Abstract
Rationale:
Giant thoracic chordoma is a highly unusual disease with no standard curative managements yet. The objective of this study is to report a very rare case of giant thoracic chordoma successfully operated by combination of thoracoscopic surgery together with posterior spinal surgery. The management of these unique cases has yet to be well-documented.
Patient concerns:
A 64-year-old man presented with a 4-month history of continuous and progressive back pain. The patient, who had been diagnosed of sacral chordoma for 2 years, received surgical treatment of posterior sacral tumor resection and instrumentation. A lytic, expanding lesion of the T5 and T6 vertebral and paraspinal region with mild epidural spinal cord compression was identified.
Diagnosis:
MRI of spine and PET/CT showed spinal cord compression secondary to the epidural component of the T5 and T6 mass, with increased metastatic marrow infiltration of the left T5 and T6 vertebral and paravertebral region, which presented as a solid tumor. Postoperative pathology confirmed the diagnosis of thoracic chordoma.
Interventions:
The patient underwent 1-stage thoracoscopic release of vertebral and paravertebral tumors, posterior resection of T5-T6 vertebral and paravertebral tumors, T4-T7 spinal canal decompression, and T2-T9 pedicle screw fixation procedure via a posterior approach.
Outcomes:
The patient's neurological deficits improved significantly after the surgery, and the postoperative period was uneventful at the 3-month and 6-month follow-up visit. There were no other complications associated with the operation during the follow-up period.
Lessons:
Taken together, the lesion's clinical features, imaging results, and pathological characteristics are unique. Combined efforts of specialists from orthopedics, thoracic surgery, neurosurgery, and medical oncology led to the successful diagnosis and management of this patient. Giant thoracic chordoma, although rare, should be part of the differential diagnosis when the patient has a history of sacral chordoma and presents with back pain and radiculopathy. We recommend the posterior approach for spinal decompression of the giant thoracic chordoma when the tumor has caused neurological deficits. One-stage thoracoscopic release or resection of vertebral and paravertebral tumor is also a good choice for surgical treatment.
Keywords: diagnosis, spinal tumor resection, stabilization, surgical treatment, thoracic chordoma, thoracoscopic surgery
1. Introduction
Chordomas are reported to be slowly growing malignant tumors arising from the remnants of the notochord.[1,2] Chordomas are the only embryonic neoplasms that commonly appear in the later decades of life, typically in the fourth to fifth decades.[3–5] In literature, the most prevalent location of chordoma is the sacrococcygeal region (50–55%), and thoracic spinal forms account for nearly 1% of all cases with chordomas.[1–3] To the best of our knowledge, this is a rare case of giant thoracic chordoma in a man presenting with back pain who was successfully treated with one-stage thoracoscopic and posterior spinal surgery. We performed a surgical exploration, thoracoscopic release of vertebral and paravertebral tumors, posterior resection of T5-T6 vertebral and paravertebral tumors, T4-T7 spinal canal decompression, and T2-T9 internal fixation procedure. In the short term, the patient's conditions improved significantly postoperatively. After reviewing pertinent literature, we discussed common perioperative considerations in patients with a giant chordoma of the thoracic spine and management considerations for these patients.
2. Case report
In June of 2018, a 64-year-old man with progressive back pain was presented to our hospital. In his history of present illness, the patient stated he has been experiencing a paroxysmal and severe back pain for approximately four months. The pain in his back could reach 7 points using visual analogue scale and could not be alleviated with rest and hot compresses. Initially, the patient attributed the pain to his overwork and thus did not seek medical attention. The patient denied experiencing any other constitutional symptoms. Upon further questioning, he recalled a history of sacral chordoma. The patient, who had been diagnosed of sacral chordoma for two years, received surgical treatment of posterior sacral tumor resection and instrumentation. No pertinent family history was identified, including, hypertension, cancer, and congenital birth difficulties.
On physical examination, the patient showed decreased sensation to pin-prick and fine-touch of left lower extremity and exhibited a 5-/5 strength in left lower extremity. Deep tendon reflexes revealed normal for both knee jerk and Achilles tendon reflexes bilaterally. Ataxia was absent. Cranial nerves, mini mental, and the rest of the neurological exam showed no abnormalities. Routine laboratory tests were ordered, including electrolytes, liver and kidney function tests, tumor markers and complete blood count. The results of the laboratory studies were almost within normal range, except that the tissue polypeptide specific antigen was significantly elevated to 83.21 U/l (normal: <80 U/l), serum carbohydrate antigen 19–9 elevated to 43.0 ng/ml (normal: <34.0 ng/ml), carbohydrate antigen 242 elevated to 25.4 ng/ml (normal: <20.0 ng/ml), and carcinoembryonic antigen elevated to 6.89 ng/ml (normal: <5.0 ng/ml). Preoperative assessments included electrocardiogram, echocardiogram, and chest x-ray (Fig. 1 A and B). Spinal MRI was ordered to visualize the thoracic lesions, to assess the stability of the vertebral column and pleural involvement, and to aid in the formulation of the surgical approach. MRI of the thoracic spine revealed widespread abnormal signal of multiple vetebras in keeping with diffuse metastatic infiltration. MRI of spine showed mild spinal cord compression secondary to the epidural componant of the T5 and T6 mass, with increased metastatic marrow infiltration of the left T5 and T6 vertebral and paravertebral regions, which presented as a solid tumor (Fig. 2 A–H). Tumor infiltrated through the T5 and T6 vetebral bodies into the left pedicles and posterior elements (Fig. 3 A and B). Extraosseous spread into the left lateral aspect of the epidural space extending posteriorly, resulting cord compression with the nerve root (Fig. 4 A–D). Bone scintigraphy indicated the skeletal abnormality of T5 and T6, with high suspicion of malignant tumor (Fig. 5). PET/CT scan also demonstrated an abnormal signal of thoracic spine with involvement of the left pleural cavity (Fig. 6).
Figure 1.
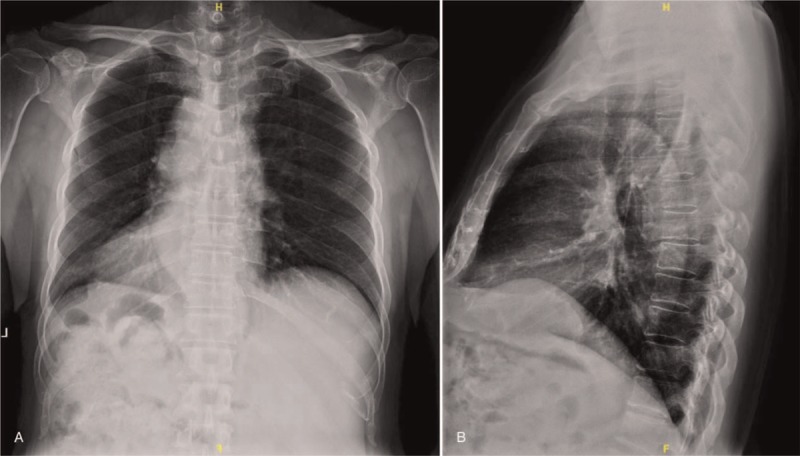
(A, B) Preoperative X-rays revealing thoracic lesions with high suspicion of spinal soft tissue tumors.
Figure 2.
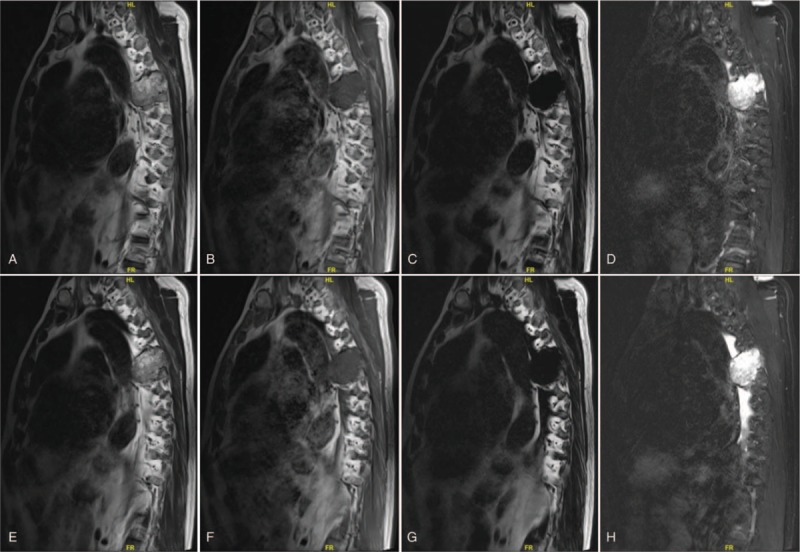
(A–H) Preoperative sagittal MRI scan revealing the density of soft tissue measuring 10 cm × 8.5 cm × 5.0 cm, obvious bony destruction in the T5 and T6, and spinal cord compression caused by thoracic chordoma, with increased metastatic marrow infiltration of the vertebra.
Figure 3.
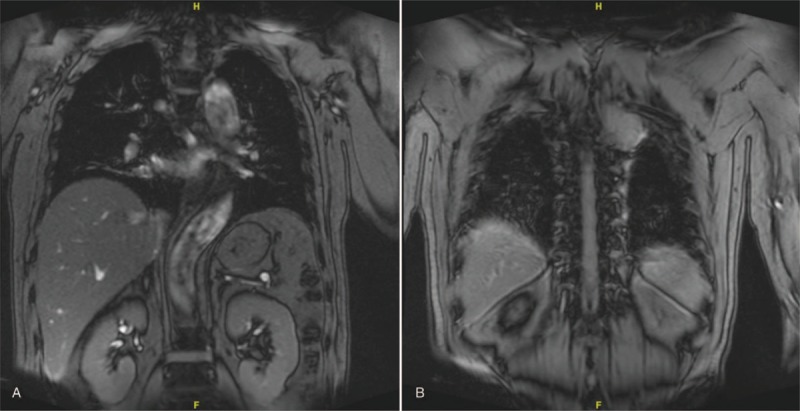
(A, B) Preoperative coronal MRI images showing the thoracic chordoma.
Figure 4.
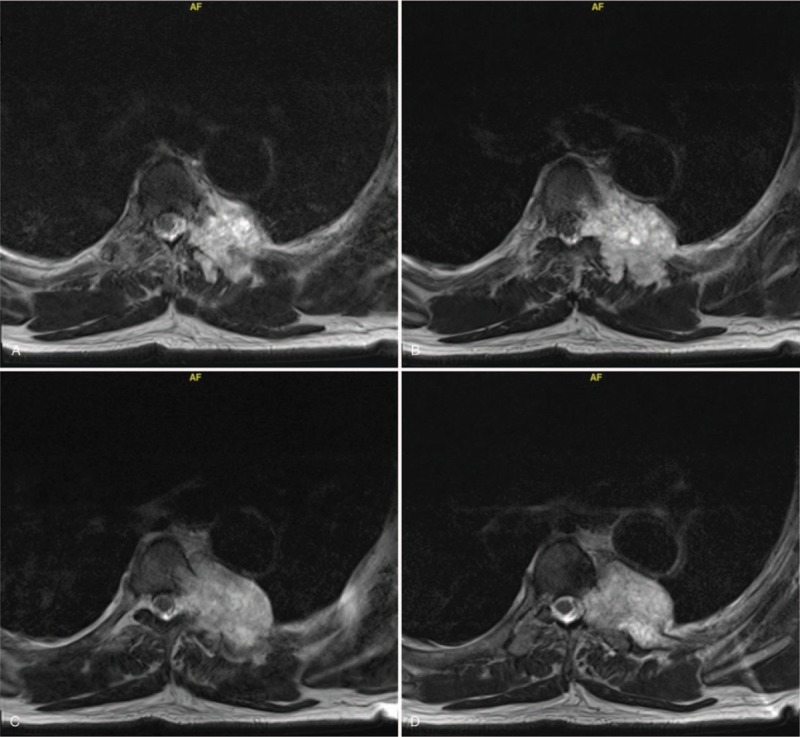
(A–D) Preoperative transverse MRI images showing the thoracic chordoma.
Figure 5.
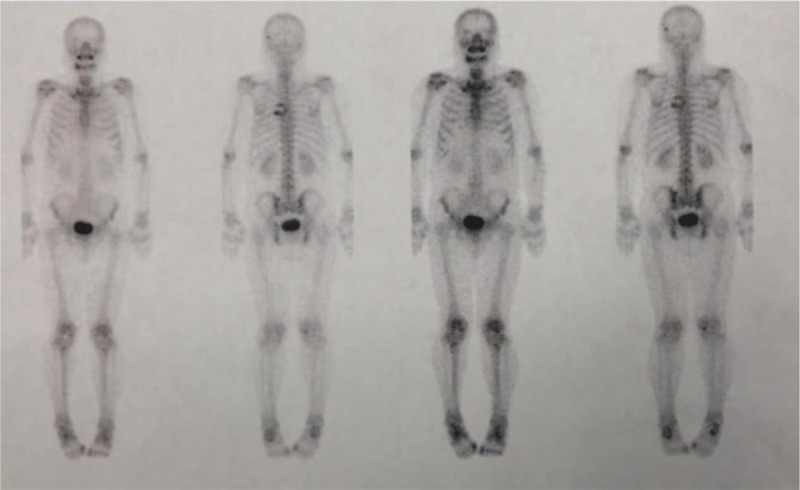
Bone scan revealed high intake in the thoracic spine, with high suspicion of malignant solid tumor.
Figure 6.
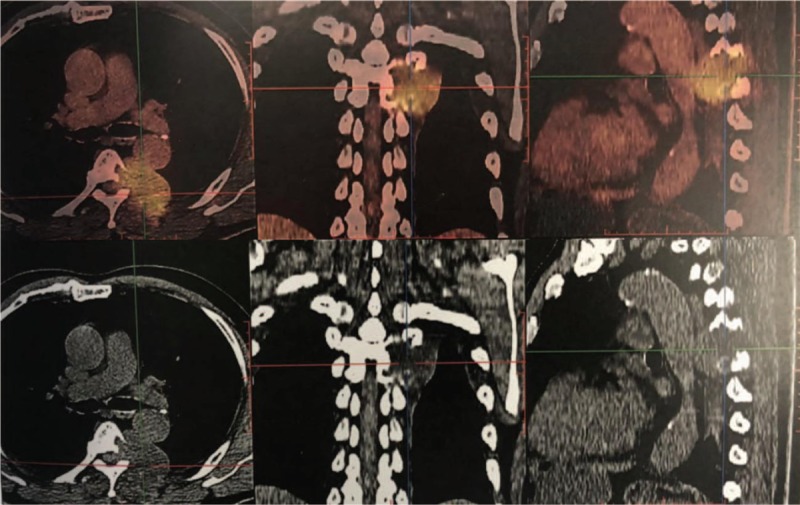
Positron emission tomography-computed tomography revealed malignant tumors of the thoracic spine with pleural involvement.
We performed a VATS to release the vertebral and paravertebral tumors infiltrating into the pleural cavity, followed by a circumferential decompression procedure of the spinal tumor to alleviate the symptoms caused by the spinal cord compression and subsequently stabilize the vertebral spine to prevent multiple vertebral bodies from collapse. Because of the size and location of the giant lesion and the extent of the invasion, the risk of surgical intervention is exceedingly high.
After successful anesthesia, the patient was placed in a right lateral position. During the exploration, no pleural effusion, no implant nodules, no obvious abnormality in the lungs was found. However, we could see a round mass at the level of the posterior mediastinal T5-T6 vertebral body with the size of 4 cm × 4 cm × 3 cm. The giant tumor with capsule and a wide base is invading into the vertebral bodies and costal vertebrae joint. There was an amount of strip adhesion between the left lower lung and the tumor. The mediastinal pleura was incised along the base of the tumor with an electric hook, thus the tumor and aorta were successfully separated with the ultrasonic knife. Hemostatic gauze was placed between the tumor and the aorta for subsequent posterior surgical identification. We indwelled a thoracic tube during the VATS operation.
Then, the patient was placed in a prone position for dorsal access to the thoracic spine. In brief, posterior circumferential decompression, tumor resection and T2 to T9 internal fixation were performed. For the posterior approach, the paraspinal muscles were detached gently on each side after a midline longitudinal incision was made over the spinous processes from T2-T9. The pedicle entry points were exposed by step-by-step bilateral dissection. At first, the pedicles of T2, T3, T4, T7, T8, T9, and the right pedicles of T5, T6 were fixed by routine screw preparation and placement needles. C-arm fluoroscopy showed that the location of pedicles was accurate and the position was satisfactory. Then, the pedicle screws were placed bilaterally, followed by pedicle screw insertion. The heads and proximal region of the left fifth and sixth ribs were exposed by dissection and resected. The tumor was located at the left paravertebral region of T5 and T6, involving the vertebral bodies and the left pedicles. The involved vertebral body of the left side of T5 and T6 and the whole lesions of the left pedicles and paravertebral lesions were resected. Because the patient did not exhibit hemodynamic instability to the placement of the pedicle screws, fixation using a screw-rod system was employed. Visual inspection using the intraoperative fluoroscopy showed optimal position of all pedicle screws. The incision was closed. Intraoperative blood loss was approximately 1500 ml, thus we used erythrocyte 4U and plasma 400 ml. Postoperatively, the patient was referred to the ICU and transferred to the general ward the next day. An x-ray after the surgery confirmed the correct positioning of the implants and no signs of displacement of the screws and rods (Fig. 7 A and B). The postoperative pathology report confirmed a thoracic chordoma. Pathological result was positive for AE1/AE3 indicating epithelial origin. Biopsy samples were positive for EMA, Vimentin, with 15% Ki-67 positive nuclei (Fig. 8 A–I).
Figure 7.
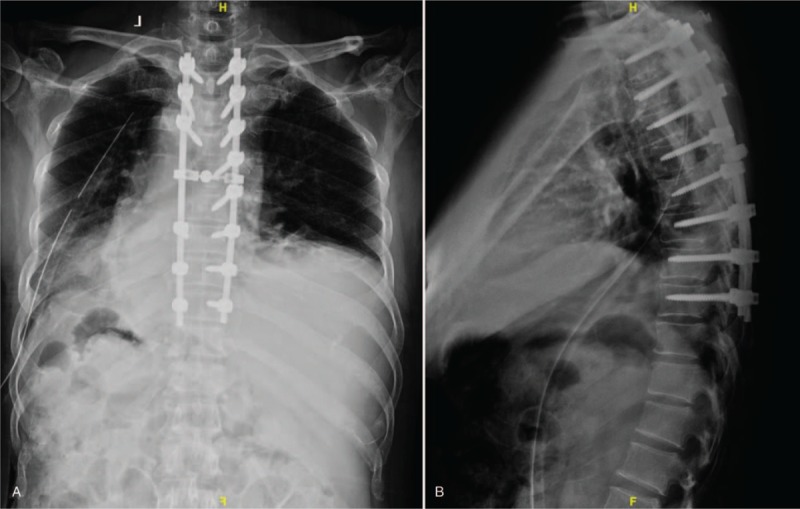
(A, B) Posteroanterior (PA) and lateral x-ray images of the thoracic spine obtained postoperatively.
Figure 8.
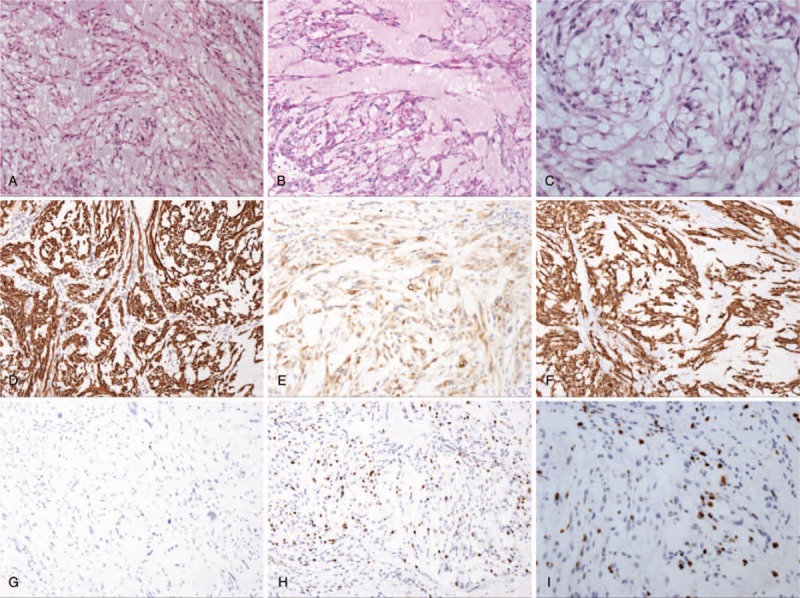
Pathologic histology of spinal tumors. (A–D) Microphotography showing characteristic nests of tumor cells separated by vascular septa (Zellballen) with cells showing significant nuclear pleomorphism with prominent nucleoli (H&E, original magnification 100×, 100×, 100×, and 200×). (E) AE1/AE3 immunostaining is strongly positive in the epithelial cells. (F) EMA immunostaining shows positive staining in the tumor cells. (G) Vimentin immunostaining shows positive staining in the tumor cells. (H, I) Ki-67 immunostaining shows 15% Ki-67 positive cells. Ki-67 staining is localized in the tumor nuclei.
One week after the operation, the patient's left lower extremities muscle strength improved to grade V compared to the preoperative status, grade V-, and the sensation to pin-prick and fine-touch of left lower extremity returned to normal. Moreover, VAS score of his back pain improved to 0 to 1 points compared to the preoperative status, 7 points. Following wound healing and removal of thoracic drainage tube, the patient underwent rehabilitation therapy and was monitored as an outpatient. The postoperative 3-month and 6-month follow-up visit showed no tumor progression and no new symptoms.
3. Discussion
Chordoma is an extraordinarily rare bone tumor which may exhibit notochord differentiation.[1,2] The tumor grows slowly, recurs locally and may metastasize later, thus, it can be difficult to diagnose and may result in devastating consequences if mismanaged.[7,8] It is a relatively rare tumor, and involvement of the thoracic spine is exceedingly rare.[1] Clinically, the tumor may be asymptomatic but usually presents with symptoms related to compression or involvement of adjacent structures and organs, including the trachea, esophagus, pleura cavity and spinal cord.[1,2,9–12] The characteristics of thoracic chordomas have not yet been fully clarified. Moreover, there are still no defined treatment protocols for thoracic chordomas due to the paucity of these patients.
Thoracic chordomas (TC) are rare tumors that were usually characterized by the invasiveness into nearby tissues.[13] Most patients with TC have clinical symptoms such as chest pain, cough, weight loss and shortness of breath.[14–16] Like most spinal disorders, TC may result in vertebral collapse, spinal instability, and progressive neurologic damage, which may cause local, radicular, or axial pain in addition to neurologic deficits from mild radicular weakness to paraparesis.[17,18] Back pain and radiculopathy can often mimic the most common cause of spinal disorders, making timely diagnosis of thoracic chordomas difficult without a high level of suspicion.[1,17,18]
In the literature, it is a rarity of chordomas of the thoracic spine presenting with back pain and radiculopathy.[1,19] Clinical studies looking at chordomas to the mobile spine is lacking due to the extremely low incidence rate. Based on our review of the case reports on PubMed,[1–5,9–24] chordomas of the thoracic spine is slightly more common during the fourth and fifth decades of life for the sporadic form.[1–5,9–24]
The location of the spinal lesion determines the neurological deficits, and there is a great deal of variability.[25,26] Compression of the cervical vertebra often exhibit symptoms of paresthesia and weakness in the upper extremities, while those located in the thoracic regions usually show symptoms of back pain, lower extremity paresthesia, weakness. In our case, the patient sought medical attention after experiencing serious back pain, lower extremity weakness. To our knowledge, this is a less-documented case of thoracic chordomas. Imaging studies including MRI, bone scan and PET/CT are non-specific, making it difficult to differentiate thoracic chordomas from other common spinal lesions.[1,19] However, imaging studies play a crucial role in the surgical intervention decision making. Imaging studies can demonstrate consecutive spinal stenosis, spinal cord or nerve root compression, compression or involvement of adjacent structures, and pathological vertebrae fractures.[1,25,26] MRI images from previous case reports demonstrate inhomogeneous lesion of spine, isointense on T1WI and iso-hyperintense on T2WI, indistinguishable from other metastatic spinal lesions.[1–18] Heterogeneous enhancement, calcification, and cystic changes are rarely observed.[27] Our patient's MRI showed isointense on T1WI and isointense lesion on T2WI, which is consistent with previous case reports. Radiographically, vertebral destruction is associated with the mediastinal mass because of the bony origin of the tumor. Lesions are usually lytic with calcifications within the tumor mass. Computed tomography scan and magnetic resonance imaging of the chest are mandatory to evaluate the extent of both the tumor and involvement of adjacent organs.
Chordomas may become malignant via their metastatic tendency, and the “gold-standard” diagnosis of chordomas relies on pathological findings. Histopathologically, chordomas are characterized by the architecture of nests of tumor epithelial cells separated by vascular septa with the cells showing significant nuclear pleomorphism with prominent nucleoli. Generally, chordomas are commonly immunoreactive for AE1/AE3, EMA.[1–3,20–29] A histological examination of our case was positive for AE1/AE3, EMA, Vimentin indicating chordoma from epithelial cells, which confirmed the diagnosis of thoracic chordoma.
Currently, no treatment guidelines exist because of the variability in treatment modalities and reported outcomes.[1–11] Surgical resection is the mainstay of treatment for TC and their metastatic lesions. We recommend surgical management of the spinal chordomas when the tumor has caused neurological deficits, spinal cord compression, destruction of spinal stability, or severe compression or involvement of adjacent structures. The best treatment for metastatic spinal chordomas causing acute paralysis and lower back pain is posterior decompression, tumor resection, and internal fixation.[1–3,24–28] This protocol accomplishes 2 objections: it alleviates the neurological deficits by decompressing the stenosis; and at the same time it provides histopathological specimens for diagnosis, which is valuable in cases where the patient presents with atypical clinical and radiological findings.[1–3,25,26] If possible, complete or grossly complete surgical resection of the primary tumor remains the treatment of the first choice. To date, surgical management of TC still remains under evaluation with no standard criteria. The more accepted approach is aggressive surgery to resect the tumor as far as possible and prevent dissemination into the surrounding tissues.[3,4] VATS combined with posterior spinal surgery might be a useful strategy to achieve rapid and sustained neurological improvements for patients with chordomas involving the thoracic spine.[12,13,30] Completion of surgical removal of the mediastinal component is directly related to its dimension. Recurrence after complete resection is a common feature and is considered to be related to the violation of tumor margins at surgical time.[29–31] The survival benefit of resection of TC is still unproven. However, such a procedure does have the benefit aiming at controlling residual tumor and is recommended for most patients.[1–11,24–31] This survival benefit of reducing the tumor burden, decompressing the spinal stenosis to alleviate radiculopathy, and facilitating subsequent chemotherapy and radiation therapy. Chordomas are known to be locally aggressive and relatively unresponsive to chemotherapy and radiation.[29] Even if not radiosensitive, radiotherapy has been used in some patients after total resection or palliative debulking. Radiotherapy does not prevent recurrence but seems to increase disease-free survival.[30]
In conclusion, we present an extremely unusual occurrence of thoracic chordoma that was successfully managed by surgical procedure, which has not been previously well reported in literature. Our focus is to emphasize the importance of considering thoracic chordoma as a diagnosis and guiding the proper management strategy upon surgical treatment.
4. Conclusion
The giant thoracic chordomas are truly rare neoplasms, particularly compared to other metastatic lesions of the spine. Although uncommon, thoracic chordomas should be part of the differential diagnosis when the patient presents with back pain or neurological deficits. To date, surgery is the mainstay of treatment in patients with good performance status prior to symptom onset and should be strongly considered in patients with the history of chordoma. One-stage thoracoscopic release or resection of vertebral and paravertebral tumor combined with spinal surgery is also a good choice for surgical treatment. With a multidisciplinary team approach, proper planning, and adequate perioperative medical management, thoracic spinal chordomas can be managed effectively.
Acknowledgments
We would like to thank our colleagues at the Department of Orthopedic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Author contributions
Conceptualization: Shuzhong Liu, An Song, Yipeng Wang, Yong Liu.
Funding acquisition: Shuzhong Liu, Yipeng Wang, Yong Liu.
Investigation: Shuzhong Liu, Xi Zhou, An Song, Siyuan Yao, Yong Liu.
Resources: Shuzhong Liu, Zhen Huo, Yong Liu.
Supervision: Yipeng Wang, Yong Liu.
Writing – original draft: Shuzhong Liu, Xi Zhou, An Song.
Writing – review & editing: Yipeng Wang, Yong Liu.
Footnotes
Abbreviations: ICU = intensive care unit, MRI = magnetic resonance imaging, PET/CT = Positron Emission Tomography-Computed Tomography, T1WI = T1-weighted image, T2WI = T2-weighted image, VAS = visual analogue scale, VATS = video-assisted thoracic surgery.
Written informed consent was obtained from the patient for publication of this article, a copy of which is available for review from the editors of Medicine. Because this article does not involve any human or animal trials, it did not require institutional ethical review and approval.
This study was supported by National Natural Science Foundation of China (Project No. 81871746; Grant recipient: YW), and Peking Union Medical College Graduate Student Innovation Fund (2018) (Project No. 2018–1002–02–08; Grant recipient: SL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Pu F, Wang B, Liu J, et al. Giant chordoma in the thoracolumbar spine: a case report and literature review. Eur Spine J 2017;26Suppl 1:95–9. [DOI] [PubMed] [Google Scholar]
- [2].Tanaka K, Sakakima H, Hida K, et al. A Case of C5 vertebral chordoma in a 73-year-old patient with more than 8 years of follow-upafter total piecemeal spondylectomy. Case Rep Orthop 2017;2017:3284131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rena O, Davoli F, Allegra G, et al. Giant chordoma of the upper thoracic spine with mediastinal involvement: a surgical challenge. Asian Spine J 2014;8:353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang Y, Li Y, Liu W, et al. The clinical outcome of recurrent sacral chordoma with further surgical treatment. Medicine (Baltimore) 2018;97:e13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xu Q, Gu H, Liu X, et al. Giant sacrococcygeal chordoma: a case report. Medicine (Baltimore) 2018;97:e13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matsumoto T, Imagama S, Ito Z, et al. Total spondylectomy following carbon ion radiotherapy to treat chordoma of the mobile spine. Bone Joint J 2013;95-B:1392–5. [DOI] [PubMed] [Google Scholar]
- [7].Fontes R, O’Toole JE. Chordoma of the thoracic spine in an 89-year-old. Eur Spine J 2012;21Suppl 4:S428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bisceglia M, D’Angelo VA, Guglielmi G, et al. Dedifferentiated chordoma of the thoracic spine with rhabdomyosarcomatous differentiation. Report of a case and review of the literature. Ann Diagn Pathol 2007;11:262–73. [DOI] [PubMed] [Google Scholar]
- [9].D’Amore T, Boyce B, Mesfin A. Chordoma of the mobile spine and sacrum: clinical management and prognosis. J Spine Surg 2018;4:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].von Witzleben A, Goerttler LT, Lennerz J, et al. In chordoma, metastasis, recurrences, Ki-67 index, and a matrix-poor phenotype are associated with patients’ shorter overall survival. Eur Spine J 2016;25:4016–24. [DOI] [PubMed] [Google Scholar]
- [11].Oppenlander ME, Maulucci CM, Ghobrial GM, et al. En bloc resection of upper thoracic chordoma via a combined simultaneous anterolateral thoracoscopic and posterior approach. Neurosurgery 2014;10Suppl 3:380–6. [DOI] [PubMed] [Google Scholar]
- [12].Conzo G, Gambardella C, Pasquali D, et al. Multifocal thoracic chordoma mimicking a paraganglioma. J Cancer Res Ther 2013;9:497–9. [DOI] [PubMed] [Google Scholar]
- [13].Akiyama T, Ogura K, Gokita T, et al. Analysis of the infiltrative features of chordoma: the relationship between micro-skip metastasis and postoperative outcomes. Ann Surg Oncol 2018;25:912–9. [DOI] [PubMed] [Google Scholar]
- [14].Ribeiro BNF, Marchiori E. Chordoma of the posterior mediastinum accompanied by synchronous lesion. Radiol Bras 2017;50:340–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fernández Carballal C, González Rodrigalvarez R, López De La Riva M, et al. Dumbbell-shaped thoracic chondroid chordoma mimicking a neurinoma. Acta Neurochir (Wien) 2010;152:325–6. [DOI] [PubMed] [Google Scholar]
- [16].Miyazawa N, Ishigame K, Kato S, et al. Thoracic chordoma: review and role of FDG-PET. J Neurosurg Sci 2008;52:117–21. [PubMed] [Google Scholar]
- [17].Wang TJ, Shu SH, Lin CW, et al. Thoracic chordoma: an unusual presentation of the spinal tumor. Am J Med Sci 2008;335:239–41. [DOI] [PubMed] [Google Scholar]
- [18].Van Kollenburg JA, De Waal Malefijt J, Gosens T, et al. Chordoma of a thoracic vertebra. A case report. Acta Orthop Belg 2007;73:812–6. [PubMed] [Google Scholar]
- [19].Demireli P, Ovali GY, Yegen G, et al. Chondroid chordoma of the thoracic spine: case report. Pathology 2007;39:280–2. [DOI] [PubMed] [Google Scholar]
- [20].Goomany A, Timothy J, Robson C, et al. En bloc resection of a thoracic chordoma is possible using minimally invasive anterior access: An 8-year follow-up. J Neurosci Rural Pract 2016;7:138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Williams BJ, Karas PJ, Rao G, et al. Laser interstitial thermal therapy for palliative ablation of a chordoma metastasis to the spine: case report. J Neurosurg Spine 2017;26:722–4. [DOI] [PubMed] [Google Scholar]
- [22].Liu S, Zhou X, Song A, et al. Successful treatment of malignant pheochromocytoma with sacrum metastases: A case report. Medicine (Baltimore) 2018;97:e12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van Wulfften Palthe ODR, Tromp I, Ferreira A, et al. Sacral chordoma: a clinical review of 101 cases with 30-year experience in a single institution. Spine J 2018;S1529-9430:31206–13. [DOI] [PubMed] [Google Scholar]
- [24].Bakker SH, Jacobs WCH, Pondaag W, et al. Chordoma: a systematic review of the epidemiology and clinical prognostic factors predicting progression-free and overall survival. Eur Spine J 2018;27:3043–58. [DOI] [PubMed] [Google Scholar]
- [25].Liu S, Song A, Zhou X, et al. Malignant pheochromocytoma with multiple vertebral metastases causing acute incomplete paralysis during pregnancy: literature review with one case report. Medicine (Baltimore) 2017;96:e8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu S, Zhou X, Song A, et al. Successful treatment of Gorham-Stout syndrome in the spine by vertebroplasty with cement augmentation: A case report and literature review. Medicine (Baltimore) 2018;97:e11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou Y, Hu B, Wu Z, et al. A giant lumbar chordoma: a case report. Medicine (Baltimore) 2018;97:e11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pillai S, Govender S. Sacral chordoma: a review of literature. J Orthop 2018;15:679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rotondo RL, Folkert W, Liebsch NJ, et al. High-dose proton-based radiation therapy in the management of spine chordomas: outcomesand clinicopathological prognostic factors. J Neurosurg Spine 2015;23:788–97. [DOI] [PubMed] [Google Scholar]
- [30].Chen YL, Liebsch N, Kobayashi W, et al. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine (Phila Pa 1976) 2013;38:E930–6. [DOI] [PubMed] [Google Scholar]
- [31].Holliday EB, Mitra HS, Somerson JS, et al. Postoperative proton therapy for chordomas and chondrosarcomas of the spine: adjuvant versus salvage radiation therapy. Spine (Phila Pa 1976) 2015;40:544–9. [DOI] [PubMed] [Google Scholar]


