Abstract
Context
Kisspeptin–neurokinin B (NKB)–dynorphin neurons are critical regulators of the hypothalamic–pituitary–gonadal axis. NKB and dynorphin are hypothesized to influence the frequency of GnRH pulses, whereas kisspeptin is hypothesized to be a generator of the GnRH pulse. How these neuropeptides interact remains unclear.
Objective
To probe the role of NKB in GnRH pulse generation and to determine the interactions between NKB, kisspeptin, and dynorphin in humans and mice with a complete absence of NKB.
Design
Case/control.
Setting
Academic medical center.
Participants
Members of a consanguineous family bearing biallelic loss-of-function mutations in the gene encoding NKB and NKB-deficient mice.
Interventions
Frequent blood sampling to characterize neuroendocrine profile and administration of kisspeptin, GnRH, and naloxone, a nonspecific opioid receptor antagonist used to block dynorphin.
Main Outcome Measures
LH pulse characteristics.
Results
Humans lacking NKB demonstrate slow LH pulse frequency, which can be increased by opioid antagonism. Mice lacking NKB also demonstrate impaired LH secretion, which can be augmented with an identical pharmacologic manipulation. Both mice and humans with NKB deficiency respond to exogenous kisspeptin.
Conclusion
The preservation of LH pulses in the absence of NKB and dynorphin signaling suggests that both peptides are dispensable for GnRH pulse generation and kisspeptin responsiveness. However, NKB and dynorphin appear to have opposing roles in the modulation of GnRH pulse frequency.
This study uses pharmacologic probes to demonstrate that endogenous GnRH-induced LH pulses can be generated in the absence of neurokinin B and dynorphin activity in humans and mice.
Despite nearly 50 years since the discovery of GnRH (1), understanding the factors that trigger GnRH neurons to drive the onset of sexual maturation and subsequently maintain reproductive function remains a challenge. Patients with idiopathic hypogonadotropic hypogonadism (IHH) are a key population to uncover these signals, as they have abnormal GnRH secretion/action (2, 3). Most patients with IHH present as teens with delayed pubertal development and suffer life-long sexual infantilism and infertility when left untreated (2, 3).
Identification of the afferent pathways through which endogenous factors (e.g., gonadal steroids, stress hormones, and nutrient signals) and external cues (e.g., social cues and day length) regulate GnRH release have recently focused on the kisspeptin–neurokinin B (NKB)–dynorphin system (4). Inactivating mutations in kisspeptin, NKB, and their respective receptors causes IHH in humans and mice, implicating these neuropeptides in the generation of GnRH pulses (5–12). Dynorphin is thought to oppose this stimulatory activity by providing critical slowing of GnRH pulse generator activity in response to progesterone during the luteal phase of the menstrual cycle (13–15). These three neuropeptides coalesce in a population of neurons in the arcuate nucleus, kisspeptin–NKB–dynorphin (KNDy) neurons, and are postulated to work in a coordinated fashion to synchronize the secretory activity of GnRH neurons to generate the pulses of GnRH secretion that are necessary to drive reproductive endocrine function (16–18).
Because biallelic loss-of-function mutations disrupt both copies of a gene, patients carrying such mutations (i.e., “human knockouts”) provide novel insights into the phenotypic consequences of gene disruption or loss. In this study, four sisters carrying biallelic, complete loss-of-function mutations in the gene encoding NKB (one of the key neuropeptides in KNDy neurons) underwent genotype-driven phenotyping. Despite an initial diagnosis of IHH, several sisters spontaneously recovered reproductive endocrine function in adult life. Studies were performed in both normal and NKB-deficient family members as well as normal and NKB-deficient mice to investigate the role of NKB in GnRH pulse generation and to dissect the interactions between NKB, kisspeptin, and dynorphin. Use of a combination of specific neuroendocrine probes revealed that the hypothalamus is capable of generating GnRH-induced LH pulses despite genetic and pharmacologic antagonism of two of the three KNDy constituents, NKB and dynorphin.
Methods
Subjects and eligibility criteria
Five women from a single consanguineous family were recruited on the basis of their genotype (Table 1). Subjects were either reproductively normal (subject 1; genotype TAC3 c.61_61delG p.A21LfsX44 heterozygote) or carried a diagnosis of hypogonadotropic hypogonadism (subjects 2, 3, 4, and 5; genotype TAC3 c.61_61delG p.A21LfsX44 homozygote). The brothers and parents were not available for study participation. IHH was defined as hypogonadal sex steroid levels (estradiol <20 pg/mL in women) in the setting of low or normal gonadotropin levels at age ≥18 years and the absence of any identifiable medical condition that could cause hypogonadotropic hypogonadism. As in our previous report (19), reversal of IHH in women was defined as: (i) fertility without use of exogenous GnRH or gonadotropin therapy; (ii) spontaneous menstrual cycling for at least 3 months in the absence of treatment; and/or (iii) LH pulse frequency and amplitude within the normal range for women. Relapse after reversal was defined as again having hypogonadal sex steroid levels (serum estradiol <20 pg/mL in women) and/or amenorrhea.
Table 1.
Study Subject Characteristics
| ID | Presentation | Initial Treatment and Subsequent Course | Research Study 2016 | ||||
|---|---|---|---|---|---|---|---|
| Protocol | FSH (IU/L) | LH (IU/L) | E2 (pg/mL) | Imaging | |||
| TAC3 c.61_61delG p.A21LfsX44 heterozygote | |||||||
| 1 | 12.6 y, menarche | 12.6 y to 35 y, regular monthly menses | i) Baseline | 4.23 | 2.18 | 20.4 | US: endometrium 6mm, multiple small follicles |
| 35 y, pregnant | ii) IVB Kiss, GnRH | ||||||
| TAC3 c.61_61delG p.A21LfsX44 homozygote | |||||||
| 2 | 15 y, 1° amenorrhea, minimal thelarche | 15–20 y, HRT with breast development, growth spurt | Not applicable | ||||
| 20 y, MPA ×10 d, positive withdrawal bleed | |||||||
| Mid-20s, HRT ×6 mo | |||||||
| Mid-20s, herbal medication | |||||||
| 31 y to present, amenorrheic | |||||||
| 3 | 14 y, 1° amenorrhea, no thelarche | 16 y 8 mo, FSH 2.1 IU/L (0.6–11), LH 1.1 IU/L (1–11), E2 <40 pmol/L | i) Baseline and IVB Kiss, GnRH | 2.12 | 0.49 | 20.3 | Normal MRI |
| 16–20 y, HRT with breast development, growth spurt | ii) Kiss infusion and IVB GnRH | US: endometrium 4 mm, all follicles <2 mm, uterus small adult size | |||||
| 22 y, FSH 7.7 IU/L (0.6–11), LH 11.9 IU/L (1–11) | |||||||
| 22 y, MPA ×1, positive withdrawal bleed | |||||||
| 22 y, spontaneous conception of healthy son, 1 month post MPA | |||||||
| 24 y, superovulation ×2 (MPA followed by CC), no pregnancies | |||||||
| 24–37 y, about every 3 mo MPA, positive intermittent withdrawal bleeds | |||||||
| 37–40 y, amenorrheic | |||||||
| 40 y to present, restarted about every 3 mo on MPA | |||||||
| 4 | 14y, 1° amenorrhea, no thelarche | 16 y 4 mo, FSH 2.4 IU/L (0.6–11), LH <0.5 IU/L (1–11), E2 49 pmol/L | i) Baseline and IVB Kiss, GnRH | 3.97 | 0.94 | 11.2 | US: endometrium 5 mm, one follicle 10 mm, uterus small adult size |
| 16–22 y, HRT with breast development | ii) NLX infusion and IVB Kiss, GnRH | ||||||
| 22–24 y, amenorrheic | |||||||
| 24 y, positive home pregnancy test followed by SAB | |||||||
| 24 y, FSH 5.5 IU/L (0.6–11), LH 5.4 IU/L (1–11) | |||||||
| 25–27 y, HRT | |||||||
| 28 y 5 mo, herbal medication, two spontaneous cycles 6 mo apart | |||||||
| 28 y, FSH, LH “normal range,” E2 “low” at 52 pmol/L | |||||||
| 29–30 y, HRT | |||||||
| 30–31 y, amenorrheic | |||||||
| 31 to present, intermittent HRT use | |||||||
| 5 | 13 y, 1° amenorrhea, no thelarche | 17–18y, HRT | i) Baseline and IVB Kiss, GnRH | 3.42 | 0.86 | 34 | Normal MRI |
| 21 y, OCPs for 6 mo | ii) NLX infusion and IVB Kiss, GnRH | US: endometrium 9 mm, cyst 3 cm | |||||
| 25 y, herbal medication, positive withdrawal bleed, repeated without effect | |||||||
| 26–28 y, amenorrheic | |||||||
| 28–29 y, regular monthly cycling (1.3 y) | |||||||
| 29–31y, every 2.5 mo cycles (2.5 y) | |||||||
| 31 y to present, yearly spontaneous spotting | |||||||
Abbreviations: CC, clomiphene citrate; E2, estradiol; HRT, hormone replacement therapy; IVB, IV bolus; Kiss, kisspeptin; MPA, medroxyprogesterone acetate; OCP, oral contraceptive pill; SAB, spontaneous abortion; US, transvaginal ultrasound.
Subjects also participated in a genetics study. Patient DNA was screened for rare sequence variants, defined as having a minor allele frequency of <1% in the Genome Aggregation Database (gnomAD), in genes known to cause IHH, as described previously (20, 21). Genes screened were CHD7 (MIM 608892), FGF8 (MIM 600483), FGFR1 (MIM 136350), GNRH1 (MIM 152760), GNRHR (MIM 138850), HS6ST1 (MIM 604846), ANOS1 (previously called KAL1, MIM 300836), KISS1 (MIM 603286), KISS1R (MIM 604161), NSMF (previously called NELF, MIM 60813), PROK2 (MIM 607002), PROKR2 (MIM 607123), TAC3 (MIM 162330), and TACR3 (MIM 162332) by PCR amplification of exons followed by Sanger sequencing. Rare sequence variants were reported when they were predicted to be damaging by at least two out of four in silico prediction programs: PolyPhen-2 (22), SIFT (23), MutationTaster (24), or Panther (25). The University of Pennsylvania Smell Identification Test scores, from a 12-item smell test, were used to classify olfactory capabilities (26, 27).
Study design
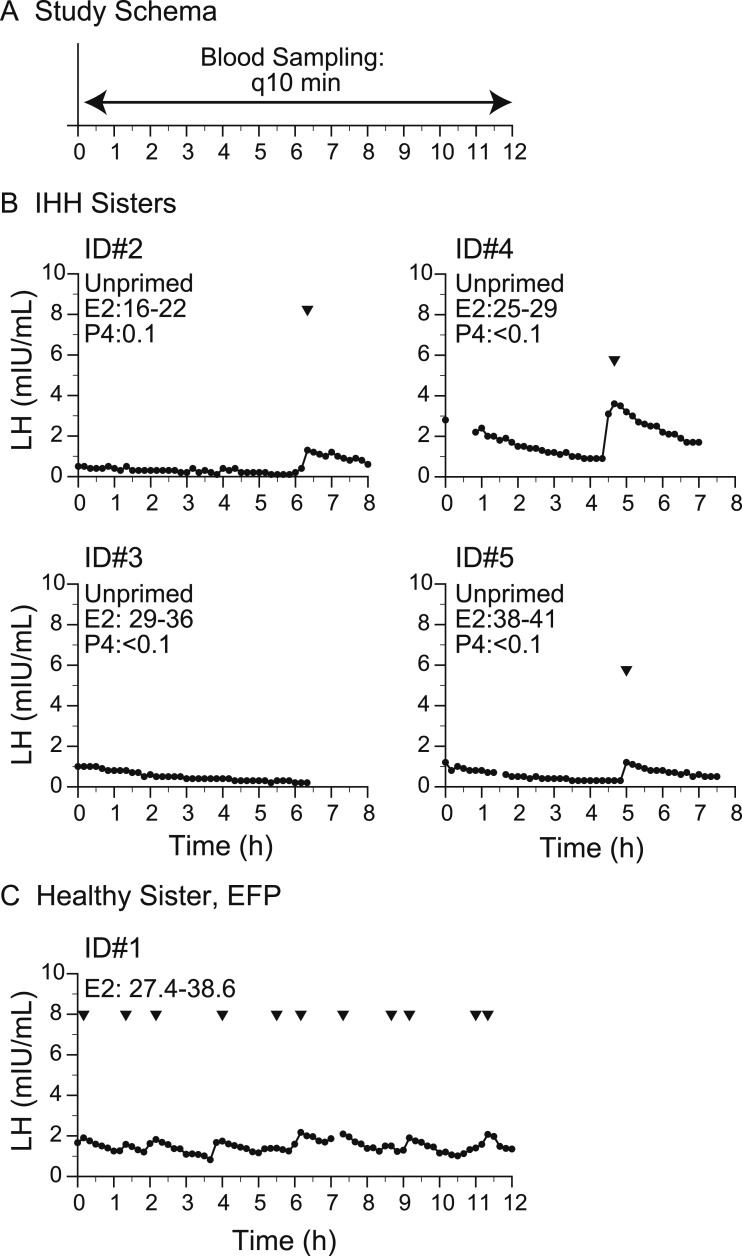
In 2010, the subjects with hypogonadotropic hypogonadism (subjects 2, 3, 4, and 5) underwent detailed neuroendocrine phenotyping in which blood sampling was performed every 10 minutes for 6 to 8 hours to map endogenous LH pulsations at the Wellcome Trust Clinical Research Facility, Cambridge, UK, under the direction of Prof. I. Sadaf Farooqi (Fig. 1A).
Figure 1.
Baseline neuroendocrine profiling. (A) Study schema. (B) Study subjects with IHH who underwent 8-h sampling between 2010 and 2011. (C) Healthy sister in early follicular phase (EFP). Arrowheads indicate LH pulses detected by the algorithm. E2, estradiol; P, progesterone; q10 min, every 10 min.
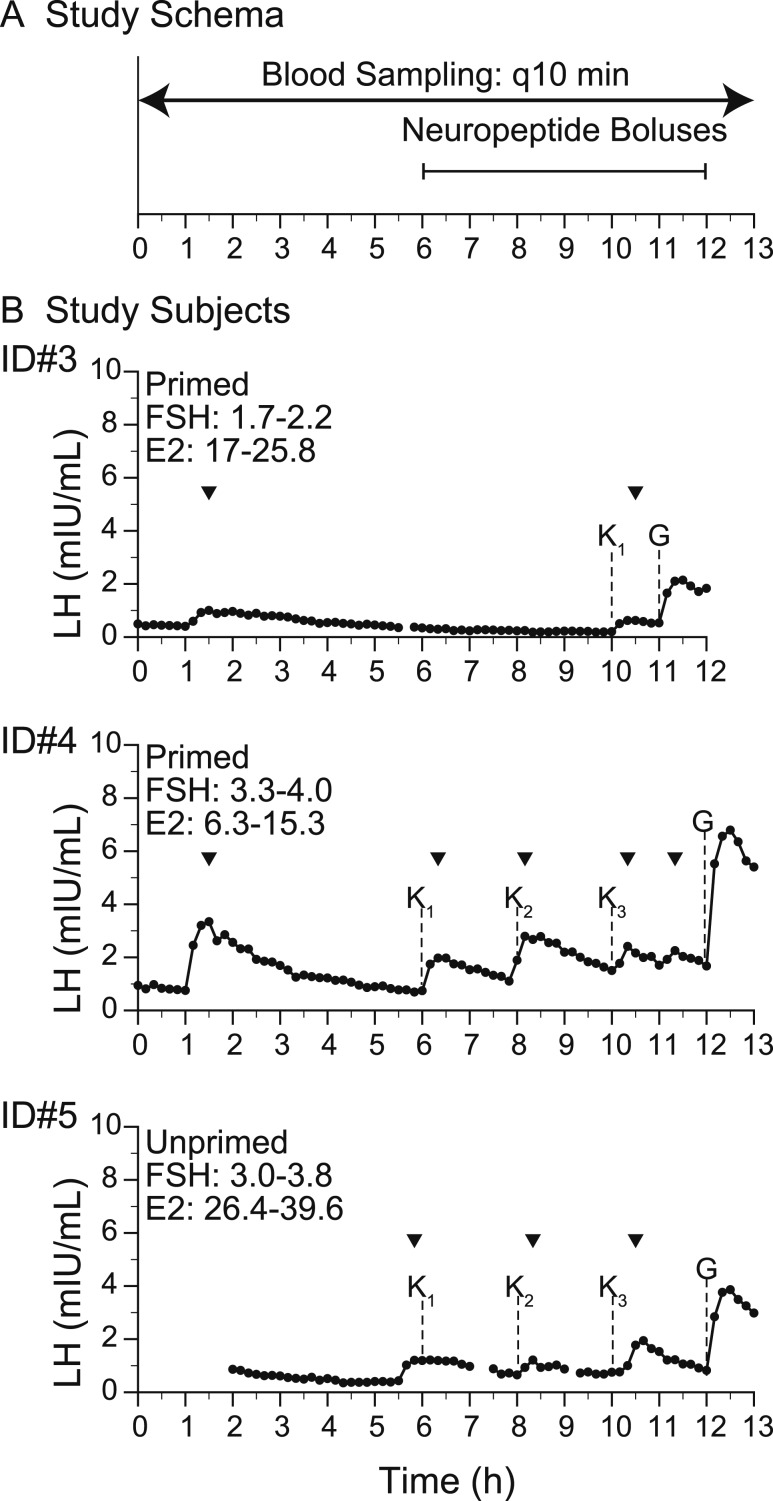
In 2016, subjects 1, 3, 4, and 5 were invited to participate in a second series of daytime studies at Massachusetts General Hospital (MGH) Clinical Research Center (CRC) to determine whether their endogenous LH pulse patterns could be modified by administration of GnRH, kisspeptin 112–121 (kp-10), and the nonspecific opioid antagonist that blocks dynorphin, naloxone (NLX) (Figs. 2A, 3A, and Fig. 4A). To ensure that the pituitary gonadotropes would be in a state of readiness, subjects 3 and 4 received exogenous pulsatile GnRH (25 ng/kg) every 2 hours by a Crono F portable infusion pump (Canè, Turin, Italy) for 3 days prior to admission to the MGH CRC (28). Subject 5 had recent evidence of some neuroendocrine activity (yearly spontaneous bleeding), so she was not primed with pulsatile GnRH (Table 1).
Figure 2.
Baseline studies with response to kisspeptin and GnRH. (A) Study schema. (B) Study subjects. Arrowheads indicate LH pulses detected by the algorithm. E2, estradiol; G, GnRH IVB (75 ng/kg); K, kp-10 by IVB (subscript indicates the dose: 1, 0.24 nmol/kg; 2, 0.72 nmol/kg; 3, 2.4 nmol/kg); q10 min, every 10 min.
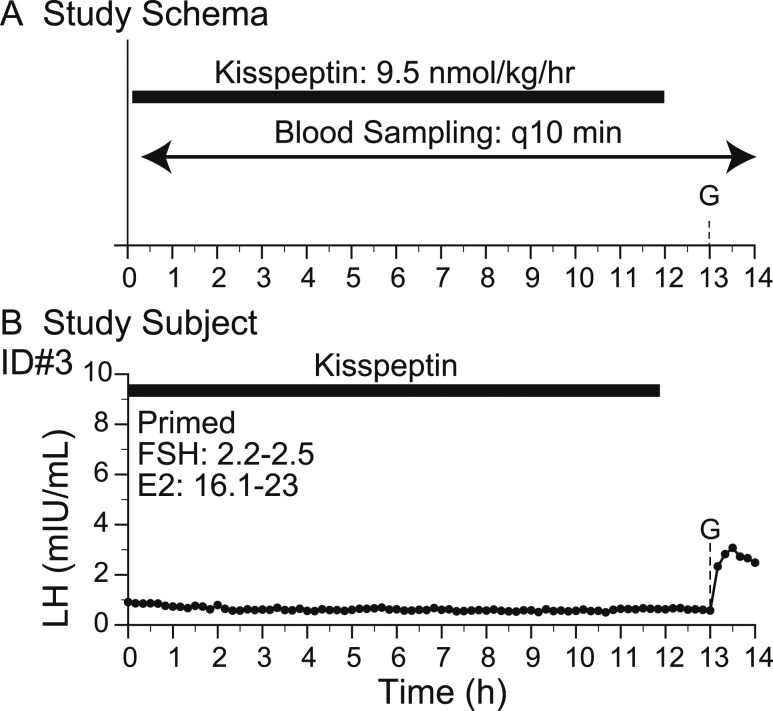
Figure 3.
Response to kisspeptin infusion and GnRH. (A) Study schema. (B) Study subject. Arrowheads indicate LH pulses detected by the algorithm. E2, estradiol; G, GnRH IVB (75 ng/kg); q10 min, every 10 min.
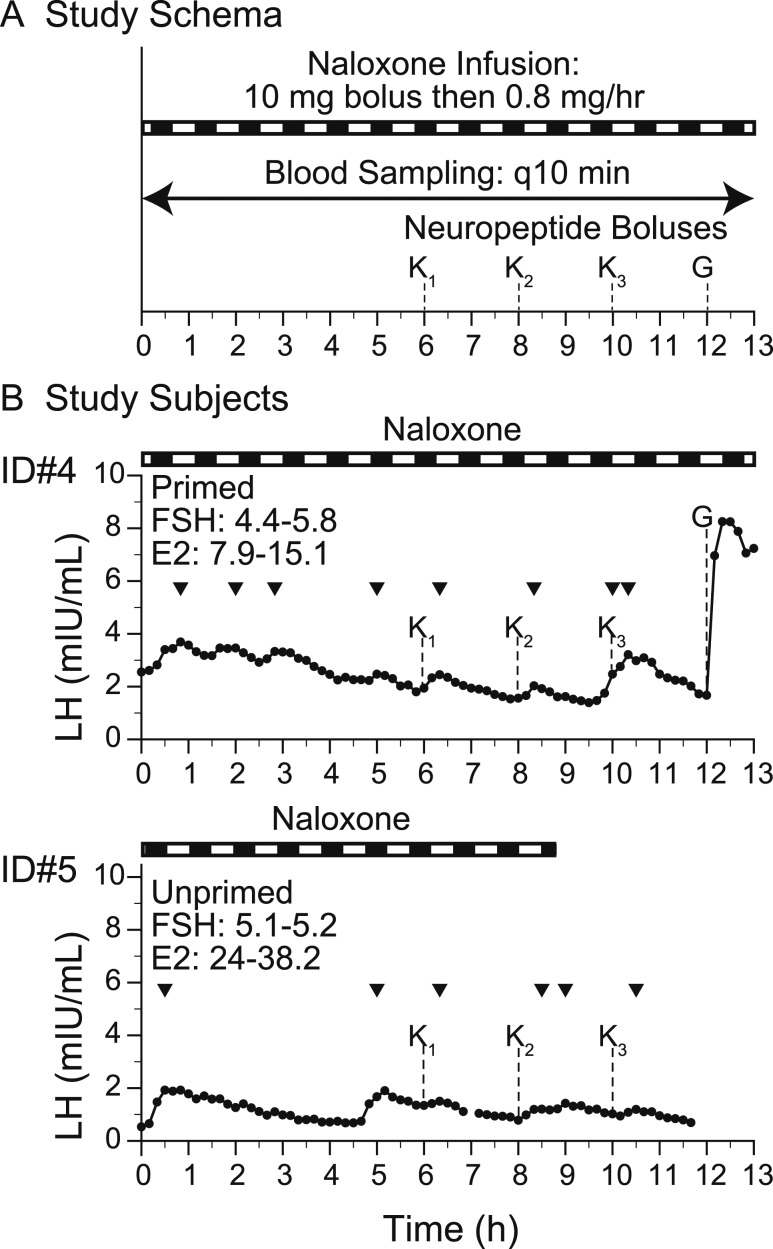
Figure 4.
Neuropeptide administration with response to kisspeptin and GnRH. (A) Study schema. (B) Study subjects. Arrowheads indicate LH pulses detected by the algorithm. E2, estradiol; G, GnRH IVB (75 ng/kg); K, kp-10 by IVB (subscript indicates the dose: 1, 0.24 nmol/kg; 2, 0.72 nmol/kg; 3, 2.4 nmol/kg); q10 min, every 10 min.
Baseline studies.
All subjects underwent blood sampling every 10 minutes for at least 6 hours to evaluate endogenous GnRH-induced LH secretion during one of their visit days to the MGH CRC (Figs. 1A and 2A).
Kisspeptin boluses.
After assessment of endogenous GnRH-induced LH secretion, subjects 3, 4, and 5, received the administration of a kp-10 0.24 nmol/kg intravenous bolus (IVB), as prior work by our group demonstrated that this dose consistently elicits GnRH-induced LH pulses of physiologic amplitude in healthy men and healthy luteal-phase women (29, 30) (Fig. 2A). Subjects 4 and 5 received subsequent kp-10 IVBs of 0.72 and 2.4 nmol/kg. Subjects 3, 4, and 5 then received a 75 ng/kg IVB of GnRH at the conclusion of these studies, as our group has previously shown that this dose results in robust GnRH-induced LH responses in individuals with intact gonadotrope function (31).
Kisspeptin infusion.
In contrast to the IVB studies, subject 3 returned to the CRC to participate in a second admission in which kp-10 was administered as a continuous infusion (9.5 nmol/kg/h) for 12 hours to determine its effect on endogenous GnRH-induced LH pulsations. Similar to the IVB studies, blood samples were drawn every 10 minutes and a GnRH 75 ng/kg IVB was administered at study conclusion (Fig. 3A).
NLX infusion, blocking dynorphin.
Subjects 4 and 5 returned to the CRC and received an NLX infusion (NLX 10 mg IVB, followed by infusion at 0.8 mg/h) for 13 hours to determine the effect of blocking dynorphin signaling with opioid antagonism on endogenous LH pulses in the absence of NKB signaling. Midway through the infusion, kp-10 and GnRH boluses (kp-10 dose range, 0.24 to 2.4 nmol/kg; GnRH, 75 ng/kg) were administered to determine whether NLX administration might enhance the response to these peptides (Fig. 4A). Again, blood samples were drawn every 10 minutes for hormone measurements. Owing to nursing error, subject 5 had the NLX infusion terminated early at hour 9.
Source of peptides
kp-10, the 10–amino acid isoform of kisspeptin (corresponding to amino acids 112–121 of the preprohormone), and GnRH were synthesized using good manufacturing practices by NeoMPS (PolyPeptide Laboratories, San Diego, CA). NeoMPS provided kp-10 under contract to the Eunice Kennedy Shriver National Institute of Child Health and Human Development. NLX was ordered from Hospira (Lake Forest, IL).
Human laboratory assays
LH for each sample and estradiol on 2-hour pools were measured by direct immunoassay using the automated Abbott Architect system (Abbott Laboratories, Abbott Park, IL) as previously described (28). Estradiol was measured by a second generation immunoassay traceable to mass spectrometry–based assays for the 2010 to 2011 studies and by Elecsys (Roche Diagnostics, Indianapolis, IN) for 2016 studies (32, 33).
Assessment of pulsatile LH release in peripubertal and adult Tac2 knockout mice
Tac2 +/− breeding pairs were generated by the Texas A&M Institute for Genomic Medicine (College Station, TX) and genotyped (34). All mice were generated and maintained on a Sv129/C57BL/6 hybrid background and group housed (three to five per cage) at the Brigham and Women’s Hospital in a temperature- and light-controlled environment with lights on from 0600 to 1800 hours and food and water provided ad libitum. Mice were handled daily for 2 to 6 weeks prior to the experiment to allow acclimation to sampling conditions.
Changes in LH secretion were assessed in sexually maturing (6-week-old) and adult (16-week-old) intact and ovariectomized (OVX) Tac2 knockout (KO) female mice and control [wild-type (WT)] littermates (n = 4 to 5 per group). Because Tac2 in mice encodes for NKB in humans, these mice are lacking NKB. Pulsatile measurements of LH secretion were assessed by repeated blood collection through a single incision at the tip of the tail. The tail was cleaned with saline and then 4 μL of blood was taken at each time point from the cut tail with a pipette. Whole blood was immediately diluted in 116 μL of 0.05% PBS with Tween 20, vortexed, and frozen on dry ice. Samples were stored at −80°C for a subsequent LH ELISA. For kp-10 administration studies, 36 sequential blood samples were collected during a 6-hour sampling period. At 170 minutes of sampling (or 180 minutes of sampling for peripubertal Tac2 KO mice), mice were injected with mouse kp-10 IP (7.5 nmol/100 μL of saline; Phoenix Pharmaceuticals). For NLX administration studies, 30 sequential blood samples were collected during a 5-hour sampling period from WT and Tac2 KO mice. WT and Tac2 KO mice were OVX to increase the frequency and amplitude of LH pulses to better determine the action of dynorphin removal in the generation of LH pulses. At 120 minutes of sampling, mice were injected with NLX IP (5 mg/kg/100 μL of saline; Sigma-Aldrich).
Data analysis
Human pulse analysis.
LH pulses were identified using a validated modification of the Santen and Bardin method (35, 36) augmented by a deconvolution algorithm (29). Pulse amplitude of kp-10–induced or GnRH-induced LH pulses was calculated as the difference between time 0 of kp-10 or GnRH administration and the peak of the pulse.
Mouse pulse analysis.
LH pulses were identified using a custom-made MATLAB code that reads the LH pulse data gathered by LH sandwich ELISA. The code includes a loop that determines a pulse based on whether (i) the height of an LH value is 20% greater than the heights of either of the two previous values as well as 10% greater than the height of the following value; (ii) the peak at the second time interval is >20% greater than the single value that comes before it to be considered a pulse.
Statistical analysis.
Paired two-way t tests were used to assess changes in mean LH, LH amplitude (nadir to peak of an LH pulse), and FSH at baseline, as defined in methods above, as compared with responses to neuropeptide interventions. All values are reports as mean ± SD, unless otherwise noted.
Study approval
All human studies were approved by the Institutional Review Board of MGH/Partners HealthCare, or by the Local Regional Ethics Committee of Cambridge, United Kingdom. All subjects gave written informed consent prior to inclusion in the studies. For the mouse studies, the Brigham and Women’s Hospital Institutional Animal Care and Use Committee approved all procedures.
Results
Study subjects initial clinical presentation and subsequent course
Subject 1 had a normal timing of menarche, normal menstrual cycles, and spontaneous pregnancy (Table 1). Her sisters, subjects 2, 3, 4, and 5, presented at 13 to 15 years with primary amenorrhea and received estrogen therapy to induce secondary sexual characteristics. Because of the lack of spontaneous sexual maturation by age 18, normal MRI, and low gonadotropins, subjects 2, 3, 4, and 5 all received a diagnosis of IHH (Table 1). None of the sisters is anosmic. Three of the four sisters with IHH demonstrated reversal of their hypogonadotropism between 22 and 28 years as evidenced by pregnancy without fertility medications (subjects 3 and 4) and regular spontaneous menstrual cycles (subject 5). However, reversal was not permanent and at the time of the physiologic studies, as subjects 3, 4, and 5 had reverted to a state of hypogonadotropic hypogonadism (Table 1).
Genetics
Sequencing of candidate genes revealed that subject 1 (normal timing of puberty and normal menstrual cycles) is heterozygous for a deletion of a single nucleotide in the gene encoding NKB (TAC3) (c.61_61delG p.A21LfsX44). This base pair deletion leads to a frameshift mutation and a premature stop codon, in the preprohormone prior to the NKB sequence, that would be predicted to result in nonsense-mediated decay. Even if the transcript were to escape nonsense-mediated decay, the frameshift mutation would disrupt the portion of the preprohormone that is processed to produce the decapeptide known as NKB. Subjects 2, 3, 4, and 5, all with hypogonadotropic hypogonadism, are homozygous for this frameshift mutation. This mutation is novel and not found in gnomAD, a normative database containing 123,136 exomes and 15,496 genomes (21). Notably, there are no individuals homozygous for any protein-truncating mutations in TAC3 in gnomAD. This family harbors no other mutations in genes known to cause IHH.
Baseline studies: slow LH pulse frequency characterizes individuals with IHH without NKB
At the time of these baseline studies, the sisters with IHH (subjects 2, 3, 4, and 5) were amenorrheic with low but detectable serum estradiol levels and low progesterone levels off hormonal medications (Table 1; Fig. 1B). All subjects with IHH had evidence of an enfeebled but organized GnRH pulse generator, as evidenced by low-frequency LH secretory events [for comparison in the physiologic early follicular phase, which is characterized by low estradiol, low progesterone: LH frequency, 7.0 ± 1.8 pulses per 12 hours; LH amplitude, 2.3 ± 1.0 IU/L (mean ± 2 SD)] (37, 38). In subjects 2, 4, and 5, one pulse was observed in the sampling interval (7 to 8 hours; mean LH amplitude, 1.5 ± 0.8 mIU/mL) (Fig. 1B). In subject 3, no pulses were observed during the study. Additionally, the LH levels of subjects 3, 4, and 5 demonstrated slow decay at the beginning of the sampling interval, suggesting that an LH secretory event had occurred before the start of the study. Thus, all subjects demonstrated an abnormally low frequency of LH secretory events. Upon repeat testing in 2016, study subjects (subjects 3, 4, and 5) again were amenorrheic with low but detectable estradiol levels off hormonal medications. All studies recapitulated the same endogenous LH patterns observed in 2010, with low-frequency LH secretory events and a mean LH amplitude of 1.3 ± 1.1 mIU/mL (Fig. 2B).
In contrast, subject 1, the healthy sister with a heterozygous protein truncating variant in TAC3, underwent blood sampling on day 4 of the menstrual cycle (early follicular phase). She exhibited 11 LH pulses in 12 hours with a mean LH pulse amplitude of 0.46 ± 0.25 mIU/mL (Fig. 1C) [healthy early follicular phase women: frequency, 7.0 ± 1.8 pulses per 12 hours; amplitude, 2.3 ± 1.0 IU/L (mean ± 2 SD)] (37, 38).
Kisspeptin boluses: individuals with IHH without NKB respond to kisspeptin
All subjects responded to kisspeptin with an LH pulse (Fig. 2B). Two study subjects received three kisspeptin boluses and demonstrated an LH pulse following kisspeptin in five of the six boluses. The one exception occurred when kisspeptin was administered immediately following an endogenous LH peak, resulting in a prolonged single peak (Fig. 2B, subject 5). Consistent with this responsiveness, all subjects demonstrated adequate pituitary priming, indicating no pituitary defect that could impair kisspeptin responsiveness (LH pulse amplitude following GnRH administration: subject 3, 1.6 mIU/mL; subject 4, 5.1 mIU/mL; subject 5, 3.0 mIU/mL).
Kisspeptin infusion: no pulsatile LH secretion
Subject 3 received a kp-10 infusion (9.5 nmol/kg/h) for 12 hours and no LH pulses were detected. There was a modest increase in mean LH during the infusion (baseline, 0.46 ± 0.24 mIU/mL; kp-10 infusion, 0.63 ± 0.08 mIU/mL; P < 0.0001) (Figs. 2B and 3B). Mean FSH levels also increased as compared with baseline (baseline, 1.9 ± 0.2 mIU/mL; kp-10 infusion, 2.4 ± 0.1 mIU/mL; P < 0.001). After the kp-10 infusion, subject 3 received an IVB of GnRH resulting in an LH pulse of comparable amplitude to that observed in the baseline study the prior day (baseline, 1.6 mIU/mL; after kp-10 infusion, 2.5 mIU/mL).
NLX infusion: blocking dynorphin with NLX increases LH and FSH secretion and LH pulse frequency, but it does not amplify kisspeptin-induced LH pulses
Subjects 4 and 5 received the nonselective opioid antagonist, NLX, as well as escalating boluses of kisspeptin (0.24, 0.72, 2.4 nmol/kg) to determine the effect of blocking dynorphin signaling on endogenous and kisspeptin-stimulated LH secretory patterns. Both studies demonstrated increased mean LH levels during NLX infusion as compared with baseline (subject 4: baseline, 1.44 ± 0.76 mIU/mL; NLX, 2.82 ± 0.54 mIU/mL; P < 0.00001; subject 5: baseline, 0.6 ± 0.25 mIU/mL; NLX, 1.1 ± 0.37 mIU/mL; P < 0.00001, across matched time points) (Figs. 2B and 4B). For the study subject in which a complete LH sampling on and off NLX infusion allowed comparison, subject 4, LH pulse frequency increased from one pulse in 6 hours (Fig. 2B) to four pulses in 6 hours (Fig. 4B). Mean FSH levels also increased as compared with baseline (subject 4: baseline, 3.7 ± 0.3 mIU/mL; NLX, 5.0 ± 0.9 mIU/mL; P < 0.01; subject 5: baseline, 3.3 ± 0.3 mIU/mL; NLX, 5.1± 0.1 mIU/mL; P < 0.0001). There was no consistent change in LH pulse amplitude (subject 4: baseline, 2.59 mIU/L; NLX, 0.45 ± 0.29 mIU/mL; subject 5: baseline, 0.82 mIU/mL; NLX, 1.22 and 1.39 mIU/mL). NLX infusions, which block dynorphin by inhibiting opioid tone, increase gonadotropin secretion and improve LH pulse frequency in individuals with IHH due to loss of NKB signaling.
Subjects 4 and 5 also received escalating boluses of kp-10 (0.24, 0.72, 2.4 nmol/kg), which were followed by an LH pulse, recapitulating results seen off NLX (Figs. 2B and 4B). There was no significant difference in the change in kisspeptin-induced LH response on or off NLX, and there was no clear dose-response relationship, although the small number of boluses at each dose limited the ability to assess such a relationship.
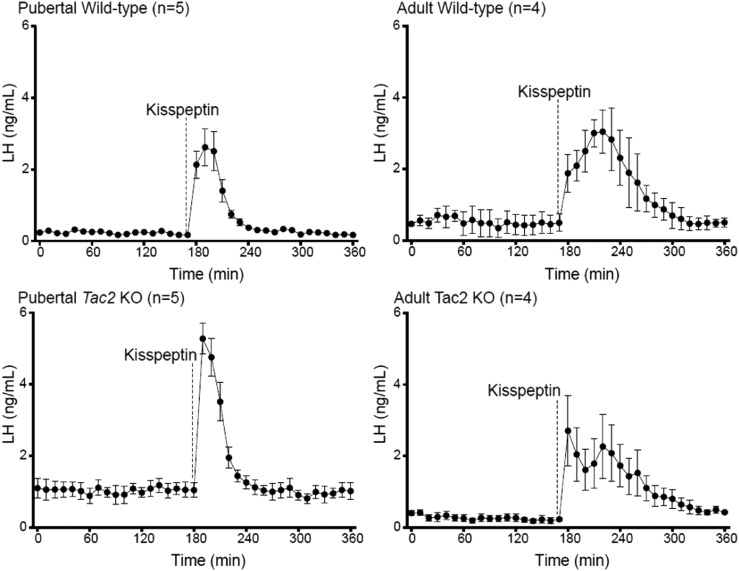
Kisspeptin boluses stimulate LH release in peripubertal and adult WT and NKB-deficient (Tac2 KO) mice
To corroborate the findings in patients with IHH, we conducted experiments in Tac2 KO and WT control female mice. Peripheral administration of kp-10 elicited a robust increase in LH in all animal groups regardless of age and genotype. Interestingly, peripubertal Tac2 KO female mice, lacking NKB, displayed a higher magnitude of LH release (5.29 ± 0.43 ng/mL, n = 5) than did control females (2.67 ± 0.48 ng/mL, n = 5; P < 0.01) (Fig. 5). However, LH returned to baseline faster in Tac2 KO mice (52 ± 3.72 minutes after injection, n = 5) than in WT control mice (68 ± 3.72 minutes, n = 5; P < 0.01). Adult WT mice displayed the expected LH pulse in response to kp-10, whereas the Tac2 KO mice that responded to kp-10 showed a biphasic response, displaying two overlapping peaks of LH (Fig. 4). In both adult groups, the induction of LH release appeared more sustained than in peripubertal mice (peripubertal WT mice, 68 ± 3.742 minutes, n = 5 vs adult WT mice, 142.5 ± 4.78 minutes, after injection, n = 4, P < 0.0001; peripubertal Tac2 KO mice, 52 ± 3.742, n = 5 vs adult Tac2 KO mice, 156.7 ± 3.33 minutes, n = 3, P = 0.07).
Figure 5.
Kisspeptin administration to Tac2 KO mice and littermate controls across sexual development. Dashed line indicates kisspeptin administration. LH values are mean ± SEM for each time point.
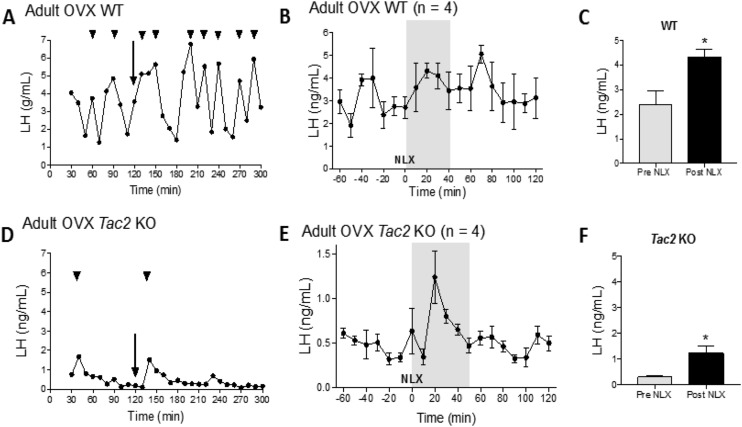
NLX increases pulsatile LH release in adult OVX WT and Tac2 KO mice
To determine the role of the opiatergic (dynorphin) influence on kisspeptin signaling in the absence of NKB, we examined the effects of NLX, which blocks dynorphin, on LH secretion. Peripheral administration of 5 mg/kg NLX induced an increase in LH in both WT (Fig. 6A–6C) and Tac2 KO female mice (Fig. 6D and 6E) within 20 minutes of administration (WT mice: 20 minutes before NLX, 2.37 ± 0.59, n = 4 vs 20 minutes after NLX, 4.31 ± 0.32, n = 4; P < 0.05. Tac2 KO mice: 20 minutes before NLX, 0.31 ± 0.06, n = 4 vs 20 minutes after NLX, 1.22 ± 0.29, n = 4, P < 0.05).
Figure 6.
LH pulse profile (A and D) and the effects of NLX (B, C, E, and F) in adult OVX WT and Tac2 KO mice. (A and D) LH pulses 120 min before NLX injection, and 180 min after NLX injection. The NLX injection is indicated by arrows. Arrowheads indicate the LH pulses. (B and E) Changes in LH secretion (mean ± SEM) 60 min before and 120 min after NLX in WT and OVX Tac2 KO mice, respectively. (C and F) The effects of NLX treatment on LH release are also shown as mean ± SEM from 20 min before (Pre NLX) and 20 min after NLX injection (Post NLX). *P < 0.05, Student t test.
After NLX administration, WT mice responded with an increase in the duration of the following LH pulses post-NLX administration (before NLX: WT mice, 25 ± 2.67 minutes, n = 3; Tac2 KO mice, 23.33 ± 2.10 minutes, n = 3, P = 0.13; after NLX: WT mice, 83.33 ± 12.02 minutes, n = 3; Tac2 KO mice, 30 ± 5.77 minutes, n = 3; P < 0.01) (Fig. 6A). Additionally, the increase in duration in the post-NLX LH pulse was accompanied by a pronounced and longer interpulse interval in WT mice (WT interpulse interval before NLX, 25.38 ±1.83 minutes; WT interpulse interval after NLX, 46.67 ± 3.33 minutes, P < 0.0002).
Tac2 KO animals displayed a markedly reduced LH baseline and number of pulses than in OVX controls (0 to 1 LH pulse in 120 minutes before NLX). The administration of NLX induced a robust LH pulse that occurred 20 minutes after treatment in all cases, with a peak that reached a twofold increase compared with baseline (before NLX, 0.31 ± 0.06 mIU/mL; after NLX, 1.2 ± 0.28 mIU/mL, P < 0.02). Although the limited number of LH pulses precluded an analysis of interpulse intervals, data suggest that NLX did not increase the duration of the LH pulse (pre-NLX Tac2 KO mice, 23.33 ± 2.10 minutes, n = 3; post-NLX Tac2 KO mice, 30 ±5.77 minutes, n = 3; P > 0.05) (Fig. 6D and 6E).
Discussion
In this study, (i) naturally occurring loss-of-function mutations in the gene encoding NKB in a consanguineous family, (ii) biochemical phenotyping, and (iii) provocative challenge testing were all employed to explore the physiologic architecture underlying GnRH pulse generation in the hypothalamus of mice and humans. Although patients with IHH carrying mutations in the gene encoding the NKB receptor (TACR3) are not uncommon, only one family with a genetic mutation leading to a complete loss of NKB (TAC3) has been reported in the literature to date (39). In this series of genotype-driven physiologic investigations, the genetic loss of NKB provided a key backdrop for baseline and provocative detailed neuroendocrine phenotyping.
Most patients with IHH have a lack of GnRH-induced LH pulsations (2). In this study, four sisters with IHH bearing homozygous loss-of-function mutations in TAC3 demonstrated a unique neuroendocrine pattern of well-articulated, but infrequent, LH pulses; this pattern showed remarkable fidelity across all four sisters and is similar to that in another published report (40). In parallel, ovariectomized Tac2 mutant mice demonstrated reduced LH pulse frequency compared with WT controls. Alternatively, the slow frequency of LH pulses speaks to the important role of NKB as a driver of normal GnRH-induced LH pulse frequency. NKB signaling has been specifically associated with GnRH pulse frequency (39), and NKB receptor antagonists have recently been shown to reduce LH pulses in postmenopausal women and patients with polycystic ovarian syndrome (41, 42). The endogenous opioid, dynorphin, potentially “unrestrained” by the pathophysiologic absence of NKB, may also have contributed to the lengthy LH interpulse interval (43). However, the observation of any LH pulses, even infrequent ones, clearly demonstrates that NKB is not essential for GnRH-induced LH pulse generation per se. The identity of the drivers of these low-frequency LH secretory events (kisspeptin, GnRH, other tachykinins, or factors yet to be discovered) requires further study (44–46).
Although loss-of-function mutations in both kisspeptin and NKB signaling have been associated with hypogonadotropic hypogonadism, there appears to be greater complexity in the phenotype associated with deficiency of NKB signaling compared with that of kisspeptin (47). Subjects 3, 4, and 5 experienced reversal of their hypogonadotropic phenotype as evidenced by their ability to have spontaneous menstrual cycles and fertility in the absence of any medications. It is tempting to speculate that their low frequency LH pulses observed in both 2010 and 2016 are related to their phenotypic reversal; that is, an intact GnRH pulse generator, even if slow, can be sped up, leading to reversal under the right circumstances. Additional studies, perhaps using opioid antagonists such as in this study, would be required to reach that conclusion with greater certitude.
The most remarkable finding of this study was the increase in LH levels during the NLX infusion in subjects with IHH. To date, the ability to stimulate endogenous GnRH-induced LH pulsations that mimic normal physiology in patients with IHH has been nonexistent. The observation of a normal LH pulse frequency in the absence of both a key driver for kisspeptin-induced/GnRH-induced LH pulsations (NKB) and a key inhibitor (dynorphin) demonstrates that both NKB and dynorphin are dispensable for GnRH pulse generation and termination. We have previously postulated that the reproductive cascade has several potential pulse generators that are capable of “standing in” when upstream inputs are dysfunctional. Possibilities include, but are not limited to, (i) pulsatile kisspeptin secretion from KNDy neurons in the absence of NKB/dynorphin autofeedback (48), (ii) other tachykinins that substitute in for NKB (45), (iii) pulsatile kisspeptin secretion from non-KNDy neurons (49), or (iv) kisspeptin-independent pulsatile GnRH secretion (50).
Considerations regarding LH pulses include the observation that subject 4 appeared to have a more pronounced response to NLX than did subject 5. Subject 4 underwent pituitary priming with exogenous GnRH and subject 5 did not, which may have amplified any effect of NLX on the LH response in subject 4. Subject 4 had also been receiving intermittent hormone replacement therapy, which may have enhanced endogenous kisspeptin action on GnRH release. This speculation is based on observations showing that periodic exposure to estradiol appears to be essential for kisspeptin action in female nonhuman primates (51). The ability to generalize these findings beyond patients with NKB pathway mutations is unclear. Prior attempts to stimulate the reproductive axis in patients with IHH (of unknown genotype) using NLX were not successful (52).
In synchrony with the human observations, LH levels increased during NLX injection in OVX WT and Tac2 mutant mice. LH pulse amplitude was clearly increased; an increase in LH pulse frequency could not be assessed due to the limited duration of the NLX injection as well as limitations of blood sampling. These findings are consistent with previous observations that NLX increases LH levels and/or pulse frequency in healthy humans and humans with hypothalamic amenorrhea, an acquired form of hypogonadism (15, 53, 54). Furthermore, these findings extend the observations regarding the effects of dynorphin on GnRH pulse termination reported in sheep, demonstrating treatment with a κ-opioid receptor–specific antagonist can prolong NKB-stimulated LH pulses (55, 56). Taken together, these studies suggest the need for further dissection of cellular events that lead to the impact of NLX on GnRH pulse generation in the presence and absence of NKB.
In prior studies, the inability of the same dose of kp-10, which effects a robust GnRH-induced LH response in healthy men and luteal-phase women, to bring about any effect in patients with IHH across a range of genotypes suggested that the functional capacity of the GnRH neuronal network is fundamentally impaired in patients with IHH (28). In contrast to these previous observations in patients with IHH with genotypes other than TAC3 or TACR3, subjects 3, 4, and 5 responded to kp-10 IVBs (28). Here, the low-frequency pulses and the ability to respond to exogenous kp-10 administration suggest that the GnRH neuronal circuitry necessary for pulse generation remains intact in patients lacking NKB. However, the ability to respond to kp-10 with LH pulses was observed only in the setting of IVB administration, and not a continuous infusion, as has been reported by others (40). Differing doses of kp-10, LH assays, and LH pulse algorithms may account for this discordance.
Comparing NKB human KOs with the female nonhuman primate receiving pharmacologic blockade of NKB receptor signaling reveals parallels in the development of hypothalamic brain circuitry. In rhesus monkeys, reciprocal signaling mechanisms between kisspeptin and NKB neurons appear to be established during the course of sexual maturation. Thus, kisspeptin-induced GnRH secretion is possible in the presence of the NK3R antagonist, SB222200, in the prepubertal state, but it is blocked in the presence of SB222200 in the pubertal state (57). Furthermore, female pubertal monkeys require the presence of circulating estradiol to respond to kp-10, whereas prepubertal monkeys do not (51). In the current study, the observation that hypogonadal female patients without endogenous NKB are capable of responding to kp-10 suggests that they too have intact hypothalamic circuitry akin to that of a prepubertal monkey.
As in the human model, the mice lacking NKB (encoded for in mice by Tac2), in both the peripubertal and adult period, responded to kp-10 with robust GnRH-induced LH pulses. Because Tac2 KO mice have an impaired reproductive axis in early life that then normalizes in adulthood, both phases of reproductive life were examined (34). In the current studies, the kp-10–stimulated, GnRH-induced LH pulse amplitude was higher in Tac2 KO mice than in WT mice and changed over time, appearing as a single pulse in sexually immature animals but biphasic in adulthood. Substance P is known to stimulate LH release and, in female animals in the setting of low sex steroids, does so during the upswing of an LH pulse, which could give the appearance of a biphasic pulse (45, 58–60). It has been hypothesized that the Tac2 KO mouse overcomes its delay in sexual maturation and establishment of normal estrus cycles due to other tachykinin inputs. As the substance P receptor is directly expressed on GnRH neurons, further research into its effect on the morphology of the LH pulse may reveal ways in which kisspeptin’s action can be augmented in mice lacking NKB.
In this series of studies, the use of a human genetic KO for NKB reveals a robust GnRH pulse generator in the absence of NKB and dynorphin signaling. Furthermore, it demonstrates the antagonistic relationship between stimulatory NKB and inhibitory dynorphin in modulation of endogenous GnRH pulse frequency. Kisspeptin is capable of stimulating GnRH-induced LH release in humans and in mice lacking NKB. Further studies are required to explore the role of antagonism of endogenous opioids in hypogonadotropic states. Nevertheless, the finding in this study that endogenous kisspeptin signaling alone is sufficient for GnRH pulse generation in human patients demonstrates the human relevance of findings from Herbison and colleagues (17, 18, 61) that optogenetic excitation of selective kisspeptin neurons induces GnRH pulses in mice. Collectively, this knowledge suggests that there may be a role for opioid antagonism in the treatment of patients with reproductive disorders due to NKB deficiency and that this may also extend to those reproductive disorders characterized by slow GnRH pulse frequency.
Acknowledgments
We thank the research subjects, members of the Massachusetts General Hospital Reproductive Endocrine Unit for discussions and reading of the manuscript, staff of the Harvard Catalyst Clinical Research Center and Wellcome–MRC Institute of Metabolic Science Translational Research Facility, Cambridge for assistance with the frequent sampling studies, the Massachusetts General Hospital Investigational Drug Service, and the Massachusetts General Hospital Clinical Laboratory Research Core.
Financial Support: This work was supported by Grants R01 HD043341, P50 HD-28138, R00 HD071970, and R01 HD090151 from the Eunice K. Shriver National Institute for Child Health and Human Development and the Harvard Catalyst/Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Awards UL1 RR 025758 and UL1 TR000170, and financial contributions from Harvard University and its affiliated academic health care centers). S.B.S. is a Robert and Laura Reynolds Research Scholar. Y.-M.C. was supported by a Doris Duke Clinical Scientist Development Award (Grant 2013110). M.F.L. was supported by a Catalyst Medical Research Investigator Training Award from Harvard Catalyst/the Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and by financial contributions from Harvard University and its affiliated academic health care centers. I.S.F. was supported by the Wellcome Trust, Cambridge National Institute for Health Research Biomedical Research Centre, and the Bernard Wolfe Endowment. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, Wellcome Trust and its affiliated academic health care centers, or the National Institutes of Health. J.E.H. and N.D.S. were supported, in part, by the Intramural Program of the NIH, National Institute of Environmental Science. W.A receives support from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham (Grant Reference Number BRC-1215-20009). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Clinical Trial Information: ClinicalTrials.gov nos. NCT00914823 (registered 5 June 2009), NCT01952782 (registered 30 September 2009), and NCT00494169 (registered 29 June 2007).
Author Contributions: Designing research studies: M.F.L., Y.-M.C., I.S.F., J.E.H., N.D.S., V.M.N., S.B.S.; conducting experiments: M.F.L., Y.-M.C., I.S.F., S.L., C.F., V.M.N., S.B.S., C.M.J., W.A., S.E.S., T.R.C.; acquiring data: M.F.L., Y.-M.C., J.E.H., S.L., C.F., V.M.N., S.B.S., C.M.J., R.T.; analyzing data: M.F.L., Y.-M.C., S.L., C.F., V.M.N., S.B.S., E.T., R.T.; providing reagents: J.E.H.; writing the manuscript: M.F.L., Y.-M.C., E.T., V.M.N., S.B.S.
Current Affiliation: J.E. Hall’s and N.D. Shaw’s current affiliation is National Institute of Environmental Health Sciences, Durham, North Carolina 27709.
Disclosure Summary: W.A. reports personal fees from Bayer AG, personal fees from Roche Diagnostics, personal fees from Spruce Biosciences, and grants and personal fees from Diurnal Ltd, outside the submitted work. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- CRC
Clinical Research Center
- gnomAD
Genome Aggregation Database
- IHH
idiopathic hypogonadotropic hypogonadism
- IVB
IV bolus
- KNDy
kisspeptin–NKB–dynorphin
- KO
knockout
- kp-10
kisspeptin 112–121
- MGH
Massachusetts General Hospital
- NKB
neurokinin B
- NLX
naloxone
- OVX
ovariectomized
- WT
wild-type
References and Notes
- 1. Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, Nair RM, Debeljuk L, White WF. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971;173(4001):1036–1038. [DOI] [PubMed] [Google Scholar]
- 2. Spratt DI, Carr DB, Merriam GR, Scully RE, Rao PN, Crowley WF Jr. The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations. J Clin Endocrinol Metab. 1987;64(2):283–291. [DOI] [PubMed] [Google Scholar]
- 3. Shaw ND, Seminara SB, Welt CK, Au MG, Plummer L, Hughes VA, Dwyer AA, Martin KA, Quinton R, Mericq V, Merino PM, Gusella JF, Crowley WF Jr, Pitteloud N, Hall JE. Expanding the phenotype and genotype of female GnRH deficiency. J Clin Endocrinol Metab. 2011;96(3):E566–E576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan YM, Broder-Fingert S, Paraschos S, Lapatto R, Au M, Hughes V, Bianco SD, Min L, Plummer L, Cerrato F, De Guillebon A, Wu IH, Wahab F, Dwyer A, Kirsch S, Quinton R, Cheetham T, Ozata M, Ten S, Chanoine JP, Pitteloud N, Martin KA, Schiffmann R, Van der Kamp HJ, Nader S, Hall JE, Kaiser UB, Seminara SB. GnRH-deficient phenotypes in humans and mice with heterozygous variants in KISS1/Kiss1. J Clin Endocrinol Metab. 2011;96(11):E1771–E1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927–4936. [DOI] [PubMed] [Google Scholar]
- 7. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 8. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357–1363. [DOI] [PubMed] [Google Scholar]
- 10. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629–635. [DOI] [PubMed] [Google Scholar]
- 12. d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104(25):10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 14. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. [DOI] [PubMed] [Google Scholar]
- 15. Shoupe D, Mishell DR Jr, Fossum G, Bopp BL, Spitz IM, Lobo RA. Antiprogestin treatment decreases midluteal luteinizing hormone pulse amplitude and primarily exerts a pituitary inhibition. Am J Obstet Gynecol. 1990;163(6 Pt 1):1982–1985. [DOI] [PubMed] [Google Scholar]
- 16. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 17. Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2015;112(42):13109–13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114(47):E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sidhoum VF, Chan YM, Lippincott MF, Balasubramanian R, Quinton R, Plummer L, Dwyer A, Pitteloud N, Hayes FJ, Hall JE, Martin KA, Boepple PA, Seminara SB. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J Clin Endocrinol Metab. 2014;99(3):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF Jr, Pitteloud N. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA. 2010;107(34):15140–15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lek M, Karczewski K, Minikel E, Samocha K, Banks E, Fennell T, O’Donnell-Luria A, Ware J, Hill A, Cummings B, Tukiainen T, Birnbaum D, Kosmicki J, Duncan L, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Cooper D, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki M, Levy Moonshine A, Natarajan P, Orozco L, Peloso G, Poplin R, Rivas M, Ruano-Rubio V, Ruderfer D, Shakir K, Stenson P, Stevens C, Thomas B, Tiao G, Tusie-Luna M, Weisburd B, Won HH, Yu D, Altshuler D, Ardissino D, Boehnke M, Danesh J, Roberto E, Florez J, Gabriel S, Getz G, Hultman C, Kathiresan S, Laakso M, McCarroll S, McCarthy M, McGovern D, McPherson R, Neale B, Palotie A, Purcell S, Saleheen D, Scharf J, Sklar P, Patrick S, Tuomilehto J, Watkins H, Wilson J, Daly M, MacArthur D. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575–576. [DOI] [PubMed] [Google Scholar]
- 25. Thomas PD, Kejariwal A, Guo N, Mi H, Campbell MJ, Muruganujan A, Lazareva-Ulitsky B. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 2006;34(Web Server issue):W645–W650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope. 1996;106(3 Pt 1):353–356. [DOI] [PubMed] [Google Scholar]
- 27. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489–502. [DOI] [PubMed] [Google Scholar]
- 28. Chan YM, Lippincott MF, Butler JP, Sidhoum VF, Li CX, Plummer L, Seminara SB. Exogenous kisspeptin administration as a probe of GnRH neuronal function in patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2014;99(12):E2762–E2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF Jr, Ren C, Chan KK, Seminara SB. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96(6):E908–E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97(8):E1458–E1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spratt DI, Chin WW, Ridgway EC, Crowley WF Jr. Administration of low dose pulsatile gonadotropin-releasing hormone (GnRH) to GnRH-deficient men regulates free alpha-subunit secretion. J Clin Endocrinol Metab. 1986;62(1):102–108. [DOI] [PubMed] [Google Scholar]
- 32. Sluss PM, Hayes FJ, Adams JM, Barnes W, Williams G, Frost S, Ramp J, Pacenti D, Lehotay DC, George S, Ramsay C, Doss RC, Crowley WF Jr. Mass spectrometric and physiological validation of a sensitive, automated, direct immunoassay for serum estradiol using the Architect. Clin Chim Acta. 2008;388(1–2):99–105. [DOI] [PubMed] [Google Scholar]
- 33. Roche Diagnostics. Elecsys estradiol III assay. Available at: https://www.rochecanada.com/content/dam/rochexx/roche-ca/products/docs/package_inserts/ESTRADIOL%20III%20_06656021190_CAN_V4_EN-final.pdf. Accessed 8 June 2017.
- 34. True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156(4):1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52(10):2617–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab. 1999;84(3):1028–1036. [DOI] [PubMed] [Google Scholar]
- 37. Filicori M, Santoro N, Merriam GR, Crowley WF Jr. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62(6):1136–1144. [DOI] [PubMed] [Google Scholar]
- 38. Hall JE, Schoenfeld DA, Martin KA, Crowley WF Jr. Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab. 1992;74(3):600–607. [DOI] [PubMed] [Google Scholar]
- 39. Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95(5):2287–2295. [DOI] [PubMed] [Google Scholar]
- 40. Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, Brailly-Tabard S, Anderson RA, Millar RP. Kisspeptin restores pulsatile LH secretion in patients with neurokinin b signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. Neuroendocrinology. 2013;97(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, Doyle C, Papadopoulou DA, Bloom SR, Mohideen P, Panay N, Hunter MS, Veldhuis JD, Webber LC, Huson L, Dhillo WS. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10081):1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313–4321. [DOI] [PubMed] [Google Scholar]
- 43. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalra SP, Crowley WR. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol. 1992;13(1):1–46. [PubMed] [Google Scholar]
- 45. Navarro VM, Bosch MA, León S, Simavli S, True C, Pinilla L, Carroll RS, Seminara SB, Tena-Sempere M, Rønnekleiv OK, Kaiser UB. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fergani C, Navarro VM. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reproduction. 2016;153(1):R1–R14. [DOI] [PubMed] [Google Scholar]
- 47. Lippincott MF, True C, Seminara SB. Use of genetic models of idiopathic hypogonadotrophic hypogonadism in mice and men to understand the mechanisms of disease. Exp Physiol. 2013;98(11):1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kelly MJ, Zhang C, Qiu J, Rønnekleiv OK. Pacemaking kisspeptin neurons. Exp Physiol. 2013;98(11):1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Comninos AN, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, Hu M, Jayasena CN, Ghatei MA, Bloom SR, Matthews PM, O’Byrne KT, Bell JD, Dhillo WS. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct. 2016;221(4):2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krsmanović LZ, Stojilković SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA. 1992;89(18):8462–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology. 2012;153(2):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Veldhuis JD, Kulin HE, Warner BA, Santner SJ. Responsiveness of gonadotropin secretion to infusion of an opiate-receptor antagonist in hypogonadotropic individuals. J Clin Endocrinol Metab. 1982;55(4):649–653. [DOI] [PubMed] [Google Scholar]
- 53. Moult PJ, Grossman A, Evans JM, Rees LH, Besser GM. The effect of naloxone on pulsatile gonadotrophin release in normal subjects. Clin Endocrinol (Oxf). 1981;14(3):321–324. [DOI] [PubMed] [Google Scholar]
- 54. Perkins RB, Hall JE, Martin KA. Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab. 1999;84(6):1905–1911. [DOI] [PubMed] [Google Scholar]
- 55. Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weems PW, Coolen LM, Hileman SM, Hardy S, McCosh RB, Goodman RL, Lehman MN. Evidence that dynorphin acts upon KNDy and GnRH neurons during GnRH pulse termination in the ewe. Endocrinology. 2018;159(9):3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Kisspeptin and neurokinin B signaling network underlies the pubertal increase in GnRH release in female rhesus monkeys. Endocrinology. 2017;158(10):3269–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Traczyk WZ, Pau KY, Kaynard AH, Spies HG. Modulatory role of substance P on gonadotropin and prolactin secretion in the rabbit. J Physiol Pharmacol. 1992;43(3):279–297. [PubMed] [Google Scholar]
- 59. Maguire CA, Song YB, Wu M, León S, Carroll RS, Alreja M, Kaiser UB, Navarro VM. Tac1 signaling is required for sexual maturation and responsiveness of GnRH neurons to kisspeptin in the male mouse. Endocrinology. 2017;158(7):2319–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Simavli S, Thompson IR, Maguire CA, Gill JC, Carroll RS, Wolfe A, Kaiser UB, Navarro VM. Substance P regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156(6):2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Campos P, Herbison AE. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2014;111(51):18387–18392. [DOI] [PMC free article] [PubMed] [Google Scholar]