Abstract
Public health authorities recommend a range of nonchemical measures to control blacklegged ticks Ixodes scapularis Say, 1821 (Ixodida: Ixodidae) in residential yards. Here we enumerate these recommendations and assess their relationship to larval tick abundance in 143 yards in Dutchess County, New York, an area with high Lyme disease incidence. We examined the relationship between larval tick abundance and eight property features related to recommendations from public health agencies: presence or absence of outdoor cats, wood piles, trash, stone walls, wood chip barriers separating lawn from adjacent forest, bird feeders, fencing, and prevalence of Japanese barberry (Berberis thunbergii DC [Ranunculales: Berberidaceae]). We assessed abundance of larval ticks using two methods, flagging for questing ticks and visual examination of ticks on white-footed mice Peromyscus leucopus Rafinesque, 1818 (Rodentia: Cricetidae). More questing larvae were found in yards where trash or stone walls were present. These effects were less pronounced as forest area increased within the yard. Counts of larvae per mouse were lower in properties with >75% of the yard fenced than in properties with less fencing. We find partial support for recommendations regarding trash, stone walls, and fencing. We did not detect effects of outdoor cats, bird feeders, barriers, wood piles, or Japanese barberry. There was low statistical power to detect effects of ground barriers (gravel, mulch, or woodchip), which were present in only two properties.
Keywords: residential yard, Ixodes scapularis, Lyme disease, integrated tick management, disease prevention
In the United States, an estimated 300,000 Lyme disease cases occur annually (Nelson et al. 2015), and the disease is expanding geographically (Schwartz et al. 2017). Throughout eastern North America, the bacterium Borrelia burgdorferi, the causative agent of Lyme disease, is transmitted by the blacklegged tick Ixodes scapularis. Exposure to infected blacklegged ticks is thought to occur peri-domestically (Dister et al. 1997, Cromley et al. 1998, Connally et al. 2006, Eisen and Dolan 2016). In the United States, most Lyme disease cases occur in the Northeast and upper Midwest (Nelson et al. 2015). In one study of 70 people bitten by ticks in Westchester County, New York, 69% reported acquiring the tick in their yard (Falco and Fish 1988). On the basis of the expectation that exposure is primarily peri-domestic, there has been widespread interest in deploying methods for controlling ticks and avoiding exposures in residential yards (Ostfeld et al. 2006a).
Many residents of areas endemic for Lyme disease undertake one or more environmental controls against ticks. In a survey of residents in three Connecticut towns in 2004, 24%–44% used a chemical pesticide in their yards against ticks, 44%–52% removed brush or leaf litter as an environmental control against ticks, 17%–29% placed wood chip or gravel barriers intended to discourage tick incursions, and 12%–25% fenced their properties to keep out deer (Gould et al. 2008). Chemical and landscaping measures entail investments by homeowners; 30%–58% of Connecticut respondents reported being willing to pay over $100 ($134 in 2018 dollars) for yard tick control. In addition to these active controls, residents might affect tick abundance inadvertently by enhancing or diminishing habitat quality for ticks or their vertebrate hosts.
Public health officials recommend an array of property management measures to control abundance of ticks and risk of exposure to tick-borne infections. The advice includes measures expected to decrease habitat for hosts of ticks, decrease tick habitat, limit tick dispersal, and decrease human contact with ticks. Advice to reduce habitat for tick hosts includes recommendations about stone walls, wood piles, bird feeders, and fences. Stone walls are recommended to be sealed (Stafford 2004). P. leucopus use stone walls to orient and to take refuge (Barry and Francq 1980). In a study in Westchester County, New York, nymphal tick abundance on unsealed, remnant stone walls was similar to the abundance on nearby forest floor (Frank et al. 1998). Wood piles, which the U.S. Centers for Disease Control (CDC) advises to keep neat and in areas of the yard away from the home (Centers for Disease Control 2018b), have been associated with increased tick-borne disease risk in some studies (Smith et al. 2001, Jones et al. 2018) but not all (Connally et al. 2009). Bird feeders, which homeowners are advised to remove altogether or keep away from the house for small mammal and bird management (Stafford 2004), have been associated with increased risk of tick encounters (Mead et al. 2018) and increased tick-borne disease risk in some studies (Smith et al. 2001, Jones et al. 2018) but not all (Townsend et al. 2003). Deer-exclusion fencing reduced tick abundance in residential forest plots (Daniels et al. 1993, Stafford 1993). Fencing is a recommended action in yards (Centers for Disease Control 2018b), where it has been correlated with reduced Lyme disease risk in one study (Fritz et al. 1997, Connally et al. 2009) but not others (Ley et al. 1995, Perkins et al. 2006, Jones et al. 2018). Recommendations to reduce tick habitat include raking of leaf litter, removal of trash (Centers for Disease Control 2018b), and avoid planting Japanese barberry (Berberis thunbergii) (Stafford 2004). Removal of leaf litter reduced immature tick abundance (Schulze et al. 1995) but has not been found to correlate with a reduction in Lyme disease incidence (Klein et al. 1996, Connally et al. 2009). Higher abundance of I. scapularis on P. leucopus has been found in nonresidential areas with denser Japanese barberry (Williams et al. 2009). Removal of Japanese barberry, which buffered microclimatic conditions, reduced I. scapularis by 60% in nonresidential forested areas (Williams and Ward 2010).
To reduce tick dispersal, public health officials have advised people to establish mulch, gravel, or wood chip barriers between forest and lawn. Past studies have found that silt barriers reduced tick dispersal (Carroll and Schmidtmann 1996). Frequent mowing is also recommended. To reduce ticks being brought into the home by pets, residents have been encouraged to keep cats and dogs out of woods (Stafford 2004). The effects of pet ownership on human risk are mixed, with cat ownership associated with increased risk in some studies (Jones et al. 2018) but not all (Ley et al. 1995, Belongia et al. 1999), and dog ownership similarly increasing risk of tick encounters (Jones et al. 2018) or disease in some studies (Fritz et al. 1997) but not others (Finch et al. 2014). For some recommendations, such as those regarding pets, wood piles, and bird feeders, associations found between the feature and human risk could arise from effects on tick abundance, human exposure, or both.
Here we asked how larval tick abundance in residential yards relates to recommended nonchemical yard management measures as environmental tick controls. We catalogued the yard management measures recommended to reduce tick bites by the CDC and the State of Connecticut’s Tick Management Handbook (Stafford 2004), which is cited by the CDC and other organizations across the United States (Harvard Health Letter 2010, UMaine Cooperative Extension 2018) and Canada (Public Health Ontario 2018). We assessed residential yards across Dutchess County, New York, for their use of strategies included in these recommendations. We do not know whether the features we documented were imposed by homeowners with the intention of controlling ticks; rather, we analyzed these features as consistent with recommended control measures. For some recommendations, such as those regarding pets, wood piles, and bird feeders, associations found between the feature and human risk could arise from effects on tick abundance, human exposure, or both. We evaluated whether the use of yard management recommendations predicted larval tick abundance. Abundance of larvae was assessed both by flagging for questing ticks and by visual counts of ticks on small mammals. Our results expand the evidence base for recommendations about how to reduce tick exposures and tick-borne disease risk in residential yards. We focused on larval ticks, because our sampling coincided with larval peak activity. While nymphal ticks are of chief concern for public health, assessing how yard features influence larval ticks is relevant to human exposure to nymphs, because larvae transition to nymphs the following year.
Materials and Methods
Study Area
The study area included residential yards of households participating in The Tick Project (Keesing and Ostfeld 2018). The Tick Project is a 5-yr study (2016–2020) in Dutchess County, New York, to determine whether controlling ticks at the neighborhood scale reduces tick-borne disease. The two tick control methods being evaluated are the fungal biopesticide Met52 and bait boxes that apply the acaricide fipronil to small mammals. In 2016, at the time of tick and small-mammal sampling for the present study, tick control treatments had not yet begun; all measurements of tick abundance in the present study were conducted in the year before any tick-control interventions were imposed. Approximately 980 households in 24 neighborhoods participate in The Tick Project; yards of a subset of participants were subject to tick sampling. Participants agreed to not apply chemicals to their yards for the purpose of insect or tick control over the course of the study, with the exception of garden areas comprising less than 10% of the yard.
Sampling Questing Ticks and Ticks on Small Mammals
We conducted tick sampling from August 11 to 22 September 2016. This period is during peak activity for larval I. scapularis in Dutchess County, after peak activity for nymphs, and before the fall peak in activity for adult ticks (Ostfeld 2011). Because the sampling period was outside the time of peak activity for nymphs, we restrict the current analyses and conclusions to larvae. Within each residential yard, we completed all tick sampling within 1 wk.
The present analysis used sampling of I. scapularis larvae in residential yards collected via flag-sampling of questing ticks (143 yards) and visual examination of larval tick burdens on small mammals (111 yards); both sampling methods were used in 89 properties. We flag-sampled using a white, 1-m2 corduroy cloth suspended by a string from a wooden dowel. Flag sampling allows the relative abundance of questing ticks to be quantified by the number of ticks collected per unit time sampling (Mather et al. 1996, Ginsberg and Zhioua 1999), and is a frequently used method for sampling I. scapularis, including in dense understory vegetation (Ginsberg and Ewing 1989, Rulison et al. 2013). For this study, one side of the cloth was moved over the ground and emergent vegetation, and all ticks were recorded and removed from both sides of the cloth in between each sample. Yard habitat types in which tick and mammal sampling occurred included forest, lawn, and shrub/garden. As a measure of questing larvae abundance, we took the mean total number of larvae recorded from up to ten 30-s flag samples (number of larvae per 30 s) for each habitat type in each property. If area permitted, we conducted 10 flagging samples in forest. If area and time permitted, we also conducted 10 flagging samples each in lawn and shrub/garden.
We conducted visual examination of larval tick burdens on small mammals using live trapping. In each yard, we set 18 Sherman live-traps, baited with crimped oats and sunflower seeds. We set traps in nine trap stations, with two traps per station, and ≥5 m between stations. We allocated trap stations proportionally to the area of each habitat type (forest, lawn, and shrub/garden) in the yard, with a minimum of two stations per habitat type. Forest was defined as at least two trees with contiguous canopy, excluding trees in lawn. We placed traps under dense vegetation where possible and under a wooden board for protection against solar radiation and rain. We trapped for two to three consecutive nights in each yard. Each trapped animal was fitted with a uniquely numbered ear tag. The number of larval ticks found on the head and ears of each animal was recorded, in addition to the individual’s species, sex, age, weight, and reproductive condition, before each animal was released at the point of capture. In prior research, we observed a stronger correlation between head-and-ear tick counts and whole-body burdens for white-footed mice Peromyscus leucopus than for eastern chipmunks Tamias striatus Linnaeus, 1758 (Rodentia: Sciuridae) (Schmidt et al. 1999), the two most common species trapped during this study. Therefore, for the current analysis, we used only larval tick counts from P. leucopus. Peromyscus leucopus transmit pathogens to I. scapularis larvae with relatively high efficiency, compared with other hosts (Hersh et al. 2014, Vuong et al. 2014), and P. leucopus abundance predicts the following year’s density of I. scapularis nymphs infected with B. burgdorferi (Ostfeld et al. 2006b). We trapped a total of 709 P. leucopus (out of a total 1018 small mammals of all species). For each property and habitat type present in a yard, we analyzed two measures of larval tick abundance on P. leucopus: first, the total number of larvae counted across all P. leucopus individuals captured and, second, the total number of larvae on mice divided by the number of P. leucopus captured. Given that our sampling took place during the larval peak in 2016, the first measure reflects the risk to residents the following year, in 2017, when 2016 larvae had transitioned to nymphs. The second measure, per capita larval tick burden, may also serve as a barometer of density of ticks in the following year. We used larval ticks on mice as a metric of tick abundance but did not examine how mice or any other specific host affected tick abundance.
Identifying and Measuring Recommended Yard Management Measures
We catalogued yard management measures provided by the CDC (Centers for Disease Control 2018a, 2018b) and in the Tick Management Handbook section on ‘Integrated Tick Management’ (Stafford 2004). Supp Table 1 (online only) provides the full set of 22 recommendations, including 10 provided by the CDC, 21 in the Tick Management Handbook, and 9 contained in both sources. We considered a statement in either source to be a recommendation if the statement used imperative language or terms such as ‘should’. We determined it to be infeasible to reliably measure adherence to certain recommendations, such as regular mowing or sealing foundations, during brief visits to yards and therefore excluded these recommendations from consideration in this study. Also excluded were measures related to human exposure to ticks rather than tick control, for example moving swing sets away from the forest edge (Stafford 2004). We identified eight features for inclusion in this study; five of these matched advice from both sources, while one feature was addressed by the CDC only and two were found only in the Tick Management Handbook. These features included the presence of a firewood pile (in apparent active use, volume approximating at least 1 m3), trash (volume of single human-made item greater than 1-gallon [3.78 liter] container), natural stone wall (at least 0.1 m in height by 2 m in length), barrier of gravel, mulch, or wood chips between forest and lawn (at least 3 feet [0.91 m] wide), bird feeder presence, and fencing (75% or greater of yard enclosed, or less than 75% enclosed). No distinction was made among different types of fencing. For logistical reasons, these yard features were recorded during visits to yards in 2018. Household ownership of a cat that spends time both indoors and outdoors was recorded by participants in an introductory survey in 2016. Finally, we addressed the recommendation to avoid planting Japanese barberry (Berberis thunbergii) by recording its presence or absence in the path taken during each flagging interval in 2018. We did not distinguish between Japanese barberry that was purposefully planted by residents versus Japanese barberry that colonized on its own. For Japanese barberry, each flagging interval represented a belt transect, a method used previously in Japanese barberry studies (Mosher et al. 2009). The plant was recorded as present if at least one plant was encountered with width at least approximately 50 cm, or one half the width of the flagging cloth. As a measure of Japanese barberry prevalence, we used the fraction of completed flagging intervals in the yard in which the plant was encountered.
Data Analysis
We separately analyzed larval tick data from flagging, total larvae on P. leucopus, and larvae per P. leucopus. For each tick dataset, we constructed a full model, including all property management variables. In addition to the property management features, the full generalized linear mixed model further included fixed effects of the habitat type in which ticks were sampled and the area of forest within the property, and random effects of neighborhood and property. As a proxy for forest area in each property, we used the number of flagging intervals conducted in forest on the property in 2018. Ten flagging intervals were conducted in properties with sufficient forest area to accommodate this number of intervals without re-sampling any area; in properties with smaller forested area, the number of intervals reflects the maximum area that could be flagged given the forest area present. The number of flagging intervals estimates relative forest area among properties with area insufficient to fit 10 intervals, although it does not allow distinctions to be made in relative forest area among properties that could accommodate 10 intervals. Habitat type and forest area were included as factors, despite not being included among public health agency recommendations, due to the importance of habitat, particularly forest, for blacklegged ticks (Stafford and Magnarelli 1993, Duffy et al. 1994, Lindsay et al. 1999). For each property management variable found to be significant in the full model, we also evaluated potential interactions between each variable and forest area, which we expected may mediate the effects of property management strategies. Each model was constructed to have an intercept of zero to derive estimates of the effects of each measure. Function ‘lmer’ in R package ‘lme4’ was used to build the models. P-values were determined by evaluating the model using package ‘lmerTest’. All analyses were carried out in R, version 3.4.4 (R Core Team 2018).
Results
Questing Larval Ticks
Trash (df = 328, t = 2.678, P = 0.008) and stone walls (df = 318, t = 2.237, P = 0.026) were each associated with increased abundance of questing larval ticks in the full model that included all property management features and random effects of neighborhood and property address (Table 1). We also observed higher abundance of questing larval ticks in forest samples (df = 195, t = 4.171, P < 0.0001). For other property management variables, we detected no significant effects on abundance of questing larvae (Supp Figs. 1–3 [online only]). Summary statistics for each habitat (mean number of ticks, standard error, range, sample size, number of properties with nymphs) are available in Supp Table 2 (online only) (questing ticks) and Supp Table 3 (online only) (ticks on mice). Counts of properties containing each management feature are available in Supp Table 4 (online only). Data are available, on questing ticks and ticks on P. leucopus, in the figshare repository (Fischhoff et al. 2018).
Table 1.
Effects of property management on questing tick abundance
| Variable | Std. error | df | t-value | P |
|---|---|---|---|---|
| Forest habitat | 0.701 | 194.61 | 4.171 | <0.0001*** |
| Lawn habitat | 0.62 | 130.728 | 0.346 | 0.73 |
| Shrub/garden habitat | 0.633 | 144.084 | 0.362 | 0.718 |
| Outside cat present | 0.713 | 357.989 | 0.158 | 0.874 |
| Wood pile present | 0.51 | 357.998 | −0.894 | 0.372 |
| Trash present | 0.521 | 327.604 | 2.678 | 0.008** |
| Stone wall present | 0.618 | 318.401 | 2.237 | 0.026* |
| Barrier present | 2.244 | 352.022 | 0.132 | 0.895 |
| Bird feeder present | 0.521 | 292.162 | −0.094 | 0.926 |
| Fencing present | 0.802 | 334.047 | −0.913 | 0.362 |
| No. of forest flagging intervals | 0.065 | 243.325 | −1.644 | 0.102 |
| Fraction of flagging intervals with barberry | 5.612 | 344.418 | 0.57 | 0.569 |
Summary of results from linear mixed model including fixed effects of property management variables, habitat, and forest area (as measured by the number of forest flagging intervals), and random effects of neighborhood and property address. Fencing is considered present if at least 75% of the property is fenced, and absent if less than 75% of the property is fenced. Trash and stone walls were each associated with increased tick abundance. Forest samples had increased tick abundance. Asterisks indicate significance: * (P < 0.05), ** (P < 0.01), *** (P < 0.001).
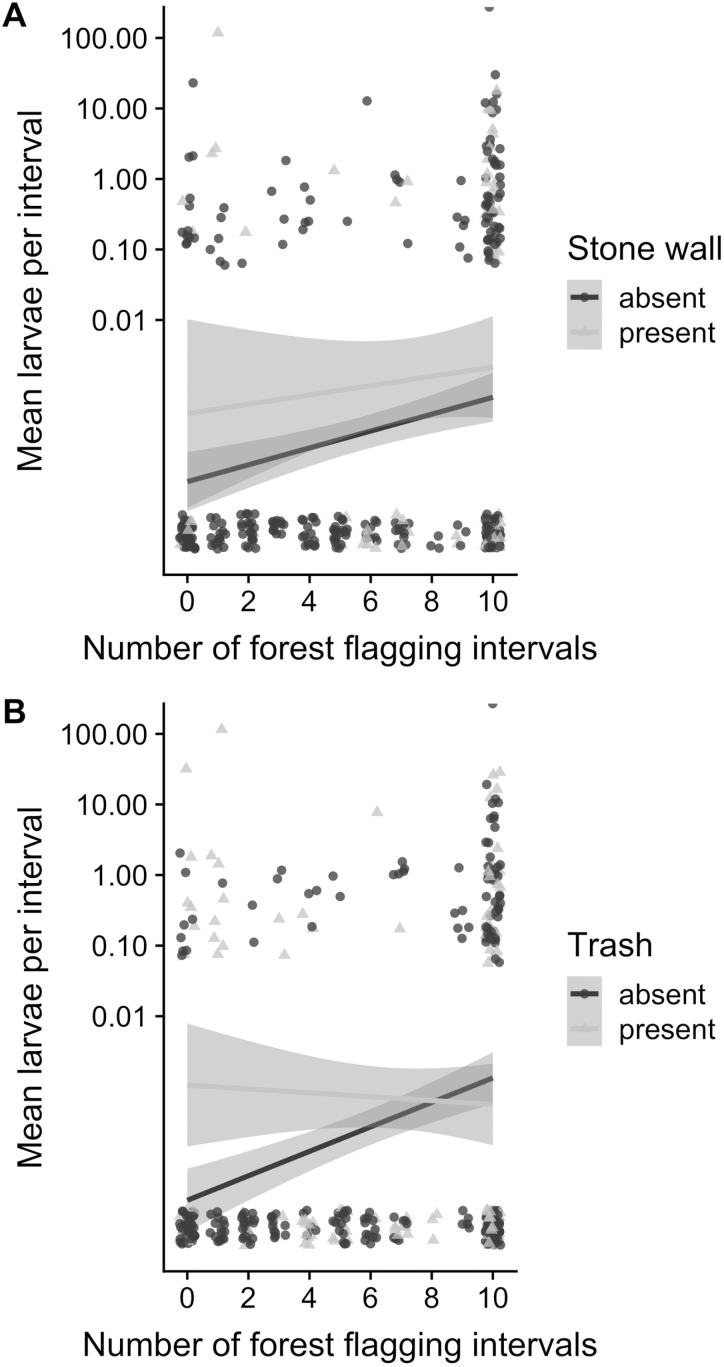
In models that included fixed effects of one property management feature, forest area, and their interaction (with random effects of neighborhood and property address), there was again a positive effect on tick abundance of the presence of trash or stone walls (trash: df = 324, t = 2.932, P = 0.004; stone wall: df = 318, t = 3.327, P = 0.001). The amount of forest habitat in a yard was found to be an important moderating effect (interactive force) on the influence of property management features. Trash or stone walls were each a less positive predictor of larval tick abundance in yards with greater forest area (trash*forest: coefficient = −0.27, df = 323, t = −2.098, P = 0.037; stone wall*forest: coefficient = −0.43, df = 358, t = −2.719, P = 0.007; Fig. 1). In the forest*trash model, we did not find a significant effect of forest area: coefficient = 0.08, df = 173, t = 1.144, P = 0.25. Neither was there a significant effect of forest in the stone wall*forest model: coefficient = 0.07, df = 173, t = 1.060, P = 0.290.
Fig. 1.
Questing larval tick abundance in relation to forest area, and the presence (1, light gray triangles) or absence (0, dark gray circles) of A) stone walls and B) trash in residential yards. Each line is based on a linear model fitted to the data; the gray bounds are 95% confidence intervals for predictions from the fitted model. To plot on log scale for greater ease of interpretation, 0.00001 has been added to each questing larval tick value. The data have been horizontally and vertically ‘jittered’ to reduce overlap of points.
Larval Ticks on White-footed Mice
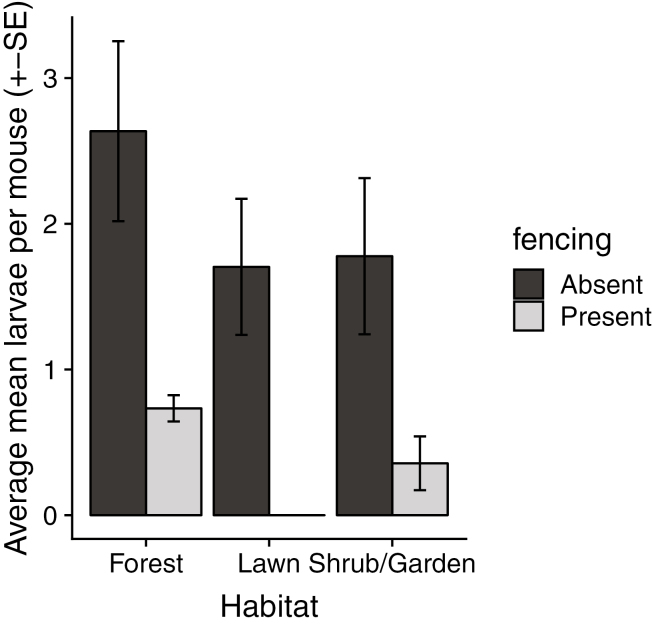
We did not detect significant effects on total larvae on mice of any property management features (Table 2, Supp Figs. 4–6 [online only]). Considering per capita tick burden on white-footed mice, the presence of fencing enclosing at least 75% of the yard had a significant negative effect on larval tick abundance (df = 74, t = −2.752, P = 0.007) (Table 3, Fig. 2; Supp Figs. 7–9 [online only]). No interaction effect was detected between fencing and forest area (fencing*forest coefficient = 0.004, df = 76, t = 0.014, P = 0.989).
Table 2.
Effects of property management on total larval ticks observed on all mice (per habitat and property)
| Variable | Std. error | df | t-value | P |
|---|---|---|---|---|
| Forest habitat | 4.476 | 117.183 | 1.321 | 0.189 |
| Lawn habitat | 4.771 | 115.961 | −0.164 | 0.87 |
| Shrub/garden habitat | 4.195 | 101.802 | 0.086 | 0.932 |
| Outside cat present | 4.822 | 115.44 | 0.652 | 0.516 |
| Wood pile present | 3.09 | 102.766 | −0.306 | 0.76 |
| Trash present | 3.48 | 113.49 | 0.145 | 0.885 |
| Stone wall present | 3.605 | 118.06 | 0.313 | 0.755 |
| Barrier present | 15.866 | 110.572 | 0.545 | 0.587 |
| Bird feeder present | 3.471 | 119.651 | 0.655 | 0.514 |
| Fencing present | 6.401 | 128.795 | −1.436 | 0.153 |
| No. of forest flagging intervals | 0.429 | 128.234 | 0.93 | 0.354 |
| Fraction of flagging intervals with barberry | 34.375 | 102.54 | 1.506 | 0.135 |
The table shows results from a linear mixed model, including fixed effects of property management variables, habitat, and forest area (as measured by the number of forest flagging intervals), and random effects of neighborhood and property address. The model was constructed to have a zero intercept. No significant effects were observed.
Table 3.
Effects of property management on per capita larval tick abundance on white-footed mice Peromyscus leucopus
| Variable | Std. error | df | t-value | P |
|---|---|---|---|---|
| Forest habitat | 1.384 | 77.497 | 1.88 | 0.064 |
| Lawn habitat | 1.456 | 87.419 | 1.205 | 0.232 |
| Shrub/garden habitat | 1.334 | 67.656 | 0.776 | 0.44 |
| Outside cat present | 1.293 | 66.155 | 0.488 | 0.627 |
| Wood pile present | 0.841 | 58.439 | −0.537 | 0.594 |
| Trash present | 0.964 | 64.248 | −1.152 | 0.254 |
| Stone wall present | 0.996 | 69.315 | 1.244 | 0.218 |
| Barrier present | 4.244 | 60.798 | 0.97 | 0.336 |
| Bird feeder present | 0.953 | 68.927 | 0.565 | 0.574 |
| Fencing present | 1.771 | 74.414 | −2.752 | 0.007** |
| No. of forest flagging intervals | 0.121 | 89.791 | 1.14 | 0.257 |
| Fraction of flagging intervals with barberry | 9.643 | 58.28 | 0.234 | 0.816 |
The linear mixed model included fixed effects of property management variables, habitat, and forest area, and random effects of neighborhood and property address. Mice from properties with fencing encircling at least 75% of the yard had lower per capita larval tick burdens than mice from yards fenced less than 75%.
Fig. 2.
Bar plot (with standard errors) of abundance of larval ticks on white-footed mice in relation to habitat and fencing. Fencing was considered present in properties with at least 75% of the yard enclosed in fencing, absent in yards with <75% fenced.
Discussion
Residential yards are thought to be the primary location at which exposure to blacklegged ticks occurs (Dister et al. 1997, Cromley et al. 1998, Connally et al. 2006, Eisen and Dolan 2016). In some communities endemic for Lyme disease, majorities of residents surveyed used nonchemical measures to control ticks in their yards, and reported willingness to pay over $100 for tick control (Gould et al. 2008). Messages from public agencies influence residents’ decisions about whether and how to manage their yards for tick control. We found that two widely used sources, the CDC and Connecticut Tick Management Handbook, together included 22 recommendations for nonchemical yard-level tick control. Given the costs of managing yards to reduce ticks, and the potential benefits of reducing ticks, it is important for public health authorities’ messages and homeowners’ decisions to be grounded in evidence about how yard management relates to tick abundance. Here we evaluated the relationship between adherence to a subset of yard management recommendations and the abundance of larval ticks, both questing and on small mammals, in residential yards. The yards are in neighborhoods in Dutchess County, New York, which ranks among the highest counties in the United States in Lyme disease incidence (Centers for Disease Control 2018c).
The presence of trash or stone walls each predicted higher abundance of questing larval ticks. Trash and stone walls may increase tick abundance by providing improved habitat for small mammals. These effects are consistent with prior research identifying positive associations between P. leucopus and stone walls and logs (Barry and Francq 1980). Natural debris provides P. leucopus with cover protection from predators in urban environments (Persons and Eason 2017), and trash benefits rodents in urban settings (Johnson et al. 2016). The effects of trash and stone walls on tick abundance were each reduced in yards with greater forest area. In yards with less forest, trash and stone walls may each substitute for the refugia that greater forest area would otherwise provide for P. leucopus and other small mammals. With increasing forest area, the benefits to small mammals of trash or stone walls may decline relative to the increasing benefits of greater forest area. The benefits to residents of removing trash or stone walls in reducing risk are greater in yards with little or no forest.
Despite the increased questing larval tick abundance in yards with stone walls and trash, we saw no effect of these features on larval abundance on white-footed mice captured in yards. One possible explanation is that abundance of larvae on mice, which may range across property boundaries, is subject to greater influence by features of neighboring yards. Fewer larvae were found per white-footed mouse in yards where at least 75% of the area is enclosed by fence, compared with yards with less or none of the property fenced. However, no effect was detected of the interaction between fencing and forest area. Thus, the effect of fencing appears to be independent of forest area.
We conclude that there is partial support for public health agency recommendations regarding trash, stone walls, and fencing. The potential benefits of each of these recommended measures needs to be weighed against each measure’s costs, both financial and in terms of the value of yards as wildlife habitat, value which may be reduced by fencing. We detected no effects of ground barriers (gravel, mulch, or woodchip), bird feeders, wood piles, or indoor/outdoor cats. In the case of barriers, only two properties had this feature, resulting in low power to detect an effect while also reflecting low adoption rates of this recommendation. Property management features evaluated had inconsistent effects between tick sampling measures. The different patterns for questing larvae versus larvae on mice, and for total versus per capita larvae on mice, indicate that other factors, in addition to the property management features measured here, affect variation in abundance of ticks and their interactions with the wildlife host community.
Experimental manipulations would be necessary to establish causal relationships between adherence to tick control recommendations and larval tick abundance. Nevertheless, our observational study accounted statistically for sources of variation that would be controlled for directly in an experimental design. During brief visits to yards we were unable to assess some recommended environmental tick control measures, such as mowing, that require longer-term monitoring of yards or surveys. Given the time available to make observations, it was necessary to define as present or absent some property features that were described by public health authorities as continuous aspects of yard management (Supp Table 1 [online only]). For example, homeowners are advised to place bird feeders in the yard away from the home; we recorded bird feeders as present or absent rather than their distance from the home. Yard features that we enumerated as binary contain heterogeneity, for example in the size or neatness of a firewood pile, or the length of a stone wall, that may have contributed variation to the results. Variation in results may also have arisen due to chemical treatments in prior years, about which we do not have information. We do not expect variation in prior year pesticide use, or spot spraying in the studied years, to have directionally influenced results with respect to any yard features.
Our study focused on larval ticks, due to sampling at the time of larval peak activity. Public health agencies are chiefly concerned with reducing exposure to nymphal ticks; larvae have not yet acquired the most common pathogens. Assessing the effects of yard management on larval ticks is relevant to controlling risk from nymphs, given that larvae transition to nymphs the following year. Moreover, the larval ticks attached to white-footed mice are more likely to survive and to become infected with tick-borne pathogens than are larvae that attach to other hosts (Keesing et al. 2009). It is possible, given the potential for hosts to transport larvae, and the potential for yard management to affect survival to the nymphal stage, that the same set of yard management features examined here may have different effects on nymphal ticks.
In addition to tick abundance, an important source of variation in risk for residents is human behavior. Our study addressed yard management measures expected to affect tick abundance, but other recommended yard management actions (e.g., placement of yard equipment away from forest edge) are based on expectations about how human behavior affects exposure to the ticks that are present.
Our study presents results on eight public health agency recommendations across a county, in 143 yards subject to the management decisions of residents. In contrast, in previous studies on the property management features examined here, data were typically collected in a more limited number of residential (Stafford 1993) or nonresidential locations (Daniels et al. 1993), on fewer interventions (Frank et al. 1998, Townsend et al. 2003, Mead et al. 2018), or relied on self-reporting about property features (Smith et al. 2001, Connally et al. 2009, Jones et al. 2018, Mead et al. 2018). Prior research often involved experimental manipulation of a single intervention (Stafford 1993, Schulze et al. 1995, Carroll and Schmidtmann 1996). The effects of certain interventions, for example trash removal, do not appear to have been previously studied in the context of tick control. To inform the decisions of residents, we suggest that when making property management recommendations, public health officials provide information on the scientific support and uncertainty about each recommendation.
Ethics statement
The authors declare no conflicts of interest. The Institutional Review Board of Cary Institute of Ecosystem Studies (CIES) approved the survey that collected data on participating households, including presence of indoor/outdoor cats. The CIES Institutional Animal Care and Use Committee approved the protocols for live-capturing small mammals.
Supplementary Material
Acknowledgments
We thank the Project Assistants of The Tick Project for their hard work in gathering the data and for providing valuable input on methods for measuring property management features. This work was supported by the Steven and Alexandra Cohen Foundation. This is a contribution to the program of Cary Institute of Ecosystem Studies.
References Cited
- Barry R. E., and Francq E. N.. . 1980. Orientation to landmarks within the preferred habitat by Peromyscus leucopus. J. Mammal. 61: 292–303. [Google Scholar]
- Belongia E. A., Reed K. D., Mitchell P. D., Chyou P. H., Mueller-Rizner N., Finkel M. F., and Schriefer M. E.. . 1999. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin. Infect. Dis. 29: 1472–1477. [DOI] [PubMed] [Google Scholar]
- Carroll J. F., and Schmidtmann E. T.. . 1996. Silt fencing as a barrier to the dispersal of Ixodes scapularis (Acari: Ixodidae) into pastures. J. Med. Entomol. 33: 921–925. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control 2018a. Lyme disease: what you need to know.https://www.cdc.gov/lyme/resources/brochure/lymediseasebrochure-P.pdf (accessed 14 May 2019). [Google Scholar]
- Centers for Disease Control 2018b. Preventing ticks in the yard. https://www.cdc.gov/ticks/avoid/in_the_yard.html (accessed 14 May 2019). [Google Scholar]
- Centers for Disease Control 2018c. Lyme disease surveillance and available data.https://www.cdc.gov/lyme/stats/survfaq.html (accessed 14 May 2019). [Google Scholar]
- Connally N. P., Ginsberg H. S., and Mather T. N.. . 2006. Assessing peridomestic entomological factors as predictors for Lyme disease. J. Vector Ecol. 31: 364–370. [DOI] [PubMed] [Google Scholar]
- Connally N. P., Durante A. J., Yousey-Hindes K. M., Meek J. I., Nelson R. S., and Heimer R.. . 2009. Peridomestic Lyme disease prevention: results of a population-based case-control study. Am. J. Prev. Med. 37: 201–206. [DOI] [PubMed] [Google Scholar]
- Cromley E. K., Cartter M. L., Mrozinski R. D., and Ertel S. H.. . 1998. Residential setting as a risk factor for Lyme disease in a hyperendemic region. Am. J. Epidemiol. 147: 472–477. [DOI] [PubMed] [Google Scholar]
- Daniels T. J., Fish D., and Schwartz I.. . 1993. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and Lyme disease risk by deer exclusion. J. Med. Entomol. 30: 1043–1049. [DOI] [PubMed] [Google Scholar]
- Dister S. W., Fish D., Bros S. M., Frank D. H., and Wood B. L.. . 1997. Landscape characterization of peridomestic risk for Lyme disease using satellite imagery. Am. J. Trop. Med. Hyg. 57: 687–692. [DOI] [PubMed] [Google Scholar]
- Duffy D. C., Clark D. D., Campbell S. R., Gurney S., Perello R., and Simon N.. . 1994. Landscape patterns of abundance of Ixodes scapularis (Acari: Ixodidae) on Shelter Island, New York. J. Med. Entomol. 31: 875–879. [DOI] [PubMed] [Google Scholar]
- Eisen L., and Dolan M. C.. . 2016. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol. 55: 1063–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco R. C., and Fish D. F.. . 1988. A survey of tick bites acquired in a Lyme disease endemic area in southern New York State. Ann. N. Y. Acad. Sci. 539: 456–457. [Google Scholar]
- Finch C., Al-Damluji M. S., Krause P. J., Niccolai L., Steeves T., O’Keefe C. F., and Diuk-Wasser M. A.. . 2014. Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS One 9: e84758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff I. R., Keesing F., Pendleton J., DePietro D., Teator M., Duerr S., Mowry S., Pfister A., Ladeau S. L., and Ostfeld R. S.. . 2018. Dataset: assessing effectiveness of recommended residential yard management measures against ticks. figshare. doi:10.6084/m9.figshare.7378313.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. H., Fish D., and Moy F. H.. . 1998. Landscape features associated with Lyme disease risk in a suburban residential environment. Landsc. Ecol. 13: 27–36. [Google Scholar]
- Fritz C. L., Kjemtrup A. M., Conrad P. A., Flores G. R., Campbell G. L., Schriefer M. E., Gallo D., and Vugia D. J.. . 1997. Seroepidemiology of emerging tickborne infectious diseases in a Northern California community. J. Infect. Dis. 175: 1432–1439. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., and Ewing C. P.. . 1989. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari: Ixodidae). Exp. Appl. Acarol 7: 313–322. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., and Zhioua E.. . 1999. Influence of deer abundance on the abundance of questing adult Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 36: 376–381. [DOI] [PubMed] [Google Scholar]
- Gould, L. H., Nelson, R. S., Griffith, K. S., Hayes, E. B., Piesman, J., Mead, P. S., Cartter, M. L. 2008. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999-2004. Vector Borne Zoonotic Dis 8: 769–776. [DOI] [PubMed] [Google Scholar]
- Harvard Health Letter 2010. More on lyme disease: tick management handbook.https://www.health.harvard.edu/newsletter_article/more-on-lyme-disease-tick-management-handbook. Accessed 14 May 2019. [Google Scholar]
- Hersh M. H., Ostfeld R. S., McHenry D. J., Tibbetts M., Brunner J. L., Killilea M. E., LoGiudice K., Schmidt K. A., and Keesing F.. . 2014. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS One 9: e99348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., Bragdon C., Olson C., Merlino M., and Bonaparte S.. . 2016. Characteristics of the built environment and the presence of the Norway rat in New York City: results from a neighborhood rat surveillance program, 2008–2010. J. Environ. Health 78: 22–29. [PubMed] [Google Scholar]
- Jones E. H., Hinckley A. F., Hook S. A., Meek J. I., Backenson B., Kugeler K. J., and Feldman K. A.. . 2018. Pet ownership increases human risk of encountering ticks. Zoonoses Public Health 65: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F., and Ostfeld R. S.. . 2018. The tick project: testing environmental methods of preventing tick-borne diseases. Trends Parasitol. 34: 447–450. [DOI] [PubMed] [Google Scholar]
- Keesing F., Brunner J., Duerr S., Killilea M., LoGiudice K., Schmidt K., Vuong H., and Ostfeld R. S.. . 2009. Hosts as ecological traps for the vector of Lyme disease. Proc. R. Soc. Lond. B Biol. Sci. 276: 3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. D., Eppes S. C., and Hunt P.. . 1996. Environmental and life-style risk factors for Lyme disease in children. Clin. Pediatr. (Phila). 35: 359–363. [DOI] [PubMed] [Google Scholar]
- Ley C., Olshen E. M., and Reingold A. L.. . 1995. Case-control study of risk factors for incident Lyme disease in California. Am. J. Epidemiol. 142: S39–S47. [DOI] [PubMed] [Google Scholar]
- Lindsay L. R., Mathison S. W., Barker I. K., McEwen S. A., and Surgeoner G. A.. . 1999. Abundance of Ixodes scapularis (Acari: Ixodidae) larvae and nymphs in relation to host density and habitat on Long Point, Ontario. J. Med. Entomol. 36: 243–254. [DOI] [PubMed] [Google Scholar]
- Mather T. N., Nicholson M. C., Donnelly E. F., and Matyas B. T.. . 1996. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol. 144: 1066–1069. [DOI] [PubMed] [Google Scholar]
- Mead P., Hook S., Niesobecki S., Ray J., Meek J., Delorey M., Prue C., and Hinckley A.. . 2018. Risk factors for tick exposure in suburban settings in the Northeastern United States. Ticks Tick. Borne. Dis. 9: 319–324. [DOI] [PubMed] [Google Scholar]
- Mosher E. S., Silander J. A., and Latimer A. M.. . 2009. The role of land-use history in major invasions by woody plant species in the northeastern North American landscape. Biol. Invasions 11: 2317–2328. [Google Scholar]
- Nelson C. A., Saha S., Kugeler K. J., Delorey M. J., Shankar M. B., Hinckley A. F., and Mead P. S.. . 2015. Incidence of clinician-diagnosed lyme disease, United States, 2005-2010. Emerg. Infect. Dis. 21: 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld R. S. 2011. Lyme disease: the ecology of a complex system. Oxford University Press, New York, NY. [Google Scholar]
- Ostfeld R. S., Price A., Hornbostel V. L., Benjamin M. A., and Keesing F.. . 2006a. Controlling ticks and tick-borne zoonoses with biological and chemical agents. Bioscience 56: 383–394. [Google Scholar]
- Ostfeld R. S., Canham C. D., Oggenfuss K., Winchcombe R. J., and Keesing F.. . 2006b. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 4: 1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. E., Cattadori I. M., Tagliapietra V., Rizzoli A. P., and Hudson P. J.. . 2006. Localized deer absence leads to tick amplification. Ecology. 87: 1981–1986. [DOI] [PubMed] [Google Scholar]
- Persons W. E., and Eason P.. . 2017. Human activity and habitat type affect perceived predation risk in urban white-footed mice (Peromyscus leucopus). Ethology. 123: 348–356. [Google Scholar]
- Public Health Ontario 2018. Lyme disease. https://www.publichealthontario.ca/en/BrowseByTopic/InfectiousDiseases/Pages/IDLandingPages/Lyme-Disease.aspx (accessed 14 May 2019). [Google Scholar]
- R Core Team 2018. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rulison E. L., Kuczaj I., Pang G., Hickling G. J., Tsao J. I., and Ginsberg H. S.. . 2013. Flagging versus dragging as sampling methods for nymphal Ixodes scapularis (Acari: Ixodidae). J. Vector Ecol. 38: 163–167. [DOI] [PubMed] [Google Scholar]
- Schmidt K. A., Ostfeld R. S., and Schauber E. M.. . 1999. Infestation of Peromyscus leucopus and Tamias striatus by Ixodes scapularis (Acari: Ixodidae) in relation to the abundance of hosts and parasites. J. Med. Entomol. 36: 749–757. [DOI] [PubMed] [Google Scholar]
- Schulze T. L., Jordan R. A., and Hung R. W.. . 1995. Suppression of subadult Ixodes scapularis (Acari: Ixodidae) following removal of leaf litter. J. Med. Entomol. 32: 730–733. [DOI] [PubMed] [Google Scholar]
- Schwartz A. M., Hinckley A. F., Mead P. S., Hook S. A., and Kugeler K. J.. . 2017. Surveillance for lyme disease – United States, 2008–2015. MMWR. Surveill. Summ. 66: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G., Wileyto E. P., Hopkins R. B., Cherry B. R., and Maher J. P.. . 2001. Risk factors for lyme disease in Chester County, Pennsylvania. Public Health Rep. 116(Suppl 1): 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford K. C., III 1993. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) with exclusion of deer by electric fencing. J. Med. Entomol. 30: 986–996. [DOI] [PubMed] [Google Scholar]
- Stafford K. C. 2004. Tick management handbook: an integrated guide for homeowners, pest control operators, and public health officials for the prevention of tick-associated disease. Connecticut Agricultural Experiment Station, New Haven, CT. [Google Scholar]
- Stafford K. C. III, and Magnarelli L. A.. . 1993. Spatial and temporal patterns of Ixodes scapularis (Acari: Ixodidae) in southeastern Connecticut. J. Med. Entomol. 30: 762–771. [DOI] [PubMed] [Google Scholar]
- Townsend A. K., Ostfeld R. S., and Geher K. B.. . 2003. The effects of bird feeders on Lyme disease prevalence and density of Ixodes scapularis (Acari: Ixodidae) in a residential area of Dutchess County, New York. J. Med. Entomol. 40: 540–546. [DOI] [PubMed] [Google Scholar]
- UMaine Cooperative Extension 2018. Tick identification lab - tick management. https://extension.umaine.edu/ipm/tickid/tick-management/ (accessed 14 May 2019). [Google Scholar]
- Vuong H. B., Canham C. D., Fonseca D. M., Brisson D., Morin P. J., Smouse P. E., and Ostfeld R. S.. . 2014. Occurrence and transmission efficiencies of Borrelia burgdorferi ospC types in avian and mammalian wildlife. Infect. Genet. Evol. 27: 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C., and Ward J. S.. . 2010. Effects of Japanese barberry (Ranunculales: Berberidaceae) removal and resulting microclimatic changes on Ixodes scapularis (Acari: Ixodidae) abundances in Connecticut, USA. Environ. Entomol. 39: 1911–1921. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Ward J. S., Worthley T. E., and Stafford K. C. III. 2009. Managing Japanese barberry (Ranunculales: Berberidaceae) infestations reduces blacklegged tick (Acari: Ixodidae) abundance and infection prevalence with Borrelia burgdorferi (Spirochaetales: Spirochaetaceae). Environ. Entomol. 38: 977–984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.