experienced physicians perform the procedures.
If a patient experiences symptomatic paroxysmal or drug-refractory persistent AF, both techniques appear to be suitable therapy options at experienced centres. However, the differences between the techniques, especially the risk of re-ablation, should be discussed with the patient in the preoperative consultation.
Limitations
The primary endpoint results were single procedure results. Information on how a repeat ablation was performed and its impact on the primary endpoint is lacking.
The study mainly evaluated patient-reported outcomes for the primary objective. Hence, data on asymptomatic AF episodes are incomplete, since Holter-ECG or continuous monitoring were not mandatory and only 63.6% of patients underwent Holter-ECG during follow-up.
Differences at baseline were expected and led to a large sample size to achieve adequate power. A potential selection bias may exist due to the cluster cohort design of the study and because the CBA and RFA centres only enrolled patients for the predefined technique. The large sample size allowed for control of potential confounders by means of statistical adjustment. More international centres contributed in the CBA group, whereas in the RFA cluster, all but one centre was located in Germany. Among the recruiting centres, differences were observed in terms of the number of patients enrolled in the study: three of 42 centres (7.1%) recruited almost 50% of patients and 10 of 42 centres (24%) recruited less than 20 patients. These differences raise the question of whether all centres recruited their patients consecutively. It has been shown that outcomes are associated with the volume of the centre and operator.24 Statistical adjustment was performed to address potential inter-operator and inter-facility differences in outcomes. Accordingly, the statistical models were calculated including a ‘random centre effect’ (Supplementary material online, Table S4).
Although a remarkable number of patients completed follow-up in both groups, completeness of follow-up was found to be different between the groups. Furthermore, a significant difference in follow-up duration was observed, which is why ‘log follow-up time’ was also included in the statistical models for adjustment. Since differences in the type of follow-up were observed between those centres inside and outside of Germany, the statistical adjustment also included the item ‘type of follow-up’.
Almost one-fourth of patients in the CBA group were treated with the first-generation CB, which is known to be less effective and is no longer available. As the recruitment started before the availability of contact force-sensing catheters and the availability of advanced RFA catheters was influenced by a safety notice and voluntary field removal by the manufacturers in the European Union in the years 2013/2014, further analysis of the outcomes according to the individual catheter type was prevented.
Conclusion
In this large cohort trial including patients with paroxysmal and persistent AF, CBA and RFA did not demonstrate significant differences in the primary endpoint of ‘atrial arrhythmia recurrence’. Procedure times were shorter and radiation exposure was higher in the CBA group. The total MACCE rates were low in both groups. Fewer ‘repeat ablations’, ‘all-cause re-hospitalizations’, and ‘adverse events after discharge’ were observed in the CBA group than in the RFA group. The higher rehospitalization rate in the RFA group was driven by the significantly higher rate of repeat ablation.
Subgroup analysis suggested a lower risk of atrial arrhythmia recurrence after CBA in paroxysmal AF. However, no difference in the primary outcome was observed between the two techniques in the subgroup of persistent AF.
Supplementary Material
Acknowledgements
The first author acknowledges funding received from the European Society of Cardiology in form of an ESC Research Grant. We thank all the local investigators and assistant personnel for their great work. We thank Drs Stephanie Fichtner and Daniel Zimmer (both Munich, Germany) for their independent work in the critical event committee.
Collaborators: Souza, J.J., Stanley, A., Spitzer, S.G., Willems, S., Dierk, T., Borchard, R., Seidl, K.H., Zahn, R., Groschup, G., Obel, I.W.P., Gerds-Li, J.H., Gopal, R.R., Schrickel, J., Lewalter, T., Stanley, A., Moshage, W., Eckardt, L., Jung, W., Kremer, P., Lubinski, A., Schumacher, B., Lickfett, L., Muenzel, T., Steinwender, C., Efremidis, M., Deneke, T., Nguyen, D.Q., Hochadel, M., and Schneider, S.
Conflict of interest: An investigator-initiated study; the non-profit foundation ‘Stiftung Institut fuer Herzinfarktforschung’ institute (Ludwigshafen, Germany) conducted the trial and was reimbursed by Medtronic GmbH, Meerbusch, Germany for their services. The participants, investigators, centres or members of the steering committee received no compensation. E.H. reports personal fees from Medtronic, outside the submitted work, and E.H. is head of the department. The department received compensation for participation in clinical research trials outside the submitted work from: Abbott, Bayer, Biotronik, Boehringer Ingelheim, Edwards, Elixier, Medtronic, and Stentys. F.S. reports personal fees from Medtronic, outside the submitted work. K.W. reports grants from Biotronik and personal fees from Boston Scientific, outside the submitted work. U.D. reports personal fees from Medtronic, outside the submitted work. M.K. reports grants from Medtronic, outside the submitted work, and fees for lectures, consulting, and advisory board activities received from Medtronic. A.G.-A. reports non-financial support from Medtronic during the time of the study and personal fees from Medtronic, outside the submitted work. J.K.R.C. reports personal fees from Medtronic, outside the submitted work. R.T. reports grants from Abbott, grants from Hansen Medical, grants and personal fees from Biosense Webster, and grants and personal fees from Medtronic during the time of the study. C.S. reports grants, personal fees, and non-financial support from Medtronic during the time of the study. A.M. reports personal fees from Medtronic, outside the submitted work. J.B. reports grants and personal fees from Medtronic, grants and personal fees from Pfizer, grants and personal fees from Biotronik, and grants and personal fees from St. Jude, during the time of the study, as well as grants from Medtronic, grants from St. Jude, and grants from Biotronik, outside the submitted work. K.H.K. reports grants and personal fees from Abbott Vascular, grants and personal fees from Medtronic, and grants and personal fees from Biosense Webster, outside the submitted work. J.S. and T.O. report unrestricted grants from Medtronic to the IHF foundation. D.A. has nothing to declare.
Contributor Information
FREEZE Cohort Study Investigators:
J J Souza, A Stanley, S G Spitzer, S Willems, T Dierk, R Borchard, K H Seidl, R Zahn, G Groschup, I W P Obel, J H Gerds-Li, R R Gopal, J Schrickel, T Lewalter, A Stanley, W Moshage, L Eckardt, W Jung, P Kremer, A Lubinski, B Schumacher, L Lickfett, T Muenzel, C Steinwender, M Efremidis, T Deneke, D Q Nguyen, M Hochadel, and s Schneider
References
- 1. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A.. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 3. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR. et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 5. Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M. et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37:2858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buiatti A, von Olshausen G, Barthel P, Schneider S, Luik A, Kaess B. et al. Cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: an updated meta-analysis of randomized and observational studies. Europace 2017;19:378–84. [DOI] [PubMed] [Google Scholar]
- 7. Mortsell D, Arbelo E, Dagres N, Brugada J, Laroche C, Trines SA. et al. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: a study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace 2019;21:581–9. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann E, Dorwarth U, Kuck KH, Vogt J, Kuniss M, Schneider S. et al. Design and baseline patient characteristics of the prospective, observational, multicenter and multinational cohort study comparing radiofrequency with cryoablation for pulmonary vein isolation in patients with atrial fibrillation—the freeze cohort study. Int J Clin Med 2014;05:1161–72. [Google Scholar]
- 9. Grimes DA, Schulz KF.. Cohort studies: marching towards outcomes. Lancet 2002;359:341–5. [DOI] [PubMed] [Google Scholar]
- 10. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R. et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 11. Furnkranz A, Bordignon S, Dugo D, Perotta L, Gunawardene M, Schulte-Hahn B. et al. Improved 1-year clinical success rate of pulmonary vein isolation with the second-generation cryoballoon in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2014;25:840–4. [DOI] [PubMed] [Google Scholar]
- 12. Reddy VY, Sediva L, Petru J, Skoda J, Chovanec M, Chitovova Z. et al. Durability of pulmonary vein isolation with cryoballoon ablation: results from the Sustained PV Isolation with Arctic Front Advance (SUPIR) Study. J Cardiovasc Electrophysiol 2015;26:493–500. [DOI] [PubMed] [Google Scholar]
- 13. Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak R. et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace 2015;17:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mesquita J, Ferreira AM, Cavaco D, Carmo P, Madeira M, Freitas P. et al. Impact of prophylactic cavotricuspid isthmus ablation in atrial fibrillation recurrence after a first pulmonary vein isolation procedure. Int J Cardiol 2018;259:82–7. [DOI] [PubMed] [Google Scholar]
- 15. Chun KRJ, Brugada J, Elvan A, Geller L, Busch M, Barrera A. et al. the impact of cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation on healthcare utilization and costs: an economic analysis from the FIRE AND ICE trial. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bode K, Ueberham L, Gawlik S, Hindricks G, Bollmann A.. Inguinal vascular complications after ablation of atrial fibrillation: an economic impact assessment. Europace 2019;21:91–8. [DOI] [PubMed] [Google Scholar]
- 17. Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, Halimi F. et al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace 2017;19:48–57. [DOI] [PubMed] [Google Scholar]
- 18. Klein G, Lickfett L, Schreieck J, Deneke T, Wieczorek M, Group F-P. et al. Comparison of ‘anatomically designed’ and ‘point-by-point’ catheter ablations for human atrial fibrillation in terms of procedure timing and costs in German hospitals. Europace 2015;17:1030–7. [DOI] [PubMed] [Google Scholar]
- 19. Bollmann A, Ueberham L, Schuler E, Wiedemann M, Reithmann C, Sause A. et al. Cardiac tamponade in catheter ablation of atrial fibrillation: German-wide analysis of 21 141 procedures in the Helios atrial fibrillation ablation registry (SAFER). Europace 2018;20:1944–51. [DOI] [PubMed] [Google Scholar]
- 20. Chun KRJ, Perrotta L, Bordignon S, Khalil J, Dugo D, Konstantinou A. et al. Complications in catheter ablation of atrial fibrillation in 3,000 consecutive procedures: balloon versus radiofrequency current ablation. JACC Clin Electrophysiol 2017;3:154–61. [DOI] [PubMed] [Google Scholar]
- 21. Steinbeck G, Sinner MF, Lutz M, Müller-Nurasyid M, Kääb S, Reinecke H.. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: a nationwide in-hospital analysis of administrative data for Germany in 2014. Eur Heart J 2018;39:4020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lilford RJ, Braunholtz DA, Greenhalgh R, Edwards SJ.. Trials and fast changing technologies: the case for tracker studies. BMJ 2000;320:43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kotaska A. Inappropriate use of randomised trials to evaluate complex phenomena: case study of vaginal breech delivery. BMJ 2004;329:1039–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sairaku A, Yoshida Y, Nakano Y, Maeda M, Hirayama H, Hashimoto H. et al. Who is the operator, that is the question: a multicentre study of catheter ablation of atrial fibrillation. Europace 2016;18:1352–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.