The locus coeruleus is the site of the earliest pathological changes in many neurodegenerative diseases. Betts et al. describe how in vivo locus coeruleus imaging can be used as a biomarker for noradrenergic dysfunction in neurodegenerative diseases, and outline a strategy for achieving reliable and biologically validated imaging approaches.
Keywords: locus coeruleus (LC), magnetic resonance imaging (MRI), neurodegeneration, noradrenaline (NA) biomarker
Abstract
Pathological alterations to the locus coeruleus, the major source of noradrenaline in the brain, are histologically evident in early stages of neurodegenerative diseases. Novel MRI approaches now provide an opportunity to quantify structural features of the locus coeruleus in vivo during disease progression. In combination with neuropathological biomarkers, in vivo locus coeruleus imaging could help to understand the contribution of locus coeruleus neurodegeneration to clinical and pathological manifestations in Alzheimer’s disease, atypical neurodegenerative dementias and Parkinson’s disease. Moreover, as the functional sensitivity of the noradrenergic system is likely to change with disease progression, in vivo measures of locus coeruleus integrity could provide new pathophysiological insights into cognitive and behavioural symptoms. Locus coeruleus imaging also holds the promise to stratify patients into clinical trials according to noradrenergic dysfunction. In this article, we present a consensus on how non-invasive in vivo assessment of locus coeruleus integrity can be used for clinical research in neurodegenerative diseases. We outline the next steps for in vivo, post-mortem and clinical studies that can lay the groundwork to evaluate the potential of locus coeruleus imaging as a biomarker for neurodegenerative diseases.
Introduction
The number of individuals over the age of 60 years is projected to rise from 841 million in 2013 to over 2 billion by 2050 (World Population Ageing report, United Nations). As the population has continued to age, age-related neurodegenerative diseases have already reached epidemic proportions. Whilst numerous therapeutic strategies are being investigated, current treatments provide only modest symptomatic relief and do not slow or halt ensuing neurodegeneration. A major priority is to develop early disease stage biomarkers that can improve the understanding of the pathophysiology of neurodegenerative diseases, enable earlier detection of pathology and facilitate the application of timely symptomatic interventions.
The locus coeruleus (LC) is the major source of noradrenaline modulation in the brain and has been shown to be involved in regulating a wide range of higher cognitive functions, such as working memory, learning and attention (Robbins, 1984; Aston-Jones and Cohen, 2005; Benarroch, 2009; Mather et al., 2016), memory consolidation and retrieval (Sterpenich et al., 2006; Sara, 2009), vigilance, arousal/wakefulness, rapid eye movement (REM) sleep behaviour, pain modulation, local blood flow and immunological mechanisms in the brain (Aston-Jones and Bloom, 1981; Benarroch, 2009; Espay et al., 2014; Heneka et al., 2015; O’Donnell et al., 2015). Conversely, age-related decline within the LC–noradrenergic system is associated with reduced cognitive abilities relating to episodic memory (Hämmerer et al., 2018, Jacobs et al., 2018b; Dahl et al., 2019) and reduced cognitive reserve (Robertson, 2013; Wilson et al., 2013; Clewett et al., 2016; Mather and Harley, 2016). In Alzheimer’s disease, tau aggregates are observed first in the LC, prior to their presence in transentorhinal/entorhinal cortex and neocortex (Braak et al., 2011; Stratmann et al., 2016; Andrés-Benito et al., 2017; Ehrenberg et al., 2017). In Parkinson’s disease, intraneuronal α-synuclein burden in the LC precedes and may be of even greater magnitude than that in the dopaminergic substantia nigra pars compacta (SNpc) (Braak et al., 2003; Dickson et al., 2008). As a result, the degree of Parkinson’s disease-related neuronal loss is likely just as severe in the LC as in the SNpc (Zarow et al., 2003; Giguère et al., 2018). Pathological changes in the LC have also been reported in other neurodegenerative and neuropsychiatric conditions (Box 1), leading to the notion that the LC may be a vulnerable target for pathology (Sharma et al., 2010). Whether this is related to LC neurons’ high metabolic need in order to maintain essential physiological functions or the close proximity of the LC to the fourth ventricle, exposing it to toxins from the CSF (Mather and Harley, 2016; Weinshenker, 2018), remains to be determined.
Box 1 Role of the LC in neurological disorders
Structural and functional changes in the human LC leading to deficits in noradrenaline may contribute to the pathophysiology and symptomatology of several neurological disorders:
Alzheimer’s disease
Deposition of tau in and degeneration of the LC occurs early in the asymptomatic phase of Alzheimer’s disease (Braak and Del Tredici, 2012; Arendt et al., 2015) suggesting that the LC is the initial site of pathology. Accumulation of tau (doubling from Braak stage 0 to 1; Ehrenberg et al., 2017) and significant volume loss precede neuronal loss in the LC during Alzheimer’s disease progression (Theofilas et al., 2017). Depletion of up to 30% of LC neurons has been reported in prodromal (mild cognitive impairment stage) Alzheimer’s disease, increasing to 55% with diagnosed dementia (Kelly et al., 2017). Neuronal loss is particularly prevalent in the rostral/middle portion of the LC (German et al., 1992; Theofilas et al., 2017), correlating with cognitive dysfunction including memory, perceptual speed, and visuo-spatial ability (Kelly et al., 2017), and with reduced noradrenaline levels in hippocampus and cortex (Lyness, 2003). Compensatory activity, such as increased metabolism in surviving neurons or reorganization of functional networks, can occur in early disease stages (Hoogendijk et al., 1999; Jacobs et al., 2015).
Synucleinopathies
In Parkinson’s disease and dementia with Lewy bodies, α-synuclein containing Lewy bodies and neuronal cell loss are seen along the entire length of the LC (German et al., 1992; Theofilas et al., 2017). Intraneuronal Lewy bodies also affect tyrosine hydroxylase (TH) activity, potentially interfering with normal catecholamine biosynthesis (Tabrez et al., 2012), which may distinguish the effects of Parkinson’s disease/dementia with Lewy bodies from Alzheimer’s disease (McMillan et al., 2011). It has also been postulated that LC burden precedes substantia nigra involvement in Parkinson’s disease (Zarow et al., 2003; Braak et al., 2004; Seidel et al., 2015), rendering the LC a good candidate for preclinical diagnosis (Liu et al., 2017). However, the field lacks a comprehensive analysis detailing the stages of neuronal loss in Parkinson’s disease.
Multiple system atrophy (MSA) is another heterogeneous α-synucleinopathy, in which autonomic dysfunction, parkinsonism, and ataxia (Stankovic et al., 2014; Walsh et al., 2018) are associated with severe neuronal loss in the LC and noradrenergic cardiorespiratory brainstem nuclei (A5, A1) (Benarroch et al., 2008). An in vivo neuromelanin-sensitive MRI study has shown that LC contrast is reduced in MSA compared to healthy controls and that the ratio of neuromelanin-sensitive MRI contrast in substantia nigra versus LC could help distinguish MSA from Parkinson’s disease (Matsuura et al., 2013).
Patients with idiopathic rapid eye movement sleep disorder (iRBD) have a strongly increased risk of developing Parkinson’s disease, dementia with Lewy bodies, MSA and mild cognitive impairment; 75.7% and 90.9% develop a neurodegenerative syndrome 10 and 14 years from the time of iRBD diagnosis, respectively. Interestingly, iRBD is associated with dysfunction of the LC-noradrenergic system (Knudsen et al., 2018) suggesting that LC imaging might serve as a valuable biomarker in patients at risk of developing neurodegenerative disease.
Chronic traumatic encephalopathy
In addition to early pathological affection of cortical sulci, substantial LC damage and tau accumulation has also been reported in early disease stages (Stein et al., 2014). LC pathology may also exacerbate clinical presentation of cognitive complaints and mood disturbances in chronic traumatic encephalopathy (Stern and Daneshvar, 2013).
Frontotemporal lobar degeneration
Modest LC neuronal loss occurs in both tau- and non-tau- forms of frontotemporal lobar degeneration (Brunnström et al., 2011; Eser et al., 2018), both in early and late disease stages (Irwin et al., 2016). Clinically, the loss and dysregulation of noradrenaline from LC degeneration may contribute to cognitive decline, including impulsivity and apathy (Passamonti et al., 2018).
Essential tremor
A neuropathological study (n = 33) revealed Lewy body pathology in the LC in 25% of the cases with essential tremor (Louis et al., 2007). Noradrenergic LC-cerebellar connections are important for the normal function of Purkinje cells and their inhibitory output (Moises et al., 1981). Degeneration and ensuing LC dysfunction may modulate Purkinje cell activity and contribute to decreased cerebellar inhibition in essential tremor (Louis et al., 2007).
Non-degenerative, neuropsychiatric illnesses
LC dysfunction, also without neuronal loss, has also been implicated in post-traumatic stress disorder (Bernard et al., 2011; Pietrzak et al., 2013), addiction (Bernard et al., 2011), depression (Berridge and Waterhouse, 2003), suicidal behaviour (Roy et al., 2017) and chronic pain (Llorca-Torralba et al., 2016; Taylor and Westlund, 2017).
With the notable exception of most of the basal ganglia, the LC projects to large portions of subcortical and cortical areas (Berridge and Waterhouse, 2003). Moreover, reminiscent of locally specific connectivity in dopaminergic nuclei (Haber and Knutson, 2010), tracing studies in the rat suggest that there might be distinct projections from rostrolateral LC to hippocampus, amygdala and septum (Van Bockstaele et al., 2006) and from caudal aspects of the LC to the spinal cord (Ennis et al., 1991). This may indicate a stronger involvement of rostrolateral LC in modulating memory encoding and caudal LC in modulating pain perception.
While the LC is among the first brain structures to demonstrate pathology in neurodegenerative diseases, it currently remains unclear how alterations in LC structure and function influence pathogenesis and symptom progression. In human studies, neuropsychiatric symptoms associated with LC activity, such as sleep dysfunction, agitation, anxiety, appetite dysfunction and depression, appear as elements of the early clinical phenotypes of neurodegenerative diseases (Assal and Cummings, 2002; Lanctôt et al., 2017; Ehrenberg et al., 2018). In animal models, lesions of the LC have been shown to exacerbate tau and amyloid-β plaque deposition, leading to depletion of noradrenaline in LC target regions and impaired cognitive function (Heneka, 2006; Kalinin et al., 2006, 2012; Jardanhazi-Kurutz et al., 2010; Chalermpalanupap et al., 2017; Rorabaugh et al., 2017). Whether the influence of LC degeneration on pathology is directly related to a dysregulation of noradrenaline, or indirectly related via neuroinflammatory mechanisms or alterations in additional LC neuromodulators, such as brain-derived neurotrophic factor or galanin, requires further investigation (Chalermpalanupap et al., 2017; Betts et al., 2018). Interestingly, there is also evidence of noradrenergic hyperactivity in early disease (as reviewed by Weinshenker et al., 2018), consistent with a number of reports of elevated levels of CSF noradrenaline and/or noradrenaline turnover in Alzheimer’s disease (Palmer et al., 1987; Hoogendijk et al., 1999). Thus, hyperactivity in a structurally impaired LC might further accelerate the propagation of neuropathology in neurodegenerative disease via noradrenergic projections (Weinshenker, 2018). As such, early detection of LC decline using in vivo imaging techniques might contribute to earlier diagnosis, and support personalized pharmacological interventions to alleviate compensatory hyperactivity of the noradrenaline system and potentially slow down disease progression.
Recent advances in non-invasive neuroimaging techniques now permit the in vivo assessment of the LC using MRI (Sasaki et al., 2006; Keren et al., 2009; Clewett et al., 2016; Betts et al., 2017; Priovoulos et al., 2018) opening the possibility to track LC changes as a biomarker for noradrenergic dysfunction. The utility of this approach for clinical research will depend on whether LC imaging in combination with established biomarkers, can help to better stage neurodegenerative diseases and characterize biomarker-positive individuals before the onset of dementia. LC imaging could also have important implications in clinical trials as a stratification tool for predicting treatment success of pharmacological intervention studies targeting the noradrenergic system. This article reports on the consensus reached at the first European Locus Coeruleus Imaging meeting held in Magdeburg in 2018. It describes the challenges for establishing LC MRI measures as a biomarker for diagnosis and targeting therapeutic interventions in neurodegeneration and outlines a strategy for obtaining reliable, biologically validated and clinically suitable imaging approaches.
As discovered over a decade ago (Sasaki et al., 2006), the LC is visualized using MRI scanning protocols that are sensitive to a paramagnetic compound called neuromelanin, which accumulates in noradrenergic neurons, and may be taken as an indicator of LC integrity (i.e. cell density). While neuromelanin-sensitive MRI is a relatively new technique, a number of studies have shown that MRI can detect signal differences in the LC between healthy controls and diseases with known LC involvement (e.g. in major depression, Parkinson’s disease and Alzheimer’s disease as recently reviewed by Liu et al., 2017). Given, however, the substantial interindividual differences in LC measures across healthy older adults (Liu et al., 2017, 2019), further studies are required to establish whether LC degeneration can be reliably detected in vivo (Liu et al., 2017; Sulzer et al., 2018). Furthermore, to clarify whether interindividual variability in LC integrity is related to varying levels of neuropathologies (e.g. tau, amyloid or α-synuclein), studies using LC MRI in combination with in vivo measurements of pathology (e.g. PET and CSF biomarkers) in cognitively normal older adults and patients are required. While these studies are currently underway, first results in ageing and early-stage Alzheimer’s disease indicate that LC MRI contrast is indeed modulated by both tau and amyloid pathology. Recent evidence demonstrating that LC MRI contrast is associated with CSF amyloid (amloid-β42/40) in early-stage Alzheimer’s disease (Betts et al., 2019) and tau pathology at higher levels of amyloid burden using tau and amyloid PET, respectively (Jacobs et al., 2018a), may partially explain the interindividual variability in LC integrity observed in old age. Interestingly, post-mortem studies using 18F-AV-1451 tau PET have also shown off-target binding properties to neuromelanin in the substantia nigra (Marquié et al., 2015; Hansen et al., 2016). Thus 18F-AV-1451 may also be capable of imaging neurodegeneration. However, such changes may be difficult to interpret because of the mixed PET signal generated by on-target binding to tau tangles and off-target binding to neuromelanin, which would likely also be age-dependent.
Practical considerations in in vivo locus coeruleus imaging
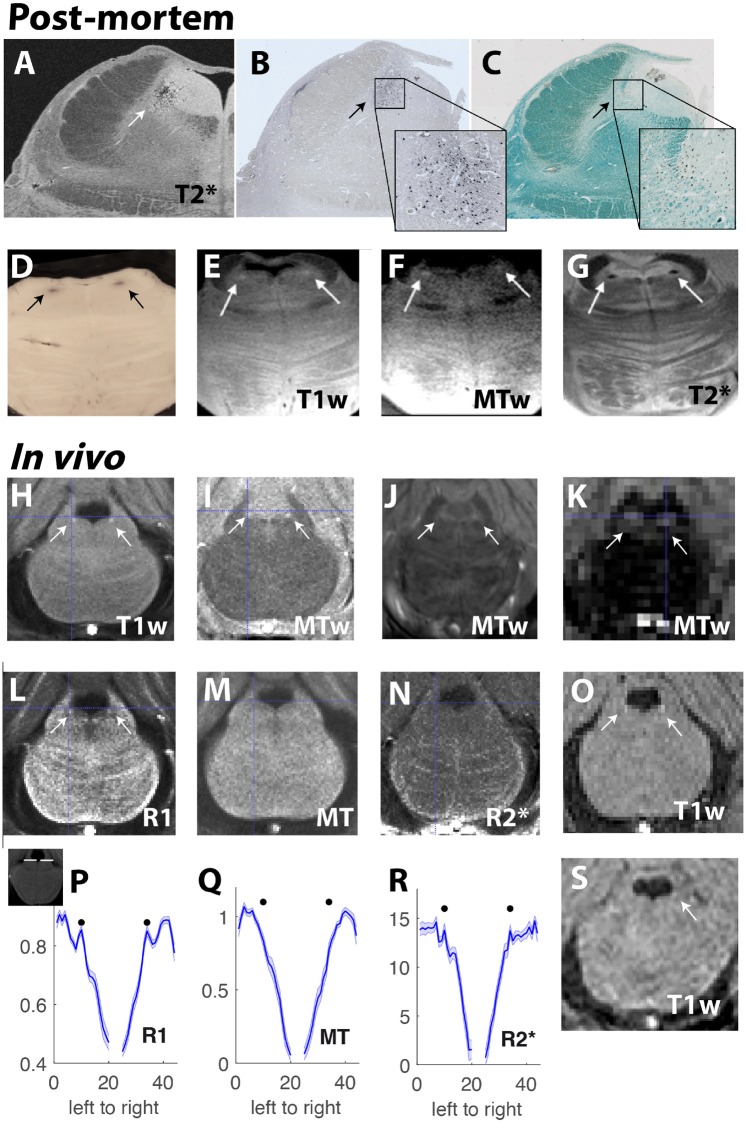
The intrinsic neuromelanin-driven contrast that allows us to visualize the LC using MRI was first identified in vivo as hyperintensity on 2D T1-weighted turbo spin echo (TSE) images (Sasaki et al., 2006). These hyperintensities correspond closely with areas of higher concentration of neuromelanin (Keren et al., 2015), suggesting that the high signal intensities in the LC are driven by neuromelanin. In support of this, a recent study has shown that the neuromelanin concentration in the substantia nigra is linearly related to neuromelanin-MRI contrast and resting blood flow in the substantia nigra (Cassidy et al., 2019). The observation that LC MRI acquisitions are subject to incidental magnetization transfer (MT) effects (Dixon et al., 1990) and that dedicated MT preparation increases neuromelanin-related contrast, have led several authors to credit MT as the primary source of LC contrast in MRI (Nakane et al., 2008; Priovoulos et al., 2018). Recent findings suggest that an interaction of (higher) intracellular water content with paramagnetic ions (such as neuromelanin) may set the LC apart from its surroundings in MT-weighted imaging (Watanabe et al., 2019). Moreover, paramagnetic ions in addition to neuromelanin may contribute to this effect as structures low in neuromelanin (e.g. periaqueductal grey matter) demonstrate similar MRI contrast to the LC using MT-weighted imaging (Cassidy et al., 2019; Watanabe et al., 2019). Despite the different approaches that have successfully visualized the LC (Fig. 1), a precise understanding of the underlying contrast mechanism is thus still lacking. As outlined in more detail below, a combination of histological and post-mortem imaging studies would provide important insights in this regard. Mechanisms involving both neuromelanin concentration and macromolecules in the LC and surrounding tissues may be active simultaneously, and a combination of mechanisms may be required to explain the contrast patterns seen in normal ageing and in pathology. A relevant aspect for imaging neuromelanin is that it is known to scavenge metals both across the lifespan and in disease states (Zecca et al., 2004; Biesemeier et al., 2016). In vitro studies, albeit with synthetic melanin, suggest that T1 shortening in the LC may be driven by compounds of neuromelanin and chelated metals, such as iron and copper (Enochs et al., 1989, 1997; Trujillo et al., 2017) impacting the macromolecule-bound pool, rather than neuromelanin or iron alone (Langley et al., 2015; Trujillo et al., 2017). It has therefore been proposed that complexes of neuromelanin-bound paramagnetic ions are the primary drivers of LC contrast (Enochs et al., 1989; Nakane et al., 2008; Trujillo et al., 2017); however, neuromelanin itself, even free of metals, is paramagnetic (Shima et al., 1997). Indeed, at least in vivo, LC contrast does not seem to benefit from typical iron-sensitive contrasts, such as quantitative susceptibility mapping (QSM) or apparent R2* (Acosta-Cabronero et al., 2016; Betts et al., 2016, respectively) (Fig. 1N and R).
Figure 1.
Overview of LC visibility using post-mortem and in vivo MRI. The LC can be imaged in post-mortem tissue using numerous MRI protocols [arrows indicate the LC, evident as dark spots in T2* (A and G) and bright spots in T1-weighted (E) and MT-weighted (F) scans] in addition to using histological techniques. (D) The LC is visible as dark spots in a post-mortem slice without any staining, due to neuromelanin deposits, which are thought to contribute to magnetic resonance visibility of the LC. (C) Myelination in the LC area. The LC and central pontine grey show very low myelination, but are surrounded by areas with very high and intermediate myelination (green areas), possibly also contributing to MR visibility of the LC. To image the LC in vivo, T1-weighted (H, O and S) and MT-weighted (I–K) MRI protocols can be used (arrows indicate the LC, evident as bright spots in T1-weighted and MT-weighted scans). Using these protocols, a decline in LC integrity in Alzheimer’s disease dementia (S) compared to healthy elderly adults (O) can be identified (Betts et al., 2019). To fine-tune these scan protocols further, it is necessary to understand the magnetic resonance contrast mechanisms that underlie LC visibility. (L–N and P–R) Quantitative maps which isolate different magnetic resonance contrast effects (R1, MT and R2* effects), show that LC visibility in T1-weighted as well as MT-weighted scans in vivo is mostly due to R1 effects (mean LC visibility across 22 healthy older adults extracted from line regions of interest; see inset on right for position of line regions of interest. Black dots indicate position of maximal signal intensity based on T1-weighted maps. Peaks in signal intensity are apparent in R1 and to some extent in R2* maps (Hämmerer et al., 2018a)]. Sequence details/ stains: (A) 7 T T2*-weighted (50 μm resolution) FLASH MRI image TE = 19 ms; (B) TH staining for LC neurons (dark); (C) Luxol fast blue staining for myelinated fibres in same slice (green); (D) Block face image after celloidin embedding (LC neurons dark); (E) 7 T T1-weighted (0.2 × 0.2 × 2 mm) TSE image (TE/TR/TI = 11/3000/825 ms); (F) 7 T MT-weighted FLASH MRI image (TE/TR = 5.1/26 ms); (G) 7 T T2*-weighted FLASH image (TE/TR = 21/30 ms) (Otaduy et al., unpublished results); (H) 3 T T1-weighted (0.4 × 0.4 × 3 mm) FLASH image (TE/TR = 3.35–16.95/27) averaged across six repetitions; (I) 3 T MT-weighted (0.4 × 0.4 × 3 mm) FLASH image (TE/TR = 3.35–16.95/30.74); (J) 7 T MT-TFL image (0.4 × 0.4 × 0.5 mm); (K) 3 T MT-weighted (1.5 mm3) SPGR image (TE/TR = 5 ms/30 ms; (O and S) 3 T T1-weighted (0.75 mm3) FLASH image (TE/TR = 5.56 ms/20 ms). Image in J is reproduced with permission from Priovoulos et al. (2018); O is reproduced with permission from Betts et al. (2017). TE/TR/TI = echo time/repetition time/inversion time.
More recently, MT preparation and/or T1-weighting have been combined, both with short repetition time spoiled gradient echo and turbo-flash readouts, to increase spatial resolution and/or shorten the acquisition time compared to TSE acquisitions (Chen et al., 2014; Betts et al., 2017; Hämmerer et al., 2018; Priovoulos et al., 2018). The small size of the LC has motivated the use of high spatial resolution, at least in-plane, while high isotropic resolution minimizes partial volume effects (i.e. inclusion of non-LC tissue within a putative LC voxel). However, this comes at the cost of relatively low signal- and contrast-to-noise ratio (SNR and CNR, respectively) due to smaller voxel sizes and higher vulnerability to subject motion, which has prompted averaging over multiple repetitions. Anisotropic voxels, which mimic the shape and orientation of the LC, have capitalized on the elongated shape of the LC to increase SNR and shorten scan times. Given the tilted and not perfectly cylindrical nature of the LC, this strategy is prone to errors at the most rostral and caudal ends of the structure leading to biases in segmentation and volumetric measurements (Liu et al., 2017). Alternatively, isotropic acquisitions that capture the entire rostrocaudal length of the LC have also been proposed (Betts et al., 2017; Priovoulos et al., 2018) and may be more consistent with ex vivo measurements compared to anisotropic scans (Liu et al., 2017). While the increased SNR with ultra-high field MRI (i.e. ≥ 7.0 T) offers higher attainable spatial resolution and/or shorter acquisition times, increased specific absorption rates means that higher power acquisitions, e.g. those using MT preparation pulses or high flip angles, might not be readily available thereby necessitating further optimization (Priovoulos et al., 2018) (see also Box 2 for recommendations on how to use currently available sequences).
Box 2 Practical suggestions for in vivo LC imaging
Sequences
The LC is most visible using T1- or MT-weighted structural sequences and can be well characterized using 3 T and 7 T MRI (see Betts et al., 2017; Priovoulos et al., 2018 and Liu et al., 2017 for a recent review). To date, there is no single ‘best’ sequence for LC imaging: there is a trade-off between non-quantitative protocols offering easier LC localization, and quantitative magnetic resonance acquisitions that provide more objective biophysical measures of brain tissue but with lower localization ability compared to dedicated LC sequences.
Voxel size
Owing to the small size of the LC (∼15 mm in length and 1–3 mm in diameter), larger voxel sizes should be avoided as they result in weaker signals owing to partial volume effects with tissue outside the LC within the voxel. On the other hand, voxel volumes lower than 0.4–0.5 mm3 run the risk of lacking sufficient signal-to-noise ratio, given the current sensitivity of neuromelanin-sensitive magnetic resoance sequences. If more accurate LC volume assessments (with less partial volume contamination) are desired, isotropic voxels sizes are preferable [e.g. 0.75 mm × 0.75 mm × 0.75 mm are possible at 3 T (Betts et al., 2017) and 0.4 mm × 0.4 mm × 0.5 mm at 7 T (Priovoulos et al., 2018)]. These, however, can make identification of individual LCs harder because of lower signal-to-noise ratio, requiring a group template approach (see below). If reliable identification of individual LCs is desired but accurate volume assessments are less critical, anisotropic voxel sizes (e.g. 0.5 mm × 0.5 mm in plane resolution × 2 mm slice thickness) can be used to exploit the quasi-cylindrical shape of the LC and maximize signal-to-noise ratio at the cost of greater partial volume errors in peripheral LC voxels (Liu et al., 2017).
Acquisition
It is recommended that the field-of-view is aligned perpendicular to the dorsal edge of the pons such that cylindrical voxels align with the LC.
Motion artefacts
Great care should be taken to reduce head motion during the MRI acquisition. A thin pillow placed on the base of the coil to ensure tight fixation and patient comfort can help minimize motion and achieve high measurement precision e.g. improving coregistration across/within individuals e.g. between structural and functional data. Shorter acquisition times which are less prone to motion artefacts can be achieved by using a reduced field-of-view comprising the brainstem only and by relying on offline-averaging of scans (Chen et al., 2014). Typical scan durations for smaller field-of-view acquisitions take approximately half the time of whole-brain acquisitions and range between 5 and 9 min at 3 T and 7 T MRI, respectively (Liu et al., 2017; Priovoulos et al., 2018). However, template-based segmentation approaches (see below) are more challenging with reduced field-of-view acquisitions than with whole brain scans (but see Dahl et al., 2019).
Post-processing
Semi-automated LC segmentation can be performed through highly iterative co-registration resulting in a high definition group-average template where the LC can be reliably identified. The resultant template LC region of interest can then be spatially transformed to each individual’s native space (Betts et al., 2017; Liu et al., 2019). When using template-based approaches however, care must be taken to minimize the impact of coregistration inaccuracies and additional user intervention might be required. There are at present differing views on whether LC segmentation should be performed manually or automatically, and on whether LC measurements should encompass the whole structure or favour partial extraction to avoid potential contamination. Chen et al. (2014) argued that automated segmentation approaches might be less error-prone and may thus lead to higher consistency across scans and studies, and that visually locating the LC as the highest-intensity voxel at either side of the fourth ventricle (on the axial plane) might be susceptible to noise-related errors. In contrast, Keren et al. (2009) argued that assessing single voxels might be more reliable in that it does not rely on boundary definitions or signal intensity thresholding, thereby leading to improved sensitivity to overall LC differences. To date, however, no study has systematically compared manual and automated methods.
Reference normalization
An important limitation of MRI is that it produces arbitrarily scaled greyscale images that are suboptimal for intersubject comparisons because normalization to a proximal control region (often the pontine tegmentum) becomes necessary (Betts et al., 2017; Liu et al., 2017; Hämmerer et al., 2018). However, the reference tissue may also change throughout the lifespan, or be abnormal in neurodegenerative diseases (Keren et al., 2009; Clewett et al., 2016). Furthermore, asymmetric signal-intensity biases in the LC and surrounding tissue have been reported both in healthy subjects and Parkinson’s disease patients (Keren et al., 2009; Garcia-Lorenzo et al., 2013; Betts et al., 2017; Tona et al., 2017), in apparent contradiction with post-mortem studies that show largely symmetric distributions of neuromelanin-pigmented cells in the LC (German et al., 1988; Baker et al., 1989; Chan-Palay and Asan, 1989; Ohm et al., 1997). It is therefore imperative to avoid instrumental biases such as transmit and receive field inhomogeneities, and ensure asymmetries are not introduced by the methodology. To this end, quantitative (parametric) MRI (Weiskopf et al., 2013) has shown promise by quantifying specific tissue properties in terms of physical units and may now be used for quantitative LC imaging (Fig. 1L–N and P–R) (Hämmerer et al., 2018a). Moving forward, one may speculate that multi-parametric mapping might be preferred to single-contrast assessments for a more complete view of LC status and greater insight into underlying contrast mechanisms, especially if performed within the population-specific time constraints.
Age comparisons and control groups
Owing to an age-related increase in neuromelanin content in LC cells as well as LC signal intensity using neuromelanin-sensitive MRI, LC imaging may be suboptimal in younger adults, likely due to reduced neuromelanin levels compared to older adults (Zecca et al., 2004, 2006; Liu et al., 2019). For the same reason, interpreting age differences in LC MRI contrast between young and older adults as age differences in LC integrity may be problematic. In a large cross-sectional dataset, a decline in LC integrity was only observed at ∼60 years of age and over (Liu et al., 2019). At present it is unclear if neuromelanin-sensitive MRI can detect a reduction in LC integrity below this age range. Moreover, within an ageing sample, control groups must be tightly matched for age to the clinical population under investigation.
Validating in vivo locus coeruleus imaging using post-mortem MRI and histology
A key aim of conducting LC imaging in clinical groups is to capture in vivo disease-related physiological and structural changes such as reductions in neuronal density. Several validation strategies, including a combination of post-mortem magnetic resonance microscopy and histological labelling (Forstmann et al., 2017), have thus been proposed to shed new light into the underlying biological processes driving in vivo imaging results. Such approaches, however, are also technically challenging. Tissue fixation and embedding media can affect the properties of post-mortem tissue and strongly influence the MRI signal (Dusek et al., 2019). Thus, the same tissue properties might be expressed differently in post-mortem and in vivo MRI. For instance, tissue fixation causes strong T1 and T2 shortening that is additionally dependent on the choice of fixative agent and fixation time (Birkl et al., 2016, 2018). Contrast changes also arise if the temperature used for post-mortem scanning differs from 37°C (Birkl et al., 2014). Differences between in vivo and post-mortem tissue could also be driven by changes in metal oxidation states during fixation or by redistribution of iron and other metals across macromolecules (e.g. proteins, neuromelanin, etc.) (Shima et al., 1997; Krebs et al., 2014). It should be considered that iron is bound in brain tissue in different molecular forms such as ferritin, neuromelanin, hemosiderin and others, each having different physico-chemical properties (Ward et al., 2014). Thus, LC contrast observed in vivo using neuromelanin-sensitive MRI at clinical field strength cannot be easily replicated with post-mortem magnetic resonance microscopy at lower temperature (T ≤ 21°C) and ultra-high magnetic field strengths (≥7.0 T). In particular, T2*/R2* measurements of the LC return markedly different values in vivo and post-mortem tissue. Whil they often afford the best visibility in post-mortem tissue (Fig. 1A and G), T2* effects are not evident in individual in vivo samples and are comparatively weaker than R1 effects in group analyses (Fig.1L–N and P–R). However, by adjusting acquisition parameters, it is still possible to observe the characteristic T1- and MT-weighted hyperintensity of the LC in in vivo (Fig. 1H–K, O and S) and ex vivo MRI (Fig. 1E and F). To address comparability issues, future post-mortem MRI studies focusing on the LC could combine quantitative T1, T2*, MT and QSM with tight control of the post-mortem fixation time and iron and metal concentrations in the fixation solution in order to relate magnetic resonance signal intensities to stereological and quantitative histological measures (e.g. myelin, lipids, neurons, iron, neuromelanin, copper, and iron- and copper-containing proteins).
Using a combination of histology and post-mortem MRI in the LC, a recent study explored whether neuromelanin-sensitive MRI contrast could serve as a proxy measure of LC ‘integrity’ (i.e. neuronal density in the LC). The authors showed that areas of LC hyperintensity in T1-weighted MRI were co-localized with neuromelanin-rich neurons in the LC region (Keren et al., 2015), suggesting that LC visibility in MRI may indeed be driven by the neuromelanin content of noradrenergic neurons. Similarly, studies using neuromelanin-sensitive MRI could show that age-related cross-sectional patterns in in vivo signal intensity across the lifespan (Shibata et al., 2006; Jacobs et al., 2018a; Liu et al., 2019) replicate the inverted U-shaped cross-sectional development of neuromelanin deposits in the LC observed in post-mortem tissue (Mann and Yates, 1974, but see Ohm et al., 1997; Zecca et al., 2004 for studies reporting a stable level of neuromelanin-containing neurons or linear increase of neuromelanin content in LC tissue across the lifespan, respectively). This inverted U-shaped pattern has been suggested to reflect increasing neuromelanin content due to continuous noradrenaline production during adulthood prior to ensuing cell death and clearance of released intracellular neuromelanin with the advent of age-related neurodegeneration and onset of disease (Mann and Yates, 1974). Indeed, post-mortem data suggest that neuromelanin may be a more suitable indicator of neuronal density in older adults (at least > 55 years) as younger adults’ LC neurons may not yet be sufficiently pigmented with neuromelanin to allow inference on cell numbers in neuromelanin-sensitive MRI (Manaye et al., 1995; Liu et al., 2019). Nonetheless, further studies are warranted to investigate whether higher signal intensities in older adults invariably represent more intact LC neurons in the LC, and whether additional mediating factors for interpreting LC-related MRI signals must be considered in different age groups or clinical populations. For instance, pathologically altered proteins, such as hyperphosphorylated or aggregated tau, which are well known markers of ageing and neurodegenerative disease as well as non-pathological proteins and lipids, are additional important constituents of neuromelanin pigments (Engelen et al., 2012; Zucca et al., 2018) that accumulate in specific organelles in the LC as occurs in substantia nigra (Zecca et al., 2004; Braak et al., 2011; Zucca et al., 2017). However, the effect of these constituents on LC MRI contrast has not yet been systematically explored.
Outlook and conclusion
A major goal of in vivo LC imaging in clinical research is to aid differential diagnosis, disease monitoring and stratification to novel treatments. As such, LC imaging has the potential to become a component of a precision medicine approach to neurodegenerative diseases. As brain function adapts to ensuing pathology, such as receptor increases in target areas of noradrenergic LC projections or increased connectivity following a decline in LC function (Herrmann et al., 2004; Ye et al., 2015), LC imaging could provide a stratification tool for predicting treatment success of pharmacological intervention studies targeting the noradrenergic system (Ye et al., 2015, see also Fig. 2). Once its relationship to pathogenic changes (e.g. α-synuclein or tau aggregation) has been determined, it could also support differential diagnosis and be added to the portfolio of longitudinal monitoring staging tools for neurodegenerative diseases. Over the next years, we expect substantial progress towards these goals through a multidisciplinary collaboration of experts in neurodegenerative diseases, imaging, neuropathology and cognition.
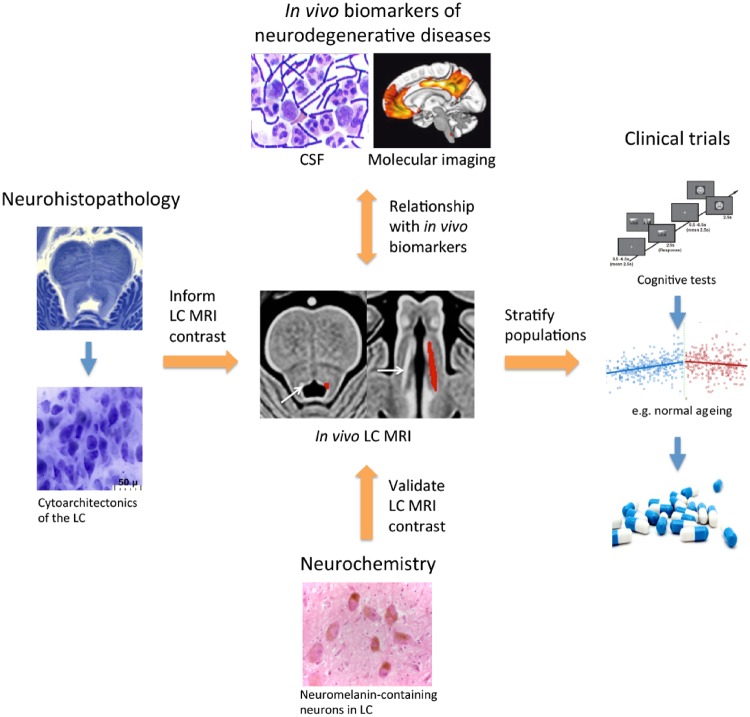
Figure 2.
Establishing LC imaging as a biomarker for noradrenergic dysfunction. LC imaging offers potential as a disease monitoring and stratification tool to predict the success of novel pharmacological ligands in clinical trials. To achieve this aim, it will be essential to validate in vivo LC MRI contrast with respect to the concentration of neuromelanin and density of noradrenergic neurons in post-mortem tissue. This will be important for developing a deeper understanding into how changes in LC MRI contrast both in vivo and in post-mortem tissue correlate with neuromelanin-bound metals in noradrenergic neurons and is influenced by the neuropathological characteristics of neurodegenerative diseases. In the clinic, longitudinal LC imaging may be combined with biomarkers (e.g. in CSF or molecular imaging) to assess how changes in LC MRI contrast may drive clinical and pathological manifestations of neurodegenerative diseases. Source of CSF image: Wikimedia Commons; molecular imaging figure is adapted from Palmqvist et al. (2017); LC integrity image (middle) adapted with permission from Betts et al. (2017); age effects on LC integrity image (right) reproduced with permission from Liu et al. (2019).
As neuromelanin accumulates in the cell bodies of LC neurons, neuromelanin-sensitive MRI can only inform on changes in cell density but not changes in synaptic density or cell activity. It is known from post-mortem studies that cell numbers in the LC decline with advancing Alzheimer’s disease pathology (Theofilas et al., 2017). However, prior to cell loss, changes in synaptic density in the LC and/or neuronal activity should be considered. Post-mortem and lesion studies suggest that noradrenaline production in remaining LC neurons may increase following degeneration of LC neurons (for a review see Weinshenker et al., 2018). At present, it is not known whether synaptic density is altered prior to or in tandem with cell loss in the LC. It would be interesting to use novel PET tracers of synaptic density (Finnema et al., 2016) in combination with LC imaging to assess how differences in LC synapse density relate to LC MRI contrast in neurodegenerative diseases. Direct and indirect functional measures of LC activity or noradrenaline release can be assessed using high resolution functional MRI, pupillometry, PET measures of noradrenergic transporter levels (Sommerauer et al., 2018), or by assessing noradrenaline levels in CSF. These measures, however, are not short of caveats. PET and functional MRI measures provide noisy assessments given the small size of the LC. Moreover, the LC is also know to release dopamine and GABA (Kempadoo et al., 2016; Takeuchi et al., 2016; Breton-Provencher and Sur, 2019), thus dopamine and GABA levels may also need to be assessed in conjunction with noradrenaline levels when characterizing LC function. Finally, it can also be difficult interpreting functional activation in the LC as an indicator of neuronal capacity since activation will not only depend on the number of neurons or synaptic density, but also on the degree in which the LC is engaged during the processing of the task at hand [i.e. the strength and the nature of the experimental manipulation, e.g. as suggested previously by Hämmerer et al. (2018)]. Nonetheless, synaptic density and functional activation may be superior indicators of cognitive impairment than cell pathology (Terry et al., 1991). As the specificity of functional and experimental manipulations for the LC increases, these measures may contribute valuable additional data on the role of the LC in neurodegenerative diseases.
One of the main tasks ahead for LC research lies in setting up carefully designed longitudinal studies in healthy ageing and disease cohorts with deep cognitive and physiological phenotyping (e.g. the DELCODE study; Jessen et al., 2018) to assess how LC integrity is related to cognitive symptoms and functional brain changes at the earliest stages of neurodegeneration. Previous work assessing the reproducibility of LC imaging by quantifying the stability of LC contrast across two or more independent scan sessions has revealed moderate to high reproducibility (Langley et al., 2017; Tona et al., 2017; Betts et al., 2017; Dahl et al., 2019). Therefore to achieve reliable longitudinal testing in the future will require an improvement in the reproducibility and reliability of LC imaging techniques. To assess how the LC modulates the function of distributed brain networks at rest and during cognitive tasks, will require further optimization of existing functional MRI sequences to overcome the challenges of brainstem imaging (Düzel et al., 2015) and inclusion of additional physiological information (see Liu et al., 2017 for recommendations). In addition, experimental tasks that elicit robust LC signals require development (Mather et al., 2016; Liu et al., 2017; Clewett et al., 2018).
Second, future studies should test whether LC imaging is related to disease progression in neurodegenerative diseases. This will require characterizing the relationship between LC MRI measures and neuropathology (e.g. with respect to amyloid, tau or alpha-synuclein using CSF biomarkers or PET) but also noradrenergic function using noradrenergic PET-tracers including those to assess noradrenergic transporter density (Sommerauer et al., 2018). However, advances in analysis (to maximize spatial resolution) and exclusion of potential off-target binding sites (Lee et al., 2018) must accompany ligand development. For optimizing and validating LC MRI further, greater synergy between in vivo, post-mortem, in vitro and clinical studies is required for a deeper understanding into how LC MRI contrast relates to the pathophysiology of different neurodegenerative diseases (Fig. 2). Such studies will also benefit from using clinically informed test beds, i.e. populations, that show a clear difference in relevant tissue properties, e.g. between young and older adults but also in neurodegenerative diseases, such as Alzheimer’s disease and beyond (Box 1).
Finally, a growing body of knowledge on similarities and differences between neuromelanin deposits in noradrenergic cells in the LC and dopaminergic cells in the substantia nigra (Zecca et al., 2004; Zucca et al., 2006; Wakamatsu et al., 2015) open further avenues for using in vivo neuromelanin-sensitive MRI to also investigate the involvement of the substantia nigra in tandem with the LC in a wide range of clinical conditions, i.e. in diseases also affecting the dopaminergic system, such as Parkinson’s disease (Sulzer et al., 2018).
In conclusion, there is rapid progress towards achieving more sensitive and high-resolution in vivo imaging of the LC. The methods are non-invasive and fast enough to be well tolerated using high (3 T) or ultra-high (≥7 T) MRI, and can have great potential to inform the effectiveness of psychopharmacological probes, therapeutic trials, and physiological monitoring in clinical populations. We anticipate rapid growth in the evidence base for developing LC imaging as a biomarker in neurodegenerative diseases.
Funding
M.B. and D.H. were supported by the Human Brain Project (SP3 WP 3.3.1) and CRC 779 (Project A7). E.D. and D.H. are also supported by the MRC MR/P012698/1. L.Z. and F.A.Z. were supported by the Italian Ministry of Education, University,and Research (MIUR) - National Research Programme (PNR) - National Research Council of Italy (CNR) Flagship “InterOmics” Project (PB.P05), by MIUR - PNR - CNR Aging program 2012-2014. L.Z. was also supported by the Grigioni Foundation for Parkinson's Disease (Milan, Italy). C.L. is supported by the MRC (MR/R006504/1) and L.P. is supported by the MRC (MR/P01271X/1). J.R. is supported by the MRC, NIHR, Wellcome Trust (103838), McDonnell Foundation, AZ-Medimmune and Janssen. M.M. is supported by the German Research Foundation (DFG) priority program PP 2041 (MO 2249/3-1) and the Alzheimer-Forschung-Inititiative e.V., (AFI # 18072). H.J. is supported by a NWO VENI grant [451-14-035], a standard grant of Alzheimer Nederland [#15007] and by European Union's Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie Grant agreement [IF-2015-GF, 706714]. N.W. received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n° 616905, and from the European Union's Horizon 2020 research and innovation programme under the grant agreement No 681094, and from the BMBF (01EW1711A & B) in the framework of ERA-NET NEURON. H.R.S. holds a 5-year professorship in precision medicine at the Faculty of Health Sciences and Medicine, University of Copenhagen, which is sponsored by the Lundbeck Foundation (Grant Nr. R186-2015-2138). L.T.G. is funded by NIH R01AG056573, K24AG053435 and the BrightFocus Foundation. R.H. is supported by the UCLH NIHR Biomedical Research Centre. K.F. is supported by the German Research Foundation (DFG) project FL715-1-3. The Wellcome Centre for Human Neuroimaging is supported by core funding from the Wellcome (203147/Z/16/Z).
Competing interests
H.R.S. has received honoraria as speaker from Sanofi Genzyme, Denmark and Novartis, Denmark, as a consultant from Sanofi Genzyme, Denmark and as senior editor (NeuroImage) from Elsevier Publishers, Amsterdam, The Netherlands. H.R.S. has also received royalties as book editor from Springer Publishers, Stuttgart, Germany. The Max Planck Institute for Human Cognitive and Brain Sciences has an institutional research agreement with Siemens Healthcare.
Glossary
Abbreviations
- LC
locus coeruleus
- MT
magnetization transfer
References
- Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A, Yang S, Nestor PJ. In vivo MRI mapping of brain iron deposition across the adult lifespan. J Neurosci 2016; 36: 364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés-Benito P, Fernández-Dueñas V, Carmona M, Escobar LA, Torrejón-Escribano B, Aso E et al. Locus coeruleus at asymptomatic early and middle Braak stages of neurofibrillary tangle pathology. Neuropathol Appl Neurobiol 2017; 43: 373–92. [DOI] [PubMed] [Google Scholar]
- Arendt T, Brückner MK, Morawski M, Jäger C, Gertz H-J. Early neurone loss in Alzheimer’s disease: cortical or subcortical? Acta Neuropathol Commun 2015; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assal F, Cummings JL. Neuropsychiatric symptoms in the dementias. Curr Opin Neurol 2002; 15: 445–50. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1981; 1: 876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 2005; 28: 403–50. [DOI] [PubMed] [Google Scholar]
- Baker KG, Törk I, Hornung J-P, Halasz P. The human locus coeruleus complex: an immunohistochemical and three dimensional reconstruction study. Exp Brain Res 1989; 77: 257–70. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The locus ceruleus norepinephrine system Functional organization and potential clinical significance. Neurology 2009; 73: 1699–704. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, Schmeichel AM, Low PA, Sandroni P, Parisi JE. Loss of A5 noradrenergic neurons in multiple system atrophy. Acta Neuropathol (Berl) 2008; 115: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD et al. Altered expression of glutamate signaling, growth factor and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 2011; 16: 634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev 2003; 42: 33–84. [DOI] [PubMed] [Google Scholar]
- Betts MJ, Acosta-Cabronero J, Cardenas-Blanco A, Nestor PJ, Düzel E. High-resolution characterisation of the aging brain using simultaneous quantitative susceptibility mapping (QSM) and R2* measurements at 7 T. NeuroImage 2016; 138: 43–63. [DOI] [PubMed] [Google Scholar]
- Betts MJ, Cardenas-Blanco A, Kanowski M, Jessen F, Düzel E. In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. NeuroImage 2017; 163: 150–59. [DOI] [PubMed] [Google Scholar]
- Betts MJ, Cardenas-Blanco A, Kanowski M, Spottke A, Teipel SJ, Kilimann I et al. Locus coeruleus MRI contrast is reduced in Alzheimer’s disease dementia and correlates with CSF Aβ levels. Alzheimers Dement Diagn Assess Dis Monit 2019; 11: 281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MJ, Ehrenberg AJ, Hämmerer D, Düzel E. Commentary: Locus Coeruleus Ablation Exacerbates Cognitive Deficits, Neuropathology, and Lethality in P301S Tau Transgenic Mice. Front Neurosci 2018; 12: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesemeier A, Eibl O, Eswara S, Audinot J-N, Wirtz T, Pezzoli G et al. Elemental mapping of Neuromelanin organelles of human Substantia Nigra: correlative ultrastructural and chemical analysis by analytical transmission electron microscopy and nano-secondary ion mass spectrometry. J Neurochem 2016; 138: 339–53. [DOI] [PubMed] [Google Scholar]
- Birkl C, Langkammer C, Golob-Schwarzl N, Leoni M, Haybaeck J, Goessler W et al. Effects of formalin fixation and temperature on MR relaxation times in the human brain: Formalin fixation MR relaxation mechanisms. NMR Biomed 2016; 29: 458–65. [DOI] [PubMed] [Google Scholar]
- Birkl C, Langkammer C, Haybaeck J, Ernst C, Stollberger R, Fazekas F et al. Temperature-induced changes of magnetic resonance relaxation times in the human brain: a postmortem study: temperature dependency of relaxation times in postmortem brain. Magn Reson Med 2014; 71: 1575–80. [DOI] [PubMed] [Google Scholar]
- Birkl C, Soellradl M, Toeglhofer AM, Krassnig S, Leoni M, Pirpamer L et al. Effects of concentration and vendor specific composition of formalin on postmortem MRI of the human brain: formalin and postmortem brain MRI. Magn Reson Med 2018; 79: 1111–5. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer's disease begin? Curr Opin Neurol 2012; 25: 708–14. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Steur ENJ, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24: 197–211. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 2004; 318: 121–34. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011; 70: 960–9. [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Sur M. Active control of arousal by a locus coeruleus GABAergic circuit. Nat Neurosci 2019; 22: 218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnström H, Friberg N, Lindberg E, Englund E. Differential degeneration of the locus coeruleus in dementia subtypes. Clin Neuropathol 2011; 30: 104–10. [DOI] [PubMed] [Google Scholar]
- Cassidy CM, Zucca FA, Girgis RR, Baker SC, Weinstein JJ, Sharp ME et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci 2019; 116: 5108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalermpalanupap T, Schroeder JP, Rorabaugh JM, Liles LC, Lah JJ, Levey AI et al. Locus coeruleus ablation exacerbates cognitive deficits, neuropathology, and lethality in P301S tau transgenic mice. J Neurosci 2017: 1483–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol 1989; 287: 373–92. [DOI] [PubMed] [Google Scholar]
- Chen X, Huddleston DE, Langley J, Ahn S, Barnum CJ, Factor SA et al. Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach. Magn Reson Imaging 2014; 32: 1301–6. [DOI] [PubMed] [Google Scholar]
- Clewett DV, Huang R, Velasco R, Lee T-H, Mather M. Locus coeruleus activity strengthens prioritized memories under arousal. J Neurosci 2018; 38: 1558–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett DV, Lee T-H, Greening S, Ponzio A, Margalit E, Mather M. Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol Aging 2016; 37: 117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Düzel S, Bodammer NC, Lindenberger U, Kühn S et al. Locus coeruleus integrity preserves memory performance across the adult life span. bioRxiv 2019. https://www.biorxiv.org/content/10.1101/332098v3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol (Berl) 2008; 115: 437–44. [DOI] [PubMed] [Google Scholar]
- Dixon WT, Engels H, Castillo M, Sardashti M. Incidental magnetization transfer contrast in standard multislice imaging. Magn Reson Imaging 1990; 8: 417–22. [DOI] [PubMed] [Google Scholar]
- Dusek P, Madai VI, Huelnhagen T, Bahn E, Matej R, Sobesky J et al. The choice of embedding media affects image quality, tissue R2*, and susceptibility behaviors in post-mortem brain MR microscopy at 7.0 T: DUSEK et al. Magn Reson Med 2019; 81: 2688–701. [DOI] [PubMed] [Google Scholar]
- Düzel E, Guitart-Masip M, Maass A, Hämmerer D, Betts MJ, Speck O et al. Midbrain fMRI: applications, limitations and challenges. In: Uludag K, Ugurbil K, Berliner L, editors. fMRI: From Nuclear Spins to Brain Functions. Boston, MA: Springer US; 2015. p. 581–609 [Google Scholar]
- Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol 2017; 43: 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg AJ, Suemoto CK, de Paula França Resende E, Petersen C, Leite REP, Rodriguez RD et al. Neuropathologic correlates of psychiatric symptoms in Alzheimer’s disease. J Alzheimers Dis 2018; 66: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen M, Vanna R, Bellei C, Zucca FA, Wakamatsu K, Monzani E et al. Neuromelanins of human brain have soluble and insoluble components with dolichols attached to the melanic structure. PLoS One 2012; 7: e48490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Behbehani M, Shipley MT, van Bockstaele EJ, Aston-Jones G. Projections from the periaqueductal gray to the rostromedial pericoerulear region and nucleus locus coeruleus: Anatomic and physiologic studies. J Comp Neurol 1991; 306: 480–94. [DOI] [PubMed] [Google Scholar]
- Enochs WS, Hyslop WB, Bennett HF, Brown III RD, Koenig SH, Swartz HM. Sources of the increased longitudinal relaxation rates observed in melanotic melanoma. An in vitro study of synthetic melanins. Invest Radiol 1989; 24: 794–804. [DOI] [PubMed] [Google Scholar]
- Enochs WS, Petherick P, Bogdanova A, Mohr U, Weissleder R. Paramagnetic metal scavenging by melanin: MR imaging. Radiology 1997; 204: 417–23. [DOI] [PubMed] [Google Scholar]
- Eser RA, Ehrenberg AJ, Petersen C, Dunlop S, Mejia MB, Suemoto CK et al. Selective vulnerability of brainstem nuclei in distinct tauopathies: a postmortem study. J Neuropathol Exp Neurol 2018; 77: 149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, LeWitt PA, Kaufmann H. Norepinephrine deficiency in Parkinson’s disease: the case for noradrenergic enhancement: norepinephrine deficiency in PD. Mov Disord 2014; 29: 1710–9. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin S, Chen M-K et al. Imaging synaptic density in the living human brain. Sci Transl Med 2016; 8: 348ra96. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, de Hollander G, van Maanen L, Alkemade A, Keuken MC. Towards a mechanistic understanding of the human subcortex. Nat Rev Neurosci 2017; 18: 57–65. [DOI] [PubMed] [Google Scholar]
- Garcia-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, Leu-Semenescu S, Gallea C, Quattrocchi G et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 2013; 136: 2120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, Woodward DJ, McIntire DD, Smith WK et al. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol 1992; 32: 667–76. [DOI] [PubMed] [Google Scholar]
- German DC, Walker BS, Manaye K, Smith WK, Woodward DJ, North AJ. The human locus coeruleus: computer reconstruction of cellular distribution. J Neurosci 1988; 8: 1776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère N, Burke Nanni S, Trudeau L-E. On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front Neurol 2018; 9: 455 https://www.frontiersin.org/article/10.3389/fneur.2018.00455/full(21 February 2019, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerer D, Callaghan MF, Hopkins A, Kosciessa J, Betts M, Cardenas-Blanco A et al. Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. Proc Natl Acad Sci 2018: 201712268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerer D, Callaghan MF, Betts MJ, Cardenas-Blanco A, Kosciessa JQ, Weiskopf N, Dolan RJ, Düzel E. Multiparametric mapping of the Locus Coeruleus in ageing. Poster presented at Dementia MRI meeting, Cambridge; 2018a. [Google Scholar]
- Hansen AK, Knudsen K, Lillethorup TP, Landau AM, Parbo P, Fedorova T et al. In vivo imaging of neuromelanin in Parkinson’s disease using18 F-AV-1451 PET. Brain 2016; 139: 2039–49. [DOI] [PubMed] [Google Scholar]
- Heneka MT. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci 2006; 26: 1343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann N, Lanctôt KL, Khan LR. The role of norepinephrine in the behavioral and psychological symptoms of dementia. J Neuropsychiatry Clin Neurosci 2004; 16: 261–76. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJG, Feenstra MGP, Botterblom MHA, Gilhuis J, Sommer IEC, Kamphorst W et al. Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann Neurol 1999; 45: 82–91. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE et al. Deep clinical and neuropathological phenotyping of Pick disease: Deep Phenotype Pick Disease. Ann Neurol 2016; 79: 272–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Becker A, Kwong K, d’Oleire Uquillas F, Sperling RA, Johnson KA. Locus coeruleus signal intensity is associated with entorhinal tau pathology at higher levels of amyloid burden. Alzheimers Dement 2018; 14: P509–10. [Google Scholar]
- Jacobs HIL, Müller-Ehrenberg L, Priovoulos N, Roebroeck A. Curvilinear locus coeruleus functional connectivity trajectories over the adult lifespan: a 7 T MRI study. Neurobiol Aging 2018; 69: 167–76. [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Wiese S, van de Ven V, Gronenschild EHBM, Verhey FRJ, Matthews PM. Relevance of parahippocampal-locus coeruleus connectivity to memory in early dementia. Neurobiol Aging 2015; 36: 618–26. [DOI] [PubMed] [Google Scholar]
- Jardanhazi-Kurutz D, Kummer MP, Terwel D, Vogel K, Dyrks T, Thiele A et al. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem Int 2010; 57: 375–82. [DOI] [PubMed] [Google Scholar]
- Jessen F, Spottke A, Boecker H, Brosseron F, Buerger K, Catak C et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers Res Ther 2018; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Polak PE, Lin SX, Sakharkar AJ, Pandey SC, Feinstein DL. The noradrenaline precursor L-DOPS reduces pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging 2012; 33: 1651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Polak PE, Madrigal JLM, Gavrilyuk V, Sharp A, Chauhan N et al. Beta-amyloid-dependent expression of NOS2in neurons: prevention by an α2-adrenergic antagonist. Antioxid Redox Signal 2006; 8: 873–83. [DOI] [PubMed] [Google Scholar]
- Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, Counts SE. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol Commun 2017; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci 2016; 113: 14835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. NeuroImage 2009; 47: 1261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm A-CE, Aston-Jones GS et al. Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. NeuroImage 2015; 113: 235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol 2018; 17: 618–28. [DOI] [PubMed] [Google Scholar]
- Krebs N, Langkammer C, Goessler W, Ropele S, Fazekas F, Yen K et al. Assessment of trace elements in human brain using inductively coupled plasma mass spectrometry. J Trace Elem Med Biol 2014; 28: 1–7. [DOI] [PubMed] [Google Scholar]
- Lanctôt KL, Amatniek J, Ancoli-Israel S, Arnold SE, Ballard C, Cohen-Mansfield J et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: new treatment paradigms. Alzheimers Dement Transl Res Clin Interv 2017; 3: 440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J, Huddleston DE, Chen X, Sedlacik J, Zachariah N, Hu X. A multicontrast approach for comprehensive imaging of substantia nigra. NeuroImage 2015; 112: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J, Huddleston DE, Liu CJ, Hu X. Reproducibility of locus coeruleus and substantia nigra imaging with neuromelanin sensitive MRI. Magn Reson Mater Physics, Biol Med 2017; 30: 121–5. [DOI] [PubMed] [Google Scholar]
- Lee CM, Jacobs HIL, Marquié M, Becker JA, Andrea NV, Jin DS et al. 18F-Flortaucipir Binding in Choroid Plexus: Related to Race and Hippocampus Signal. J Alzheimers Dis 2018; 62: 1691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Acosta-Cabronero J, Cardenas-Blanco A, Loane C, Berry AJ, Betts MJ et al. In vivo visualization of age-related differences in the locus coeruleus. Neurobiol Aging 2019; 74: 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Marijatta F, Hämmerer D, Acosta-Cabronero J, Düzel E, Howard RJ. Magnetic resonance imaging of the human locus coeruleus: a systematic review. Neurosci Biobehav Rev 2017; 83: 325–55. [DOI] [PubMed] [Google Scholar]
- Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience 2016; 338: 93–113. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel J-PG, Honig LS, Rajput A, Robinson CA et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007; 130: 3297–307. [DOI] [PubMed] [Google Scholar]
- Lyness S. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol Aging 2003; 24: 1–23. [DOI] [PubMed] [Google Scholar]
- Manaye KF, McIntire DD, Mann D, German DC. Locus coeruleus cell loss in the aging human brain: A non-random process. J Comp Neurol 1995; 358: 79–87. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Yates PO. Lipoprotein pigments—Their relationship to ageing in the human nervous system II. The melanin content of pigmented nerve cells. Brain 1974; 97: 489–498. [DOI] [PubMed] [Google Scholar]
- Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue: validation of PET Tracer. Ann Neurol 2015; 78: 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav Brain Sci 2016; 39: e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Harley CW. The locus coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cogn Sci 2016; 20: 214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Maeda M, Yata K, Ichiba Y, Yamaguchi T, Kanamaru K et al. Neuromelanin Magnetic resonance imaging in Parkinson’s disease and multiple system atrophy. Eur Neurol 2013; 70: 70–77. [DOI] [PubMed] [Google Scholar]
- McMillan PJ, White SS, Franklin A, Greenup JL, Leverenz JB, Raskind MA et al. Differential response of the central noradrenergic nervous system to the loss of locus coeruleus neurons in Parkinson’s disease and Alzheimer’s disease. Brain Res 2011; 1373: 240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moises HC, Waterhouse BD, Woodward DJ. Locus coeruleus stimulation potentiates Purkinje cell responses to afferent input: The climbing fiber system. Brain Res 1981; 222: 43–64. [DOI] [PubMed] [Google Scholar]
- Nakane T, Nihashi T, Kawai H, Naganawa S. Visualization of neuromelanin in the substantia nigra and locus ceruleus at 1.5 T Using a 3D-gradient echo sequence with magnetization transfer contrast. Magn Reson Med Sci 2008; 7: 205–10. [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Ding F, Nedergaard M. Distinct functional states of astrocytes during sleep and wakefulness: is norepinephrine the master regulator? Curr Sleep Med Rep 2015; 1: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm TG, Busch C, Bohl J. Unbiased estimation of neuronal numbers in the human nucleus coeruleus during aging. Neurobiol Aging 1997; 18: 393–9. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Wilcock GK, Esiri MM, Francis PT, Bowen DM. Monoaminergic innervation of the frontal and temporal lobes in Alzheimer’s disease. Brain Res 1987; 401: 231–8. [DOI] [PubMed] [Google Scholar]
- Palmqvist S, Schöll M, Strandberg O, Mattsson N, Stomrud E, Zetterberg H et al. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun 2017; 8 http://www.nature.com/articles/s41467–017–01150-x(21 February 2019, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Lansdall C, Rowe J. The neuroanatomical and neurochemical basis of apathy and impulsivity in frontotemporal lobar degeneration. Curr Opin Behav Sci 2018; 22: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Gallezot J-D, Ding Y-S, Henry S, Potenza MN, Southwick SM et al. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatry 2013; 70: 1199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Priovoulos N, Jacobs HIL, Ivanov D, Uludag K, Verhey FRJ, Poser BA. High-resolution in vivo imaging of human locus coeruleus by magnetization transfer MRI at 3 T and 7 T. NeuroImage 2018; 168: 427–36. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Cortical noradrenaline, attention and arousal1. Psychol Med 1984; 14: 13. [DOI] [PubMed] [Google Scholar]
- Robertson IH. A noradrenergic theory of cognitive reserve: implications for Alzheimer’s disease. Neurobiol Aging 2013; 34: 298–308. [DOI] [PubMed] [Google Scholar]
- Rorabaugh JM, Chalermpalanupap T, Botz-Zapp CA, Fu VM, Lembeck NA, Cohen RM et al. Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain 2017; 140: 3023–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Wang Q, Palkovits M, Faludi G, Dwivedi Y. Altered miRNA expression network in locus coeruleus of depressed suicide subjects. Sci Rep 2017; 7: 4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 2009; 10: 211–23. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport 2006; 17: 1215–8. [DOI] [PubMed] [Google Scholar]
- Seidel K, Mahlke J, Siswanto S, Krüger R, Heinsen H, Auburger G et al. The Brainstem pathologies of Parkinson’s Disease and dementia with lewy bodies: the brainstem in iPD and DLB. Brain Pathol 2015; 25: 121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y, Xu T, Graf WM, Fobbs A, Sherwood CC, Hof PR et al. Comparative anatomy of the locus coeruleus in humans and nonhuman primates. J Comp Neurol 2010; 518: 963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E, Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S et al. Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 tesla. Magn Reson Med Sci 2006; 5: 197–200. [DOI] [PubMed] [Google Scholar]
- Shima T, Sarna T, Swartz HM, Stroppolo A, Gerbasi R, Zecca L. Binding of iron to neuromelanin of human substantia nigra and synthetic melanin: an electron paramagnetic resonance spectroscopy study. Free Radic Biol Med 1997; 23: 110–9. [DOI] [PubMed] [Google Scholar]
- Sommerauer M, Fedorova TD, Hansen AK, Knudsen K, Otto M, Jeppesen J et al. Evaluation of the noradrenergic system in Parkinson’s disease: an 11 C-MeNER PET and neuromelanin MRI study. Brain 2018; 141: 496–504. [DOI] [PubMed] [Google Scholar]
- Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG et al. Cognitive impairment in multiple system atrophy: a position statement by the neuropsychology task force of the MDS multiple system atrophy (MODIMSA) study group: COGNITIVE IMPAIRMENT IN MSA. Mov Disord 2014; 29: 857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TD, Alvarez VE, McKee AC. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther 2014; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Daneshvar DH. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013; 81: 1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich V, D’Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewalle G et al. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. J Neurosci 2006; 26: 7416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann K, Heinsen H, Korf H-W, Del Turco D, Ghebremedhin E, Seidel K et al. Precortical phase of Alzheimer’s Disease (AD)-related tau cytoskeletal pathology: precortical phases of AD. Brain Pathol 2016; 26: 371–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cassidy C, Horga G, Kang UJ, Fahn S, Casella L et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Park Dis 2018; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrez S, R. Jabir N, Shakil S, H. Greig N, Alam Q, M. Abuzenadah A et al. A synopsis on the role of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol Disord - Drug Targets 2012; 11: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016; 537: 357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Westlund KN. The noradrenergic locus coeruleus as a chronic pain generator: Is the LC a Neuropathic Pain Generator? J Neurosci Res 2017; 95: 1336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R et al. Physical basis of cognitive alterations in alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991; 30: 572–80. [DOI] [PubMed] [Google Scholar]
- Theofilas P, Ehrenberg AJ, Dunlop S, Di Lorenzo Alho AT, Nguy A, Leite REP et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: a stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement 2017; 13: 236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona K-D, Keuken MC, de Rover M, Lakke E, Forstmann BU, Nieuwenhuis S et al. In vivo visualization of the locus coeruleus in humans: quantifying the test–retest reliability. Brain Struct Funct 2017; 222: 4203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo P, Summers PE, Ferrari E, Zucca FA, Sturini M, Mainardi LT et al. Contrast mechanisms associated with neuromelanin-MRI: Neuromelanin-MRI Contrast. Magn Reson Med 2017; 78: 1790–800. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol 2006; 10: 743–58. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Tabuchi K, Ojika M, Zucca FA, Zecca L, Ito S. Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J Neurochem 2015; 135: 768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RR, Krismer F, Galpern WR, Wenning GK, Low PA, Halliday G et al. Recommendations of the global multiple system atrophy research roadmap meeting. Neurology 2018; 90: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 2014; 13: 1045–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Tan Z, Wang X, Martinez-Hernandez A, Frahm J. Magnetic resonance imaging of noradrenergic neurons. Brain Struct Funct 2019; 244: 1609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D. Long road to ruin: noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci 2018; 41: 211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Suckling J, Williams G, Correia MM, Inkster B, Tait R et al. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3T: a multi-center validation. Front Neurosci 2013; 7 http://journal.frontiersin.org/article/10.3389/fnins.2013.00095/abstract(30 October 2018, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS et al. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 2013; 80: 1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T et al. Improving response inhibition in Parkinson’s disease with atomoxetine. Biol Psychiatry 2015; 77: 740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 2003; 60: 337. [DOI] [PubMed] [Google Scholar]
- Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci USA 2004; 101: 9843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca FA, Bellei C, Giannelli S, Terreni MR, Gallorini M, Rizzio E et al. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. J Neural Transm 2006; 113: 757–67. [DOI] [PubMed] [Google Scholar]
- Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 2017; 155: 96–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca FA, Vanna R, Cupaioli FA, Bellei C, De Palma A, Di Silvestre D et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson’s disease. NPJ Park Dis 2018; 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]