Abstract
Latent Epstein-Barr virus (EBV) infection can clinically reactivate in immunosuppressed individuals causing lymphoproliferative disease and rarely hepatitis. In this study, we provide in vivo and in vitro evidence that Treponema pallidum infection can cause EBV reactivation with hepatitis in an immunocompetent patient. We report the diagnostic challenges and immunological findings of coinciding syphilis and EBV-associated hepatitis. Using an in vitro EBV-reactivation assay, we demonstrate that T pallidum reactivates latent EBV in a Toll-like receptor (TLR)2/B-cell receptor signaling-dependent manner. Epstein-Barr virus-associated reactivation or lymphoproliferation should be considered in infections with pathogens that activate TLR2.
Keywords: EBV, reactivation, syphilis, TLR, Treponema pallidum
Epstein-Barr virus (EBV) is the causative agent of infectious mononucleosis. Almost all adults are latently EBV-infected. Epstein-Barr virus-infected memory B cells are tightly controlled by EBV-specific T-cell immunity. Significant EBV-reactivation or EBV-associated lymphoproliferation can occur in patients with impaired cellular immune functions, eventually resulting in lymphoproliferative disease or hemophagocytic lymphohistiocytosis [1]. In rare cases, loss of immunological control over EBV may be associated with hepatitis as the leading clinical manifestation [2]. Low-level EBV viral loads—as a result of lymphoproliferation—can also occur in response to minor stressors. The immunological mechanisms triggering clinical EBV reactivation are ill-defined.
Syphilis is a sexually transmitted infection caused by the spirochete Treponema pallidum. Approximately 5 million new cases are diagnosed each year [3], with a maximum incidence in high-risk groups for sexually transmitted diseases and intravenous drug abusers. Primary syphilis manifests with genital ulceration. During the secondary stage, clinical presentation is dominated by a nonitching rash on the trunk and extremities and rarely includes hepatitis. The immune response is dominated by a T cell-mediated delayed-type hypersensitivity reaction, phagocytosis by macrophages, and a prominent B-cell activation [4]. A high number of plasmablasts is the hallmark finding in skin histology [4]. Treponema pallidum has a different phospholipid composition than common Gram-negative bacteria and lacks lipopolysaccharides (LPSs) [3]. Toll-like receptor (TLR)-2 is a pivotal immune receptor to sense spirochete [5], and data from a mouse model supports that TLR signaling is critical for the immune response against T pallidum [6].
MATERIAL AND METHODS
Laboratory Analyses and Histopathology
Diagnostic EBV polymerase chain reaction (PCR) was done in the clinical laboratory on deoxyribonucleic acid (DNA) extracted from whole blood and using TaqMan-based quantitative PCR (qPCR) for BALF5 (Supplementary Table S1), quantified against the World Health Organization standard. The test has a detection range of 1000–109 copies/mL. Liver biopsy was stained with hematoxylin and eosin, Sirius Red, Diastase-PAS, and Perls’ Prussian Blue to assess morphology. Immunohistochemistry for Spirochetes was performed using an anti-T pallidum antibody (CP135C; Biocare Medical). Epstein-Barr virus was detected by EBV encoding region (EBER) in situ hybridization (ISH) (Ventana, Roche Diagnostics). Immune-phenotyping included immunohistochemistry staining for CD3 (2GV6), CD20 (L26), CD38 (SP149), EBV latent membrane protein ([LMP] CS1-4) (all Ventana), and Epstein-Barr nuclear-associated antigen-2 (EBNA2) (PE2; Abcam).
Epstein-Barr Virus In Vitro Reactivation Assay
All experiments were done on fresh peripheral blood mononuclear cells (PBMCs) from EBV+ healthy blood donors. Peripheral blood mononuclear cells were depleted from T cells, natural killer (NK) cells, or monocytes using the flow-through of the respective MACS positive-selection kits. B cells were isolated using the MACS negative-selection kit (all Miltenyi). For in vitro T pallidum-specific stimulation, we used gelatin particles coated with purified pathogenic T pallidum from the Nichols Strain (Serodia-TP-PA(100T); Fujirebio Europe). Other stimulus used were as follows: B-cell receptor (BCR) cross-linking (5 μg/mL AffiniPure F(ab’)₂ Fragment Goat Anti-Human IgA + IgG + IgM(H + L); Jackson ImmunoResearch), TLR2 (1 μg/mL Pam3CSK4; InvivoGen), TLR4 (1 μg/mL LPS; Sigma), TLR7/8 (0.1 μg/mL R848 [Resiquimod]; InvivoGen), and TLR9 (0.5 μg/mL CpG; InvivoGen). Cells in media alone were used as negative control. Epstein-Barr virus-ribonucleic acid (RNA) was quantified using reverse-transcription PCR for BZLF-1, EBNA, LMP1, EBER, and BamHI-W and normalization to 2 housekeeping genes (GAPDH and PKG1) using the method described in [7] (Supplementary Table S1 and Supplementary Methods). To allow data normalization, we set the unstimulated condition to 40 cycles.
Toll-Like Receptor Reporter Cell Lines
HEK-Blue human TLR (hTLR)2 and hTLR4 reporter cells (InvivoGen) were stimulated with the T pallidum-coated gelatin particles for 9 hours using the indicated dilutions. Toll-like receptor 2 (1 μg/mL Pam3CSK4) and TLR4 (1 μg/mL LPS) were used as positive controls for the respective HEK lines. Toll-like receptor activation was visualized using the HEK-Blue detection medium according to the manufacturers’ instructions.
Retrospective Serological Study
The local ethics committee approved the study. We considered all patients serologically tested for both syphilis and EBV between 2008 and 2018. Epstein-Barr virus serological testing was within a maximum of ±30 days from the syphilis test. We extracted the peak value for leucocyte counts and liver enzyme tests, as well as viral capsid antigen (VCA)-immunoglobulin (Ig)M, VCA-IgG, and EBNA-IgG antibody results. Subjects with and without a positive syphilis test were compared using non-parametric t tests.
RESULTS
Epstein-Barr Virus Hepatitis in an Immunocompetent Patient With Syphilis
We report EBV-associated hepatitis that occurred in an immunocompetent 28-year-old woman with concomitant syphilis infection. Three months after giving birth to a healthy child, she was hospitalized because of fatigue, nausea, and upper abdominal pain. One week earlier, she had started oral contraception. At initial presentation, mild jaundice, a slight macular rash, and dark urine 2 days before were noted. She had markedly elevated liver function tests (Figure 1A and Supplementary Figure S1) but normal white blood cell counts and C-reactive protein (Supplementary Table S2). Abdominal ultrasound revealed borderline splenomegaly and a slightly thickened gall bladder wall. Magnetic resonance cholangiopancreatography confirmed free liquid around the gall bladder but was otherwise normal. Serology for hepatitis viruses (A, B, C, and E) and human immunodeficiency virus was negative. She was seropositive for EBV (EBNA-IgG+, VCA-IgG+) and cytomegalovirus (CMV) (CMV-IgM+, CMV-IgG+). Epstein-Barr virus-IgM was not tested at that time. Autoimmune serology was negative (Supplementary Table S3). Primary CMV infection with hepatitis was suspected, and she was discharged for outpatient follow-up.
Figure 1.
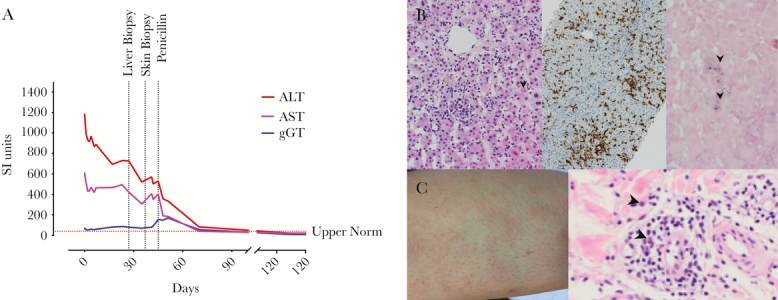
Clinical findings of an immunocompetent patient with syphilis and Epstein-Barr virus (EBV) hepatitis. (A) Longitudinal levels of transaminases (red and pink), gamma-glutamyl transferase ([gGT] blue) levels are shown. Day 0 indicates initial hospitalization. Horizontal dotted lines indicate upper normal values for aspartate aminotransferase (AST). (B) Liver biopsy showing acute lobular hepatitis with sinusoidal infiltrating lymphocytes and circumscribed small perivenular necrosis (arrows; left panel, hematoxylin and eosin staining). Infiltrating lymphocytes were CD3 positive (middle panel). Immunohistochemistry combined with in situ hybridization detected EBV encoding region-positive intrasinusoidal lymphocytes (arrow head; right panel). (C) Skin rash on the leg (left panel) showing perivascular infiltrate consisting of lymphocytes, histiocytes, and plasma cells (arrow heads) in the skin biopsy (right panel). ALT, alanine aminotransferase.
Because of persistent fatigue, nausea, and transient fever, she presented at our outpatient clinic10 days later. She still had a minimal rash but no jaundice. We completed the virology assessment by an EBV- and CMV-PCR. Cytomegalovirus was undetectable (<137 IU/mL). Epstein-Barr virus PCR was positive at 3900 genome equivalents (GEq)/mL, which we confirmed in a second blood draw (5500 GEq/mL). B-cell counts in the peripheral blood were normal with a high frequency of naive B cells, ie, without obvious evidence for memory B-cell lymphoproliferation (Supplementary Table S3). She had no history of severe or recurrent infections, immunosuppression, or drug abuse. In the immunological work-up, we found no evidence for a primary immunodeficiency (Supplementary Table S3). A liver biopsy was performed because of persistent hepatitis and equivocal virus serology. Histopathology showed acute lobular hepatitis with small circumscribed perivenular necrosis and sinusoidal infiltrating lymphocytes lining-up in a “string-of-beads”-pattern. In situ hybridization detected EBER-positive intrasinusoidal lymphocytes (Figure 1B). Latent membrane protein and EBNA2 were negative. Diagnosis of hepatitis associated with EBV reactivation was made with pregnancy-associated immunosuppression as a potential trigger [8]. In absence of other causes of hepatopathy, EBV-associated lymphoproliferation alone likely would not have explained the liver findings.
Throughout the next 2 weeks, she developed a progressive fine-stained itchy rash (Figure 1C), flank pain, sweating, and headaches. We excluded a complicated urinary tract infection. Skin biopsy showed inflammation with numerous plasma cells, compatible with secondary syphilis (Figure 1C). Serological testing confirmed the diagnosis (venereal disease research laboratory [VDRL] and T pallidum particle agglutination [TPPA] positive). Borrelia spp serology, cerebral magnetic resonance imaging, and spinal tap (PCR for EBV, Enterovirus, HSV-1/2, CMV; and serology against T pallidum, Borrelia, and FSME) revealed no other diagnosis. Liver biopsy was re-examined but showed no evidence for treponemes.
Syphilis was treated with a single injection of long-acting Benzathine penicillin G (2.4 million units intramuscularly). The rash and sweating resolved rapidly. A minor headache and fatigue persisted for 4–5 months. Because syphilis screening during pregnancy was negative 11 months earlier, infection must have occurred in the meantime. During follow-up, VDRL titers became negative and TPPA titers dropped from 1:40 960 to 1:10 240 (Supplementary Figure S2). Her partner tested positive for syphilis and was treated accordingly. The child was healthy and developed normally. Serological testing for syphilis was negative in all her children.
Treponema pallidum Induces Epstein-Barr Virus (EBV) Reactivation In Vitro: A Potential Explanation of EBV Hepatitis in Syphilis
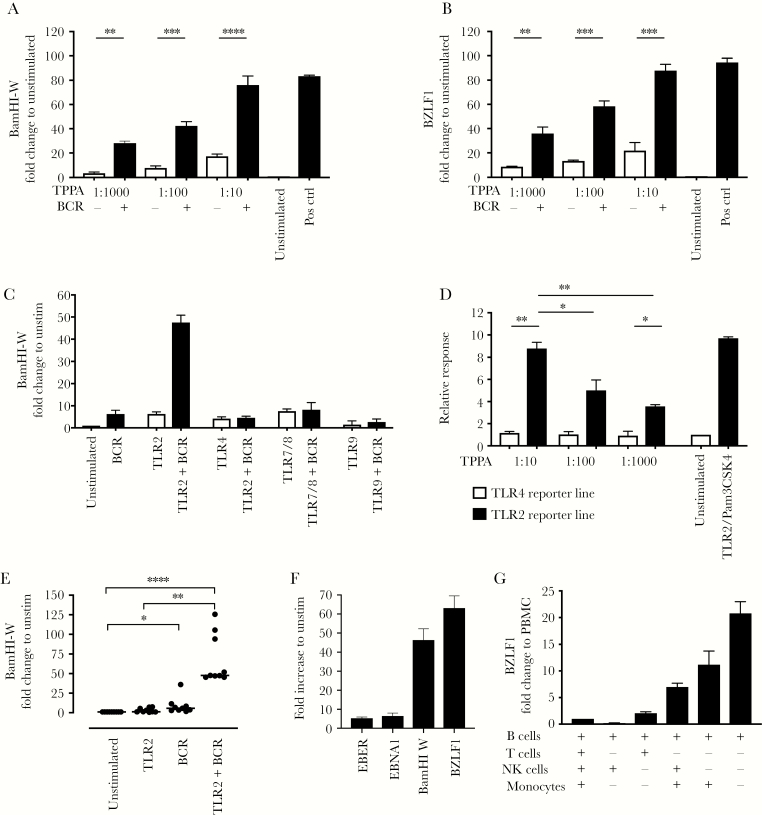
In the immunologic workup, we found increased systemic plasma blasts (ie, activated B cells) accounting for 4.5% of all B cells (Supplementary Table S3). We hypothesized that T pallidum-mediated B-cell activation might have triggered EBV reactivation. To test this, we established a qPCR-based in vitro EBV reactivation assay. Indeed, stimulation of T cell-depleted PBMCs from latently EBV-infected healthy blood donors with T pallidum proteins resulted in robust and dose-dependent EBV reactivation in vitro as evidenced by an increase transcription of the early lytic EBV gene BZLF-1 (Figure 2A and B and Supplementary Figure S3). This was even more pronounced when we stimulated with T pallidum proteins in combination with a BCR cross-linking antibody.
Figure 2.
Mechanism of Treponema pallidum induced Epstein-Barr virus (EBV) reactivation in vitro. (A and B) T cell-depleted peripheral blood mononuclear cells (0.5 million cells) of healthy EBV+ subjects (n = 5) were stimulated with T pallidum protein-loaded beads (at dilution from 1:10 to 1:1000) with or without B-cell receptor (BCR) costimulation. After 96 hours, quantitative polymerase chain reaction for BamHI-W (A) and the early lytic gene BZLF1 (B) was performed. Experiments were done in triplicates. Epstein-Barr virus gene expression was normalized to 2 housekeeping genes: GAPDH and PGK1 (for details see Methods and Supplementary Methods). Relative expression was normalized to the unstimulated condition. (C) Testing a set of Toll-like receptor (TLR) agonists (TLR2, TLR4, TLR7/8, or TLR9) with or without BCR cross-linking in n = 2 healthy subjects shows that TLR2/BCR synergizes to induce EBV-ribonucleic acid expression. Conditions were similar to A and B. (D) Treponema pallidum particle agglutination (TPPA) proteins activate TLR2-specific, but not TLR4-specific, reporter cell lines. Summary data of n = 3 experiments. Optical density (OD) was normalized to the unstimulated condition (= media alone = set as 1). Median and interquartile range of triplicates corrected for background (= OD of non-T pallidum-coated beads) is displayed. Toll-like receptor 2 ligand (1 μg/mL Pam3CSK4) was used as a positive control and comparator. (E) Toll-like receptor 2/BCR-mediated EBV replication was confirmed in a total of n = 9 healthy EBV+ subjects. (F) Toll-like receptor 2/BCR stimulation resulted in significantly higher upregulation of BZLF-1 and BamHI-W, compared with EBV encoding region (EBER) or Epstein-Barr nuclear-associated antigen (EBNA)1 (** for comparisons). BZLF1 expression was significantly higher than BamHI-W (*). (G) Epstein-Barr virus reactivation of B cells in the presence of various other (autologous) immune cell subsets showed that TLR2/BCR-induced EBV reactivation was B-cell intrinsic (n = 2 donors). Statistics were limited to data with ≥3 subjects/experiments. For all comparisons, one-way analysis of variance with correction for multiple comparisons was performed. *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001.
Epstein-Barr Virus Reactivation Can Be Induced Through Toll-Like Receptor 2/B-Cell Receptor Activation
Toll-like receptor ligands are strong B-cell activators and are pivotal in the immunity against spirochetes. Therefore, we assessed how different TLR ligands affect EBV reactivation in this in vitro system. Toll-like receptor-2, -4, and -7/8 all resulted in a minor increase of EBV DNA. In contrast, the costimulation of TLR2 and BCR cross-linking increased EBV DNA by more than 40-fold, which was not observed in combination with other TLR ligands (Figure 2C). Using TLR-specific reporter cell lines, we could indeed show that T pallidum activated TLR2 signaling to a comparable extent as a synthetic TLR2 ligand (Figure 2D). Toll-like receptor 2/BCR-induced EBV RNA increase was consistent (Figure 2E) and focused on BZLF-1 and BamHI-W (Figure 2F). Cell-depletion experiments confirmed that the effect was B-cell intrinsic, whereas in the presence of NK cells and T cells EBV reactivation was robustly inhibited (Figure 2G). Both TLR2 and BCR stimulation were able to modulate the TLR2 and BCR expression, potentially explaining the synergistic effect of TLR2 and BCR stimulation (Supplementary Figure S4).
Hepatopathy Is Frequent in Syphilis, but Epstein-Barr Virus Reactivation Is Rarely Considered as a Cause
Given the very high prevalence of latent EBV in the population and our in vitro findings, EBV-associated hepatitis should frequently occur in syphilis. In our hospital, of the subjects with a positive syphilis test and available EBV serology between 2008 and 2018, 41.7% had liver tests above 3 times the normal upper limit versus 9.4% in the syphilis negative controls (P = .0035; odds ratio = 6.9 [2.5–19.8]). Epstein-Barr virus-IgM was only requested in 20% (455 of 2280) of the tests, and EBV-PCR was rarely requested in this population. None of the EBV-IgM or EBV-PCR were done in the syphilis-positive subjects, thus precluding an analysis of the frequency of clinical EBV reactivation in syphilis-infected subjects in our clinic (Supplementary Figure S5).
DISCUSSION
We provide in vitro (BZLF1 increase) and in vivo (EBV-associated hepatitis) evidence that T pallidum can induce EBV reactivation. In vitro EBV reactivation was mediated via stimulation of TLR2, especially in the context of concomitant BCR stimulation. Specifically, we provide in vitro evidence that T pallidum proteins (1) induce lytic EBV gene expression to the same extend as TLR2/BCR costimulation and (2) directly activate a TLR2-reporter cell line. In our retrospective clinical study, we could not determine whether clinical EBV reactivation occurs frequently in syphilis. In line with our data, clinical Leptospirosis infection—another TLR2-activating spirochete—has been reported to cause EBV reactivation with hepatitis [9]. Group A streptococci also activate B cells via TLR2 and trigger EBV replication in tonsils [10].
Immunopathogenesis of EBV hepatitis during immunosuppression remains ill-understood, but it is attributed to EBV reactivation [11]. The liver biopsy showed the typical findings of EBV hepatitis including the presentation of the infiltrate and EBER positivity in ISH. It is worthy to note that EBER-positive cells are often scattered, typically sparse, and may be overlooked or missed in small biopsies [12, 13]. As in our case, LMP is typically negative in EBV hepatitis and not useful in the diagnostic process [13]. In our patient, we found no evidence for lues hepatitis: T pallidum was not detectable in the liver biopsy, and the histopathology was typical for EBV. Treponema pallidum hepatitis shows granulocyte infiltrations around bile ducts, and granuloma formation and lymphocytic vasculitis can be observed [14]; all of which were absent in our patient. The decline of the liver enzymes before antibiotic treatment could have indicated regained EBV control and reduction of EBV-induced immunopathology. A drug reaction with eosinophilia and systemic symptoms (DRESS) to the oral contraception was excluded in the absence of eosinophilia and based on the histology. The IgM against EBV and CMV was likely explained by cross-reactive or polyclonal B-cell activation; a common feature of herpes viruses [15].
Because the EBV-PCR used in the clinical laboratory measures a lytic EBV gene only at the DNA level, we cannot exclude that the systemically detected increased EBV genome in our patient was due to lymphoproliferation (ie, increased number of infected B cells) rather than lytic replication. However, our experimental in vitro studies indicated that latent EBV can be reactivated using an activation pathway that is very likely active in T pallidum infection in vivo. Therefore, these data provide a potential mechanistic insight that could explain the in vivo phenotype of our patient.
Our retrospective analysis of patients serologically tested for syphilis and EBV indicates a high prevalence of hepatopathy in syphilis patients. However, hepatopathy in this setting may have various causes including T pallidum hepatitis, drug-induced hepatopathy, and others. The true prevalence of EBV hepatitis in syphilis needs to be addressed in appropriate, prospective clinical cohorts. Moreover, our retrospective analysis suggests that in clinical practice, doctors in our hospital rarely consider EBV reactivation as a cause of hepatitis in the context of syphilis and consequently do not appropriately test for EBV reactivation (EBV-PCR and anti-EBV-IgM). We propose that EBV reactivation should be considered in the differential diagnosis of hepatopathy in syphilis and other infections with TLR2-activating pathogens.
CONCLUSIONS
In conclusion, the clinical findings and histopathology combined with the experimental data argue for a bona fide EBV reactivation in the context of syphilis. Pregnancy-associated inhibited T-cell activity [8] and syphilis-induced inhibition of T-cell proliferation [16] may have synergized with TLR2/BCR-induced B-cell activation to enable EBV reactivation or lymphoproliferation. Here, the in vitro assay to study EBV reactivation has various potential applications, including drug testing or studying viral replication and kinetics.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Klaus Baumgartl from the IT Department of the University Hospital Basel for help with the data extraction for the retrospective clinical analysis.
Author contributions. J. R. H., P. S. F., P. H., and L. T. performed experiments/generated data and analyzed data. B. M.-D. provided cells and material. T. D., M. R., and H. H. H. analyzed and interpreted data. C. T. B. designed experiments, analyzed data, and funded the study. J. R. H., P. S. F., and C. T. B. drafted the manuscript. All authors contributed to the writing of the manuscript.
Financial support. This work was funded by the L. & Th. La Roche Stiftung and the Margot und Erich Goldschmidt & Peter René Jacobson-Stiftung (both to C. T. B.). M. R. is supported by a professorship from the Swiss National Science Foundation (PP00P3_173186).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV-lessons from the clinic. J Exp Med 2017; 214:269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sondermann W, Baba HA, Korber A. Hepatitis due to EBV-reactivation under infliximab in a psoriasis patient. Dermatol Ther 2017; 30:e12525. [DOI] [PubMed] [Google Scholar]
- 3. Peeling RW, Mabey D, Kamb ML, et al. Syphilis. Nat Rev Dis Primers 2017; 3:17073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlson JA, Dabiri G, Cribier B, Sell S. The immunopathobiology of syphilis: the manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am J Dermatopathol 2011; 33:433–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schröder NW, Eckert J, Stübs G, Schumann RR. Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology 2008; 213:329–40. [DOI] [PubMed] [Google Scholar]
- 6. Silver AC, Dunne DW, Zeiss CJ, et al. MyD88 deficiency markedly worsens tissue inflammation and bacterial clearance in mice infected with Treponema pallidum, the agent of syphilis. PLoS One 2013; 8:e71388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hellemans J, Mortier G, De Paepe A, et al. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 2007; 8:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haeri S, Baker AM, Boggess KA. Prevalence of Epstein-Barr virus reactivation in pregnancy. Am J Perinatol 2010; 27:715–9. [DOI] [PubMed] [Google Scholar]
- 9. Karrasch M, Herfurth K, Kläver M, et al. Severe leptospirosis complicated by Epstein-Barr Virus reactivation. Infection 2015; 43:763–9. [DOI] [PubMed] [Google Scholar]
- 10. Ueda S, Uchiyama S, Azzi T, et al. Oropharyngeal group A streptococcal colonization disrupts latent Epstein-Barr virus infection. J Infect Dis 2014; 209:255–64. [DOI] [PubMed] [Google Scholar]
- 11. Negro F. The paradox of Epstein-Barr virus-associated hepatitis. J Hepatol 2006; 44:839–41. [DOI] [PubMed] [Google Scholar]
- 12. Schechter S, Lamps L. Epstein-Barr virus hepatitis: a review of clinicopathologic features and differential diagnosis. Arch Pathol Lab Med 2018; 142:1191–5. [DOI] [PubMed] [Google Scholar]
- 13. Suh N, Liapis H, Misdraji J, et al. Epstein-Barr virus hepatitis: diagnostic value of in situ hybridization, polymerase chain reaction, and immunohistochemistry on liver biopsy from immunocompetent patients. Am J Surg Pathol 2007; 31:1403–9. [DOI] [PubMed] [Google Scholar]
- 14. Lee RV, Thornton GF, Conn HO. Liver disease associated with secondary syphilis. N Engl J Med 1971; 284:1423–5. [DOI] [PubMed] [Google Scholar]
- 15. Gupta E, Bhatia V, Choudhary A, et al. Epstein-Barr virus associated acute hepatitis with cross-reacting antibodies to other herpes viruses in immunocompetent patients: report of two cases. J Med Virol 2013; 85:519–23. [DOI] [PubMed] [Google Scholar]
- 16. Kantor FS. Infection, anergy and cell-mediated immunity. N Engl J Med 1975; 292:629–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.