Abstract
Androgens (testosterone and DHT) increase adult hippocampal neurogenesis by increasing survival of new neurons in male rats and mice via an androgen receptor pathway, but it is not known whether androgens regulate neurogenesis in female rats and whether the effect is age-dependent. We investigated the effects of DHT, a potent androgen, on neurogenesis in young adult and middle-aged male and female rats. Rats were gonadectomized and injected with the DNA synthesis marker bromodeoxyuridine (BrdU). The following day, rats began receiving daily injections of oil or DHT for 30 days. We evaluated cell proliferation (Ki67) and survival of new neurons (BrdU and BrdU/NeuN) in the hippocampus of male and female rats by using immunohistochemistry. As expected, DHT increased the number of BrdU+ cells in young males but surprisingly not in middle-aged males or in young and middle-aged females. In middle age, DHT increased the proportion of BrdU/NeuN cells, an effect driven by females. Androgen receptor expression also increased with aging in both female and male rats, which may contribute to a lack of DHT neurogenic effect in middle age. Our results indicate that DHT regulates adult hippocampal neurogenesis in a sex- and age-dependent manner.
Neurogenesis, the production of new neurons, in the hippocampus continues through the life span of most mammals studied to date (1). Sex hormones (estrogens and androgens) regulate different aspects of hippocampal neurogenesis, such as proliferation and/or survival of these new neurons in rodents (2, 3). There is also evidence of sex differences in how hormones regulate neurogenesis. For example, estradiol regulates cell proliferation and survival of new neurons in female but not male rats (4). We have previously shown that androgens (testosterone and DHT) increase the survival of new neurons but not cell proliferation in the hippocampus of male rats and mice via an androgen receptor (AR) pathway (5–7). However, it is not known whether androgens regulate any aspects of adult neurogenesis in females. Given that ARs are expressed in the female hippocampus (8), this suggests that androgens may modulate neurogenesis in females too. In addition to sex, age can also modulate the effects of hormones on hippocampal neurogenesis. In middle age, neurogenesis decreases (9), and reduction of corticosteroid levels (by adrenalectomy) (10) and exercise (11) can restore neurogenesis levels in aged rodents. With aging, the hippocampus also loses its ability to respond to estrogens in female rats (12, 13). For instance, estradiol increases cell proliferation in the hippocampus in young but not middle-aged nulliparous female rats (12, 13). The objective of this study was to investigate the effects of DHT on hippocampal neurogenesis (proliferation and survival of new neurons) in young and middle-aged male and female rats.
At age 2 months (∼70 days old, young) and 11 to 12 months (middle-aged), male and female Sprague-Dawley rats were gonadectomized and allowed to recover for 1 week (n = 5 to 8 per group) (4–6, 14). One week allows for circulating gonadal hormone levels to decrease to very low or undetectable levels (4, 5, 15, 16). One day after ovariectomy, estradiol levels are undetectable in female rats (15); ∼5 days after gonadectomy, circulating estradiol and testosterone decrease to ∼10% of their original levels or to undetectable levels in males (17, 18). We chose 11 to 12 months as middle-age because rats can live up to 24 months, at 12 months the levels of neurogenesis are substantially decreased compared with those in young adults (9, 19–21), and at 12 months sexual motivation and fecundity are significantly reduced in both sexes (22–24).
After the 1-week recovery period, all animals received a single intraperitoneal injection of bromodeoxyuridine (BrdU; 200 mg/kg) to label dividing cells and their progeny (6). The following day, chronic hormone or vehicle treatment began. Males and females were injected subcutaneously with 0.25 mg DHT (DHT in 0.1 mL of sesame oil) or an equivalent volume of sesame oil for 30 days. The dose of DHT chosen in this study was the lowest dose examined that increased neurogenesis in castrated young adult male rats (5, 6). Twenty-four hours after the final injection, animals were given an overdose of sodium pentobarbital and perfused with 4% paraformaldehyde. Brains were then collected; sectioned by using a freezing microtome; processed for BrdU (survival of 30-day-old cells), Ki67 (cell proliferation marker), androgen receptor (AR); and colabeled for BrdU/NeuN (new neurons using NeuN, a marker for mature neurons) immunohistochemistry.
The following primary antibodies were used with diaminobenzidine chromogen: mouse anti-BrdU monoclonal [1:200; Roche; catalog no. 11170376001 (25)], rabbit anti-Ki67 polyclonal [1:3000; Vector Laboratories; catalog no. VP-K451 (26)], and rabbit anti-AR monoclonal [1:100; Abcam; catalog no. ab133273 (27)]. For fluorescence double labeling, the following primary antibodies were used: rat anti-BrdU [1:500; Bio-Rad/ABD Serotec; catalog no. OBT0030S (28)] and mouse anti-NeuN [1:250; Millipore; catalog no. MAB377 (29)]. Detailed protocols are found elsewhere (6, 7).
Thus, in this experiment, BrdU+ cells were 30-day-old daughter cells from progenitor cells that had been synthesizing DNA for a 2-hour period 31 days before euthanasia and prior to any hormone treatment. A subset of samples were used to measure DHT levels in serum collected on the day of perfusion (stored at −20°C) by using a commercial ELISA kit [IBL-America; catalog no. IB59116 (30)]. All samples were run in duplicate following the manufacturer’s protocol. The DHT antibody is highly specific with 8.7% cross-reactivity with testosterone and 0.2% cross-reactivity with androstenedione; the sensitivity is 6.0 pg/mL. Average intra-assay coefficient of variation was <15%. All protocols were approved by the Animal Care Committee at the University of British Columbia and conformed to the guidelines set out by the Canadian Council on Animal Care.
A researcher blinded to experimental conditions counted all BrdU+ and Ki67+ cells in both hemispheres for each section for the entire rostrocaudal extent of the granule cell layer (GCL) including the subgranular zone, defined as the 50-µm band between the GCL and the hilus (13 to 15 sections in total per animal). We used a modified optical fractionator method (31, 32) to estimate the total number of BrdU+ and Ki67+ cells in the GCL and hilus, as has been used before (5, 7, 33–37). Total cells were calculated by multiplying the total number of cells counted by 10 to account for the fact that we used 1/10 series of sections for each immunohistochemistry procedure. GCL and hilus volumes were quantified from digitized images by using the Cavalieri’s Principle, multiplying the sum of the area of each section by the section thickness (40 µm) (38). Densities of BrdU+ and Ki67+ were calculated by dividing the total number of cells by the GCL volume. Densities (total cells per unit volume) were used because there are sex differences in the volume of the dentate gyrus in rats (39, 40).
We assessed expression of AR by quantitative densiometric analysis using ImageJ software (US National Institutes of Health, Bethesda, MD). Photomicrographs of four hippocampal sections per animal were taken at 40× magnification by using the same exposure and gain settings. Optical density (OD) was assessed in the CA1, CA3, and GCL regions by placing six circles (40 µm diameter) along these regions. OD levels were corrected for background levels using areas with no immunoreactivity (i.e., molecular layer of the dentate gyrus and radiatum layer of the hippocampus). To determine whether BrdU+ cells were of a neuronal phenotype, in a subset of brains, BrdU+ cells were examined for colabeling with NeuN (neuronal marker) for 50 cells (young adult group) or all cells (middle-aged group).
All analyses were performed by using Statistica software, version 8.0 (StatSoft Inc., Tulsa, OK). Volume of the dentate gyrus (GCL and hilus) and density of BrdU+ cells were analyzed using repeated-measures ANOVA, with age (young, middle-aged), sex (male, female), and treatment (DHT, oil) as between-subjects factors and region (GCL, hilus) as a within-subjects factor. The OD of AR was analyzed by using repeated-measures ANOVA, with age (young, middle-aged), sex (male, female), and treatment (DHT, oil) as between-subjects factors and region (CA1, CA3, GCL) as a within-subjects factor. Density of Ki67+ cells, proportion of BrdU/NeuN colabeled cells, and serum DHT concentrations were analyzed by using ANOVA, with age (young, middle-aged), sex (male, female), and treatment (DHT, oil) as between-subjects factors. When appropriate, post hoc analysis was performed by using the Neuman-Keul procedure. Test statistics were considered significant at P ≤ 0.05.
Regardless of age and treatment, the volume of the GCL and hilus were larger in males than females [main effect of sex; F(1,42) = 4.42; P < 0.05] (Table 1). The volume of the GCL and hilus increased with age irrespective of sex and treatment [main effect of age; F(1,42) = 47.37; P < 0.0001]. In the case of the hilus, the volume increased with aging more so in males than in females [interaction between region, age, and sex; F(1,42) = 20.20; P < 0.0001]. As expected, the volume of the hilus was larger than the volume of the GCL [main effect of region; F(1,44) = 1450.14; P < 0.0001]. Treatment with DHT did not significantly affect GCL or hilus volumes (all P > 0.08). To account for the sex differences in GCL and hilus volumes, we present BrdU+ and Ki67+ cell counts as densities (total cells per unit volume).
Table 1.
Mean (SEM) Volume of the GCL and Hilus and BrdU Density in Hilus in Gonadectomized Young and Middle-Aged Male and Female Rats Treated With Oil or DHT
| Age, Sex, and Treatment | Sample Size, n | GCL Volume, mm3 | Hilus Volume, mm3 | Hilus BrdU Density, per mm3 |
|---|---|---|---|---|
| Young | ||||
| Females | ||||
| Oil | 7 | 2.51 (0.12) | 6.09 (0.19) | 85.14 (15.34) |
| DHT | 8 | 2.46 (0.08) | 6.00 (0.28) | 76.06 (7.06) |
| Males | ||||
| Oil | 5 | 2.31 (0.19) | 5.32 (0.49) | 96.18 (23.29) |
| DHT | 6 | 2.62 (0.21) | 5.37 (0.51) | 134.70 (17.38) |
| Middle age | ||||
| Females | ||||
| Oil | 6 | 2.33 (0.09) | 7.67 (0.42) | 20.69 (7.89) |
| DHT | 6 | 2.45 (0.15) | 7.33 (0.28) | 6.40 (2.49) |
| Males | ||||
| Oil | 6 | 2.94 (0.25) | 9.05 (0.79) | 6.06 (1.41) |
| DHT | 6 | 2.87 (0.22) | 10.05 (0.60) | 4.65 (1.63) |
The volume of the hilus was larger than the volume of the GCL (main effect of region; P < 0.0001). Males had a larger volume of GCL and hilus than females (main effect of sex; P < 0.05). The volume of the GCL and hilus increased with age (main effect of age; P < 0.0001) and in the hilus, the volume increased with aging more so in males than females (interaction between region, age, and sex; P < 0.0001). DHT treatment did not affect the volume of the GCL and hilus (all P > 0.08). The density of BrdU+ cells was not significantly affected by age, sex, or treatment (all P > 0.9). Sample size is number of individuals.
As expected, DHT treatment increased serum levels of DHT in males and females regardless of age [main effect of treatment; F(1,17) = 36.67; P < 0.0001]. The mean (±SEM) DHT level was 38.55 ± 2.99 pg/mL in oil-treated rats and 73.64 ± 8.70 pg/mL in DHT-treated rats. DHT levels were significantly higher in females than males regardless of treatment [main effect of sex; F(1,17) = 26.6; P < 0.0001]. The mean (±SEM) DHT level was 69.58 ± 8.66 pg/mL in DHT-treated females and 40.02 ± 3.79 pg/mL in DHT-treated males. No significant differences were detected in DHT levels with age (P > 0.3).
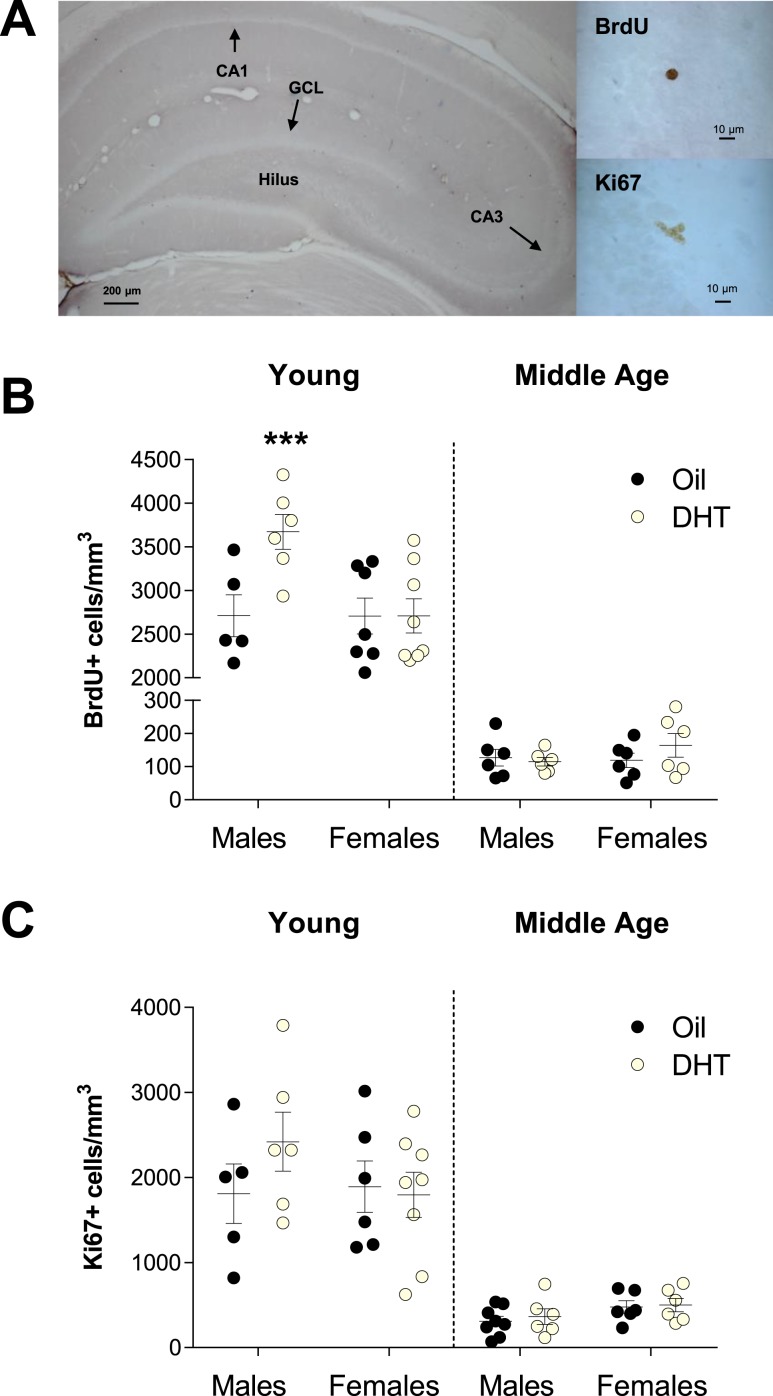
DHT treatment increased the density of BrdU+ cells in the GCL in young males (P < 0.001) but not in young females or middle-aged rats of both sexes [all P > 0.9; interaction between age, sex, treatment and region; F(1,42) = 4.03; P = 0.05] (Fig. 1). The density of BrdU+ cells in the GCL was significantly higher in the young than in the middle-aged rats irrespective of sex and treatment, as expected [main effect of age; F(1,42) = 647.85; P < 0.0001]. In the hilus, the density of BrdU+ cells was not significantly affected by age, sex, or treatment (all P > 0.9; Table 1). To determine how many BrdU+ cells were neurons, we examined the colabeling of BrdU and NeuN (a mature neuronal marker; BrdU/NeuN) in the GCL. Aging decreased the proportion of BrdU/NeuN colabeled cells in the dentate gyrus [main effect of age; F(1,39) = 27.98; P < 0.0001]. In the young group, sex and treatment did not affect the percentage of BrdU/NeuN colabeled cells (all P > 0.18; Table 2). In middle age, DHT treatment increased the proportion of BrdU/NeuN cells [interaction between age and treatment; F(1,39) = 4.77; P = 0.03], and this effect was driven by the middle-aged females (P = 0.005) compared with the middle-aged males (P = 0.48).
Figure 1.
Chronic DHT treatment increases the density of BrdU+ cells in the dentate gyrus of young male adult rats but has no significant effects in middle-aged male and young and middle-aged female rats. (A) Photomicrographs of a representative section of dentate gyrus with the GCL, hilus, CA1, and CA3 regions and representative BrdU+ and Ki67+ cells in the subgranular zone. (B) Mean ± SEM density of BrdU+ cells in the GCL of the dentate gyrus in young and middle-aged male and female rats. In young males, DHT increased the density of BrdU+ cells relative to the oil treatment group (***P < 0.001; interaction between age, sex, treatment, and region; P = 0.05). (C) Mean ± SEM density of Ki67+ cells in the GCL of the dentate gyrus in young and middle-aged male and female rats. Chronic DHT treatment did not affect cell proliferation in the hippocampus of young or middle-aged male and female rats. Circles represent individual data points (number of individuals).
Table 2.
Mean (SEM) Percentage of Cells Coexpressing BrdU and NeuN in GCL in Gonadectomized Young and Middle-Aged Male and Female Rats Treated With Oil or DHT
| Age, Sex, and Treatment | Sample Size, n | BrdU/NeuN, % |
|---|---|---|
| Young | ||
| Females | ||
| Oil | 5 | 87.89 (2.27) |
| DHT | 8 | 87.16 (1.10) |
| Males | ||
| Oil | 4 | 88.68 (1.94) |
| DHT | 5 | 84.24 (3.12) |
| Middle age | ||
| Females | ||
| Oil | 6 | 63.80 (3.43) |
| DHT | 6 | 81.32 (5.82)a |
| Males | ||
| Oil | 6 | 67.03 (5.39) |
| DHT | 6 | 70.88 (7.00) |
The proportion of BrdU+ cells colabeled with NeuN was significantly higher in young compared with middle-aged animals (main effect of age; P < 0.0001). In middle-age, but not in young adults, DHT increased the proportion of BrdU/NeuN colabeled cells relative to the oil treatment group (interaction between age and treatment), but this effect was driven by the middle-aged females (P = 0.005) compared with the middle-aged males (P = 0.48). Sample size is number of individuals.
P < 0.01 relative to oil treatment group.
DHT treatment did not affect the density of Ki67+ cells in young or middle-aged males and females (all P > 0.25; Fig. 1). However, as expected, there were more Ki67+ cells in the GCL of young compared to middle-aged rats [main effect of age; F(1,43) = 97.18; P < 0.0001] irrespective of sex and treatment.
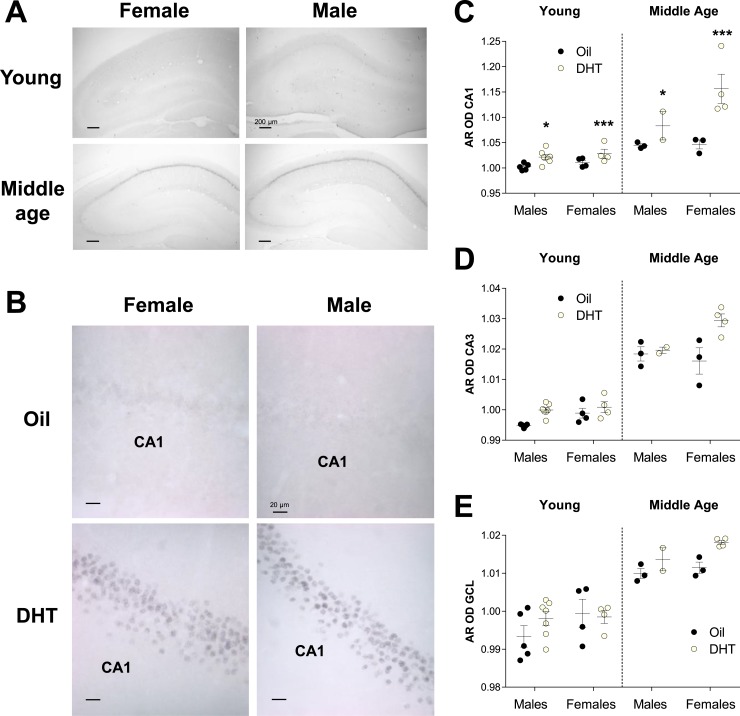
Finally, we performed a qualitative and quantitative (OD) analysis of AR expression in the hippocampus, and results were similar with both methods (Table 3; Fig. 2). AR OD increased significantly with aging in the CA1, CA3, and GCL, regardless of sex, and DHT treatment increased the expression of AR in the CA1 in middle-aged rats of both sexes [interaction between region, age, and treatment; F(2,48) = 8.1; P < 0.001] (Fig. 2). However, DHT treatment increased AR OD in the CA1, regardless of age, more so in females (P = 0.0001) than in males (P = 0.056) [interaction between region, sex, and treatment; F(2,48) = 3.3; P < 0.05] (Fig. 2). Qualitatively, in oil-treated animals, AR-ir cells were absent throughout the GCL in young males and female rats but were expressed at low levels in middle-aged male and female rats. In the CA3, we found low to absent levels of AR-ir cells in young rats of both sexes, and expression increased in middle-aged rats. In the CA1, we found low to intermediate levels of AR-ir in oil-treated young rats and levels increased with aging and DHT treatment in both sexes (Fig. 2).
Table 3.
AR Expression in Hippocampus Using Relative Rating Scaling in GCL, CA1, and CA3 Regions in Young and Middle-Aged Male and Female Rats Treated With Oil or DHT
| Age, Sex, and Treatment | AR Expression | ||
|---|---|---|---|
| GCL | CA1 | CA3 | |
| Young | |||
| Females | |||
| Oil | 0 | + | 0/+ |
| DHT | 0 | ++/+++ | 0 |
| Males | |||
| Oil | 0 | 0/+ | 0 |
| DHT | 0 | ++ | 0 |
| Middle age | |||
| Females | |||
| Oil | 0/+ | ++ | + |
| DHT | + | +++ | +/++ |
| Males | |||
| Oil | 0/+ | ++ | + |
| DHT | + | ++/+++ | + |
Relative rating scale refers to absent (0), light (+), intermediate (++), and robust (+++) expression.
Figure 2.
Chronic DHT treatment increases AR optical density in the CA1 region of the hippocampus in young and middle-aged male and female rats. (A) Representative images of AR expression in the hippocampus of young and middle-aged gonadectomized oil-treated female and male rats. AR expression increased with aging in the CA1, CA3, and GCL in both sexes. (B) Representative images of AR expression in the CA1 region of the hippocampus in young male and female rats treated with oil or DHT. AR expression is low in the CA1 of gonadectomized oil-treated animals. DHT increases the expression of AR in both female and male rats. OD for AR (mean ± SEM) was measured in the (C) CA1, (D) CA3, and (E) GCL regions. AR OD increased significantly with aging in the CA1, CA3, and GCL, regardless of sex, and DHT treatment increased the expression of AR in the CA1 in young and middle-aged rats of both sexes (interaction between region, age, and treatment; P < 0.001). DHT treatment increased AR OD in the CA1, regardless of age, more so in females than in males (interaction between region, sex, and treatment; P < 0.05). Asterisks denote significant differences between oil and DHT treatment (*P ≤ 0.05; ***P < 0.001). Circles represent individual data points (number of individuals).
In the current study, we found that chronic (30 days) DHT increased survival of new neurons but not cell proliferation in the hippocampus of gonadectomized young adult male rats, consistent with our previous research in male rats and mice (5–7). This is also in line with previous work showing that castration decreases survival of new neurons 24 to 30 days after BrdU injection but has no effect on cell proliferation (5, 41). Shorter testosterone treatment (3, 15, or 21 days) has no effect on hippocampal neurogenesis (42–45), indicating that a longer exposure (30 days) to androgens is required to increase neurogenesis in the hippocampus, at least in physiological doses, because higher doses can decrease neurogenesis (46, 47). Thus, collectively, although longer-term exposure to androgens increases survival of new neurons in the dentate gyrus, androgens do not appear to influence cell proliferation in male rats [this study and others (5, 6)], mice (7), or voles (48).
Unlike in young adult males, DHT did not affect survival of new neurons or cell proliferation in gonadectomized young adult female rats. We previously found that estradiol modulates cell proliferation and survival of new neurons in young adult female rats (4, 49–52) but has no significant effect on neurogenesis in adult male rats (4, 5). In the current study, we found that females had higher serum DHT levels than males regardless of treatment or age. The higher DHT levels in females were likely due to the dose being somewhat higher in females than males. Thus, it is possible that these higher circulating DHT levels resulted in an eliminated response to neurogenesis, although we observed an increase in cell fate (BrdU/NeuN) with DHT in middle-aged females (discussed below). Together, our results suggest that sex steroids have sex-specific effects on hippocampal neurogenesis with androgens modulating neurogenesis in young adult males and estrogens modulating neurogenesis in young adult females. Although these findings may not seem surprising, it is important to understand that both sexes have ARs and estrogen receptors (ERs), but these receptors are responding to respective hormones in a sex-specific way to modulate neurogenesis in the hippocampus. Both ERα and ERβ are expressed in the hippocampus (CA1, CA3, GCL) in males and females, and no sex differences exist in their expression (53, 54). Intriguingly, there are more ERs in the GCL than there are ARs in both sexes, and ERs have been detected on proliferating cells (Ki67+ or BrdU+ cells) and immature neurons (doublecortin-expressing cells) in adult male and female rats (50, 55, 56), but to our knowledge ARs have not been detected on proliferating cells or immature neurons in the dentate gyrus in male rats and mice (6, 7). Our findings are unlikely to involve ERs (α or β) because of the use of DHT in this study. DHT is a nonaromatizable androgen that binds with high affinity to AR. However, 5α-androstane-3β, 17β-diol, a DHT metabolite, has been shown to function as an ERβ ligand (57). To avoid any possible ERβ activation, we used the lowest possible dose of DHT that increases neurogenesis in young males (5). From previous work, estradiol does not increase neurogenesis in males and decreases neurogenesis (survival of new neurons) in females (4). In addition, in young male rats, the same dose of DHT regulates neurogenesis via ARs as blocking AR with flutamide eliminates the DHT-induced increase in new neuron survival (6). Together this suggests that the DHT regulation of hippocampal neurogenesis is mediated by the AR.
Perhaps surprisingly, the effect of DHT to modulate survival of new neurons was absent in middle-aged males and females. This effect is consistent with the recent work of Moser et al. (58), who found that testosterone did not affect the number of immature neurons (using doublecortin), in middle-aged (13 months) or aged (23 months) male rats. This would suggest that, with aging, the dentate gyrus loses its ability to respond to sex steroids. Indeed, in females, acute estradiol increases cell proliferation in young but not middle-aged nulliparous rats (12, 13). Intriguingly, in females, previous reproductive experience (pregnancy and motherhood) can rescue the hippocampus response to estrogens later in middle-age, as acute estrogens increased cell proliferation in multiparous rats (13). Thus, it is possible that experience, reproductive or otherwise, may restore the ability of androgens to upregulate hippocampal neurogenesis in males in middle age. In our study, the proportion of BrdU/NeuN colabeled cells was affected by age and treatment. DHT increased the proportion of BrdU/NeuN colabeled cells only in middle-aged females. Overall, as expected, the proportion of new cells that express mature neuronal protein decreased with age. In middle-aged female mice, letrozole, an aromatase inhibitor blocking the conversion of androgens to estrogens, increases hippocampal neurogenesis [through use of the immature marker doublecortin (59)]. Together with our findings, this suggests that the hippocampus may still be able to respond to sex steroid hormones in middle age, an effect that varies by sex as in both studies, middle-aged females showed increased neurogenesis levels with androgens.
Surprisingly, we found that AR expression increased with aging in both sexes in all hippocampal regions. We found that ARs were expressed at high and moderate levels in the CA1 and CA3 regions of the hippocampus, respectively, in both young and middle-aged rats. But, consistent with previous research, we did not find expression of ARs in the GCL in young gonadectomized male rats (6, 8, 60); although there is conflicting evidence which may be due to strain, species, and age differences, as we report AR expression in the GCL at middle age but not in young adults (see below). In Wistar rats, Moghadami et al. (61) and Brännvall et al. (46) found AR expression in the GCL in young gonadectomized and intact male rats. In gonadectomized male mice, ARs are not expressed in the GCL but in males receiving DHT treatment, granule cells did express AR (7). In other rat strains (Bruce-Spruce Long-Evans, Fischer 344, and Sprague-Dawley), most studies have found that ARs are not expressed in the GCL (6, 8, 60); however, one study found low to medium AR expression by using qualitative analysis in the GCL of intact young male Sprague-Dawley rats (62). We also found that DHT increases the expression of ARs in the CA1 in males in line with previous research in young male rats (6) and male mice (7) and in other brain regions (preoptic-hypothalamic regions) (24). In females and males, 2 days of testosterone treatment also showed increased AR expression in the CA1 region (8), but to our knowledge no other studies have investigated AR expression in the female hippocampus after chronic DHT treatment. In female rats, AR expression varies with the estrous cycle (62) as AR expression was highest when estradiol levels are low in the CA1, CA3, and dentate gyrus (62). Furthermore, 7-day DHT treatment decreased AR expression in most brain regions in intact female rats (62), suggesting that DHT in the presence of gonadal hormones downregulates AR expression in the female hippocampus. In the current study, DHT increased AR expression in gonadectomized females (as well as males), suggesting that gonadal hormones interact to regulate brain levels of AR. As discussed above, previous work has found that androgens increase neurogenesis via the AR. However, in young male rats and mice, ARs are not found in immature neurons (doublecortin-expressing cells) in the GCL of the hippocampus (6, 7) but instead ARs are expressed in mature neurons (NeuN expressing cells) in the hippocampus (62). We have previously proposed that one mechanism of action is that androgens bind to ARs in the CA3 region and this initiates a retrograde response of a survival factor that targets newborn neurons in the GCL (63). In the current study, we found ARs were expressed in the CA1 and CA3 regions in young and middle-aged male and female rats and their expression increases after DHT treatment in both sexes at both ages. However, we observed an effect of DHT only on new neuron survival in young males. In young females and middle-aged males and females, ARs are expressed in the CA1 and CA3, but binding of DHT to these ARs does not result in the modulation of neurogenesis, possibly indicating different downstream mechanisms of bound AR to modulate neurogenesis in young adult females, and middle-aged males.
We found higher expression of AR in the hippocampus of middle-aged compared with young rats of both sexes. In addition, in contrast to young rats, we detected AR-ir cells in the GCL in middle-aged rats. AR mRNA expression also increases in the hippocampus with aging in males (60), but our study showed that hippocampal AR expression increases with aging in females. It may seem paradoxical that although DHT increases neurogenesis via the AR in males (6), despite the increase in AR expression with middle age, DHT no longer increases neurogenesis in middle-aged rats. However, we have previously found that overexpression of the AR in the brain (Nestin-AR) resulted in a failure of DHT to increase neurogenesis in young adult male mice (7). This suggests a “dose response” of AR expression, with optimal levels needed for DHT to increase neurogenesis. In the preoptic area, the number of AR-ir cells increase with aging in intact male rats (64), but not in castrated males (24), and these are negatively correlated with circulating testosterone levels (64). In young rats, AR expression depends on androgen levels, and gonadectomy decreases AR expression in the hippocampus (8, 60, 61). Circulating androgens decrease with age in male rats (65), and therefore we would expect AR expression to decrease with age. Our findings and the ones by Wu et al. (64) are surprising, and it is possible that with aging the relationship between androgens and DHT changes. Indeed, we observed that DHT treatment increased AR expression in the CA1 in middle-aged males and females indicating that exogenous regulation of AR expression is similar in both ages. As outlined above, high doses of AR in the brain (in transgenic male mice) result in an abolished neurogenic response to DHT (7). It is possible that the increase in AR expression with aging is responsible for the lack of DHT mediated increase in new neuron survival.
To summarize, our study demonstrates that DHT increases neurogenesis in young adult males but not in young adult females and that aging eliminates the ability of DHT to enhance neurogenesis in males showing sex and age differences in the neurogenic response to DHT. However, in middle-aged rats, DHT treatment increased the proportion of surviving cells expressing the mature neuronal protein (an effect seen in the females only). AR expression in the hippocampus increases with aging and DHT treatment in both sexes, suggesting that ARs do respond to DHT but this does not result in a neurogenic response in females and middle-aged males. Altogether our current and previous research (3) indicates that androgens and estrogens have sex-specific and age-specific effects on hippocampal neurogenesis.
Acknowledgments
We thank Dr. Mark Martindale (Whitney Laboratory for Marine Bioscience, University of Florida) for access to an epifluorescent microscope.
Financial Support: This work was funded by the Canadian Institutes of Health Research (Grant MOP102568) (to L.A.M.G.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AR
androgen receptor
- BrdU
bromodeoxyuridine
- ER
estrogen receptor
- GCL
granule cell layer
- OD
optical density
References and Notes
- 1. Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, Gould E, Hen R, Abrous DN, Toni N, Schinder AF, Zhao X, Lucassen PJ, Frisén J. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duarte-Guterman P, Yagi S, Chow C, Galea LAM. Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Horm Behav. 2015;74:37–52. [DOI] [PubMed] [Google Scholar]
- 3. Mahmoud R, Wainwright SR, Galea LAM. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front Neuroendocrinol. 2016;41:129–152. [DOI] [PubMed] [Google Scholar]
- 4. Barker JM, Galea LAM. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152(4):888–902. [DOI] [PubMed] [Google Scholar]
- 5. Spritzer MD, Galea LAM. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67(10):1321–1333. [DOI] [PubMed] [Google Scholar]
- 6. Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LAM. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology. 2013;154(9):3294–3304. [DOI] [PubMed] [Google Scholar]
- 7. Swift-Gallant A, Duarte-Guterman P, Hamson DK, Ibrahim M, Monks DA, Galea LAM. Neural androgen receptors affect the number of surviving new neurones in the adult dentate gyrus of male mice. J Neuroendocrinol. 2018;30(4):e12578. [DOI] [PubMed] [Google Scholar]
- 8. Xiao L, Jordan CL. Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat hippocampus. Horm Behav. 2002;42(3):327–336. [DOI] [PubMed] [Google Scholar]
- 9. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2(10):894–897. [DOI] [PubMed] [Google Scholar]
- 11. van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiba S, Suzuki M, Yamanouchi K, Nishihara M. Involvement of granulin in estrogen-induced neurogenesis in the adult rat hippocampus. J Reprod Dev. 2007;53(2):297–307. [DOI] [PubMed] [Google Scholar]
- 13. Barha CK, Galea LAM. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol Aging. 2011;32(11):2091–2095. [DOI] [PubMed] [Google Scholar]
- 14. Barha CK, Lieblich SE, Chow C, Galea LAM. Multiparity-induced enhancement of hippocampal neurogenesis and spatial memory depends on ovarian hormone status in middle age. Neurobiol Aging. 2015;36(8):2391–2405. [DOI] [PubMed] [Google Scholar]
- 15. Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336(2):293–306. [DOI] [PubMed] [Google Scholar]
- 16. Barker JM, Galea LAM. Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. Gen Comp Endocrinol. 2009;164(1):77–84. [DOI] [PubMed] [Google Scholar]
- 17. Andò S, Giacchetto C, Canonaco M, Aquila S, Valenti A, Beraldi E, Piro A, Dessì-Fulgheri F. Effects of castration on androstenedione, testosterone and dihydrotestosterone plasma levels in adult male rats. Horm Res. 1986;23(2):122–127. [DOI] [PubMed] [Google Scholar]
- 18. Ma L, Li W, Zhu H-P, Li Z, Sun Z-J, Liu X-P, Zhao J, Zhang J-S, Zhang Y-Q. Localization and androgen regulation of metastasis-associated protein 1 in mouse epididymis. PLoS One. 2010;5(11):e15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21(2):464–476. [DOI] [PubMed] [Google Scholar]
- 20. Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5(6):545–558. [DOI] [PubMed] [Google Scholar]
- 21. Eid RS, Chaiton JA, Lieblich SE, Bodnar TS, Weinberg J, Galea LAM. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1–17. [DOI] [PubMed] [Google Scholar]
- 22. Chambers KC, Phoenix CH. Sexual behaviors of aging female rats: influence of age and hormonal state of male partners. Neurobiol Aging. 1986;7(3):165–171. [DOI] [PubMed] [Google Scholar]
- 23. Mattheij JA, Swarts JJ. Quantification and classification of pregnancy wastage in 5-day cyclic young through middle-aged rats. Lab Anim. 1991;25(1):30–34. [DOI] [PubMed] [Google Scholar]
- 24. Wu D, Gore AC. Changes in androgen receptor, estrogen receptor alpha, and sexual behavior with aging and testosterone in male rats. Horm Behav. 2010;58(2):306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. RRID:AB_514483, https://scicrunch.org/resolver/AB_514483.
- 26. RRID:AB_2314701, https://scicrunch.org/resolver/AB_2314701.
- 27. RRID:AB_11156085, https://scicrunch.org/resolver/AB_11156085.
- 28. RRID:AB_609570, https://scicrunch.org/resolver/AB_609570.
- 29. RRID:AB_2298772, https://scicrunch.org/resolver/AB_2298772.
- 30. RRID:AB_2801465, https://scicrunch.org/resolver/AB_2801465.
- 31. West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. [DOI] [PubMed] [Google Scholar]
- 32. West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14(4):275–285. [DOI] [PubMed] [Google Scholar]
- 33. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. [DOI] [PubMed] [Google Scholar]
- 34. Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. [DOI] [PubMed] [Google Scholar]
- 35. Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481(3):252–265. [DOI] [PubMed] [Google Scholar]
- 36. Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29(46):14484–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22(4):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96(5):379–394. [DOI] [PubMed] [Google Scholar]
- 39. Chow C, Epp JR, Lieblich SE, Barha CK, Galea LAM. Sex differences in neurogenesis and activation of new neurons in response to spatial learning and memory. Psychoneuroendocrinology. 2013;38(8):1236–1250. [DOI] [PubMed] [Google Scholar]
- 40. Yagi S, Chow C, Lieblich SE, Galea LAM. Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus. 2016;26(1):87–101. [DOI] [PubMed] [Google Scholar]
- 41. Wainwright SR, Lieblich SE, Galea LAM. Hypogonadism predisposes males to the development of behavioural and neuroplastic depressive phenotypes. Psychoneuroendocrinology. 2011;36(9):1327–1341. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Z, Yang R, Zhou R, Li L, Sokabe M, Chen L. Progesterone promotes the survival of newborn neurons in the dentate gyrus of adult male mice. Hippocampus. 2010;20(3):402–412. [DOI] [PubMed] [Google Scholar]
- 43. Spritzer MD, Ibler E, Inglis W, Curtis MG. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011;195:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carrier N, Kabbaj M. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Horm Behav. 2012;61(5):678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wainwright SR, Workman JL, Tehrani A, Hamson DK, Chow C, Lieblich SE, Galea LAM. Testosterone has antidepressant-like efficacy and facilitates imipramine-induced neuroplasticity in male rats exposed to chronic unpredictable stress. Horm Behav. 2016;79:58–69. [DOI] [PubMed] [Google Scholar]
- 46. Brännvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci. 2005;21(4):871–878. [DOI] [PubMed] [Google Scholar]
- 47. Zhang W, Cheng J, Vagnerova K, Ivashkova Y, Young J, Cornea A, Grafe MR, Murphy SJ, Hurn PD, Brambrink AM. Effects of androgens on early post-ischemic neurogenesis in mice. Transl Stroke Res. 2014;5(2):301–311. [DOI] [PubMed] [Google Scholar]
- 48. Fowler CD, Freeman ME, Wang Z. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57(3):257–269. [DOI] [PubMed] [Google Scholar]
- 49. Ormerod BK, Lee TT-Y, Galea LAM. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55(2):247–260. [DOI] [PubMed] [Google Scholar]
- 50. Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LAM. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141(4):1793–1800. [DOI] [PubMed] [Google Scholar]
- 51. Barha CK, Lieblich SE, Galea LAM. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol. 2009;21(3):155–166. [DOI] [PubMed] [Google Scholar]
- 52. Duarte-Guterman P, Lieblich SE, Chow C, Galea LAM. Estradiol and GPER activation differentially affect cell proliferation but not GPER expression in the hippocampus of adult female rats. PLoS One. 2015;10(6):e0129880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388(4):603–612. [DOI] [PubMed] [Google Scholar]
- 54. Kalita K, Szymczak S, Kaczmarek L. Non-nuclear estrogen receptor β and α in the hippocampus of male and female rats. Hippocampus. 2005;15(3):404–412. [DOI] [PubMed] [Google Scholar]
- 55. Isgor C, Watson SJ. Estrogen receptor α and β mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience. 2005;134(3):847–856. [DOI] [PubMed] [Google Scholar]
- 56. Pérez SE, Chen E-Y, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145(1):117–139. [DOI] [PubMed] [Google Scholar]
- 57. Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moser VA, Christensen A, Liu J, Zhou A, Yagi S, Beam CR, Galea L, Pike CJ. Effects of aging, high-fat diet, and testosterone treatment on neural and metabolic outcomes in male brown Norway rats. Neurobiol Aging. 2019;73:145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chaiton JA, Wong SJ, Galea LA. Chronic aromatase inhibition increases ventral hippocampal neurogenesis in middle-aged female mice. Psychoneuroendocrinology. 2019;106:111–116. [DOI] [PubMed] [Google Scholar]
- 60. Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136(8):3213–3221. [DOI] [PubMed] [Google Scholar]
- 61. Moghadami S, Jahanshahi M, Sepehri H, Amini H. Gonadectomy reduces the density of androgen receptor-immunoreactive neurons in male rat’s hippocampus: testosterone replacement compensates it. Behav Brain Funct. 2016;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feng Y, Weijdegård B, Wang T, Egecioglu E, Fernandez-Rodriguez J, Huhtaniemi I, Stener-Victorin E, Billig H, Shao R. Spatiotemporal expression of androgen receptors in the female rat brain during the oestrous cycle and the impact of exogenous androgen administration: a comparison with gonadally intact males. Mol Cell Endocrinol. 2010;321(2):161–174. [DOI] [PubMed] [Google Scholar]
- 63. Galea LAM, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25(11):1039–1061. [DOI] [PubMed] [Google Scholar]
- 64. Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor α in male rats. J Comp Neurol. 2009;512(5):688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rosario ER, Chang L, Beckett TL, Carroll JC, Paul Murphy M, Stanczyk FZ, Pike CJ. Age-related changes in serum and brain levels of androgens in male Brown Norway rats. Neuroreport. 2009;20(17):1534–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]