Abstract
Rationale:
Seizures are rare during the perioperative period; in most cases, there is a previous history of epilepsy or surgery-associated seizures. Febrile convulsions may occur when the body temperature rises above 38°C; this is the most common cause of seizures in children. Febrile convulsions after general anesthesia in the postanesthetic care unit (PACU) without a past or family history are rare. Some reviews suggest that since anesthesia changes immunity, elective surgery should be postponed three weeks after live vaccination.
Patient:
A 12-month-old female with bilateral hearing loss underwent cochlear implantation under general anesthesia. She did not have any history of convulsions or developmental disorders. However, 1 week before surgery, measles-mumps-rubella (MMR) vaccination was given as a regular immunization.
Diagnoses:
Forty minutes after arrival at the PACU, sudden generalized tonic-clonic movement occurred during recovery and the patient's measured body temperature exceeded 38.0°C.
Interventions:
Thiopental sodium was administered intravenously as an anticonvulsant, and the tonic-clonic movement stopped immediately. Endotracheal intubation was performed to secure the airway, and tepid massage and diclofenac β-dimethylaminoethanol administration were performed to lower the patient's body temperature.
Outcomes:
There was no further fever and no seizures, and no other neurological deficits were observed until discharge.
Lessons:
The anesthesiologist should check the recent vaccination history even if the patient has not developed particular symptoms after vaccination. It is important to know that febrile convulsions may occur in patients who have recently received MMR vaccination.
Keywords: febrile convulsions, general anesthesia, MMR vaccination
1. Introduction
Although rare, seizures may occur during the perioperative period.[1] Only 2 cases of convulsions were reported among a total of 24,156 children who received anesthesia.[2] A previous history of epilepsy and surgery-associated seizures are risk factors for postoperative seizures.[3] Most perioperative seizures in patients with a preexisting seizure disorder are likely related to the patient's underlying condition, including changes in antiepileptic drug levels, fatigue, stress, and sleep deprivation.[4] Febrile convulsions may occur when the body temperature rises above 38°C; this is the most common cause of seizures in children.[5] There are reports of febrile convulsions occurring after general anesthesia, but the patients had a relevant past or family history.[6] In addition, in some documents, anesthesia and surgery may affect the immune response, so it is recommended that surgery be performed after a certain period of time.[7] Here, we report a case of febrile convulsions in the postanesthetic care unit (PACU) although a normal body temperature was maintained during anesthesia in a patient measles-mumps-rubella (MMR) vaccinated a week ago without a history of seizures.
1.1. Ethical review
This case report was approved by the Clinical Ethics Committee of Chonnam National University Hospital (CNUH-EXP-2019-012). The parents of the patient provided informed consent for publication of the case.
2. Case presentation
A 12-month-old female underwent both cochlear implantation under general anesthesia due to bilateral hearing loss. Her weight was 9 kg, her height was 73 cm, and she was on no regular medications. She was born at 38 weeks of gestation with a birthweight of 3.5 kg via cesarean section to a mother who had no underlying disease. She showed no congenital anomalies or developmental disorders. She received scheduled MMR vaccination 1 week before surgery and did not have any symptoms of upper respiratory infection or fever. All results of preoperative laboratory tests were within the respective normal ranges, including an electrocardiogram, chest X-ray, and blood and urine examinations. Brain magnetic resonance imaging showed no contraindications for cochlear implantation mapping. The patient entered the operating room without any premedication. After entering the operating room, monitoring was initiated, including noninvasive blood pressure, electrocardiogram activity, oxygen saturation, and pediatric bispectral index (BIS). Anesthesia was induced with 50 mg of thiopental sodium and 10 μg of fentanyl, and 5 mg of rocuronium was administered intravenously to facilitate intubation. Endotracheal intubation was performed using a tube (internal diameter, 3.5 mm) with a cuff, and both lungs sounded well. An esophageal thermometer and forced air blanket were applied to manage the patient's body temperature. Her initial core body temperature was 36.4°C, and it was maintained between 36.1°C and 36.5°C (Fig. 1). Anesthesia was maintained with 1.5 to 2.0 vol% sevoflurane (with 50% oxygen and 50% nitrous oxide) at a target BIS range of 40 to 60; the end tidal carbon dioxide tension was in the range of 35 to 37 mmHg. The surgery proceeded normally without any adverse events; the total anesthesia time was 3 hours and 20 minutes. Fentanyl (10 μg) was administered intravenously 10 minutes before the end of surgery for pain control and to prevent emergence agitation. At the end of the operation, 2.5 mg of pyridostigmine and 0.1 mg of glycopyrrolate were added to restore neuromuscular blockade. The patient was transferred to the PACU after confirming full recovery of spontaneous eye opening and spontaneous respiration. The vital signs measured at the PACU were normal except for a temperature of 37.2°C measured at the axilla; a tympanic thermometer could not be used as both ears were covered after the operation, so the patient's body temperature was measured at the axilla. Due to the elevated body temperature, we decided to observe without further heating. Oxygen was supplied at a flow rate of 5 L/minute via a simple facial mask. The patient remained in the PACU for 40 minutes and recovered well from anesthesia. The patient had a Modified Aldrete Score of 9/10, and was scheduled to move to the general ward. However, she suddenly became lethargic and showed difficulty in breathing. The oxygen supplied to the mask was increased to 10 L/minute and a jaw-thrust maneuver was performed. The patient showed tonic-clonic movements. Thiopental sodium (50 mg) was administered intravenously and the seizures ceased. Endotracheal intubation and positive pressure ventilation were performed to prevent respiratory depression due to thiopental sodium. During intubation, the practitioner felt that the patient was abnormally warm. The body temperature measured at the axilla was 37.7°C, and the patient was immediately treated with tepid massage. An esophageal thermometer was inserted to allow continuous monitoring of her body temperature, which was shown to be 38.1°C. Despite tepid massage for 20 minutes, the patient's body temperature increased further to 39.0°C (Fig. 2). Diclofenac β-dimethylaminoethanol (20 mg) was injected intramuscularly to prevent further body temperature elevation. Consciousness and spontaneous respiration were recovered 1 hour after the seizure. The patient's body temperature as measured with an esophageal thermometer had decreased to 37.5°C. In addition, her oxygen saturation was maintained at >97% after extubation. There was no evidence of atelectasis or pneumonia on a chest X-ray performed the following day. She had no additional seizures while in the general ward, and 60 mg of dexibuprofen was administered orally for 3 days to prevent any increase in body temperature. There was no epileptic wave on electroencephalography (EEG) performed on the fourth postoperative day, and she was discharged without convulsions or focal neurological symptoms on the fifth day.
Figure 1.
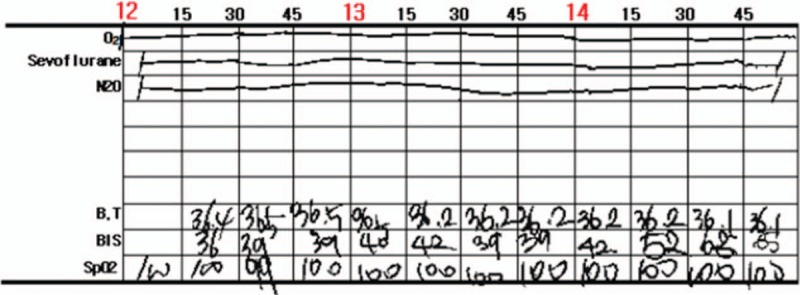
Intraoperative esophageal body temperature maintained between 36.1°C and 36.5°C.
Figure 2.
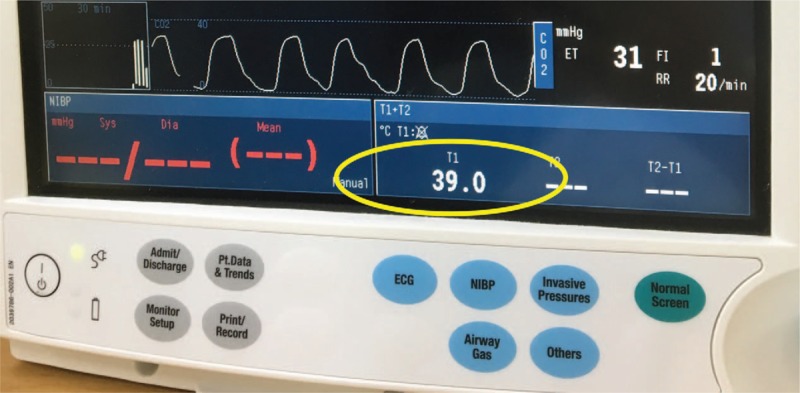
Increased body temperature at PACU despite tepid massage after febrile convulsions. The English in this document has been checked by at least 2 professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/FJlWEj.
3. Discussion
The possible causes of the seizure in this case include the use of volatile anesthetic agents, such as sevoflurane and an increase in body temperature. First, the use of sevoflurane is known to change normal EEG into an epileptiform pattern.[8] Most seizures associated with sevoflurane occur during anesthesia induction or emergence.[9] Such seizures are thought to be due to changes in the serum concentrations of anticonvulsants following fasting and drug interactions with sevoflurane.[10] Second, febrile convulsions occurred in association with increases in body temperature. Febrile convulsions are the most common seizure disorder in childhood, affecting 2% to 5% of children between the ages of 6 and 60 months.[11] Most febrile convulsions occur within 24 hours of the onset of fever.[5]
The patient in this case had no known cause of seizures other than fever. The patient and her parents had no history of febrile convulsions or seizure episodes. Surgery was not associated with convulsive movements. There was no regular medication or electrolyte imbalance before the induction of anesthesia. In this case, tonic-clonic movements occurred suddenly 40 minutes after arrival at the PACU, and the measured body temperature continued to rise to 39.0°C. Although the direct cause of the tonic-clonic movement in this case could not be determined, hyperthermia was considered to be a major contributing factor.
Yano et al[6] reported 2 pediatric patients with personal and family histories of febrile convulsions who showed seizure-like movements during general anesthesia. They suggested that the interaction of increased temperature and sevoflurane may elicit neuronal excitability in patients susceptible to febrile convulsions, particularly in those with a positive family history. In previous reports,[9] most seizures associated with sevoflurane occurred during anesthesia induction or emergence. In those cases, however, the patients had past and family histories of febrile convulsions. In addition, fever occurred after 40 minutes after the end of anesthesia.
The cause of fever, which appeared to be responsible for the seizures, is unclear. Hyperthermia is any elevation in core temperature, which can result from excessive heating, excessive heat production, inadequate heat loss, or set point elevation. Postoperative fever is common; the cause can be determined according to the timing of fever onset.[12] Immediate postoperative fever can be caused by medication, transfusion, preoperative infection, and trauma.[13] However, our patient had none of these risk factors.
It is possible that body temperature will increase due to the use of a warming blanket under anesthesia. Hyperthermia is rare in adults using modern warming systems, but occasionally occurs in infants and children.[14] Another possible cause of hyperthermia is the impairment of heat control ability due to anesthesia. All volatile intravenous anesthetics and opioids affect thermoregulatory control.[15] However, the patient in this case was monitored continuously during anesthesia. Malignant hyperthermia is a rare possible cause of a rise in body temperature. However, the partial pressure of carbon dioxide in the exhaled gas was normal and a postoperative examination revealed no increase in creatine kinase.[16]
The remaining possible explanation for the patient's elevated body temperature was MMR vaccination 1 week before surgery. It has been reported that the incidences of fever and febrile convulsions increase after MMR vaccination.[17] The MMR vaccine is a combined vaccine that protects against 3 separate illnesses—measles, mumps, and rubella (German measles)—in a single injection. Among 10- to 24-month-olds receiving their first dose of a measles-containing vaccine, the incidence rates of fever and seizures were reported to be elevated 7 to 10 days after vaccination.[18] Despite the lack of consensus in the delay of surgery after vaccination, Pablo Ingelmo et al recommend elective surgery should be waiting for a week after inactive vaccination and 3 weeks after live vaccination.[19]
The exact cause of febrile seizure is not known, but inflammation is known to be involved. Interleukin (IL)-1β plays a role in the inflammatory response associated with the pathogenesis of febrile convulsions. These findings support the reported significantly increased rate of biallelic polymorphisms in the IL-1 promoter at position 511, which increases the secretion of IL-1, in children showing febrile convulsions with a duration of more than 15 minutes.[20] The injection of IL-1β into the ventricles of 14-day-old rats results in febrile seizures at lower body temperatures and IL-1β knockout mice are more resistant to heat, suggesting that IL-1β plays a role in triggering febrile convulsions.[21]
Immunomodulation by anesthesia other than surgical stimulation has been reported.[22] The fever in our patient may have been the result of changes in the immune system caused by anesthesia or surgery. Anesthesia after vaccination may also reduce immunoreactivity.[23] The incidence of postoperative complications was reported to be higher in children receiving live attenuated vaccines, including those for polio, mumps, measles, and rubella.[24] Immunization-provoked seizures suggest roles for systemic inflammation and genetic susceptibility in the induction of febrile seizures after vaccination.[25] In this case, anesthesia and surgery may have influenced the immune response to vaccination. Thus, the normally regulated body temperature may have risen due to the altered immune response. Elevated body temperature and increased inflammation may have caused febrile convulsions in our patient.
4. Conclusion
In conclusion, convulsive movement can occur in the PACU following even uneventful general anesthesia in patients without risk factors. If a child shows convulsive movement without a history of epilepsy, his or her body temperature should be checked immediately. In addition, the anesthesiologist should check the recent vaccination history before anesthesia induction even if the patient has not developed particular symptoms after vaccination, especially the MMR vaccine.
Author contributions
Conceptualization: Hong-Beom Bae.
Investigation: Sungmin Kim.
Project administration: Taehee Pyeon.
Supervision: Jeong Il Choi.
Writing – original draft: Hyung Gon Lee, Joungmin Kim.
Writing – review & editing: Joungmin Kim.
Joungmin Kim orcid: 0000-0003-1135-1968.
Footnotes
Abbreviations: BIS = bispectral index, EEG = electroencephalography, MMR = measles-mumps-rubella, PACU = postanesthetic care unit.
The authors have no funding and conflicts of interests to disclose.
References
- [1].Hines R, Barash PG, Watrous G, et al. Complications occurring in the postanesthesia care unit: a survey. Anesth Analg 1992;74:503–9. [DOI] [PubMed] [Google Scholar]
- [2].Murat I, Constant I, Maud’huy H. Perioperative anaesthetic morbidity in children: a database of 24,165 anaesthetics over a 30-month period. Paediatr Anaesth 2004;14:158–66. [DOI] [PubMed] [Google Scholar]
- [3].Akavipat P, Rungreungvanich M, Lekprasert V, et al. The Thai Anesthesia Incidents Study (THAI Study) of perioperative convulsion. J Med Assoc Thai 2005;88Suppl 7:S106–12. [PubMed] [Google Scholar]
- [4].Niesen AD, Jacob AK, Aho LE, et al. Perioperative seizures in patients with a history of a seizure disorder. Anesth Analg 2010;111:729–35. [DOI] [PubMed] [Google Scholar]
- [5].Leung AK, Hon KL, Leung TN. Febrile seizures: an overview. Drugs Context 2018;7:212536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yano T, Okubo S, Naruo H, et al. Two cases with past and family history of febrile convulsion developed seizure-like movements during sevoflurane anesthesia. Anesthesiology 2008;109:571. [DOI] [PubMed] [Google Scholar]
- [7].Siebert JN, Posfay-Barbe KM, Habre W, et al. Influence of anesthesia on immune responses and its effect on vaccination in children: review of evidence. Paediatr Anaesth 2007;17:410–20. [DOI] [PubMed] [Google Scholar]
- [8].Voss LJ, Sleigh JW, Barnard JP, et al. The howling cortex: seizures and general anesthetic drugs. Anesth Analg 2008;107:1689–703. [DOI] [PubMed] [Google Scholar]
- [9].Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth 2005;15:266–74. [DOI] [PubMed] [Google Scholar]
- [10].Perks A, Cheema S, Mohanraj R. Anaesthesia and epilepsy. Br J Anaesth 2012;108:562–71. [DOI] [PubMed] [Google Scholar]
- [11].Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics 2008;121:1281–6. [DOI] [PubMed] [Google Scholar]
- [12].Galicier C, Richet H. A prospective study of postoperative fever in a general surgery department. Infection control 1985;6:487–90. [DOI] [PubMed] [Google Scholar]
- [13].Narayan M, Medinilla SP. Fever in the postoperative patient. Emerg Med Clin North Am 2013;31:1045–58. [DOI] [PubMed] [Google Scholar]
- [14].Sessler DI. Perioperative thermoregulation and heat balance. Lancet 2016;387:2655–64. [DOI] [PubMed] [Google Scholar]
- [15].Negishi C, Lenhardt R. Fever during anaesthesia. Best Pract Res Clin Anaesthesiol 2003;17:499–517. [DOI] [PubMed] [Google Scholar]
- [16].Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet J Rare Dis 2015;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev 2012;15:Cd004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ma SJ, Xiong YQ, Jiang LN, et al. Risk of febrile seizure after measles-mumps-rubella-varicella vaccine: a systematic review and meta-analysis. Vaccine 2015;33:3636–49. [DOI] [PubMed] [Google Scholar]
- [19].Bertolizio G, Astuto M, Ingelmo P. The implications of immunization in the daily practice of pediatric anesthesia. Curr Opin Anaesthesiol 2017;30:368–75. [DOI] [PubMed] [Google Scholar]
- [20].Kanemoto K, Kawasaki J, Yuasa S, et al. Increased frequency of interleukin-1beta-511T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia 2003;44:796–9. [DOI] [PubMed] [Google Scholar]
- [21].Dube C, Vezzani A, Behrens M, et al. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol 2005;57:152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rossaint J, Zarbock A. Perioperative inflammation and its modulation by anesthetics. Anesth Analg 2018;126:1058–67. [DOI] [PubMed] [Google Scholar]
- [23].Short JA, van der Walt JH, Zoanetti DC. Immunization and anesthesia - an international survey. Paediatr Anaesth 2006;16:514–22. [DOI] [PubMed] [Google Scholar]
- [24].Currie J. Vaccination: is it a real problem for anesthesia and surgery? Paediatr Anaesth 2006;16:501–3. [DOI] [PubMed] [Google Scholar]
- [25].Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J 2008;49:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


