Abstract
The immune system responds to invaders (pathogenic microbes and cancer cells) but is tightly controlled to prevent harmful reactions against self tissues, commensal microbes, and the fetus. Elucidation of the molecular basis of these control mechanisms has been one of the most impressive recent advances in Immunology. Two of these mechanisms are particularly important and are being targeted therapeutically — inhibitory receptors (so-called checkpoint molecules) and a population of CD4+ T cells called regulatory T cells. This article summarizes how defining these mechanisms has opened new avenues for therapeutic manipulation of immune responses, and how experimental models, including transgenic and knockout mice we and others have used, have contributed to developing the critical knowledge base.
INTRODUCTION
The mammalian immune system has the remarkable capacity to react against and eradicate the countless infectious pathogens we encounter in our everyday lives, as well as cancer cells that arise because of accumulation of somatic mutations. The ability to exploit the defense mechanisms of immunity was brought to brilliant realization by Edward Jenner's effective vaccination against smallpox in 1796. Although Jenner was not the first to try this procedure, he was the first to scientifically establish its effectiveness by challenging the vaccinated boy with smallpox and confirming that the dreaded disease did not develop. The success of vaccination focused the attention of the immunology community on ways to stimulate immune responses, the fundamental basis of vaccination. Through the early part of the 20th century, many fundamental observations were made about the specificity and protective capacity of antibodies and lymphocytes, culminating in the identification of B and T lymphocytes as the two basic components of the adaptive immune system.
In the 1960s and 1970s, immunologists began to realize that the immune system must have ways of controlling itself so it does not make damaging responses against harmless self and environmental antigens, and disruption of these mechanisms must underlie autoimmune and allergic diseases. Therapeutic targeting requires understanding mechanisms at a molecular level and defining the signals that activate or inhibit these pathways. The two control mechanisms that have been most successfully targeted are regulatory T cells and inhibitory receptors of T cells.
RESULTS AND DISCUSSION
Regulatory T Cells and Interleukin 2
Although the search for cells that suppressed immune responses had been ongoing since the 1960s, it had proved a daunting challenge to clearly identify these cells, purify them to homogeneity, and define their mechanisms of action. In 1995, Sakaguchi et al. (1) showed, in a landmark publication, that mice contained a population of CD4+ T cells that controlled immune responses and the absence of these cells was associated with the development of systemic, multi-organ autoimmunity. The authors named these cells regulatory T cells (Tregs). Although this association was intriguing, it did not prove the biological significance of Tregs because the link with autoimmunity was only a correlation. Shortly after the discovery of Tregs, a transcription factor called Foxp3 was identified as highly and preferentially expressed in these cells compared to all others (2). Coincidentally, it was known that a severe systemic autoimmune disease that developed in boys was associated with mutations in FOXP3, an X-linked gene. This disease goes by the acronym IPEX, for immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (3). Soon thereafter, the foxp3 gene was knocked out in mice, and it was found that the mice developed an IPEX-like disease. Importantly, these mice lacked Foxp3+ Tregs, and the disease could be prevented by introducing into the mice Foxp3+ Tregs from healthy animals (4). Thus, Koch's postulates were proved, showing that Foxp3+ Tregs are essential for maintaining unresponsiveness to self antigens and thus for preventing autoimmune diseases.
One of the surface markers that the Sakaguchi laboratory had identified on Tregs was the α chain of the interleukin 2 (IL-2) receptor, CD25. IL-2 had been discovered as a T cell growth factor. Its receptor consists of three chains — the β and γ chains are the signaling chains that are expressed constitutively on most T cells and the α chain increases the affinity of the receptor for IL-2 and is expressed transiently on recently activated T cells and constitutively on Tregs, allowing these cells to respond to physiologic levels of the growth factor (5). The high expression on Tregs suggested that these cells depend on IL-2 for their maintenance. This hypothesis was proved by the findings that in mice, knockout of IL-2 or the α or β chain of the receptor led to loss of Tregs and the development of systemic autoimmunity (6-8). Rare patients with mutations in CD25 also develop a systemic autoimmune disease (9). Thus, IL-2 is an unusual cytokine in that it has opposing functions — it stimulates immune responses by enhancing proliferation of recently activated T cells and it suppresses immune responses by promoting Treg function.
To address how this balance is maintained, we have developed transgenic mouse models in which a known antigen, ovalbumin (Ova), is expressed either systemically all the time or in the skin under the control of an inducible promoter. Transfer of Ova-specific CD4+ T cells into these mice induces a systemic or cutaneous inflammatory disease (10, 11). Surprisingly, these mice spontaneously recover over time, even though the antigen continues to be expressed and the T cells are present. The basis of the recovery is a dramatic switch in the nature of the antigen-specific T cells. The acute inflammatory disease is associated with the development of Foxp3- effector T cells that produce pro-inflammatory cytokines, and resolution is caused by the subsequent development of Foxp3+ Tregs. Thus, the immune response shows sequential phases, activation followed by Treg-mediated control. If the T cells are unable to produce IL-2 (because of deletion of the il2 gene), the initial inflammatory disease is mild, reflecting the role of IL-2 in activating effector cells. More strikingly, recovery fails to occur because of a failure to generate and maintain Tregs. These experimental studies have established the dual functions of IL-2 and emphasized its obligatory role as a cytokine for Tregs.
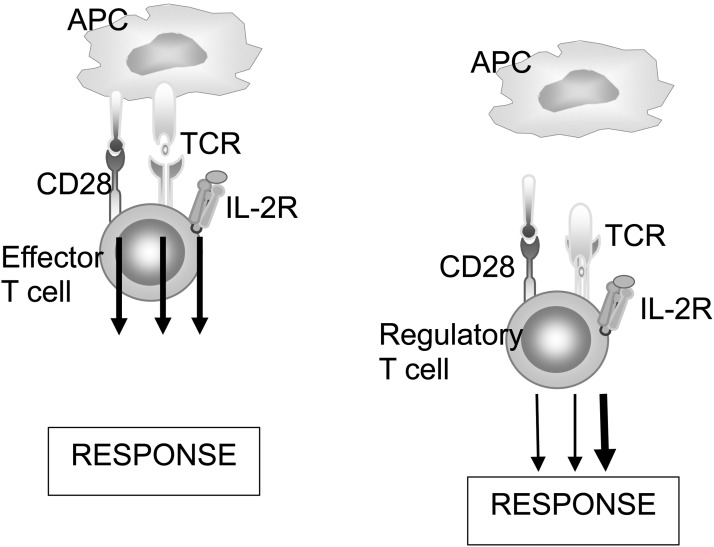
Defining the basis of this dual function has been instructive. Tregs are more sensitive to IL-2 than effector T cells because, as mentioned earlier, Tregs constitutively express high levels of the high-affinity αβγ IL-2 receptor. Tregs also appear to have a signaling pathway that is different from that of other T cells. The activation of most T cells requires a combination of signals from the antigen receptor, costimulators that respond to danger signals expressed upon encounter with pathogenic microbes, and growth factors such as IL-2. Tregs, on the other hand, have tuned down signaling from the antigen receptor and costimulators and are much more dependent on IL-2–mediated signals (Figure 1). The biological significance of this dependence on IL-2 is that whenever T cells respond to foreign antigens and produce IL-2, the cytokine boosts Tregs, thus preventing simultaneous autoimmunity and maintaining control on the response (12).
Fig. 1.
Signaling pathways in effector and regulatory T cells. Conventional Foxp3- effector T cells rely on signals from multiple receptors for their responses, whereas Foxp3+ Tregs are highly dependent on signals from the interleukin 2 (IL-2) receptor. APC, antigen-presenting cell; TCR, T cell receptor.
The realization that IL-2 is a critical Treg-maintaining cytokine (13) has led to an astonishing revision in our view of the therapeutic potential of this growth factor. Initial attempts to use IL-2 in patients were based on its originally defined function, to stimulate immune responses. High doses of the cytokine were given to cancer patients to boost anti-tumor immune responses (14). This approach suffered from toxic effects of administering large amounts of the cytokine, and the treatment was beneficial in a small minority of patients, so this strategy for cancer therapy was largely abandoned. More recently, investigators hypothesized that low doses of IL-2 would act preferentially on Tregs because of the greater sensitivity of these cells to the cytokine, and could be used to treat inflammatory diseases by suppressing immune responses. Initial small proof-of-concept trials indicated that this was the case in patients with a virus-associated vasculitis and in steroid-resistant graft-versus-host disease (15, 16). Subsequent larger studies in patients with lupus have also shown beneficial effects (17, 18). Thus, the field has undergone a surprising turn — a cytokine that was once used to stimulate immune responses is now used to suppress inflammatory diseases. More recently, academic institutions and biotech and pharmaceutical companies are developing chemically modified versions of IL-2 that bind to the α chain of the receptor and thus have more selective action on Tregs (19).
The Principle of Immune Checkpoint Blockade
It has been known for decades that antigen is not enough to stimulate a productive immune response; that is why purified protein vaccines must be administered with adjuvants. The search for second signals led to the identification of CD28 expressed on T cells as a receptor that recognized B7 molecules expressed on antigen-presenting cells. This was soon followed by the identification of two CD28-like molecules expressed on T cells, CTLA-4 and programmed death 1 (PD-1) (arcane historical names that do not reflect their functions). Surprisingly, whereas CD28 activated T cells, CTLA-4 and PD-1 inhibited T-cell responses. Because of these actions, CD28 is called a costimulator and CTLA-4 and PD-1 are coinhibitors. We showed that blocking CTLA-4 with an antibody or knocking the gene out in mice led to strong responses to aqueous antigens, which normally were not immunogenic (20, 21). These and many other such studies established the roles of the coinhibitors as controllers of immune responses. A major breakthrough came from the finding that blocking CTLA-4 also led to strong T-cell responses against some tumors and subsequent rejection of the tumors (22). This seminal observation has led to the development of so-called checkpoint blockade for cancer immunotherapy, which uses blocking antibodies against CTLA-4, PD-1, or the ligand for PD-1 to remove the brakes on immune responses and thus treat a variety of cancers (23). This is the first truly successful immunotherapy that has been developed for cancer, and is changing current practices of cancer care.
One of the unfortunate consequences of checkpoint blockade therapy is that it unleashes immune responses not only against cancer cells but against all other antigens that are encountered by the patient's immune system, including self antigens. As a result, and predictably, patients treated with anti–PD-1/programmed death ligand 1 or anti-CTLA-4 frequently develop autoimmune diseases that are severe enough in over a quarter of the patients that the treatment must be curtailed. The next generation of immune modulation is likely to combine checkpoint blockade with vaccination with tumor antigens, in an attempt to preferentially boost antitumor responses. Thus, the field of immunology will have come full circle, using Jenner's principles of vaccination to remove the brakes on specific immune responses. The same principle, combining low-dose IL-2, discussed earlier, with specific antigen therapy may be useful for stimulating Tregs against specific antigens — in this case, to suppress, not enhance, immune responses.
CONCLUSIONS
The studies discussed herein show the biological significance and therapeutic potential of understanding immune regulation. Defining the molecular characteristics of regulatory T cells and checkpoint molecules has led to successful therapies that promote Tregs by administering IL-2 to suppress immune responses and treat inflammatory diseases, or, conversely, to block checkpoint molecules with antibodies to stimulate immune responses to treat cancers. These successful therapies dramatically show the value of basic research — what started as a search for control mechanisms has culminated in novel therapies. There are few, if any, as successful examples of the bed-to-bedside approach that drives modern biomedical research. The future will tell if more regulatory pathways remain to be discovered and targeted therapeutically. In immunology, the future has never been more exciting and full of promise.
ACKNOWLEDGMENTS
The author's research has been supported by grants from the National Institutes of Health.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Tweardy, Houston: The anti–PD-1 and anti-CDL4 antibodies are being used to treat many, many cancers. I was wondering if you could speculate how maybe perhaps a low-dose IL-2 could be used in combination with those to further improvement the immune response against tumors — perhaps the low-dose IL-2 or activating Tregs?
Abbas, San Francisco: So, the low-dose IL-2 is actually being used for autoimmune disease. It's the opposite. But there is a lot of interest in combining the receptor–beta chain targeted IL-2 — the high-dose equivalent of IL-2 — with anti–PD-1. There's one clinical trial that is very early stage. It's a crazy product that they have made and it's not very promising at the moment. It's got bad vibes. But they're many, many companies that are trying to combine anti–PD-1 with the version of IL-2 that stimulates effector T-cells. And you really want it to stimulate effector T-cells, because you don't want to have too many regulatory T-cells. So, that's why people are making the beta chain–targeted IL-2.
Vierling, Houston: Superb talk, very provocative. When you look at the clinical utility of this, you mentioned what was happening already in lupus. My question is whether or not to exploit this further in other autoimmune diseases. One needs first to know the antigen in order to stimulate a regulatory population to then have this dominant effect. If that's the case, does it lead you to be able to immunize with antigens to create a regulatory environment to prevent the development of autoimmune disease?
Abbas, San Francisco: Excellent question. So, the two autoimmune diseases in which we do have a good understanding of the antigen are multiple sclerosis and type one diabetes; lupus is not that great. There are attempts to combine peptide therapy with IL-2. But IL-2 the low-dose IL-2, is being used now is agnostic about the antigen. We don't care what the antigen is. Let the patient deal with what the antigen is. We'll just help the patient generate more regulatory T cells. I have a good friend in Paris — he's a clinical immunologist named David Klatzmann (I'm on the advisory board of his academic program and his company), and David is doing what we call a basket trial. He doesn't know — we don't know — what do you mean by low dose? And the low dose for rheumatoid arthritis may not be a low dose for lupus. We just don't know. So, David is doing a basket trial on 10 different diseases including one that I find incredibly provocative, and we don't know where it is going to go. There is a suspicion that in pregnant women the fetus is tolerated because of regulatory T cells. Then the next argument follows whether recurrent abortion may be a defect in regulatory T cells. So, he's treating 10 women who have had recurrent pregnancy loss with low-dose IL-2. No idea whether it's going to pan out or not pan out. He's doing a very large basket trial, and lupus is the poster child in his basket trial but also 10 other diseases.
Selker, Boston: Wonderful talk. Along the same lines as we just heard — this is almost like chemotherapy in that we just slug a process. The immune response as I understand it, and I'm an epithelial biologist, is more tailored to individual antigens. How do we take this — instead of just upregulating or downregulating all Tregs or attacking cells — how can we make it antigen specific?
Abbas, San Francisco: So, you know the anti–PD-1 is also agnostic about the antigen, right? You're just blasting — letting the immune response go and not worrying about what — and let the patient deal with the specificity. The next generation will be combining the anti–PD-1 with the tumor vaccine. This is all about to happen. Then it's going to do exactly what you're saying. You help the immune system even more by boosting the correct response. Because right now what's happening with the anti–PD-1 therapy is predictably those patients are getting nasty autoimmune diseases. Some of them are so bad that they have to withdraw the therapy. So, this is entirely predictable. You are just unleashing the immune response against anything including self. But if we can target it better — exactly the way you're saying — antigen plus an immune modulator is by far the best way to go.
REFERENCES
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 2.Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nat Rev Immunol. 2014;14:343–9. doi: 10.1038/nri3650. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 4.Häri S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 5.Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc Natl Acad Sci U S A. 2006;103:2788–93. doi: 10.1073/pnas.0511161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadlack B, Merz H, Schorle H, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Kündig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–6. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 8.Willerford DM, Chen J, Ferry JA, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 9.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A. 1997;94:3168–71. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2–dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–86. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–42. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Gorman WE, Dooms H, Thorne SH, et al. The initial phase of an immune response functions to activate regulatory T cells. J Immunol. 2009;183:332–9. doi: 10.4049/jimmunol.0900691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinen T, Kannan AK, Levine AG, et al. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol. 2016;17:1322–33. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saadoun D, Rosenwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–77. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 17.He J, Zhang K, Wei Y, et al. Low-dose interleukin-2 selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22:991–3. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 18.Humrich JY, Riemekasten G. Restoring regulation — IL-2 therapy in systemic lupus erythematosus. Expert Rev Clin Immunol. 2016;12:1153–60. doi: 10.1080/1744666X.2016.1199957. [DOI] [PubMed] [Google Scholar]
- 19.Abbas AK, Trotta E, Simeonov DR, Marson A, Bluestone JA. Revisiting IL-2: biology and therapeutic prospects. Sci Immunol. 2018;3:pii–eaat1482. doi: 10.1126/sciimmunol.aat1482. [DOI] [PubMed] [Google Scholar]
- 20.Perez VL, Van Parijs L, Biuckians A, Zheng X, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 21.Walker LS, Ausubel LJ, Chodos A, Bekarian N, Abbas AK. CTLA-4 differentially regulates T cell responses to endogenous tissue protein versus exogenous immunogen. J Immunol. 2002;169:6202–9. doi: 10.4049/jimmunol.169.11.6202. [DOI] [PubMed] [Google Scholar]
- 22.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 23.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]