ABSTRACT
Background
Western diets are associated with increased incidences of obesity, hypertension, diabetes, and hypercholesterolemia, whereas Mediterranean diets, richer in polyphenols, monounsaturated fats, fruits, vegetables, poultry, and fish, appear to have cardiometabolic health benefits. Previous work has included population-based studies with limited evidence for causation or animal studies focused on single macro- or micronutrients; therefore, primate animal models provide an opportunity to determine potential mechanisms underlying the effects of dietary patterns on health and disease.
Objective
The aim of this study was to determine the effects of whole dietary patterns, either a Western or Mediterranean diet, on skeletal muscle mitochondrial bioenergetics in cynomolgus macaques.
Methods
In this study, 22 adult female cynomolgus macaques (∼11–14 y by dentition) were fed either a Western or Mediterranean diet for 30 mo. The Western diet was designed to mimic the diet of a middle-aged American woman and the Mediterranean diet included key aspects of Mediterranean diets studied in humans, such as plant-based proteins and fat, complex carbohydrates, and fiber. Diets were matched on macronutrient composition (16% protein, 54% carbohydrate, and 31% fat) and cholesterol content. Skeletal muscle was collected for high-resolution respirometry, citrate synthase activity, and western blot measurements. Pearson correlation analysis between respirometry measures and measures of carbohydrate metabolism was also performed.
Results
We found that consumption of a Western diet resulted in significantly higher mitochondrial respiration with fatty acid oxidation (FAO) (53%), FAO + complex I (52%), complex I + II (31%), max electron transport system (ETS) (31%), and ETS rotenone sensitive (31%) than did consumption of a Mediterranean diet. In addition, measures of respiration in response to fatty acids were significantly and positively correlated with both insulin resistance and plasma insulin concentrations.
Conclusions
This study highlights the importance of dietary composition in mitochondrial bioenergetics and that diet can influence skeletal muscle mitochondrial respiration independently of other factors such as macronutrient composition.
Keywords: Western, Mediterranean, diet, skeletal muscle, mitochondria, bioenergetics, nonhuman primates
Introduction
Typical Western diets contain high amounts of saturated fats, sucrose and fructose, proteins from red meats, and sodium, and low amounts of monounsaturated and polyunsaturated fats. Consumption of a Western diet has been associated with increased incidences of obesity, all-cause mortality, cancer, kidney disease, osteoporosis, hypertension, type 2 diabetes, and hypercholesterolemia (1–3). In contrast, Mediterranean diets are composed of high amounts of monounsaturated fats from mainly plant sources such as olive oil, fruits, vegetables, and proteins from poultry and fish sources. Consumption of a Mediterranean diet has been associated with reduced risk of developing diabetes, cancer, cardiovascular disease, and Alzheimer disease (4–7). These associations with dietary patterns have been elucidated primarily by population-based epidemiological studies; therefore, there is limited evidence for causation and a need for mechanistic insights.
Animal studies have reported that a high-fat diet increases fatty acid– but not pyruvate-, malate-, or glutamate-mediated respiration of mitochondria isolated from skeletal muscle (8–10). Increased expressions of oxidative phosphorylation proteins and uncoupling protein 3 were also observed. When mice were fed a high-fat and high-sucrose diet, increased mitochondrial respiration in permeabilized skeletal muscle fibers, increased citrate synthase (CS) and carnitine palmitoyl transferase (CPT) activity, and increased expression of uncoupling protein 3, CS, and complex I were observed (11). Rodents in these studies were obese and insulin resistant, but not overtly diabetic. In humans, previous studies demonstrate decreased mitochondrial enzyme activity in subjects with type 2 diabetes and obesity. Obese subjects had lower succinate dehydrogenase (complex II) activity (12), lower CPT activity (13), and lower cytochrome c oxidase activity (complex IV) (14). When mitochondrial function was examined by respirometry, no differences in respiration between obese and lean subjects were observed (15). Diabetic subjects had lower NADH:ubiquinone oxidoreductase (complex I) activity and CS activity (16). Diabetics also exhibited lower skeletal muscle mitochondrial respiration than healthy controls (17). These subjects also had decreased levels of mitochondrial DNA and CS activity, suggesting that there was lower mitochondrial density in their skeletal muscle, a potential cause for their decreased mitochondrial respiration.
In this study, we have examined the effects of whole dietary patterns, either a Mediterranean or a Western diet, on skeletal muscle mitochondrial bioenergetics. It is unlikely that the negative effects of a Western diet or the positive effects of a Mediterranean diet are due to a single dietary component. To our knowledge, the study presented here is the first to report on the effects of whole dietary patterns on skeletal muscle mitochondrial bioenergetics. We utilized cynomolgus macaques to examine the effects of whole diets administered for 30 mo, equivalent to ∼8 human years. We performed bioenergetic profiling of skeletal muscle comprised of high-resolution respirometry of permeabilized muscle fibers with and without fatty acid substrates, tissue CS activity, and western blot analyses.
Methods
Experimental model and study design
The ancillary study described here examined 22 randomly selected female cynomolgus macaques (Macaca fascicularis) ranging in age from ∼11 to 14 y that were available from a larger parent study. The parent study included 42 female cynomolgus macaques and was designed to test the effects of Mediterranean compared with Western diet on a broad range of health outcomes (18).
Animals were housed in small social groups of 3–4 in pens measuring 3.3 m × 3.3 m × 3.3 m, on a 12 h/12 h light/dark schedule. Animals were fed either a Western or Mediterranean diet designed and produced by the Primate Nutrition and Diet Laboratory at Wake Forest School of Medicine for 30 mo. For the parent study, animals were randomly assigned to either diet treatment using stratified randomization, balanced on pretreatment characteristics that reflect overall health, including body weight, BMI, basal cortisol, and plasma lipid concentrations. Diet composition is shown in Supplemental Table 1 and macronutrient composition is shown in Supplemental Table 2. These semipurified diets were matched on protein, fat, carbohydrate, and cholesterol content. The Western diet was designed to be similar to consumption of middle-aged American women, with protein and fat derived from mainly animal sources and high in saturated fats and sodium (19). The Mediterranean diet was designed to mimic key aspects of the Mediterranean diet, including protein and fats from mainly plant sources, higher amounts of monounsaturated fats, high in complex carbohydrates and fiber, and lower amounts of sodium (20, 21). In particular, the Mediterranean diet included English walnut powder and extra virgin olive oil, which were key ingredients included in the PREDIMED study (22). Water was available ad libitum. Skeletal muscle tissue (Vastus lateralis) for bioenergetics analyses was collected at the time of necropsy. Animals were first sedated with intramuscular ketamine hydrochloride (15 mg/kg body weight), then intravenous sodium pentobarbital (∼13 mg/kg body weight) was administered to achieve surgical anesthesia, and animals were exsanguinated in accordance with guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. All procedures were conducted in compliance with state and federal laws, standards of the US Department of Health and Human Services, and the Animal Care and Use Committee of Wake Forest School of Medicine. All procedures and protocols were reviewed and approved by the Wake Forest School of Medicine Animal Care and Use Committee.
Body mass measurements
Body length (suprasternal notch to pubic symphysis) was measured during month 27 of the treatment phase and body weight was measured on the day of necropsy. BMI (in kg/m2) was calculated as described previously (23).
Insulin, glucose, and insulin sensitivity measurements
Intravenous glucose tolerance tests with insulin responses were done during treatment phase month 26 as previously described (24). Briefly, animals were sedated with intramuscular ketamine hydrochloride (15 mg/kg body weight) after being deprived of food for 18 h, then administered 500 mg dextrose/kg body weight. Blood samples were taken at 0, 5, 10, 20, 30, 40, and 60 min. Glucose AUC and k were calculated as previously described (23). Insulin AUC was calculated using insulin responses between 10 and 40 min. Glucose concentrations were determined by colorimetric assay using reagents and instrumentation (ACE Alera autoanalyzer) from Alfa Wasserman Diagnostic Technologies (25). Insulin was determined by ELISA (Mercodia). HOMA-IR was calculated as described previously (26).
Energy intake measurements
Energy intake was measured in the parent study (18). Briefly, each monkey was offered 120 kcal diet per kilogram of body weight per day for the duration of the study (30 mo) and experimental diets were weighed before and after the meal to calculate calories consumed. In addition, all monkeys were provided enrichment several times per week, including flavored noncaloric ice cubes 2 times/wk, rice krispies 1 time/wk, and low-calorie vegetables such as celery 1 time/wk.
Preparation of permeabilized skeletal muscle fibers
Immediately after killing, skeletal muscle tissue (Vastus lateralis) was removed. Approximately 10–15 mg tissue was selected for mechanical separation as described previously (27). The tissue was placed in ice-cold BIOPS (10 mM Ca-EGTA buffer, 0.1 µM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM potassium morpholineethanesulfonic acid, 0.5 mM DTT, 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phosphocreatine, pH 7.1). The tissue was cut into smaller bundles of ∼5 mg and separated mechanically with sharp angular forceps under magnification, permeabilized with saponin (30 mg/mL) for 30 min on ice, and washed with MiR05 (110 mM sucrose, 60 mM K+-lactobionate, 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 1 mg/mL BSA, pH 7.1) or buffer Z (105 mM K-MES, 30 mM KCl, 1 mM EGTA, 10 mM K2HPO4, 5 mM MgCl2·6H2O, 0.5 mg/mL BSA, pH 7.4) for 15 min on ice before analysis.
High-resolution respirometry of permeabilized skeletal muscle fibers
Two substrate-uncoupler-inhibitor titration (SUIT) protocols were used to examine mitochondrial bioenergetics with and without fatty acid oxidation (FAO). Approximately 2.5 mg of tissue was added to each chamber and steady-state rate of respiration measurements were obtained after every substrate addition and expressed as picomoles per second per milligram.
For the SUIT protocol with fatty acids, high-resolution oxygen flux measurements were conducted in 2 mL MiR06Cr (MiR05 containing 20 mM creatine and 280 U/mL catalase) using the Oroboros Oxygraph-2k (O2k; Oroboros Instruments). This protocol was adapted from Pesta and Gnaiger (27) and completed as follows: 7.5 mM ADP, 0.5 mM octanoylcarnitine, 2 mM malate, 10 μM cytochrome c to test for mitochondrial membrane integrity, 5 mM pyruvate, 10 mM glutamate, 50 mM succinate, 2 additions of 0.25 μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) followed by a titration of 0.5 μM FCCP to obtain maximal electron transport system (ETS) capacity, 10 mM glycerol-3-phosphate, 0.5 μM rotenone (Rot), and 5 μM antimycin-A.
For the SUIT protocol without fatty acids, high-resolution oxygen flux measurements were conducted in 2 mL buffer Z containing 20 mM creatine and 25 μM blebbistatin to inhibit contraction (28). This protocol was completed as follows: 2 mM malate, 4 mM ADP, 20 mM pyruvate, 10 mM glutamate, 10 mM succinate, 10 μM cytochrome c to test for mitochondrial membrane integrity, 2 additions of 0.25 μM FCCP followed by a titration of 0.5 μM FCCP to obtain maximal ETS capacity, 0.5 μM Rot, and 5 μM antimycin-A.
Respiration parameters measured in these SUIT protocols are summarized in Supplemental Table 3.
To further examine the effects of diet on mitochondrial bioenergetics we also calculated flux control ratios. Flux control ratios were calculated by dividing each respiration parameter by maximum respiration (27).
CS activity assay
CS activity was determined according to the manufacturer's instructions (Citrate synthase assay kit, Sigma CS0720). Briefly, skeletal muscle (Vastus lateralis) was homogenized in cold CelLytic MT (Sigma C3228) at pH 7.4 and a protease inhibitor cocktail (Sigma P8340). The homogenized sample was centrifuged at 12,000 × g for 10 min at 4ºC and the supernatant containing the protein was collected. Protein concentration was determined by bicinchoninic acid protein assay (Pierce). CS activity was measured by continuous spectrophotometric rate determination at 412 nm. Each sample was run in triplicate.
Western blotting
Skeletal muscle (Vastus lateralis) homogenate was loaded at a concentration of 30 μg total protein per well for separation by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked at room temperature for 1 h in 5% nonfat dry milk in tris-buffered saline containing 0.1% Tween-20. Blots were probed overnight at 4°C with primary antibodies (VDAC/Porin, Abcam, ab15895; GAPDH, Abcam, ab9484; CPT1b, Invitrogen, PA5-12218) and incubated with the appropriate HRP-conjugated anti-IgG antibody. Antibody-bound protein was detected by enhanced chemiluminescence and quantified by densitometry with ImageJ (National Institutes of Health).
Statistical analysis
Distributions of all variables were examined before any further analysis. Normality was assessed by Shapiro–Wilk tests and any variables that were not normally distributed were log transformed to achieve a normal distribution (FAO + complex I, FAO max ETS capacity, FAO ETS Rot sensitive, body weight, fasting glucose, fasting insulin, glucose AUC, and insulin AUC). Statistical significance between groups was evaluated by unpaired 2-tailed Student's t tests using Microsoft Excel software. Significance between groups was defined as P ≤ 0.05. Pearson correlation coefficients were assessed between all variables and partial correlations were adjusted for age and weight. Analysis was performed using SAS Enterprise Guide version 7.12 (SAS Institute Inc.).
Results
Demographic and bioenergetic characteristics of nonhuman primate subjects
Age, weight, BMI, HOMA-IR, fasting blood glucose, fasting blood insulin, glucose AUC, and insulin AUC for the subcohort of animals utilized for this ancillary study are summarized in Table 1. Cross-sectionally, there were no statistically significant differences between groups for any of these characteristics. However, this ancillary study was not adequately powered for between-group differences in these characteristics. In the larger parent study, body weight was significantly increased in the Western diet group at all time points after 6 mo on the diet; these changes were not observed in the Mediterranean diet group (18). In addition, the parent study found that Western diet resulted in increased body fat, activity, energy expenditure, TG concentrations, insulin resistance, and hepatosteatosis when compared with the Mediterranean diet (18).
TABLE 1.
Characteristics at necropsy of female cynomolgus macaques fed either a Western diet or Mediterranean diet1
| Western diet | Mediterranean diet | P value | |
|---|---|---|---|
| Age, y | 12.3 ± 0.2 (11.5–13.2) | 12.2 ± 0.2 (11.1–13.7) | 0.75 |
| Weight, kg | 3.65 ± 0.40 (2.59–7.10) | 3.19 ± 0.33 (2.42–5.24) | 0.32 |
| BMI, kg/m2 | 46.7 ± 3.4 (36.3–73.9) | 41.3 ± 2.5 (31.7–56.4) | 0.18 |
| HOMA-IR | 13.4 ± 3.2 (1.0–34.2) | 11.4 ± 2.4 (2.9–26.2) | 0.62 |
| Fasting blood glucose, mg/dL | 78 ± 3 (61–96) | 91 ± 7 (64–133) | 0.12 |
| Fasting blood insulin, mIU/L | 66.4 ± 16.7 (6.4–175) | 48.4 ± 9.5 (16.9–117) | 0.80 |
| Glucose AUC, mg · dL−1 · min | 2950 ± 430 (1720–6070) | 2830 ± 159 (1960–3680) | 0.84 |
| Insulin AUC, mg · dL−1 · min | 6820 ± 2450 (1480–28,900) | 4520 ± 1540 (1060–18,700) | 0.43 |
Values are mean ± SEM (range), n = 11 per group. Differences between groups were evaluated by unpaired 2-tailed Student's ttests. Glucose and insulin measurements were obtained from plasma samples.
Overall energy intake for animals in this ancillary study was not significantly different between the Western or Mediterranean diet groups, both in mean calories consumed (P = 0.30) and in mean calories consumed per kilogram of body weight (P = 0.83) (Supplemental Figure 1).
Representative bioenergetic profiles from a single animal are shown in Figure 1. All bioenergetic measurements of permeabilized muscle fiber respiration and CS activity for both diet groups are summarized in Supplemental Table 4.
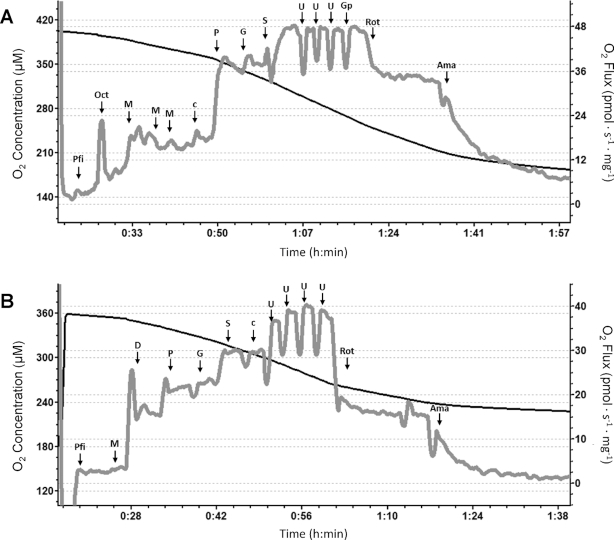
FIGURE 1.
Representative traces of high-resolution respirometry of permeabilized skeletal muscle fibers from a single female cynomolgus macaque. (A) High-resolution respirometry of permeabilized fibers with the SUIT protocol with fatty acids. (B) High-resolution respirometry of permeabilized fibers with the SUIT protocol without the addition of fatty acids. Ama, antimycin-A; c, cytochrome c; D, ADP; G, glutamate; Gp, glycerol-3-phosphate; M, malate; Oct, octanoylcarnitine; P, pyruvate; Pfi, permeabilized fibers; Rot, rotenone; S, succinate; SUIT, substrate-uncoupler-inhibitor titration; U, FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone).
Diet induces bioenergetic changes in permeabilized fibers
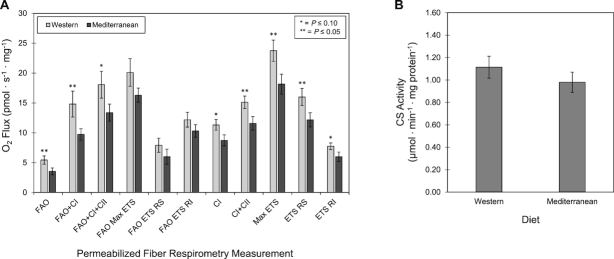
Western diet–fed primates exhibited higher oxygen flux across all respirometry measurements in permeabilized fibers than did primates fed a Mediterranean diet (Figure 2A). There was significantly higher respiration with FAO, FAO + complex I, complex I + II, max ETS, and ETS Rot sensitive. There were also trends for higher FAO + complex I + II, complex I, and ETS Rot insensitive.
FIGURE 2.
Effects of Western or Mediterranean diet on skeletal muscle bioenergetics of female cynomolgus macaques. (A) High-resolution respirometry of permeabilized muscle fibers. (B) CS activity. *,**Different from Mediterranean group: *P ≤ 0.10, **P ≤ 0.05. Data are presented as mean ± SEM, n = 11. C, complex; CS, citrate synthase; ETS, electron transport system; FAO, fatty acid oxidation; RI, rotenone insensitive; RS, rotenone sensitive.
To examine potential mechanisms of action, we measured CS activity, a marker of mitochondrial content (29, 30). Mean CS activities were similar for the Western and Mediterranean diet groups ( Figure 2B). This finding was confirmed by performing western blots for VDAC/Porin, a mitochondrial structural protein. Mean VDAC/Porin protein expression relative to GAPDH was similar for the Western and Mediterranean diet groups (Supplemental Figure 2). To examine differences in the relative contributions of substrates to mitochondrial respiration, we calculated flux control ratios for key respiratory parameters. We found trends for higher FAO flux control ratio (P = 0.08) and FAO + complex I flux control ratio (P = 0.10) in the Western diet group than in the Mediterranean diet group. To further examine these differences in FAO capacity, we performed western blots for CPT1, the rate-limiting step in the transport of fatty acids into mitochondria and β-oxidation (Supplemental Figure 1). Although there was no significant difference between the 2 groups, we found a trend for increased expression of CPT1 in the Mediterranean diet group (P = 0.09).
Correlations between permeabilized muscle fiber bioenergetics and insulin sensitivity, glucose, and insulin measurements
Pearson correlation coefficients were used to assess relationships between bioenergetic parameters and insulin sensitivity, glucose, and insulin measurements by diet group and are shown in Table 2 (Western diet) and Table 3 (Mediterranean diet); relationships with both groups combined are shown in Supplemental Table 5. For correlation analysis, our primary metabolic outcome was insulin sensitivity calculated by HOMA-IR.
TABLE 2.
Correlations between carbohydrate metabolism phenotypes and respirometry of permeabilized muscle fibers of female cynomolgus macaques fed a Western diet1
| Western diet | FAO | FAO + Complex I | FAO + Complex I + II | FAO Max ETS Capacity | FAO ETS Rot Sensitive | FAO ETS Rot Insensitive | Complex I | Complex I + II | Max ETS Capacity | ETS Rot Sensitive | ETS Rot Insensitive |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson | |||||||||||
| HOMA-IR | 0.77** | 0.86*** | 0.84** | 0.77** | 0.74* | 0.75** | 0.30 | 0.19 | 0.32 | 0.41 | −0.01 |
| Fasting blood glucose | 0.20 | 0.18 | 0.11 | 0.02 | 0.03 | 0.05 | 0.46 | 0.38 | 0.53 | 0.50 | 0.42 |
| Fasting blood insulin | 0.57 | 0.63* | 0.63* | 0.58 | 0.58 | 0.60 | 0.30 | 0.15 | 0.37 | 0.47 | 0.02 |
| Glucose AUC | 0.25 | 0.18 | 0.11 | 0.09 | 0.11 | 0.03 | −0.36 | −0.15 | 0.00 | 0.03 | −0.09 |
| Insulin AUC | 0.64* | 0.68* | 0.65* | 0.66* | 0.66* | 0.59 | −0.17 | −0.03 | −0.10 | −0.05 | −0.17 |
| Partial for age + weight | |||||||||||
| HOMA-IR | 0.81** | 0.90** | 0.86** | 0.81* | 0.73* | 0.82** | 0.30 | 0.18 | 0.47 | 0.62 | −0.01 |
| Fasting blood glucose | 0.09 | 0.11 | 0.00 | −0.05 | −0.11 | 0.03 | 0.48 | 0.36 | 0.70 | 0.71 | 0.46 |
| Fasting blood insulin | 0.66 | 0.68 | 0.66 | 0.62 | 0.56 | 0.65 | 0.27 | 0.15 | 0.53 | 0.69 | 0.01 |
| Glucose AUC | 0.03 | −0.05 | −0.05 | 0.00 | 0.16 | −0.08 | 0.02 | −0.02 | 0.34 | 0.42 | 0.07 |
| Insulin AUC | 0.86** | 0.83** | 0.85** | 0.88** | 0.92*** | 0.78** | 0.51 | 0.60 | 0.71 | 0.26 | −0.12 |
Values are Pearson correlation coefficients (r) for relationships between measures of carbohydrate metabolism and measures of respiration of permeabilized skeletal muscle fibers. Partial correlations were controlled for age and weight at time of necropsy. *,**,***Significant correlation: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. ETS, electron transport system; FAO, fatty acid oxidation; Rot, rotenone.
TABLE 3.
Correlations between carbohydrate metabolism phenotypes and respirometry of permeabilized muscle fibers of female cynomolgus macaques fed a Mediterranean diet1
| Mediterranean diet | FAO | FAO + Complex I | FAO + Complex I + II | FAO Max ETS Capacity | FAO ETS Rot Sensitive | FAO ETS Rot Insensitive | Complex I | Complex I + II | Max ETS Capacity | ETS Rot Sensitive | ETS Rot Insensitive |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson | |||||||||||
| HOMA-IR | −0.04 | 0.13 | 0.06 | 0.15 | −0.25 | 0.34 | −0.33 | −0.32 | −0.21 | 0.02 | −0.48 |
| Fasting blood glucose | 0.44 | −0.10 | −0.07 | 0.47 | 0.41 | 0.00 | −0.11 | −0.13 | 0.03 | 0.14 | −0.14 |
| Fasting blood insulin | −0.26 | 0.04 | −0.03 | −0.05 | −0.30 | 0.21 | −0.43 | −0.36 | −0.30 | −0.07 | −0.54 |
| Glucose AUC | 0.14 | −0.09 | 0.20 | 0.24 | 0.04 | 0.19 | −0.14 | −0.27 | 0.01 | 0.24 | −0.36 |
| Insulin AUC | −0.52 | −0.14 | −0.12 | −0.54 | −0.32 | −0.14 | −0.24 | −0.09 | −0.38 | −0.39 | −0.22 |
| Partial for age + weight | |||||||||||
| HOMA-IR | −0.10 | 0.07 | 0.08 | 0.12 | −0.27 | 0.37 | −0.48 | −0.43 | −0.40 | −0.14 | −0.65 |
| Fasting blood glucose | 0.38 | −0.39 | −0.09 | 0.45 | 0.54 | −0.12 | −0.36 | −0.33 | −0.24 | −0.11 | −0.36 |
| Fasting blood insulin | −0.26 | 0.09 | 0.01 | −0.05 | −0.36 | 0.29 | −0.43 | −0.33 | −0.39 | −0.17 | −0.57 |
| Glucose AUC | 0.18 | −0.07 | 0.17 | 0.31 | 0.09 | 0.19 | 0.03 | −0.16 | 0.22 | 0.46 | −0.29 |
| Insulin AUC | −0.73* | 0.19 | −0.30 | −0.75* | −0.62 | −0.16 | 0.17 | 0.43 | 0.05 | −0.01 | 0.13 |
Values are Pearson correlation coefficients (r) for relationships between measures of carbohydrate metabolism and measures of respiration of permeabilized skeletal muscle fibers. Partial correlations were controlled for age and weight at time of necropsy. *Significant correlation, P ≤ 0.05. ETS, electron transport system; FAO, fatty acid oxidation; Rot, rotenone.
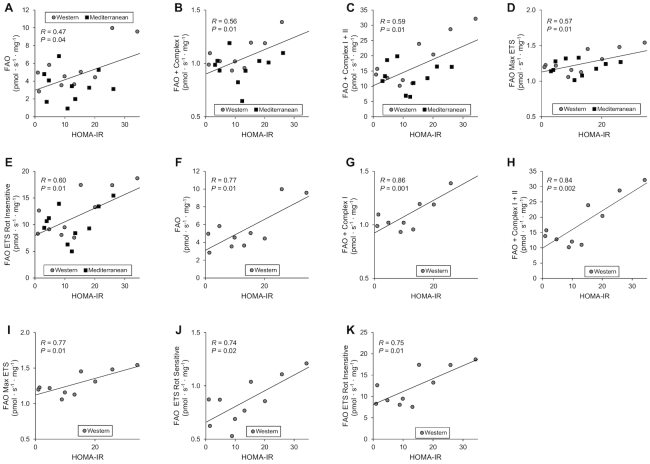
Regression plots illustrating the statistically significant relationships with HOMA-IR are shown in Figure 3A–E for both groups combined. HOMA-IR was significantly positively correlated with FAO ( Figure 3A), FAO + complex I (Figure 3B), FAO + complex I + II (Figure 3C), FAO max ETS (Figure 3D), and FAO ETS Rot insensitive (Figure 3E). These relationships between measures of FAO and HOMA-IR were strongest in the Western diet group and are shown in Figure 3F–K. HOMA-IR was positively correlated with FAO (Figure 3F), FAO + complex I (Figure 3G), FAO + complex I + II (Figure 3H), FAO max ETS (Figure 3I), FAO ETS Rot sensitive (Figure 3J), and FAO ETS Rot insensitive (Figure 3K).
FIGURE 3.
Correlations between permeabilized muscle fiber bioenergetics and insulin sensitivity of female cynomolgus macaques. (A–E) Plots of FAO measures against HOMA-IR for all animals in the study. (F–K) Plots of FAO measures against HOMA-IR for animals in the Western diet group. Pearson correlation coefficients (R) and P values are shown on each plot. ETS, electron transport system; FAO, fatty acid oxidation; Rot, rotenone.
In addition, with both groups combined, fasting blood insulin was positively correlated with FAO + complex I + II (Supplemental Figure 3A) and FAO ETS Rot sensitive (Supplemental Figure 3B). Insulin AUC was positively correlated with FAO + complex I + II (Supplemental Figure 3C). In the Western diet group, fasting blood insulin was positively correlated with FAO + complex I (Supplemental Figure 3D) and FAO + complex I + II (Supplemental Figure 3E). Insulin AUC was significantly positively correlated with FAO (Supplemental Figure 3F), FAO + complex I (Supplemental Figure 3G), FAO + complex I + II (Supplemental Figure 3H), FAO max ETS (Supplemental Figure 3I), and FAO ETS Rot sensitive (Supplemental Figure 3J).
Discussion
We observed significantly higher respiration both with and without FAO in permeabilized skeletal muscle fibers from animals fed a Western compared with a Mediterranean diet. These changes were independent of CS activity, a marker of mitochondrial volume or content (29, 30), and expression of VDAC/Porin. These data suggest that the increase in respiration with a Western diet is likely not due to differences in mitochondrial content and could be the result of alterations in other factors such as mitochondrial morphology and composition, mitochondrial quality control, or other intracellular interactions. For example, differences in mitochondrial morphology have been linked to differences in respiration: more fragmented mitochondria are associated with nutrient-rich environments and inefficient energy production, whereas elongated mitochondria are associated with periods of starvation and more efficient production of ATP (31).
We also observed a trend for increased FAO and FAO + complex I flux control ratios in the Western diet group. To examine a potential mechanism for this observation, we looked at the expression of CPT1 by western blot. Interestingly, we found a trend for increased CPT1 expression in the Mediterranean diet group. For the measurement of FAO, we used a combination of octanoylcarnitine and malate to induce respiration from FAO. This combination bypasses the rate-limiting step of conversion of acyl-CoAs to acylcarnitines by CPT1, because octanoylcarnitine can freely cross the mitochondrial membrane (32). If we had used octanoyl-CoA and carnitine separately as substrates to induce FAO, we may have observed different results. It is important to consider that this study was not able to examine the stimulation of FAO by longer fatty acids such as palmitate, MUFAs such as oleate, or PUFAs such as linoleic acid. These limitations may account for the lower FAO observed in the Mediterranean diet group despite marginally higher CPT1 expression in skeletal muscle. Indeed, muscle has different capacities for the oxidation of various types of fatty acids (33). Notably, other studies have reported that oleate increases both gene and protein expression of CPT1 (34, 35), which is consistent with our observation that a Mediterranean diet rich in monounsaturated fats increased expression of CPT1 in skeletal muscle.
Previous studies of mitochondrial bioenergetics have reported that high-fat diets in rodents are associated with increased mitochondrial respiration in response to both fatty acid and carbohydrate substrates, increased mitochondrial biogenesis, and increased mitochondrial content, despite increased insulin resistance (36–39). However, these studies included very-high-fat diets with 45–60% of total calories from fat that were compared with control diets that contained 8–12% of calories from fat. In addition, the composition of fats in these studies varies. Some studies included flax seed oil and olive oil as sources of fat, whereas others had lard as the major source. It should be noted that the study presented here does not compare high-fat and low-fat diets. Both the Western and Mediterranean experimental diets contain 31% of calories from fat. Indeed, we matched the experimental diets on protein, carbohydrate, and total fat content. The Western experimental diet has a larger amount of saturated fat, whereas the Mediterranean experimental diet has a larger percentage of monounsaturated and polyunsaturated fats. Our results suggest that manipulations of the types of fats consumed can alter mitochondrial bioenergetics independently of other factors such as macronutrient composition, energy intake, and obesity.
Previous studies have linked Western diets to chronic low-grade inflammation with elevated concentrations of biomarkers such as C-reactive protein, IL-6 and IL-18, and fibrinogen (40). In the context of obesity and insulin resistance, increased concentrations of TNF-α, IL-6, and C-reactive protein have also been reported in both animal models and humans (41). Inflammation is associated with increased mitochondrial respiration in brown adipose tissue, adipocytes, and fibroblasts (42–44). Lark et al. (41) have suggested that high-fat diets that increase mitochondrial respiration and partially oxidized lipid metabolites also increase production of reactive oxygen species and H2O2 that could lead to the induction of proinflammatory cascades and prolonged inflammation. In the present study, the lower mitochondrial respiration we observed with the Mediterranean diet may be linked to reduced reactive oxygen species, with the potential to lower the systemic inflammatory burden. This diet is high in n–3 fatty acids and has a lower ratio of n–6:n–3 fatty acids; both of these have been associated with anti-inflammatory effects and could play a role in the differences in mitochondrial bioenergetics observed (45, 46). Future studies designed to investigate the effects of Western and Mediterranean dietary patterns on inflammation will need to be conducted in order to determine how these relate to differences in mitochondrial metabolism.
We observed strong positive correlations between FAO and HOMA-IR and fasting blood insulin. These results suggest that there is a potential link between increased FAO of octanoylcarnitine and the development of insulin resistance. Interestingly, we did not observe significant correlations between these parameters when respiration was examined in the absence of octanoylcarnitine. Within-group relationships were strongest in the Western diet group and generally nonsignificant in the Mediterranean diet group, perhaps reflecting greater heterogeneity in responses to diet in the Western diet group. However, when both diet groups are combined, similar relationships to the Western diet group persist. Partial correlations controlling for body weight and age suggest that insulin AUC was positively associated with FAO and FAO max ETS capacity in the Western diet group and negatively associated in the Mediterranean diet group, suggesting a complex diet × body weight interaction. In light of these correlations, further studies are necessary to understand the link between FAO and insulin resistance. It should also be noted that we made multiple comparisons in this study, which increases the risk of type I errors and may lead to significant correlations that are false or incorrect. To mitigate these risks, we chose insulin sensitivity calculated by HOMA-IR as our primary metabolic outcome for correlation analysis. If we reduce the significance threshold to P ≤ 0.01, many of these relationships are still significant including the relationships between measures of FAO and HOMA-IR. In addition, these results are consistent; not only do measures of FAO significantly and positively correlate with HOMA-IR, but so do other surrogate measurements of insulin resistance: fasting blood insulin and insulin AUC.
It should be noted that human studies of diabetics and obese individuals have reported reductions in skeletal muscle mitochondrial bioenergetics (12–17). In this study, metabolic perturbations associated with the Western diet may be related to a prediabetic state in humans. A significantly longer study would be required to determine the temporal relationship between bioenergetic changes associated with diet and the development of overt diabetes. Our results may reflect an early adaptation to insulin resistance which may be followed by bioenergetic decline upon disease progression.
Major strengths of this study include the use of high-resolution mitochondrial respirometric profiling with and without fatty acid substrate, the comparison of whole dietary patterns rather than manipulation of a single macronutrient, and the use of a well-established nonhuman primate model highly translatable to humans that allowed for tight experimental control that cannot otherwise be achieved. This study utilized similarly aged premenopausal female cynomolgus monkeys, so additional studies would be required to determine the effects of age, sex, and menopause. A major limitation of this study is the absence of a baseline measurement of mitochondrial respiration or a “control” diet. Muscle samples were obtained only after necropsy. Pre–post diet effects would require multiple biopsies and would, therefore, require an experimental design that is different from the parent study utilized here. Although baseline differences are less likely in animal studies than in human trials, the animals were monitored at baseline and randomly assigned to diet groups with stratified randomization that ensured the groups were balanced based on pretreatment characteristics (body weight, BMI, basal cortisol, and plasma lipid concentrations). The experimental diets were also carefully matched on macronutrient composition (16% protein, 54% carbohydrate, and 31% total fat) and cholesterol so that the only variables were fat composition and the sources of diet components: more animal sources for the Western diet and more plant sources for the Mediterranean diet.
We report that the consumption of a Western diet resulted in higher skeletal muscle mitochondrial respiration than consumption of a Mediterranean diet and that FAO significantly and positively correlated with insulin resistance, particularly in the Western diet group. Although future investigation is warranted, including analysis of mitochondrial function pre- and postdietary manipulation, these studies suggest a potential link between high skeletal muscle mitochondrial respiration, the consumption of a Western diet, and the development of insulin resistance. In addition, these results highlight the importance of dietary composition in the study of mitochondrial bioenergetics. We have shown that changes in the types of fat consumed can influence skeletal muscle mitochondrial respiration independently of other factors such as macronutrient composition.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—JLG-A: played a key role in the conceptualization of this ancillary study, performed all the respirometric analyses of permeabilized muscle fibers, and played a lead role in data analysis and manuscript preparation; JLG-A and CAS: played a key role in the development of this ancillary study; ZG: performed the citrate synthase activity analyses; SEA and MZV: participated in the design of the project; SEA: designed the diets in collaboration with the other investigators and supervised the diet laboratory that formulated them; SEA and KTM: participated in the monitoring of animal health; MZV: advised on the translation of human to nonhuman primate diets during the diet development process; KTM: collected samples; TCR: participated in the design and conduct of the project, diet development, and supervised the Clinical Chemistry and Endocrinology Laboratory that performed the insulin, glucose, and insulin sensitivity measurements and manuscript preparation; CAS: was the principal investigator of the parent study and played a key role in data analyses and manuscript preparation; AJAM: was responsible for the development of this study, provided oversight for all mitochondrial assessments, worked directly with the study team to coordinate the experimental plan, and supervised data analyses and manuscript preparation; and all authors: read and approved the final manuscript.
Notes
Supported by NIH grants R21 AG051077 (to AJAM), R01 HL087103 (to CAS), and R01 HL122393 (to TCR), AHA grant 15MCPRP25680019 (to AJAM), and Wake Forest Claude Pepper Older Americans Independence Center grant P30 AG21332.
Author disclosures: JLG-A, ZG, SEA, MZV, KTM, TCR, CAS, and AJAM, no conflicts of interest.
Supplemental Tables 1–5 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CPT, carnitine palmitoyl transferase; CS, citrate synthase; ETS, electron transport system; FAO, fatty acid oxidation; FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; Rot, rotenone; SUIT, substrate-uncoupler-inhibitor titration.
References
- 1. Heidemann C, Schulze MB, Franco OH, Van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118(3):230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81(2):341–54. [DOI] [PubMed] [Google Scholar]
- 3. Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Ren Physiol. 2011;301(5):919–31. [DOI] [PubMed] [Google Scholar]
- 4. Martínez-González MÁ, De La Fuente-Arrillaga C, Nunez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z, Benito S, Tortosa A, Bes-Rastrollo M. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336:1348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knoops KTB, De Groot LCPGM, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, Van Staveren WA. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. J Am Med Assoc. 2004;292(12):1433–9. [DOI] [PubMed] [Google Scholar]
- 6. De Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–85. [DOI] [PubMed] [Google Scholar]
- 7. Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69(11):1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cole MA, Murray AJ, Cochlin LE, Heather LC, McAleese S, Knight NS, Sutton E, Jamil AA, Parassol N, Clarke K. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Res Cardiol. 2011;106(3):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoeks J, de Wilde J, Hulshof MFM, van den Berg SAA, Schaart G, van Dijk KW, Smit E, Mariman ECM. High fat diet-induced changes in mouse muscle mitochondrial phospholipids do not impair mitochondrial respiration despite insulin resistance. PLoS One. 2011;6:e27274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jørgensen T, Grunnet N, Quistorff B. One-year high fat diet affects muscle-but not brain mitochondria. J Cereb Blood Flow Metab. 2015;35(6):943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stephenson EJ, Camera DM, Jenkins TA, Kosari S, Lee JS, Hawley JA, Stepto NK. Skeletal muscle respiratory capacity is enhanced in rats consuming an obesogenic Western diet. AJP Endocrinol Metab. 2012;302(12):E1541–9. [DOI] [PubMed] [Google Scholar]
- 12. He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50(4):817–23. [DOI] [PubMed] [Google Scholar]
- 13. Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13(14):2051–60. [DOI] [PubMed] [Google Scholar]
- 14. Kelley DE, Goodpaster B, Wing RR, Simoneau J-A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Metab. 1999;277(6):E1130–41. [DOI] [PubMed] [Google Scholar]
- 15. Ara I, Larsen S, Stallknecht B, Guerra B, Morales-Alamo D, Andersen JL, Ponce-González JG, Guadalupe-Grau A, Galbo H, Calbet JAL et al.. Normal mitochondrial function and increased fat oxidation capacity in leg and arm muscles in obese humans. Int J Obes. 2011;35:99–108. [DOI] [PubMed] [Google Scholar]
- 16. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–50. [DOI] [PubMed] [Google Scholar]
- 17. Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50(4):790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shively CA, Appt SE, Vitolins MZ, Uberseder B, Michalson KT, Silverstein-Metzler MG. Mediterranean versus Western diet effects on caloric intake, obesity, metabolism, and hepatosteatosis in nonhuman primates. Obesity. 2019;27:(5):777–84. doi:10.1002/oby.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhodes DG, Clemens JC, Goldman JD, LaComb RP, Moshfegh AJ. 2011–2012 What We Eat in America, NHANES tables 1–40. [Internet] Beltsville (MD): Food Surveys Research Group; 2014; [cited 2019 Feb 24]. Available from: www.ars.usda.gov/services/docs.htm?docid=18349. [Google Scholar]
- 20. Bédard A, Riverin M, Dodin S, Corneau L, Lemieux S. Sex differences in the impact of the Mediterranean diet on cardiovascular risk profile. Br J Nutr. 2012;108(8):1428–34. [DOI] [PubMed] [Google Scholar]
- 21. Kafatos A, Verhagen H, Moschandreas J, Apostolaki I, Van Westerop JJM. Mediterranean diet of Crete: foods and nutrient content. J Am Diet Assoc. 2000;100(12):1487–93. [DOI] [PubMed] [Google Scholar]
- 22. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J et al.. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(34):1–14. [DOI] [PubMed] [Google Scholar]
- 23. Jayo JM, Shively CA, Kaplan JR, Manuck SB. Effects of exercise and stress on body fat distribution in male cynomolgus monkeys. Int J Obes Relat Metab Disord. 1993;17(10):597–604. [PubMed] [Google Scholar]
- 24. Shively CA, Clarkson TB. Regional obesity and coronary artery atherosclerosis in females: a non‐human primate model. Acta Med Scand. 1987;222(S723):71–8. [DOI] [PubMed] [Google Scholar]
- 25. Adams MR, Golden DL, Williams JK, Franke AA, Register TC, Kaplan JR. Soy protein containing isoflavones reduces the size of atherosclerotic plaques without affecting coronary artery reactivity in adult male monkeys. J Nutr. 2005;135(12):2852–6. [DOI] [PubMed] [Google Scholar]
- 26. Silverstein-Metzler MG, Shively CA, Clarkson TB, Appt SE, Carr JJ, Kritchevsky SB, Jones SR, Register TC. Sertraline inhibits increases in body fat and carbohydrate dysregulation in adult female cynomolgus monkeys. Psychoneuroendocrinology. 2016;68:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. In: Palmeira CM, Moreno AJ, editors. Mitochondrial bioenergetics: methods and protocols. Dordrecht: Springer; 2012. pp. 25–58. [DOI] [PubMed] [Google Scholar]
- 28. Perry CGR, Kane DA, Lin C-T, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ et al.. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J. 2011;437(2):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Picard M, Ritchie D, Wright KJ, Romestaing C, Thomas MM, Rowan SL, Taivassalo T, Hepple RT. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell. 2010;9(6):1032–46. [DOI] [PubMed] [Google Scholar]
- 30. Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F et al.. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590(14):3349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17(4):491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ojuka E, Andrew B, Bezuidenhout N, George S, Maarman G, Madlala HP, Mendham A, Osiki PO. Measurement of β-oxidation capacity of biological samples by respirometry: a review of principles and substrates. Am J Physiol Endocrinol Metab. 2016;310(9):E715–23. [DOI] [PubMed] [Google Scholar]
- 33. Reubsaet FAG, Veerkamp JH, Trijbels JMF, Monnens LAH. Total and peroxisomal oxidation of various saturated and unsaturated fatty acids in rat liver, heart and m. quadriceps. Lipids. 1989;24(11):945–50. [DOI] [PubMed] [Google Scholar]
- 34. Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008;283(17):11107–16. [DOI] [PubMed] [Google Scholar]
- 35. Henique C, Mansouri A, Fumey G, Lenoir V, Girard J, Bouillaud F, Prip-Buus C, Cohen I. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J Biol Chem. 2010;285(47):36818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lanza IR, Blachnio-Zabielska A, Johnson ML, Schimke JM, Jakaitis DR, Lebrasseur NK, Jensen MD, Sreekumaran Nair K, Zabielski P. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. Am J Physiol Endocrinol Metab. 2013;304(12):E1391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56(8):2085–92. [DOI] [PubMed] [Google Scholar]
- 38. Hancock CR, Han D-H, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A. 2008;105(22):7815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia-Roves P, Huss JM, Han D-H, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci U S A. 2007;104(25):10709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27. [DOI] [PubMed] [Google Scholar]
- 41. Lark DS, Fisher-Wellman KH, Neufer PD. High-fat load: mechanism(s) of insulin resistance in skeletal muscle. Int J Obes Supl. 2012;2:S31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alcalá M, Calderon-Dominguez M, Bustos E, Ramos P, Casals N, Serra D, Viana M, Herrero L. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci Rep. 2017;7:16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, Moretti M, De Smaele E, Beg AA, Tergaonkar V et al.. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13:1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM et al.. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. J Am Med Assoc. 2004;292(12):1440–6. [DOI] [PubMed] [Google Scholar]
- 46. White PJ, Arita M, Taguchi R, Kang JX, Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes. 2010;59(12):3066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.