Abstract
Development of an improved tuberculosis (TB) vaccine is a high worldwide public health priority. Bacillus Calmette-Guerin (BCG), the only licensed TB vaccine, provides variable efficacy against adult pulmonary TB, but why this protection varies is unclear. Humans are regularly exposed to non-tuberculous mycobacteria (NTM) that live in soil and water reservoirs and vary in different geographic regions around the world. Immunologic cross-reactivity may explain disparate outcomes of BCG vaccination and susceptibility to TB disease. Evidence supporting this hypothesis is increasing but challenging to obtain due to a lack of reliable research tools. In this review, we describe the progress and bottlenecks in research on NTM epidemiology, immunology and heterologous immunity to Mtb. With ongoing efforts to develop new vaccines for TB, understanding the effect of NTM on vaccine efficacy may be a critical determinant of success.
Keywords: nontuberculous mycobacteria, BCG, tuberculosis, vaccines, innate immunity, adaptive immunity
In this review article, we summarize the current knowledge of nontuberculous mycobacteria interaction with M. tuberculosis, including exposure, clinical manifestations, and evidence for heterologous immune responses, as well as offer suggestions for future research directions in this area.
Tuberculosis remains a leading cause of death worldwide despite the availability of effective chemotherapy for over 60 years [1]. BCG (Mycobacterium bovis bacille Calmette-Guérin) vaccination has variable efficacy (from nonexistent to >80% durable protection) with unknown correlates of immunity [2]. Hypotheses include heterogeneity of the BCG strain, exposure to ultraviolet light, vitamin D levels, helminthic or chronic viral coinfection, and effects of poor nutrition. One prominent hypothesis is that exposure or infection with nontuberculous mycobacteria (NTM) modulates the immunogenicity and efficacy of BCG. Since the last major review of heterologous immunity between NTM, BCG, and Mycobacterium tuberculosis, new advances have been made in understanding tissue immunology, the epidemiology of NTM species, NTM genomics, and cross-protective immune epitopes in infectious diseases [3]. In this review, we will evaluate whether NTM induce heterologous immunity to M. tuberculosis and discuss the following issues in the field: (1) epidemiology of environmental reservoirs and geographic variation in NTM, (2) effects of NTM on innate and adaptive immune responses, and (3) human data suggesting a role for NTM in modifying the efficacy of BCG vaccination. Last, we will offer suggestions for future research directions to test the effect of heterologous immunity between NTM and M. tuberculosis.
Several knowledge and clinical care gaps exist currently. First, does an individual’s exposure to NTM generate innate or adaptive responses that impair or augment the response to BCG or other tuberculosis vaccines? Heterologous immunity could be mediated by trained innate immunity, CD4+ T cells, CD8+ T cells, nonclassically restricted T cells (eg, CD1, MR1), or antibodies. Second, could studies of heterologous mycobacterial immunity offer insight into new tuberculosis vaccine strategies and change the selection of adjuvants and antigens or the target age of vaccination? Whole cell vaccines, including BCG, demonstrate great promise as tuberculosis vaccines, but they may interact with environmental NTM in ways that are currently unpredictable. Third, can immunologic assays be developed to diagnose NTM exposure or disease to enhance clinical care and provide insight into questions of heterologous immunity? Current tools for diagnosing exposure to NTM are nonspecific and limit the understanding of NTM exposure and host responses.
NTM ENVIRONMENTAL RESERVOIRS AND CORRELATION WITH EXPOSURE
NTM are generally nonpathogenic bacteria with varied environmental niches, based on species. NTM are commonly isolated from water (natural and human-engineered sources like swimming pools and drinking water systems), and soils [4, 5] where they can form biofilms that resist some disinfectant and heat treatment methods [6]. Risk factors for NTM infection include drinking water derived from piped systems, compared to well water, proximity to natural water bodies [6], and a possible link to soil exposure activities [7]. In the United States, 70% of NTM pulmonary disease is associated with ocean coastlines and NTM prevalence estimates are higher in southern compared to northern states [8, 9]. The highest rates of NTM pulmonary disease are found in the Hawaiian Islands, where a study collected samples from household water sources (eg, showerheads, faucets), soil, and clinical samples, to determine the species specificity of NTM in this region. M. chimaera (a species in the M. avium complex) was the most common species of NTM isolated from both environmental and respiratory specimens [10]. No M. avium was isolated in this study, unlike in the continental United States. Although these prevalence studies are confounded by ascertainment bias and imprecise clinical phenotyping (not distinguishing colonization vs disease), the data suggest regional and geographic differences in NTM rates in the United States.
NTM WORLDWIDE PATTERNS OF EXPOSURE AND INFECTION
Variation in NTM exposures based upon geographic and environmental factors may partly explain the disparate outcomes of BCG vaccination. Although one theory suggests that NTM exposure is higher in the tropics, the preponderance of evidence indicates otherwise [2]. Environmental sampling studies, skin tests in asymptomatic individuals, and rates of disseminated M. avium complex in HIV-positive populations all suggest that NTM infections are not more common in equatorial regions compared to northern latitude regions [2, 11]. In fact, a major source of NTM exposure is in potable water which has historically been more prevalent in northern latitudes. The worldwide NTM variation is more likely due to exposure to different NTM species rather than overall magnitude of exposure. Sputum culture data demonstrate broad worldwide geographic variation in NTM species associated with clinical disease. The NTM-NET study included pulmonary isolates from 30 countries on 6 continents and found variation by global region and by country within regions [12]. Only 9.8% of the NTM isolates were from Asia, none of which came from high tuberculosis burden countries, suggesting an opportunity for future study. Similarly, although 30.0% of isolates were from sub-Saharan Africa, they were all submitted by South African laboratories, potentially underestimating NTM diversity across the continent [13]. Differences in NTM sensitization and disease may persist despite migration to a different region. Non-US born individuals displayed higher levels of sensitization to M. intracellulare compared to US born [14]. A study of NTM isolates in Washington State found that unusual NTM species in the United States were more commonly isolated from non-US born individuals, reflecting the NTM patterns in their countries of origin [15].
NTM CLINICAL DIAGNOSTICS, INFECTION, AND DISEASE
The clinical spectrum of NTM is poorly understood. Several lines of evidence suggest that tuberculosis pathogenesis evolves, often slowly, along a continuum of infection to disease states. Although it is well known that NTM cause disease in many body sites, the period of time from exposure to infection to disease is not known, partly due to the frequent environmental exposures of humans to NTM and the lack of NTM-specific diagnostic tests of exposure. The most common and best studied NTM in humans are part of the M. avium complex, which includes M. avium subspecies, M. intracellulare, and M. chimaera, which can cause colonization and disease. Delayed-type hypersensitivity (DTH) skin testing for M. avium complex in the United States suggests exposure rates of 16%–48% [14], although this was based on a poorly characterized assay with crude whole cell M. intracellulare extracts [14, 16]. It is not known if DTH immune responses to M. intracellulare antigens are an indicator of asymptomatic infection or previous exposure/infection with subsequent clearance of all organisms. Without adequate diagnostic reagents, the relationship of exposure, colonization, asymptomatic infection, and progression to disease for all NTM is poorly understood. Clinical assays available for diagnosing NTM-related disease or colonization include acid-fast bacilli stains, cultures, and DNA-based molecular diagnostics. However, no immunologic assays reliably measure prior exposure or current infection with NTM. The lack of specific NTM immunodiagnostic tools has hampered clinical care and research efforts.
MICROBIOLOGIC FEATURES
The genus Mycobacterium includes the M. tuberculosis complex species, Mycobacterium leprae, and NTM, among others. Genetic characterization of NTM species and their comparison to M. tuberculosis and M. bovis BCG are limited. Many NTM species are still not sequenced and as a consequence the taxonomic and phylogenetic structure of the genus remains partially analyzed [17]. Speciation of members of the Mycobacterium genus is mainly based on the 16S rRNA sequences and, to a lesser extent, on shared phenotypic characteristics [17]. A recent comparative genomic analysis of M. komanii, M. malmesburii, M. nonchromogenicum, M. fortuitum, M. tuberculosis, and M. bovis demonstrated wide divergence between NTM and M. tuberculosis, with M. komanii showing 18.6% homology to M. tuberculosis, M. fortuitum 10.6% homology, and M. malmesburii with 8.9% homology [18]. Other comparative genomic analyses indicate that there are clusters of proteins that are present in NTM but not M. tuberculosis and contain immunogenic T-cell epitopes [19]. However, despite genomic differences, there are also many conserved regions among Mycobacteria. Multiple copies of genes encoding homologous proteins and M. tuberculosis-derived immunogenic proteins, including the Esx family and PE/PPE, are found in most mycobacterial genomes, suggesting a possible mechanism for shared T-cell responses and cross-protective immunity to these bacteria [18]. The lack of available sequences for a diverse set of NTM from different geographical locations results in major bottlenecks for epitope determinations and conservation studies.
MACROPHAGES, INNATE IMMUNITY, AND TRAINED IMMUNE RESPONSES TO NTM
The mechanisms by which NTM induce innate immune responses are not as well understood as for BCG or M. tuberculosis, but the genus Mycobacteria does share many similarities, including a complex and lipid-rich cell wall [20]. In M. tuberculosis and BCG, Toll-like receptors (TLR) act as first-line pattern recognition receptors for the innate immune response [20]. NTM lipomannans and lipoarabinomannans differ structurally across species and can influence TLR2-dependent and independent signaling [21]. C-type lectins are critical for phagocytosis of multiple pathogens, including M. tuberculosis. An intriguing set of studies demonstrate that M. abscessus activates both TLR2 and dectin-1 [22]. The activation of dectin-1 suggests that NTM have potential to influence macrophage function broadly, as dectin-1 signaling after BCG vaccination or zymosan (dectin-1 ligand) stimulation influences epigenetic imprinting and durably modulates innate immune responses, a concept known as trained immunity [23, 24]. This pathway represents a possible innate mechanism where NTM could affect immune responses to M. tuberculosis and impact drug and vaccine development.
NTM persist and replicate within macrophages, but their survival strategies are variable and generally different from M. tuberculosis. NTM display widely variable intracellular replication rates in murine macrophages and can persist for prolonged periods in these animals in vivo [25]. Within macrophages, both M. tuberculosis and NTM induce autophagy variably [26, 27]. Inhibition of autophagy by M. abscessus is correlated with virulence, suggesting that in selected NTM, evolutionary strategies to evade intracellular killing are required for persistent macrophage infection [28]. NTM species also vary in their capacity to induce cell death [29]. In summary, the species-specific heterogeneity of activation of innate immune responses may influence the induction and maintenance of NTM-specific adaptive immune responses and modulation of BCG efficacy.
T-CELL RESPONSES AGAINST NTM
Although most people are regularly exposed to NTM in their environment, immune studies are limited. The lack of NTM-specific reagents hampers immunologic studies, which could help address fundamental questions about how NTM exposure influences tuberculosis vaccines. Selected studies used NTM purified protein derivatives (NTM PPDs) to measure NTM-specific immune responses [30]. However, PPDs derived from NTM contain several proteins that overlap with M. tuberculosis-PPD [31] and thus prevent definitively attributing immune response to any particular NTM species. Interferon-gamma (IFN-γ) release assays (IGRAs), which are commonly used to diagnose M. tuberculosis infection, depend on T-cell responses against the M. tuberculosis-derived antigens ESAT-6, CFP10, and TB7.7. These antigens are absent from M. bovis BCG and from the majority of common NTM (exceptions include M. kansasii, M. szulgai, and M. marinum), and thus can distinguish prior or current M. tuberculosis infection from BCG vaccination and most NTM. Several studies described cross-reactive immune responses between NTM and M. tuberculosis [30, 32]. Interestingly, some individuals with negative PPD and IGRA results and no history of BCG vaccination react to M. tuberculosis-derived antigens and NTM PPDs [30, 32]. Furthermore, DTH studies of mycobacterial PPDs demonstrate that, after BCG vaccination, British children have increased skin reactions to M. tuberculosis extracts compared with M. fortuitum [33]. In a study of PPD reactions in a low endemic setting, the majority of small M. tuberculosis-PPD reactions could be attributed to NTM exposure or disease [34]. Cross-reactivity between NTM and M. tuberculosis antigens may also explain higher baseline reactivity in M. tuberculosis-negative adults as compared to children [35]. In this regard, cross-reactivity at the epitope level correlated with sequence conservation of the epitopes in NTM [30].
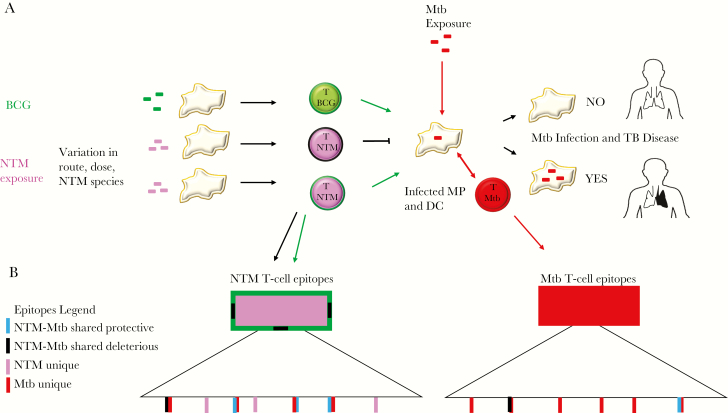
Interactions between the immune system and its environment are highly complex and do not fit a simple paradigm in which exposure to a single species elicits a species-specific response. The degree of sequence identity required to activate cross-reactive T-cell responses is not easily predicted, and highly divergent epitopes can elicit cross-reactive responses [36]. In fact, similar levels of sequence identity can result in both epitopes that are cross-reactive and those that are not (Figure 1). In addition to Mycobacteria, other pathogens like herpesviruses demonstrate cross-reactivity and interspecies conservation [37]. Indeed, epitope conservation can exist between structurally unrelated antigens with little sequence homology [38]. Some amino acid substitutions are more likely to disrupt cross-reactivity than others, in particular nonconservative substitutions and those that target anchor residues for MHC binding and key residues for T-cell recognition. Together, these studies suggest that we can identify well-defined species-specific T-cell epitopes through the combined use of bioinformatics and high-throughput cellular assays.
Figure 1.
Model of NTM, BCG, and Mycobacterium tuberculosis immunologic interactions. A, BCG vaccination is usually administered at birth and NTM exposure occurs regularly throughout life via multiple routes with variable doses. These NTM-specific innate and adaptive immune responses may influence the response to BCG vaccination and susceptibility to M. tuberculosis. B, T-cell epitopes that are common between NTM and M. tuberculosis may mediate protective or deleterious immune responses: blue rectangle, T-cell epitopes that are shared between NTM and M. tuberculosis and lead to cross-protection between species; black rectangle, T-cell epitopes that are shared between NTM and M. tuberculosis but are not cross-protective and may impair immune responses to M. tuberculosis; pink rectangle, T-cell epitopes unique to NTM; red rectangle, T-cell epitopes unique to M. tuberculosis. Abbreviations: Mtb, Mycobacterium tuberculosis; NTM, nontuberculous mycobacteria; TB, tuberculosis; MP, macrophage; DC, dendritic cell.
B-CELL RESPONSES AGAINST NTM
While some effort has been committed to study T-cell responses in NTM disease and exposure, very little progress has been made to define B-cell and antibody responses to NTM. Recent studies identified important roles for B cells and antibodies in tuberculosis pathogenesis, including the discovery that variation in human antibodies can distinguish latent tuberculosis infection from tuberculosis disease [39, 40]. These studies may spark more research in humoral immunity and its role in the context of M. tuberculosis and NTM.
ANIMAL MODELS OF NTM INFECTION, BCG VACCINATION, AND MTB CHALLENGE
Animal models of the effect of NTM on M. tuberculosis pathogenesis and BCG vaccine responses display the complex interactions between each of these bacteria and the host immune response. Early experiments consistently demonstrated that NTM infections protect animals from subsequent challenge with M. tuberculosis [41]. Adoptively transferred T cells from mice infected with M. tuberculosis provided protection from challenge with selected NTM, supporting the concept that cross-protective T cells exist among different Mycobacteria species [42]. Studies of the effect of NTM on BCG vaccine responses are heterogeneous and challenging to interpret for multiple reasons, including different NTM exposure strategies (subcutaneous, intravenous, intraperitoneal, oral, or aerosol), the quantity and duration of NTM exposure, and the species of NTM used for heterologous immunity experiments. Nevertheless, many studies suggest that NTM do not interfere with the protective effects of BCG vaccination. Experiments conducted in the 1980s found that prior aerogenic infection with M. kansasii, M. simiae, M. avium, and M. scrofulaceum protected mice from subsequent M. tuberculosis challenge and did not alter the capacity for BCG to influence M. tuberculosis bacterial burden [43, 44]. Interestingly, this effect was not uniform among all species of NTM; protection was conferred against M. avium and M. kansasii but not M. simiae aerosol challenge. Other models suggest that NTM may interfere with BCG efficacy. The compartment of infection, species of NTM, capacity for NTM to replicate intracellularly, and duration of NTM exposure may play an important role in influencing cross-protection. For example, mice exposed intravenously to M. avium (which can replicate in mice), but not M. fortuitum or M. cheloneae (which cannot), developed reduced protective immune response to subsequent BCG vaccination, cleared the live BCG vaccine more rapidly, and were more susceptible to M. tuberculosis challenge following BCG vaccination [45]. Several studies found that oral M. avium exposure decreases BCG efficacy [46], including evidence that repeated oral M. avium exposure increases the proportion of antigen-specific CD4+FoxP3+ T cells [47]. The route of BCG administration may mitigate the immunologic effects of oral NTM exposure. Price et al [48] demonstrated that oral NTM influenced T-cell responses to intradermal but not inhaled BCG. Variation in NTM exposure, including dose, species, and strain, influences BCG responses and M. tuberculosis heterologous immunity (Figure 1) [45, 48, 49]. Further detailed animal studies with antigen-specific reagents and consistent exposure strategies of NTM and BCG may provide insight into the specific epitopes shared in mice and the effects of NTM on M. tuberculosis immunity and BCG effectiveness.
HETEROLOGOUS MYCOBACTERIAL IMMUNITY
A systematic review of BCG vaccine trials demonstrated that the protective effect of BCG against pulmonary tuberculosis was greater in trials conducted farther from the equator, when BCG was given in infancy or childhood, and with stringent tuberculin testing to exclude individuals with evidence of sensitization to mycobacteria [50]. Although the overall burden of NTM may not vary geographically (see comments above), multiple lines of epidemiologic evidence suggest that species-specific exposure to NTM varies by region, likely reflecting prevailing climate and ecological niches for environmental NTM species. The variability of NTM species exposure may differentially influence immune responses to both BCG and M. tuberculosis [50]. In support of this hypothesis, greater prevaccine baseline IFN-γ responses to PPD were noted in Malawi, but a greater increase in IFN-γ responsiveness after vaccination was observed in the UK [51]. A reanalysis of the pediatric population in the Chingleput BCG trial from India, which failed to show protective efficacy from BCG in its primary analysis, suggested that BCG has partial efficacy (32%, P value = .03) in individuals without evidence of exposure/infection to M. tuberculosis and M. intracellulare (using PPD from each bacterial species) [52]. Although this secondary analysis is suggestive of an NTM effect, the definitions of infection (varying levels of induration to PPD-B and PPD-S, which are purified protein derivatives of NTM and Mtb, respectively) were poorly justified [52]. In a study of 625 000 BCG-naive navy recruits with serial skin testing using PPDs from M. tuberculosis and M. intracellulare, the incidence of active tuberculosis disease was lower in individuals with an M. intracellulare skin test result that was greater in magnitude than the M. tuberculosis skin test [16]. The mechanism by which NTM may influence BCG vaccine efficacy may be due to either (1) blocking, whereby prior mycobacterial infection diminishes BCG replication, reducing the magnitude and duration of protective T-cell responses, or (2) masking, where vaccination leads to diminished overall protection due to heterologous immune responses from prior mycobacterial exposure, diminishing the overall boosting effect [2]. Although the data are not conclusive, the findings suggest that prior NTM exposure and sensitization could provide a protective immune response against tuberculosis disease (ie, heterologous mycobacterial immunity) and/or impair BCG-induced vaccine protection. However, these previous studies were focused on a limited number of immune responses (blood IFN-γ release assays or DTH in skin), employed whole cell reagents rather than single proteins or peptide pools, evaluated a limited number of NTM species in only 2 geographic regions, and/or were underpowered. With ongoing efforts to develop new vaccines for tuberculosis, understanding the role of NTM exposure and heterologous immune responses may be critical to success.
Epidemiologic evidence supports the hypothesis that NTM induce protective effects from prior infection with M. tuberculosis. NTM rates are increasing worldwide [53], coincident with a decline in tuberculosis, potentially indicating that tuberculosis disease and infection with M. tuberculosis may provide some protection against NTM disease [54]. A systematic review that included studies from middle- and high-income settings found an increase in the proportion of NTM disease related to coincident decreases in tuberculosis and increases in NTM exposure [54]. Several studies demonstrated that pediatric lymphadenitis due to NTM has increased with changes to BCG policies, and others confirmed that BCG is associated with protection from NTM lymphadenitis, M. ulcerans, and M. leprae [55]. Three NTM-derived vaccines (DAR-901, M. vaccae, and M. indicus pranii) are in advanced tuberculosis vaccine trials and support the concept that NTMs confer heterologous immunity to tuberculosis. Together, these data support a role for one or more NTM species influencing tuberculosis pathogenesis A summary of our conclusions is provided in Table 1.
Table 1.
Summary of Major Conclusions
| Topic | Summary |
|---|---|
| Epidemiology | Different populations are exposed to different NTM species |
| Cohorts | More NTM cohorts with detailed clinical characterization and immunologic data are needed to develop reagents and understand pathogenesis |
| Environmental data | More NTM environmental sampling studies are needed to assess nature and magnitude of NTM exposure |
| Animal models | The type of NTM and route of exposure influence mycobacterial heterologous immune responses to BCG and Mycobacterium tuberculosis infection |
| NTM genomics, bioinformatics, and diagnostics | Additional genomic data are needed to understand NTM species differences and opportunities to develop NTM diagnostic reagents |
| Heterologous immunity | Improved diagnostics and immunologic reagents are needed to harness insights from heterologous immunity to develop more effective tuberculosis vaccines |
Abbreviations: BCG, Bacillus Calmette-Guerin; NTM, nontuberculous mycobacteria.
BOTTLENECKS IN RESEARCH ON NTM AND HETEROLOGOUS IMMUNITY TO MTB
To understand whether NTM provide heterologous immunity to M. tuberculosis, a number of research barriers need to be addressed, including the following.
NTM Cohorts
There are very few clinically characterized NTM cohorts with cryopreserved peripheral blood mononuclear cells (PBMCs) available for developing reagents and assessing immunologic questions. Optimal study cohorts require several features, including sufficient sample size and NTM diversity. Although M. avium complex is the most common NTM, many other important NTM need examination to capture the most common species across the world. Once a M. avium complex or NTM IGRA is developed, PBMCs from large tuberculosis cohorts (vaccine and natural history) will be needed to test concepts of heterologous immunity.
Human NTM Reservoirs
Current knowledge of the natural history of NTM infections is limited. We do not understand the anatomic location or bacillary level of human reservoirs of NTM. This information is critical for understanding the size of the antigenic challenge that is present and what is needed to induce an immune response. Is the reservoir only in the lungs with people with pulmonary disease? Or is it present in other reservoirs such as the blood, gastrointestinal tract (oral to colon, ie, part of the microbiome), or other mucosal sites? Is there a latent state similar to M. tuberculosis? If NTM cohorts are developed, these questions could be addressed with reservoir sampling strategies.
Environmental NTM Reservoirs
Another source of NTM antigen exposure is the environment. If the exposures are frequent and large enough, there may be sufficient exposure to induce a T-cell response. NTM are commonly described as present in water and soil reservoirs and more prevalent in the tropics. But the data underlying this concept are quite limited and based on modest sampling strategies. Understanding sources of environmental exposure could help illuminate how often humans are exposed, the NTM involved, and the heterogeneity of these variables in different geographic settings around the world.
Animal Models of NTM-M. tuberculosis Interaction
The past 10 years of immunology study has dramatically improved our understanding how the route of exposure plays on the induction of differential T-cell responses and tolerance. Prior animal studies demonstrated intriguing but incomplete data on the role of NTM dose and route on M. tuberculosis pathogenesis as well as BCG responses. Understanding this concept with further animal studies will help bridge the gap between environmental exposures to NTM and the influence these commensals may play in vaccine design.
NTM Genomics and Bioinformatics
Developing NTM peptide reagents requires defining unique and conserved NTM epitopes when comparing NTM to M. tuberculosis and to each other. This method requires whole genome sequence data from a diverse repertoire of NTM and ideally from several representatives of each species. Currently, there are only a small number of available sequences from a limited number of NTM.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers R21 AI 125777 to J. A. S., C. L. A., D. J. H., A. S., and T. R. H.; K24 AI137310 to T. R. H.; and R01 AI 136921 to J. A. S.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers 2016; 2:16076. [DOI] [PubMed] [Google Scholar]
- 2. von Reyn CF. Correcting the record on BCG before we license new vaccines against tuberculosis. J R Soc Med 2017; 110:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346:1339–45. [DOI] [PubMed] [Google Scholar]
- 4. Honda JR, Virdi R, Chan ED. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front Microbiol 2018; 9:2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu Y, Yu X, Zhao D, et al. Isolation of nontuberculous mycobacteria from soil using Middlebrook 7H10 agar with increased malachite green concentration. AMB Express 2017; 7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falkinham JO. Impact of human activities on the ecology of nontuberculous mycobacteria. Future Microbiol 2010; 5:951–60. [DOI] [PubMed] [Google Scholar]
- 7. Dirac MA, Horan KL, Doody DR, et al. Environment or host?: a case-control study of risk factors for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012; 186:684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strollo SE, Adjemian J, Adjemian MK, Prevots DR. The burden of pulmonary nontuberculous mycobacterial disease in the United States. Ann Am Thorac Soc 2015; 12:1458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adjemian J, Daniel-Wayman S, Ricotta E, Prevots DR. Epidemiology of nontuberculous mycobacteriosis. Semin Respir Crit Care Med 2018; 39:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Honda JR, Hasan NA, Davidson RM, et al. Environmental nontuberculous mycobacteria in the Hawaiian Islands. PLoS Negl Trop Dis 2016; 10:e0005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fordham von Reyn C, Arbeit RD, Tosteson AN, et al. The international epidemiology of disseminated Mycobacterium avium complex infection in AIDS. International MAC Study Group. AIDS 1996; 10:1025–32. [DOI] [PubMed] [Google Scholar]
- 12. Hoefsloot W, van Ingen J, Andrejak C, et al. ; Nontuberculous Mycobacteria Network European Trials Group The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013; 42:1604–13. [DOI] [PubMed] [Google Scholar]
- 13. Okoi C, Anderson STB, Antonio M, Mulwa SN, Gehre F, Adetifa IMO. Non-tuberculous mycobacteria isolated from pulmonary samples in sub-Saharan Africa - a systematic review and meta analyses. Sci Rep 2017; 7:12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med 2007; 176:306–13. [DOI] [PubMed] [Google Scholar]
- 15. Ford ES, Horne DJ, Shah JA, Wallis CK, Fang FC, Hawn TR. Species-specific risk factors, treatment decisions, and clinical outcomes for laboratory isolates of less common nontuberculous mycobacteria in Washington State. Ann Am Thorac Soc 2017; 14:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmer CE, Edwards LB. Identifying the tuberculous infected. The dual-test technique. JAMA 1968; 205:167–9. [PubMed] [Google Scholar]
- 17. Fedrizzi T, Meehan CJ, Grottola A, et al. Genomic characterization of nontuberculous mycobacteria. Sci Rep 2017; 7:45258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gcebe N, Michel A, Gey van Pittius NC, Rutten V. Comparative genomics and proteomic analysis of four non-tuberculous Mycobacterium species and Mycobacterium tuberculosis complex: occurrence of shared immunogenic proteins. Front Microbiol 2016; 7:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Checkley AM, Wyllie DH, Scriba TJ, et al. Identification of antigens specific to non-tuberculous mycobacteria: the Mce family of proteins as a target of T cell immune responses. PLoS One 2011; 6:e26434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alderwick LJ, Harrison J, Lloyd GS, Birch HL. The mycobacterial cell wall–peptidoglycan and arabinogalactan. Cold Spring Harb Perspect Med 2015; 5:a021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quesniaux VJ, Nicolle DM, Torres D, et al. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol 2004; 172:4425–34. [DOI] [PubMed] [Google Scholar]
- 22. Shin DM, Yang CS, Yuk JM, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol 2008; 10:1608–21. [DOI] [PubMed] [Google Scholar]
- 23. Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014; 345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaufmann E, Sanz J, Dunn JL, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018; 172:176–90.e19. [DOI] [PubMed] [Google Scholar]
- 25. Orme IM, Collins FM. Resistance of various strains of mycobacteria to killing by activated macrophages in vivo. J Immunol 1983; 131:1452–4. [PubMed] [Google Scholar]
- 26. Zullo AJ, Lee S. Mycobacterial induction of autophagy varies by species and occurs independently of mammalian target of rapamycin inhibition. J Biol Chem 2012; 287:12668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cardenal-Muñoz E, Arafah S, López-Jiménez AT, et al. Mycobacterium marinum antagonistically induces an autophagic response while repressing the autophagic flux in a TORC1- and ESX-1-dependent manner. PLoS Pathog 2017; 13:e1006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim SW, Subhadra B, Whang J, et al. Clinical Mycobacterium abscessus strain inhibits autophagy flux and promotes its growth in murine macrophages. Pathog Dis 2017; 75: doi: 10.1093/femspd/ftx107. [DOI] [PubMed] [Google Scholar]
- 29. Ganbat D, Seehase S, Richter E, et al. Mycobacteria infect different cell types in the human lung and cause species dependent cellular changes in infected cells. BMC Pulm Med 2016; 16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindestam Arlehamn CS, Paul S, Mele F, et al. Immunological consequences of intragenus conservation of Mycobacterium tuberculosis T-cell epitopes. Proc Natl Acad Sci U S A 2015; 112:E147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prasad TS, Verma R, Kumar S, et al. Proteomic analysis of purified protein derivative of Mycobacterium tuberculosis. Clin Proteomics 2013; 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Black GF, Dockrell HM, Crampin AC, et al. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J Infect Dis 2001; 184:322–9. [DOI] [PubMed] [Google Scholar]
- 33. Weir RE, Black GF, Nazareth B, et al. The influence of previous exposure to environmental mycobacteria on the interferon-gamma response to bacille Calmette-Guérin vaccination in southern England and northern Malawi. Clin Exp Immunol 2006; 146:390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Reyn CF, Horsburgh CR, Olivier KN, et al. Skin test reactions to Mycobacterium tuberculosis purified protein derivative and Mycobacterium avium sensitin among health care workers and medical students in the United States. Int J Tuberc Lung Dis 2001; 5:1122–8. [PubMed] [Google Scholar]
- 35. Scriba TJ, Tameris M, Mansoor N, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol 2010; 40:279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Birnbaum ME, Mendoza JL, Sethi DK, et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell 2014; 157:1073–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiu C, McCausland M, Sidney J, et al. Broadly reactive human CD8 T cells that recognize an epitope conserved between VZV, HSV and EBV. PLoS Pathog 2014; 10:e1004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ishizuka J, Grebe K, Shenderov E, et al. Quantitating T cell cross-reactivity for unrelated peptide antigens. J Immunol 2009; 183:4337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Achkar JM, Chan J, Casadevall A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol Rev 2015; 264:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu LL, Chung AW, Rosebrock TR, et al. A functional role for antibodies in tuberculosis. Cell 2016; 167:433–43.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palmer CE, Long MW. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am Rev Respir Dis 1966; 94:553–68. [DOI] [PubMed] [Google Scholar]
- 42. Orme IM, Collins FM. Crossprotection against nontuberculous mycobacterial infections by Mycobacterium tuberculosis memory immune T lymphocytes. J Exp Med 1986; 163:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Orme IM, Roberts AR, Collins FM. Lack of evidence for a reduction in the efficacy of subcutaneous BCG vaccination in mice infected with nontuberculous mycobacteria. Tubercle 1986; 67:41–6. [DOI] [PubMed] [Google Scholar]
- 44. de Lisle GW, Wards BJ, Buddle BM, Collins DM. The efficacy of live tuberculosis vaccines after presensitization with Mycobacterium avium. Tuberculosis (Edinb) 2005; 85:73–9. [DOI] [PubMed] [Google Scholar]
- 45. Brandt L, Feino Cunha J, Weinreich Olsen A, et al. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun 2002; 70:672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flaherty DK, Vesosky B, Beamer GL, Stromberg P, Turner J. Exposure to Mycobacterium avium can modulate established immunity against Mycobacterium tuberculosis infection generated by Mycobacterium bovis BCG vaccination. J Leukoc Biol 2006; 80:1262–71. [DOI] [PubMed] [Google Scholar]
- 47. Poyntz HC, Stylianou E, Griffiths KL, Marsay L, Checkley AM, McShane H. Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis. Tuberculosis (Edinb) 2014; 94:226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Price DN, Kusewitt DF, Lino CA, McBride AA, Muttil P. Oral tolerance to environmental mycobacteria interferes with intradermal, but not pulmonary, immunization against tuberculosis. PLoS Pathog 2016; 12:e1005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hernandez-Pando R, Pavön L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun 1997; 65:3317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014; 58:470–80. [DOI] [PubMed] [Google Scholar]
- 51. Black GF, Weir RE, Floyd S, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 2002; 359:1393–401. [DOI] [PubMed] [Google Scholar]
- 52. Narayanan PR. Influence of sex, age & nontuberculous infection at intake on the efficacy of BCG: re-analysis of 15-year data from a double-blind randomized control trial in South India. Indian J Med Res 2006; 123:119–24. [PubMed] [Google Scholar]
- 53. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 2015; 36:13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis 2014; 18:1370–7. [DOI] [PubMed] [Google Scholar]
- 55. Zimmermann P, Finn A, Curtis N. Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis. J Infect Dis 2018; 218:679–87. [DOI] [PubMed] [Google Scholar]