Abstract
The field of sexual medicine, in reference to the science of the sexual response and the clinical management of sexual dysfunctions, has evolved remarkably in the last 25 years. Erection biology has been central in driving this progress and is measured considerably by the discovery, study, and clinical translation of a simple gaseous molecule, nitric oxide, which is operative in the penis. Nitric oxide functions extraordinarily as a neurotransmitter and molecular signal transducer. It is now well understood to be the principal molecular mediator of penile erection and to be a critical element involved in dysregulatory mechanisms of erection disorders ranging from erectile dysfunction to priapism. It is most familiarly associated with the scientific development of oral medications for treating erectile dysfunction, which has modernized the clinical management of this condition.
INTRODUCTION
This year marks the 20th anniversary of the regulatory agency approval and commercial release of Viagra (sildenafil citrate; Pfizer, New York, New York) for the treatment of erectile dysfunction, defined as “the consistent inability to attain and maintain a penile erection sufficient for sexual satisfaction” (1). The timing is momentous for reflecting on the impact of this therapeutic agent for its almost miraculous role in the treatment of disorders of penile erection as well as its influence in the field of sexual health more broadly. In many respects, the drug is revolutionary because of its place in re-inaugurating a sexual revolution over the past 2 decades, with affirmation that good sexual health constitutes a barometer of overall general health and sense of well being of both men and women.
Despite the ready recognition of sildenafil citrate and similarly active medications known as phosphodiesterase type 5 (PDE5) inhibitor agents used for treating erectile dysfunction today, less conversational is the chemical substance underlying this phenomenon, nitric oxide. Nitric oxide, an evanescent and seemingly trivial gaseous molecule, is the centerpiece in the scientific development and clinical application of these agents. Succinctly stated, the scientific discovery that nitric oxide represents the key chemical factor in regulating penile erection critically undergirded the advancement of PDE5 inhibitors clinically.
This essay serves to present the role of nitric oxide in the biology of the penis that has impacted the contemporary practice of sexual medicine. It covers the original scientific work in the field that identified the function of nitric oxide in the mechanism of penile erection and acknowledges key observations for applying PDE5 inhibitors as a systemic treatment that phenomenally targets the penis with therapeutic efficacy. It also briefly reviews the current science of erection biology, the place of PDE5 inhibitor therapy in clinical management schemes of erection disorders at present, and the clinical and societal impacts of PDE5 inhibitor therapy.
MATERIALS AND METHODS
This exercise consisted of a current literature search using the National Library of Medicine PubMed Services and a survey of proceedings from national and international conferences referable to the topic. References were made to key words such as nitric oxide, gaseous molecule, neurotransmitter, signal transduction, penis, erection, and erectile dysfunction.
RESULTS
Basic science research and clinical studies have supported the understanding that nitric oxide is the key molecular mediator of penile erection. Evidence has accumulated regarding the effects of this chemical in the penis inclusive of molecular signaling, regulation of alternative signaling pathways, and activity in oxidative/nitrosative stress biology. Its functional roles in the penis pertain to erection biology and penile homeostasis with influence on the contractile state of corporal muscle, metabolic functions, and tissue structural health. Discoveries surrounding nitric oxide biology in the penis have served to institute and promote therapeutic prospects in pharmaceutical arenas and other innovative ways as well.
DISCUSSION
The science of penile erection was advanced significantly in the 1980s, commencing with the recognition that physical causes associated with cardiovascular and other health comorbidity states substantially account for the pathophysiology of erectile dysfunction (2,3). Before that time, erectile function was largely related to psychological factors, and erectile dysfunction was believed to be primarily a psychologically based disorder. Accordingly, the field rapidly evolved to recognize that the physiologic process of penile erection involves a complex interplay of vascular and neurologic events, modulated by hormonal factors (4). Further acceptance of the organic biology of penile erection resulted from demonstrations that vasoactive drugs delivered to the penis by local injection (e.g., alprostadil, papaverine, and phentolamine) rapidly elicit penile erection (5). Although investigators were unable to prove that these agents operated in the actual physiology of penile erection, the clinical pharmacologic era of erections was inaugurated. Fervent interest was stoked both to find the elusive chemical effector of this sexual response and refine a simpler mode of therapy such as oral administration that would target the penis for erectile dysfunction management.
The advent of truly efficacious oral pharmacotherapy for erectile dysfunction may well have come about in the 1990s through serendipitous events. In clinical trial work performed in the early 1990s, scientists at Pfizer, Inc., made the observation that a drug under study for treating angina actually facilitated spontaneous erections (6). This drug, originally known as UK-92480, was developed as a selective PDE5 inhibitor. The observation then prompted further study by Pfizer scientists that confirmed the expression of PDE5 in corporal tissue, but not so appreciably in the myocardium (7,8). The course of events virtually salvaged a drug development program with a completely different paradigm shift from its original focus.
Occurring at about the same time frame as Pfizer's research, original scientific work was conducted by several scientists independently investigating the mechanism of penile erection. In line with a common investigative approach in the field at that time, organ bath studies of isolated ex vivo corporal tissue obtained from various animal species and humans showed that the tissue contractile effects are inhibited by the administration of inhibitors of nitric oxide production while these inhibitory effects are reversed by the nitic oxide precursor, L-arginine (9-11). Complementary in vivo studies in dogs showed that the PDE5 inhibitor, zaprinast, injected into the penis enhanced pelvic nerve-stimulated penile erections (12). However, the notion of delivering zaprinast to humans beyond local injection was not appreciated at that time, and research endeavors continued with interest to identify the factor required to initiate the erection response. Critically supportive evidence of nitric oxide functioning as a physiologic mediator of erection was understood with in vivo erection studies showing that inhibitors of nitric oxide production blocked electrically stimulated penile erection (13). Localizations of nitric oxide synthetic enzymes to autonomic neuroregulatory nerves in the penis as well as to vascular and trabecular endothelium (13) added convincing evidence as to the sources of nitric oxide and the behavior of this chemical in mediating the penile erection response.
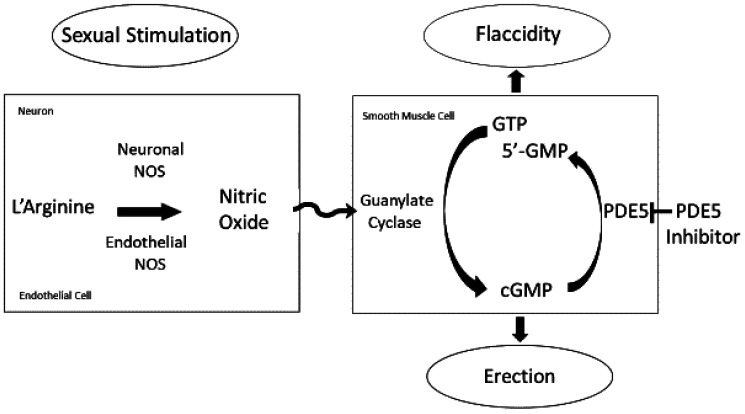
These investigations contributed to the emergence of nitric oxide as a relevant biologic mediator and aligned with characterizations of its biologic sources and actions elsewhere in the body. As for other organ systems, the genitourinary tract was affirmed to be a locus for the diverse functions of nitric oxide. Early characterized roles of nitric oxide as a reactive oxidative stress species, neurotransmitter, and vasodilator readily applied to the penis (14). The action of the molecule in the penis typifies its classical manner of physiologic signaling (Figure 1). Constitutive nitric oxide synthase isoforms (i.e., neuronal and endothelial synthetic enzymes of nitric oxide production) generate nitric oxide from its precursor amino acid L-arginine that exerts a signal transduction mechanism involving downstream effector actions (15,16). These enzymes are localized to neurostimulated nerve endings and blood flow–activated endothelium within the penis, and the orchestrated effect of penile erection in a sustained manner involves specialized phosphorylative mechanisms acting on both nitric oxide sources (17). Following sexual excitation, nitric oxide is generated and released, and then it diffuses locally to corporal smooth muscle cells whereby it activates the enzyme guanylate cyclase. Activation of this enzyme enables it to catalytically convert guanosine triphosphate to 3', 5'-cyclic guanosine monophosphate (cGMP), which then acts as a second messenger molecule. cGMP activates protein kinase G, alternatively known as cGMP-specific protein kinase I (18). This signal ultimately executes a cascade of biological processing events that aggregately result in erectile tissue relaxation, which is the linchpin for penile erection to occur. Signal transduction via single-reactive thiols in the penis represents a secondary mechanism of nitric oxide signaling in the penis (19). PDE5, an enzyme localized to corporal smooth cells, hydrolyzes cGMP to its inactive form 5'-GMP, thereby terminating nitric oxide/cGMP signaling (20,21).
Fig. 1.
Schematic diagram of the nitric oxide signaling pathway in the penis for mediating the erection response. The diagram shows the molecular basis for erection physiology that may transition between flaccidity (erectile tissue contraction) and erection (erectile tissue relaxation) depending upon the extent of sexual stimulation. Nitric oxide is generated constitutively from L-arginine by catalysis of neuronal nitric oxide synthase (NOS) and endothelial NOS localized to neurons and endothelial cells, respectively. After its release from generator cells in the penis, nitric oxide diffuses locally to corporal smooth muscle cells whereby it activates guanylate cyclase to convert 5′-guanosine triphosphate (GTP) to 3′, 5′-cyclic guanosine monophosphate (cGMP). This cyclic nucleotide then exerts downstream effects resulting in penile erection. Phosphodiesterase type 5 (PDE5) degrades cGMP to its inactive form, 5′-GMP, which is subsequently reformed into GTP. A PDE5 inhibitor blocks PDE5 activity, thereby potentiating effector actions of cGMP.
The biology of nitric oxide in the penis extends beyond its central role in corporal smooth muscle relaxation with relevance for mediating the erection response. Investigations soon to follow established its involvement in multiple other molecular interactions in the penis with influence on erectile tissue health in various other ways. The molecule is linked with oxidative/nitrosative stress mechanisms in the penis and thereby applies to such pathologic scenarios as vasculogenic derangements and fibrotic conditions occurring in the penis (22-24). It has been found to regulate androgen metabolism and modulate cellular transduction pathways in the penis that are associated with hypogonadal effects on the penis (25,26) and excessive, uncontrolled penile erection (priapism) (27,28).
The culmination of these investigations revealed the astonishing basis by which PDE5 inhibitor therapy delivered by an oral systemic approach yields penile erection. Key features are the release of nitric oxide locally into corporal tissue with a mechanism of action resulting in erectile tissue relaxation and the tissue-specific effect of PDE5 that is inhibitable. The “sweet spot” of PDE5 inhibitor therapy for the management of erectile dysfunction is that the intervention operates conditionally to enhance the sexually stimulated erection mechanism by targeting PDE5 that is highly localized to corporal tissues. In other words, under conditions of sexual stimulation, the presence of sildenafil or any PDE5 inhibitor augments nitric oxide/cGMP signaling and thereby promotes the erection response.
In line with the rapid scientific progress in this field, PDE5 inhibitor therapy for the treatment of erectile dysfunction in the United States achieved Food and Drug Administration regulatory agency approval in the form of sildenafil citrate (Viagra) in 1998, vardenafil hydrochloride (Levitra, Bayer Schering Pharma AG, Berlin, Germany), tadalafil (Cialis, Lilly LLC, Indianapolis, Indiana) in 2003, and avanafil (Stendra, Vivus Inc., Mountain View, California) in 2012. Numerous clinical trials have established efficacy and tolerability of medications in this class (29). This therapy is acknowledged to possess an approximately 70% efficacy rate in producing successful sexual intercourse in general (30). Consensus guidelines acknowledge the place of PDE5 inhibitor therapy as first-line treatment for erectile dysfunction, irrespective of etiology (31,32). However, transient side effects of the therapy (e.g., headaches, facial flushing, and indigestion) or contraindications (i.e., nitrate use) may limit its use for all patients such that other standard options for erectile dysfunction management (e.g., vacuum erection devices, penile injections, intraurethral suppositories, and penile prostheses) are available and can be offered.
PDE5 inhibitor therapy was later shown to serve as an intervention for recurrent ischemic priapism when used according to a daily therapeutic scheme unassociated with sexual stimulation (33,34). Further study of the science of nitric oxide signaling in the penis instigated this treatment in a molecular modulatory mode that reverses PDE5 dysregulation in the penis, found to be a pathophysiologic mechanism for priapism (35). It has also been contended that PDE5 inhibitor therapy offers an antifibrotic benefit for such penile fibrotic conditions as Peyronie's disease, largely supported by preclinical investigations in the field (36).
The modern era of the science and practice of sexual medicine may well credit the scientific discoveries of nitric oxide signaling in the penis for launching PDE5 inhibitory therapy. In the realm of basic science, erection biologic studies surrounding nitric oxide paved the way for a host of subsequent investigations exploring alternative cellular and molecular mechanisms of the sexual response. Clinical research in sexual medicine ramped up with rigorous investigations of erectile dysfunction parameters including its etiologies, severities, associated clinical disease states, and affected populations. Expanded interest in understanding and investigating all forms of sexual dysfunction for both men and women was generated, and it arguably contributed to progress in the field of study of female sexual dysfunctions. The clinical practice of sexual medicine was advanced, promoting public dialogue about sexual health problems and galvanizing the medical community beyond urologists and sexual health specialists to become aware and offer treatment for these problems. Furthermore, the modern science of erections has propelled forward the conviction that sexual health is a public health concern, with evidence that its attention and treatment achieve life quality improvement and overall health maintenance (32,37,38).
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Spiegel, New York City: That was an excellent talk. Tell us about side effects, in addition to 4-hour long erections. Specifically, do visual side effects — off-target effects — of PDE-6 inhibitors occur?
Burnett, Baltimore: Certainly there have been visual side effects. PDE-6 is localized in the retina, and there is some cross reactivity. We have seen about 3% of patients with some transient visual side effects, such as halos around objects and blue spots. All of these side effects are thought to be transient and not a permanent effect on vision. Although there has been a description of a visual syndrome called NAION — nonarteritic anterior ischemic optic neuropathy — but that occurs in men who have severe diabetes and very severe vascular disease that may change some of the vascular constriction in vessels of the eye.
Crowley, Boston: Given the frequency of this, the issue comes up as to whether or not there's a genetic component. Since you have a 20% affected rate, has anybody done a GWAS (genome-wide association study) on affected males compared to unaffected males to see if there are any loci that might give rise to other pathways and targets?
Burnett, Baltimore: There have been studies looking at the genetic polymorphisms for some of the different enzymes — PDE-5 and others — that may be variable in different patients. In fact, that's why we have more than one product out there, because maybe there are slight differences in the structure of these agents that resemble the enzyme agonists. Therefore, there may be differences in different individuals. So, that work is underway. It's not taking off in a huge way in this field, but I think that may be exciting for the future to consider these options.
References
- 1.McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the fourth international consultation on sexual medicine 2015. J Sex Med. 2016;13:135–43. doi: 10.1016/j.jsxm.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–7. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 5.Brindley GS. Pilot experiments on the actions of drugs injected into the human corpus cavernosum penis. Br J Pharmacol. 1986;87:495–500. doi: 10.1111/j.1476-5381.1986.tb10191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzenstein L, Grossman EB. Viagra (Sildenafil Citrate): The Remarkable Story of the Discovery and Launch. New York, New York: Medical Information Press; 2001. [Google Scholar]
- 7.Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 8.Wallis RM, Corbin JD, Francis SH, et al. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83((5A)):3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro LJ, Bush PA, Buga GM, et al. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Bichem Biophys Res Commun. 1990;170:843–50. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 10.Rajfer J, Aronson WJ, Bush PA, et al. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 11.Kim N, Azadzoi KM, Goldstein I, et al. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J Clin Invest. 1991;88:112–8. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trigo-Rocha F, Aronson WJ, Hohenfellner M, et al. Nitric oxide and cGMP: mediators of pelvic nerve-stimulated erection in dogs. Am J Physiol. 1993;264((2 Pt 2)):H419–22. doi: 10.1152/ajpheart.1993.264.2.H419. [DOI] [PubMed] [Google Scholar]
- 13.Burnett AL, Lowenstein CJ, Bredt DS, et al. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–3. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 14.Burnett AL, Musicki B. The nitric oxide signaling pathway in the penis. Curr Pharm Des. 2005;11:3987–94. doi: 10.2174/138161205774913381. [DOI] [PubMed] [Google Scholar]
- 15.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 16.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298((Pt 2)):249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurt KJ, Sezen SF, Lagoda GF, et al. Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2012;109:16624–9. doi: 10.1073/pnas.1213790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedlund P, Aszodi A, Pfeifer A, et al. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2349–54. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Gillespie JS, Martin W. Non-adrenergic, non-cholinergic relaxation of the bovine retractor penis muscle: role of S-nitrosothiols. Br J Pharmacol. 1994;111:1287–95. doi: 10.1111/j.1476-5381.1994.tb14885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 21.Rybalkin SD, Yan C, Bornfeldt KE, et al. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–91. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 22.Jones RW, Rees RW, Minhas S, et al. Oxygen free radicals and the penis. Expert Opin Pharmacother. 2002;3:889–97. doi: 10.1517/14656566.3.7.889. [DOI] [PubMed] [Google Scholar]
- 23.Munarriz R, Park K, Huang YH, et al. Reperfusion of ischemic corporal tissue: physiologic and biochemical changes in an animal model of ischemic priapism. Urology. 2003;62:760–4. doi: 10.1016/s0090-4295(03)00484-9. [DOI] [PubMed] [Google Scholar]
- 24.Azadzoi KM, Schulman RN, Aviram M, et al. Oxidative stress in arteriogenic erectile dysfunction: prophylactic role of antioxidants. J Urol. 2005;174:386–93. doi: 10.1097/01.ju.0000161209.39959.67. [DOI] [PubMed] [Google Scholar]
- 25.Traish AM, Munarriz R, O'Connell L, et al. Effects of medical or surgical castration on erectile function in an animal model. J Androl. 2003;24:381–7. doi: 10.1002/j.1939-4640.2003.tb02686.x. [DOI] [PubMed] [Google Scholar]
- 26.Morelli A, Filippi S, Mancina R, et al. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology. 2004;145:2253–63. doi: 10.1210/en.2003-1699. [DOI] [PubMed] [Google Scholar]
- 27.Burnett AL, Chang AG, Crone JK, et al. Noncholinergic penile erection in mice lacking the gene for endothelial nitric oxide synthase. J Androl. 2002;23:92–7. doi: 10.1002/j.1939-4640.2002.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 28.Lagoda G, Sezen SF, Cabrini MR, et al. Molecular analysis of erection regulatory actors in sickle cell disease-associated priapism in human penis. J Urol. 2013;189:762–8. doi: 10.1016/j.juro.2012.08.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257–80. doi: 10.1111/j.1464-410X.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- 30.Khera M, Goldstein I. Erectile dysfunction. BMJ Clin Evid. 2011;2011:pii–1803. [PMC free article] [PubMed] [Google Scholar]
- 31.Porst H, Burnett A, Brock G, et al. SOP conservative (medical and mechanical) treatment of erectile dysfunction. J Sex Med. 2013;10:130–71. doi: 10.1111/jsm.12023. [DOI] [PubMed] [Google Scholar]
- 32.Burnett AL, Nehra A, Breau RH, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–41. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Bivalacqua TJ, Musicki B, Hsu LL, et al. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS One. 2013;8:e68028. doi: 10.1371/journal.pone.0068028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnett AL, Anele UA, Trueheart IN, et al. Randomized controlled trial of sildenafil for preventing recurrent ischemic priapism in sickle cell disease. Am J Med. 2014;127:664–8. doi: 10.1016/j.amjmed.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Champion HC, Bivalacqua TJ, Takimoto E, et al. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661–6. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Cadavid NF, Rajfer J. Treatment of Peyronie's disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol. 2010;7:215–21. doi: 10.1038/nrurol.2010.24. [DOI] [PubMed] [Google Scholar]
- 37.Krane RJ, Goldstein I, Saenz de Tejada I. Impotence. N Engl J Med. 1989;321:1648–59. doi: 10.1056/NEJM198912143212406. [DOI] [PubMed] [Google Scholar]
- 38.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159–66. doi: 10.1046/j.1525-1497.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]