Abstract
Context
Studies suggest many essential trace metal(loid)s are involved in glucose metabolism, but the associations among pregnant women are unclear.
Objective
To assess associations between early pregnancy plasma zinc, selenium, copper, and molybdenum levels and blood glucose levels later in the second trimester.
Design
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies‒Singleton Cohort is a prospective cohort study conducted between July 2009 and January 2013.
Setting
Twelve academic research hospitals in the United States.
Patients
A total of 1857 multiracial, nonobese, healthy women.
Main Outcome Measure
Blood glucose levels from 1-hour 50-g gestational load test (GLT) at 24 to 28 weeks of gestation.
Results
Higher concentrations of first-trimester copper were associated with higher glucose levels from the GLT (i.e., every 50% increase in copper concentration was related to 4.9 mg/dL higher glucose level; 95% CI: 2.2, 7.5 mg/dL) adjusted for maternal sociodemographic characteristics and reproductive history. In contrast, every 50% increase in molybdenum concentration was associated with 1.2 mg/dL lower mean glucose level (95% CI: −2.3, −0.1 mg/dL). The magnitude of these associations was greater at the upper tails of glucose level distribution based on quantile regressions of the 10th, 50th, and 90th percentiles.
Conclusions
Higher copper and lower molybdenum concentrations could increase the risk of glucose dysregulation during pregnancy, with women at higher risk of gestational diabetes mellitus potentially affected to a greater extent. Further work is needed to understand the mechanisms involved with early pregnancy essential metal(loid)s to inform clinical diagnosis and prevention of glucose intolerance during pregnancy.
Among 1857 pregnant US women, we found higher copper and lower molybdenum could potentially increase the risk of glucose dysregulation, with those at higher risk of GDM affected to a greater extent.
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. The prevalence of GDM has increased over the past few decades, affecting ∼5% to 9% of all pregnancies in the United States (1–4). Elevated glucose level in pregnancy due to insulin resistance coupled with relatively insufficient insulin production is an indication of GDM (5). Accumulating evidence suggests that any form of pregnancy hyperglycemia, even without meeting the clinical criteria for GDM, is associated with increased risks of a variety of adverse health outcomes, including preeclampsia, high birth weight, and primary cesarean section (6). Therefore, it is critical to identify modifiable factors that may potentially lower glucose levels in pregnancy.
Environmental factors may contribute to the development of glucose intolerance in pregnancy (7). Among these factors, essential trace metal(loid)s may be important with respect to glucose intolerance in pregnancy, given their involvement in glucose homeostasis and the potential to modify their levels through dietary management. Accumulating data from animal and metabolic studies demonstrated that a number of essential trace metal(loid)s, including zinc, selenium, chromium, iron, manganese, copper, and molybdenum, can affect glucose metabolism and insulin sensitivity with downstream effects on hyperglycemia and GDM (8–10). In fact, some of these metal(loid)s are considered potential preventive and treatment agents for diabetes (11). However, when in excess of cellular needs, some metal(loid)s (e.g., selenium, iron, and copper) may generate reactive oxygen species and induce insulin resistance (12, 13). A number of studies have evaluated zinc and selenium as they relate to GDM, with studies showing inconsistent results for zinc and relatively consistent inverse associations for selenium and GDM risk (10, 14). Few studies have investigated the association between copper, molybdenum, and glucose levels in pregnancy. Even fewer have evaluated these essential metal(loid)s in early pregnancy as they relate to GDM and glycemic dysregulation in later pregnancy.
Therefore, we aimed to examine the association between essential metal(loid)s and glucose levels in pregnancy in a prospective multiracial cohort of pregnant US women. We evaluated the associations between early pregnancy essential metal(loid)s that had detectable concentrations in the study population (i.e., zinc, selenium, copper, molybdenum) and glucose levels measured late in the second trimester, commonly used as an indicator of GDM risk. In addition, we evaluated shifts in the distribution of glucose levels as they related to early pregnancy concentrations of these metal(loid)s.
Methods
Study population
The study used data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies‒Singleton Cohort. The full cohort consisted of 2344 healthy, nonobese, low-risk pregnant women from 12 US clinical sites between July 2009 and January 2013. Women were excluded if they had preexisting chronic diseases or medical conditions and past pregnancy complications, had smoked within the past 6 months, or were consuming at least one alcoholic drink per day. Women were enrolled between 8 and 13 weeks’ gestation, with five study visits occurring throughout pregnancy. At each visit, an interview was conducted using a standardized and structured questionnaire to collect information, including maternal demographic characteristics, reproductive and pregnancy history, and health behavior. Blood specimens and maternal anthropometric measurements were collected at each visit. Additional details of the study can be found elsewhere (15).
For this analysis, women were included if they provided blood samples with plasma metal(loid) measurements in the late first trimester and underwent a 1-hour 50-g gestational load test (GLT) late in the second trimester. Among the 2344 women recruited into the study, 83 did not have any metal(loid) measurement on enrollment. In addition, 404 women did not have a glucose measurement from the GLT. This resulted in a final analytical population of 1857 women (79.2%).
Informed consent was obtained before data collection, and institutional review board approvals were obtained for all participating clinical sites, the data coordinating center, and the the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Metal(loid) data measurement
Concentrations of zinc, selenium, copper, and molybdenum were measured in blood plasma samples collected during the late first trimester of pregnancy (median: 12 weeks’ gestation) and were stored at −70°C pending analysis. Plasma specimens were shipped to the Wadsworth Center, New York State Department of Health, for trace element analysis using inductively coupled plasma-mass spectrometry, a multi-element method optimized for serum/plasma samples and validated for use in biomonitoring studies. The inductively coupled plasma-mass spectrometry instrument was calibrated with matrix-matched standards traceable to the National Institute of Standards and Technology. Three levels of serum internal quality control materials were included in each analytical run, and 2% of all samples were analyzed in duplicate. Typical between-run precision (for a total of 79 independent days of analysis) varied from 3.7% for an internal quality control running mean concentration of 730 µg/L to 3.6% at 2960 µg/L for zinc, 3.9% at 119 µg/L to 3.4% at 270 µg/L for selenium, 3.4% at 1080 µg/L to 3.2% at 3000 µg/L for copper, and 17.7% at 1.57 µg/L to 4.4% at 11.3 µg/L for molybdenum. Method validation was established using National Institute of Standards and Technology standard reference materials and method performance assessed by successful participation in external proficiency testing programs for serum/plasma trace elements operated by the Center de Toxicologie du Quebéc, UK NEQAS for Trace Elements, German EQUALM, and the New York State proficiency testing program for trace elements. All metal(loid) measurements were above the limit of detection calculated according to the International Standards Organization / the International Union of Pure and Applied Chemistry harmonized guidelines.
Glucose data measurement
The primary outcome of interest was blood glucose levels obtained from the nonfasting 1-hour 50-g GLT during the late second trimester (median: 27 weeks’ gestation), utilizing the first step of the Carpenter-Coustan criteria for GDM screening (16). In addition to assessing glucose levels continuously, for clinical relevance we also used two other binary outcomes. First, because a glucose level ≥140 mg/dL denotes additional screening for GDM using the Carpenter-Coustan criteria, we dichotomized <140 mg/dL vs ≥140 mg/dL as a secondary outcome. For conciseness, we denoted glucose levels ≥140 mg/dL as abnormal GLT glucose levels. Second, we diagnosed GDM on the basis of a diagnostic 100-g 3-hour oral glucose tolerance test (OGTT) according to the Carpenter-Coustan criteria (17).
Covariates
Information on demographic factors, medical history, and lifestyle factors was obtained at baseline through an in-person interview, and physical activity information was collected through self-administered questionnaires. A priori, we selected the following variables as potential confounders and strong predictors of GDM on the basis of a previous literature review: maternal age at enrollment, self-identified race/ethnicity, prepregnancy body mass index (BMI), physical activity, family history of type 2 diabetes, parity, gestational week at GLT, and socioeconomic status (assessed by maternal educational level, employment status, and marital status) on the basis of a previous literature review as established risk factors of GDM (18–20). Prepregnancy BMI was calculated from self-reported prepregnancy weight and measured height upon enrollment. We also considered controlling for early gestational weight gain, defined as the difference between the measured weight upon enrollment (median: 12 weeks’ gestation) and the next visit (median: 19 weeks’ gestation) and clinical sites in sensitivity analyses.
Statistical analysis
Demographic characteristics of the study participants were reported using mean ± SD for continuous variables and number (percentage) for categorical variables. Distributions of the plasma metal(loid) concentrations were reported using geometric means and SD.
We first fitted linear regression models to evaluate the associations between each metal(loid) and mean glucose level from the GLT. Metal(loid) levels were log-transformed because of right-skewness. Although natural log-transformation is commonly used, for these data, 1-unit differences in the metal(loid)s on the natural log-transformed scale corresponded to a much wider range than the interquartile range on the original scale, which would have limited the interpretability and clinical relevance of the estimated effect sizes. Therefore, base 1.5 logarithm function was chosen to ensure 1-unit change on the transformed scale to be within or close to the interquartile range of the original metal(loid) concentrations. The following covariates were included in the final models: maternal age in years at enrollment (continuous), self-identified race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander), maternal educational level (high school or less, some college or associate degree, college degree or higher), full-time employed or student (yes/no), married or living with a partner (yes/no), family history of diabetes (yes/no), nulliparity (yes/no), prepregnancy BMI (kg/m2, continuous), moderate to vigorous level of physical activity (hours per week, continuous), and gestational week at blood specimen collection (weeks, continuous). Gestational week at GLT was not included because of its nonsignificant difference between women with normal GLT and those with abnormal GLT (P value = 0.31) and additional missingness (n = 27).
To evaluate shifts of glucose level distribution in addition to the mean and to detect associations that occur primarily at the tails of the distribution, quantile regression was performed (21). Specifically, we focused on the 10th, 50th, and 90th percentiles of glucose levels from the GLT. For each percentile, we built a linear regression model on each metal(loid), adjusting for the same set of covariates mentioned previously. To evaluate changes in glucose level distribution with each metal(loid), estimated coefficients with their 95% CIs were reported.
To allow for more flexibility in the exposure-outcome associations and to explore potential nonlinear associations, we used splines models. For each metal(loid), we performed restricted cubic spline transformations with three unspecified knots estimated using default quantiles and then regressed the mean glucose level and percentiles of glucose level distribution on transformed metal(loid) levels (22). Deviations from linearity were evaluated statistically by testing whether the coefficient of the second spline transformation was significantly different from 0.
Because of the relatively high prevalence of women with abnormal GLT results (14.2%), in secondary analyses, log-binomial models were used to estimate the risk ratios of abnormal GLT in relation to the levels of each metal(loid). Logistic regression models were used to estimate the OR of diagnosed GDM, which had a low prevalence (3.6%) in this study population. Finally, we conducted two sensitivity analyses to assess the possibility of confounding and/or mediation by early gestational weight gain, as well as the clustering effect within each clinical site. For early gestational weight gain, we added it to the linear regression models; for clinical site, we fitted mixed-effect models with a random intercept for each clinic site.
All analyses were performed in R version 3.4.1. Quantile regression models were performed with R package quantreg (23), and spline models were performed with R package rms (24).
Results
Baseline characteristics of the study population are shown in Table 1. Our study population included 1857 women, of whom 264 (14.2%) had glucose levels ≥140 mg/dL from the 1-hour 50-g GLT (recognized as abnormal GLT results) despite their low-risk antenatal profiles. The distribution of glucose levels from the GLT was approximately normally distributed, with a mean of 110.5 mg/dL and an SD of 26.9 mg/dL. A greater percentage of Asian/Pacific Islander women had abnormal GLT (24% compared with 14% in the total population). Women who were missing GLT or metal(loid) measurement data had baseline characteristics similar to those included for the present analysis, with the exception of Asian/Pacific Islander race/ethnicity (i.e., this group made up 26% of the missing population compared with 20% of the full cohort). Among the 264 women with abnormal GLT results, 58 were diagnosed with GDM in the OGTT. An additional 76 women with normal GLT results also underwent an OGTT, among whom nine were diagnosed as having GDM.
Table 1.
Maternal Characteristics of the Study Population
| Total | GLT <140 mg/dL | GLT ≥140mg/dL | |
|---|---|---|---|
| N = 1857 (100%) | n = 1593 (85.8%) | n = 264 (14.2%) | |
| Age at pregnancy, mean ± SD, y | 28.1 ± 5.5 | 27.8 ± 5.5 | 29.7 ± 5.4 |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 513 (28) | 452 (28) | 61 (23) |
| Non-Hispanic black | 482 (26) | 435 (27) | 47 (18) |
| Hispanic | 512 (27) | 441 (28) | 71 (27) |
| Asian/Pacific Islander | 350 (19) | 265 (17) | 85 (32) |
| Educational level, n (%) | |||
| High school or less | 539 (29) | 459 (29) | 80 (30) |
| Some college or associate degree | 544 (29) | 461 (29) | 83 (31) |
| College degree or higher | 774 (42) | 673 (42) | 101 (39) |
| Full-time employed/student, n (%) | 1365 (74) | 1179 (74) | 186 (70) |
| Married/living with a partner, n (%) | 1392 (75) | 1177 (74) | 215 (81) |
| Family history of diabetes, n (%) | 357 (19) | 293 (18) | 64 (24) |
| Nulliparity, n (%) | 925 (50) | 810 (51) | 115 (44) |
| Prepregnancy BMI, kg/m2 | |||
| Mean ± SD | 23.6 ± 3.0 | 23.6 ± 2.9 | 23.7 ± 3.0 |
| Normal (<25), n (%) | 1249 (67) | 1081 (68) | 168 (64) |
| Overweight (≥25), n (%) | 608 (33) | 512 (32) | 96 (36) |
| Gestational week at GLT, mean ± SD, wk | 27.0 ± 2.5 | 27.0 ± 2.5 | 26.8 ± 2.7 |
| Early gestational weight gain,a mean ± SD, kg | 2.9 ± 2.3 | 3.0 ± 2.2 | 2.8 ± 2.4 |
| Physical activity,b mean ± SD, h/wk | 18.8 ± 19.3 | 19.0 ± 19.3 | 17.5 ± 18.9 |
Gestational weight gain from enrollment (median: 12 weeks’ gestation) to the next visit (median: 19 weeks’ gestation).
Moderate to vigorous level of physical activity.
Levels of plasma zinc and selenium among the study population (medians of 806 µg/L and 123 µg/L, respectively) were comparable to those measured among nonpregnant US women of reproductive age in the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2014, whereas the levels of plasma copper (median = 1874 µg/L) were much higher in our study population than the serum copper levels reported in the NHANES (Table 2). Molybdenum has not been evaluated in plasma/serum in the NHANES. Levels of metal(loid)s varied significantly among different racial/ethnic groups and educational levels and with regard to other maternal characteristics (25). All four metal(loid)s were weakly correlated with pairwise Spearman correlations below 0.2.
Table 2.
Distribution of Metal(loid)s
| Metal(loid) | Study Population (N = 1857) | NHANES (N = 514)a | |||
|---|---|---|---|---|---|
| Geometric Mean | Median | 25th Percentile | 75th Percentile | Median | |
| Zn | 804 | 806 | 714 | 901 | 773 |
| Se | 122 | 123 | 113 | 132 | 124 |
| Cu | 1851 | 1874 | 1637 | 2105 | 1284 |
| Mo | 1.9 | 1.9 | 1.4 | 2.7 | — |
Data are presented as μg/L.
Levels of metal(loid)s among nonpregnant women at reproductive age (18–45 y) from the NHANES 2011 to 2014 data. In NHANES, zinc (Zn), selenium (Se), and copper (Cu) were measured in serum; serum molybdenum (Mo) levels were not measured.
Linear associations between metal(loid) concentrations and glucose levels
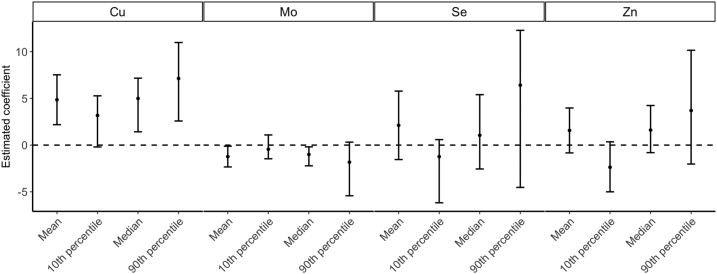
Multivariable adjusted linear regression models showed that a 50% increase in copper concentration (corresponding to a 1-unit increase on the log1.5 scale) was related to a 4.9-mg/dL (95% CI: 2.2, 7.5 mg/dL) increase in mean glucose level from the GLT. For molybdenum, there was a 1.2-mg/dL (95% CI: −2.3, −0.1 mg/dL) decrease in mean glucose level for every 50% increase in molybdenum. No evidence of synergistic effect was observed for copper and molybdenum. On the basis of quantile regression analyses at selected percentiles of the glucose distribution, the magnitudes of these associations were observed to be greater at the upper tail of the glucose level distribution (Fig. 1). For example, for every 50% increase in copper, women in the 90th percentile of the glucose distribution were expected to have 7.1 mg/dL (95% CI: 2.6, 11.0) higher glucose levels, which was greater than that for women in the median of the glucose distribution [5.0 mg/dL (95% CI: 1.4, 7.2)], and that for women in the 10th percentile of the glucose distribution [3.2 mg/dL (95% CI: −0.2, 5.3)]. For both zinc and selenium, we observed nonsignificant positive associations with glucose levels from the GLT (the mean, median, and 90th percentile), with the exception of negative associations with the 10th percentile of glucose distribution.
Figure 1.
Distribution changes in glucose level from the 1-hour glucose load test as a function of 50% increase in each metal(loid). Results were obtained by running multivariable adjusted linear regression and quantile regressions on the 10th, 50th, and 90th percentiles of the glucose level. All models were adjusted for age, race/ethnicity, educational level, full-time employed/student, marital status, family history of diabetes, parity, prepregnancy BMI, physical activity, and gestational week at blood sampling. The error bars represent the 95% CIs of the estimates. Cu, copper; Mo, molybdenum; Se, selenium; Zn, zinc.
Nonlinear assessment of metal(loid) concentrations and glucose levels
In the restricted cubic spline analysis, the likelihood ratio tests of the second spline transformation variables were not rejected at the 0.05 significance level for any of the metal(loid)s and regression models, suggesting no significant violations of the linearity assumptions.
Metal(loid)s in association with abnormal GLT glucose levels and GDM
We also evaluated the risk of abnormal GLT results in association with each metal(loid) in both crude models and multivariable adjusted models (Table 3). Women had a 1.53 increased risk of abnormal GLT for every 50% increase in copper concentration (95% CI: 1.19, 1.97). For molybdenum, every 50% increase of molybdenum was associated with a 14% reduced risk of abnormal GLT [relative risk: 0.86 (95% CI: 0.78, 0.96)]. Similar results for copper and molybdenum were found for diagnosed GDM, and there was suggestive evidence of positive associations between selenium concentrations and GDM (Table 3).
Table 3.
Association Between 50% Increase in Metal(loid) Concentration and Abnormal GLT and Diagnosed GDM
| Abnormal GLTa | Diagnosed GDMb | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude Model | Adjusted Modelc | Crude Model | Adjusted Modelc | |||||
| Metal(loid) | Risk Ratio (95% CI) | P | Risk Ratio (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Zn | 1.10 (0.90, 1.35) | 0.34 | 1.18 (0.98, 1.42) | 0.09 | 0.98 (0.59, 1.51) | 0.94 | 1.02 (0.60, 1.58) | 0.94 |
| Se | 0.99 (0.70, 1.38) | 0.94 | 1.25 (0.90, 1.74) | 0.19 | 1.93 (0.95, 3.78) | 0.06 | 1.79 (1.33, 5.68) | 0.01 |
| Cu | 1.35 (1.06, 1.71) | 0.01 | 1.53 (1.19, 1.97) | <0.01 | 1.76 (1.05, 2.97) | 0.03 | 1.67 (0.95, 2.98) | 0.08 |
| Mo | 0.92 (0.82, 1.02) | 0.11 | 0.86 (0.78, 0.96) | 0.01 | 0.73 (0.58, 0.92) | 0.01 | 0.72 (0.56, 0.92) | 0.01 |
Abbreviations: Cu, copper; Mo, molybdenum; Se, selenium; Zn, zinc.
Glucose level from GLT ≥140 mg/dL.
Diagnosed using the Carpenter-Coustan criteria.
Adjusted for age, race/ethnicity, educational level, full-time employed/student, marital status, family history of diabetes, parity, prepregnancy BMI, physical activity, and gestational week at blood sampling.
Sensitivity Analyses
In sensitivity analyses that accounted for the potential clustering effect of clinic sites by fitting a mixed-effect model with a random intercept for each clinic site (12 in total), similar estimates were found for the associations between each metal(loid) and glucose levels from the GLT. Variances explained by clinic sites were around 3%, suggesting little clustering effect. Adjusting for early pregnancy weight gain to examine potential sources of bias also yielded similar results (not shown), suggesting early pregnancy weight gain was not a strong confounder or mediator in this analysis.
Discussion
In a prospective multiracial study of pregnant women in the United States, we found that higher concentrations of plasma copper in early pregnancy were prospectively associated with higher glucose levels assessed in the late second trimester as part of a GDM screening test. On the other hand, higher concentrations of plasma molybdenum in early pregnancy were associated with lower glucose levels from the same GDM screening test. Furthermore, the magnitudes of these associations were greater on the upper tail of glucose level distributions. These findings suggest that higher copper and lower molybdenum levels in blood plasma can potentially increase the risk of glucose dysregulation during pregnancy and may affect women at higher risk of GDM to a greater extent.
In the current study, we evaluated this association prospectively in a pregnant population. Few epidemiological studies have evaluated the association between copper and glucose levels in pregnant women. One case-control study found higher serum copper concentrations among women with GDM, similar to our results (26), whereas two other case-control studies found no difference in copper concentrations (27, 28). Various reasons could explain the inconsistencies in previous studies, including limited power because of small sample size, cross-sectional design, and methodological differences in copper level assessment, as well as residual confounding and variations in baseline risk of GDM. Our findings are consistent with those of a growing number of studies among nonpregnant populations that found increased copper concentrations among patients with diabetes (29, 30), suggesting a positive relationship between copper and glucose levels.
Copper enters the human body mainly through drinking water, diet and supplements, skin contact, or inhalation of air containing copper particulates. The majority of serum copper is transported bound to ceruloplasmin. The biological mechanism through which copper may affect glucose levels is complex. Animal studies showed that free copper ions are highly redox active and can elicit reactive oxygen species production (13, 31). This induced oxidative stress may contribute to cell damage, including damage to pancreatic β cells that are responsible for insulin secretion. Copper may also impair insulin sensitivity through disturbance of hormone-dependent signal transduction (32). Conversely, diabetes may perturb copper metabolism (33). Although blood specimens for copper measurement in the current study were collected before the GDM screening test conducted during mid to late pregnancy, it is possible that the rise in copper levels indicates early-stage impairments in glucose metabolism. Further complicating this issue is the continuous increase of copper accumulation throughout pregnancy, as a result of its important role in the formation of various enzymatic and other processes within the developing fetus (34, 35). Thus, it is unclear whether higher copper exposure in early pregnancy is a contributor to higher glucose levels later in pregnancy or whether some underlying causes of higher glucose levels are associated with greater accumulation of copper. Additional work is needed to provide insights to (i) the causality of the copper-glucose metabolism relationship, (ii) whether this relationship is similar among nonpregnant and pregnant populations, (iii) whether this relationship depends on the timing of copper measurements, and (iv) whether this relationship differs with different forms of copper (ceruloplasmin-bound and non–ceruloplasmin-bound copper).
Our study showed an inverse association between plasma molybdenum and glucose levels in pregnancy. Molybdenum is an effective antihyperglycemic agent, shown to decrease blood glucose levels by mimicking insulin activity, promoting lipogenesis and increasing insulin sensitivity in animal models (8). Its association with GDM risk has been evaluated in only a small case-control study (27), in which no significant difference was observed. Although deficiency in molybdenum is rare in the general population (36), pregnant women may be more prone to molybdenum insufficiency because of a greater demand for molybdenum (37). Moreover, exposure to molybdenum comes almost entirely through food, especially from above-ground plants such as legumes, leafy vegetables, and cauliflower (36). It is possible that the association shown in this study is partially due to intake of food high in fiber, vegetable protein, and vitamins, which were previously related to lower GDM risk (38, 39). Future studies are needed to better understand whether molybdenum has an effect on glucose regulation during pregnancy.
Finally, our results showed that all observed associations were not constant over the entire glucose level distribution but were consistently stronger in the upper tails. This suggests that women with a higher risk of glucose dysregulation in pregnancy may be more susceptible to the effect of high copper and low molybdenum levels as they relate to their glucose metabolism. Because this study was done among nonobese women with no known preexisting conditions, it would be worthwhile to further examine the effects of these metal(loid)s among other populations at higher risk of GDM.
In addition to the aforementioned major findings, we also detected suggestive positive associations between selenium and GDM, a result that differs from previous studies (10, 27, 40–43). These studies (27, 41–43) were conducted outside the United States, with exposures to selenium that were lower than exposures in our study population (mean serum/plasma selenium concentrations below 100 μg/L vs 123.9 μg/L, respectively). Evidence from in vivo studies and animal studies suggested that the role of selenium in glucose metabolism depends on the concentration and the compound type (12, 44–46). In fact, one epidemiological study involving 8876 nonpregnant US adults (NHANES III participants) found the relationship between selenium and the prevalence of diabetes to be nonlinear and generally positive in higher levels of selenium (47). Therefore, it is possible that a protective effect of selenium is limited to only a lower range and that higher selenium levels exceeding certain levels can exert a hyperglycemic effect.
The current study has several limitations. First, nonfasting glucose levels from GLT can be affected by the collection date and timing of the last meal; therefore, glucose levels may not truly represent the level of insulin resistance of the women. Second, a single measurement of the metal(loid)s at enrollment may or may not capture the true exposure levels during early pregnancy. This is of less concern with selenium and copper, for which we found high correlations between measurements at enrollment and measurements during the early second trimester (gestational weeks 16 to 22) among a subsample of 101 women who were followed up longitudinally (Spearman correlation = 0.72 and 0.75, respectively). Third, adjusting for gestational weeks at blood collection may not fully account for variations in plasma volume expansion during pregnancy (48). Nevertheless, all metal(loid)s were collected within a narrow range (8 to 13 weeks’ gestation), when little increase in plasma volume occurs. In addition, it is important to note that most of the sources of error mentioned previously are likely to be nondifferential, therefore leading to bias toward the null.
The recruitment criteria of the study population may limit the generalizability of our results. For example, women who were obese as well as those who had preexisting chronic conditions, psychiatric disorders, or previous pregnancy complications were excluded from the current study. Although this allowed us to evaluate risk factors independently of baseline reproductive health status, caution should be taken when generalizing these results to other populations with higher risk of pregnancy complications, who may be more susceptible to pregnancy exposures. In addition, although certain racial/ethnic groups with higher prevalence of GDM, such as Asian/Pacific Islanders (49), were oversampled, exploration of potential effect modification by race/ethnicity was not well powered in this study because of limited sample sizes in each racial/ethnic group. Finally, future studies should also take into account interactions among the metal(loid)s.
Despite these limitations, this study has several strengths. First, this study is among the few to evaluate prospective associations between specific essential metal(loid)s with glucose levels during pregnancy. The prospective design and early measurements of metal(loid)s minimize the possibility of reverse causation and differential measurement errors. Second, the use of quantile regression allowed us to fully capture the associations between essential metal(loid)s and glucose levels. We were able to observe shifts in the distribution that would not have been detectable using classic regression methods focusing solely on the mean. In addition, quantile regression does not require any distributional assumptions (e.g., normality of the outcome) and is not sensitive to the presence of outliers. Third, we were able to evaluate and adjust for a number of potential confounders as well as important risk factors of GDM, reducing the chance of bias. Fourth, we assessed the association in a relatively large multiracial/ethnic population of US women from multiple sites.
Conclusion
In this study, we found that elevated plasma copper level was associated with higher glucose level and abnormal GLT results on routine GDM testing used as part of standard obstetrical care, suggesting higher risk of GDM. In contrast, higher concentrations of molybdenum in plasma were associated with decreased glucose levels. Future studies are needed to replicate these findings, as well as to investigate the link between essential metal(loid)s and glucose metabolism during pregnancy in more detail among populations with different baseline risks and across different ranges of exposure. If replicated and well quantified, these findings could provide early markers of potentially modifiable environmental exposures that are related to glucose dysregulation in pregnancy.
Acknowledgments
Financial Support: This work is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Intramural Research Program (contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, HHSN275291199991I, and HHSN275201000001Z) and the National Institute of Environmental Health Sciences (contract number P30ES000002).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- GDM
gestational diabetes mellitus
- GLT
gestational load test
- NHANES
National Health and Nutrition Examination Survey
- OGTT
oral glucose tolerance test
Reference and Notes
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis. 2014;11:130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bardenheier BH, Elixhauser A, Imperatore G, Devlin HM, Kuklina EV, Geiss LS, Correa A. Variation in prevalence of gestational diabetes mellitus among hospital discharges for obstetric delivery across 23 states in the United States. Diabetes Care. 2013;36(5):1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care. 2007;30(Suppl 2):S105–S111. [DOI] [PubMed] [Google Scholar]
- 6. The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 7. Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. [DOI] [PubMed] [Google Scholar]
- 8. Mwiti Kibiti C, Jide Afolayan A. The biochemical role of macro and micro-minerals in the management of diabetes mellitus and its associated complications: a review. Int J Vitam Nutr Res. 2015;85(1-2):88–103. [DOI] [PubMed] [Google Scholar]
- 9. Kaur B, Henry J. Micronutrient status in type 2 diabetes. Adv Food Nutr Res. 2014:71:55–100. [DOI] [PubMed] [Google Scholar]
- 10. Kong F-J, Ma L-L, Chen S-P, Li G, Zhou J-Q. Serum selenium level and gestational diabetes mellitus: a systematic review and meta-analysis. Nutr J. 2016;15(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. González-Villalva A, Colín-Barenque L, Bizarro-Nevares P, Rojas-Lemus M, Rodríguez-Lara V, García-Pelaez I, Ustarroz-Cano M, López-Valdez N, Albarrán-Alonso JC, Fortoul TI. Pollution by metals: is there a relationship in glycemic control? Environ Toxicol Pharmacol. 2016;46:337–343. [DOI] [PubMed] [Google Scholar]
- 12. Mueller AS, Bosse A, Pallauf J. Selenium, an ambivalent factor in diabetes? Established facts, recent findings and perspectives. Curr Nutr Food Sci. 2006;2(2):151–168. [Google Scholar]
- 13. Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–1208. [DOI] [PubMed] [Google Scholar]
- 14. Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT. Association between maternal zinc status, dietary zinc intake and pregnancy complications: a systematic review. Nutrients. 2016;8(10):641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grewal J, Grantz KL, Zhang C, Sciscione A, Wing DA, Grobman WA, Newman RB, Wapner R, D’Alton ME, Skupski D, Nageotte MP, Ranzini AC, Owen J, Chien EK, Craigo S, Albert PS, Kim S, Hediger ML, Buck Louis GM. Cohort profile: NICHD fetal growth studies-singletons and twins. Int J Epidemiol. 2018;47(1):25–25l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta Y, Kalra B, Baruah MP, Singla R, Kalra S. Updated guidelines on screening for gestational diabetes. Int J Womens Health. 2015;7:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773. [DOI] [PubMed] [Google Scholar]
- 18. Galtier F. Definition, epidemiology, risk factors. Diabetes Metab. 2010;36(6 Pt 2):628–651. [DOI] [PubMed] [Google Scholar]
- 19. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115(3):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bouthoorn SH, Silva LM, Murray SE, Steegers EA, Jaddoe VW, Moll H, Hofman A, Mackenbach JP, Raat H. Low-educated women have an increased risk of gestational diabetes mellitus: the Generation R Study. Acta Diabetol. 2015;52(3):445–452. [DOI] [PubMed] [Google Scholar]
- 21. Koenker R, Hallock KF. Quantile regression. J Econ Perspect. 2001;15(4):143–156. [Google Scholar]
- 22. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 23. Koenker R. quantreg: quantile regression. 2017. Available at: https://CRAN.R-project.org/package=quantreg. Accessed 18 December 2018.
- 24. Harrell FE., Jr rms: Regression modeling strategies. 2017. Available at:https://CRAN.R-project.org/package=rms. Accessed 27 January 2019.
- 25. Zheng Y, Zhang C, Weisskopf M, Williams PL, Parsons PJ, Palmer CD, Buck Louis GM, James-Todd T. Data from: A prospective study of early pregnancy essential metal(loid)s and glucose levels late in the second trimester. figshare 2018. Deposited 24 December 2018. 10.6084/m9.figshare.7505324.v2. [DOI] [PMC free article] [PubMed]
- 26. Wang Y, Tan M, Huang Z, Sheng L, Ge Y, Zhang H, Jiang M, Zhang G. Elemental contents in serum of pregnant women with gestational diabetes mellitus. Biol Trace Elem Res. 2002;88(2):113–118. [DOI] [PubMed] [Google Scholar]
- 27. Al-Saleh E, Nandakumaran M, Al-Shammari M, Al-Harouny A. Maternal fetal status of copper, iron, molybdenum, selenium and zinc in patients with gestational diabetes. J Matern-Fetal Neonatal Med. 2004;16(1):15–21. [DOI] [PubMed] [Google Scholar]
- 28. Loven A, Romem Y, Pelly IZ, Holcberg G, Agam G. Copper metabolism: a factor in gestational diabetes? Clin Chim Acta. 1992;213(1-3):51–59. [DOI] [PubMed] [Google Scholar]
- 29. Abou-Seif MA, Youssef A-A. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346(2):161–170. [DOI] [PubMed] [Google Scholar]
- 30. Atari-Hajipirloo S, Valizadeh N, Khadem-Ansari M-H, Rasmi Y, Kheradmand F. Altered concentrations of copper, zinc, and iron are associated with increased levels of glycated hemoglobin in patients with type 2 eiabetes mellitus and their first-degree relatives. Int J Endocrinol Metab. 2016;14(2):e33273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cooper GJS, Chan Y-K, Dissanayake AM, Leahy FE, Keogh GF, Frampton CM, Gamble GD, Brunton DH, Baker JR, Poppitt SD. Demonstration of a hyperglycemia-driven pathogenic abnormality of copper homeostasis in diabetes and its reversibility by selective chelation: quantitative comparisons between the biology of copper and eight other nutritionally essential elements in normal and diabetic individuals. Diabetes. 2005;54(5):1468–1476. [DOI] [PubMed] [Google Scholar]
- 32. Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology: copper. Biomed Pharmacother. 2003;57(9):386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hordyjewska A, Popiołek Ł, Kocot J. The many “faces” of copper in medicine and treatment. Biometals. 2014;27(4):611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vukelić J, Kapamadzija A, Petrović D, Grujić Z, Novakov-Mikić A, Kopitović V, Bjelica A. Variations of serum copper values in pregnancy. Srp Arh Celok Lek. 2012;140(1-2):42–46. [PubMed] [Google Scholar]
- 35. McArdle HJ. The metabolism of copper during pregnancy: a review. Food Chem. 1995;54(1):79–84. [Google Scholar]
- 36. Agency for Toxic Substances and Disease Registry. Toxicological profile for molybdenum. April 2017. Available at: https://www.atsdr.cdc.gov/ToxProfiles/tp212.pdf. Accessed 19 July 2017. [PubMed]
- 37. Lammi-Keefe CJ, Couch SC, Philipson E, eds. Handbook of Nutrition and Pregnancy. Totowa, NJ: Humana Press; 2008. [Google Scholar]
- 38. Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29(10):2223–2230. [DOI] [PubMed] [Google Scholar]
- 39. Bao W, Bowers K, Tobias DK, Olsen SF, Chavarro J, Vaag A, Kiely M, Zhang C. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: a prospective cohort study. Am J Clin Nutr. 2014;99(6):1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Askari G, Iraj B, Salehi-Abargouei A, Fallah AA, Jafari T. The association between serum selenium and gestational diabetes mellitus: a systematic review and meta-analysis. J Trace Elem Med Biol. 2015;29:195–201. [DOI] [PubMed] [Google Scholar]
- 41. Tan M, Sheng L, Qian Y, Ge Y, Wang Y, Zhang H, Jiang M, Zhang G. Changes of serum selenium in pregnant women with gestational diabetes mellitus. Biol Trace Elem Res. 2001;83(3):231–237. [DOI] [PubMed] [Google Scholar]
- 42. Bo S, Lezo A, Menato G, Gallo ML, Bardelli C, Signorile A, Berutti C, Massobrio M, Pagano GF. Gestational hyperglycemia, zinc, selenium, and antioxidant vitamins. Nutrition. 2005;21(2):186–191. [DOI] [PubMed] [Google Scholar]
- 43. Kilinc M, Guven MA, Ezer M, Ertas IE, Coskun A. Evaluation of serum selenium levels in Turkish women with gestational diabetes mellitus, glucose intolerants, and normal controls. Biol Trace Elem Res. 2008;123(1-3):35–40. [DOI] [PubMed] [Google Scholar]
- 44. Spallholz JE, Palace VP, Reid TW. Methioninase and selenomethionine but not Se-methylselenocysteine generate methylselenol and superoxide in an in vitro chemiluminescent assay: implications for the nutritional carcinostatic activity of selenoamino acids. Biochem Pharmacol. 2004;67(3):547–554. [DOI] [PubMed] [Google Scholar]
- 45. Drake EN. Cancer chemoprevention: selenium as a prooxidant, not an antioxidant. Med Hypotheses. 2006;67(2):318–322. [DOI] [PubMed] [Google Scholar]
- 46. Mueller AS, Pallauf J. Compendium of the antidiabetic effects of supranutritional selenate doses: in vivo and in vitro investigations with type II diabetic db/db mice. J Nutr Biochem. 2006;17(8):548–560. [DOI] [PubMed] [Google Scholar]
- 47. Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30(4):829–834. [DOI] [PubMed] [Google Scholar]
- 48. Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr. 2017;106(Suppl 6):1620S–1625S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chu SY, Abe K, Hall LR, Kim SY, Njoroge T, Qin C. Gestational diabetes mellitus: all Asians are not alike. Prev Med. 2009;49(2-3):265–268. [DOI] [PubMed] [Google Scholar]