ABSTRACT
We uncovered the neurotransmitter N-acetyl-aspartyl-glutamate (NAAG) as a reservoir providing glutamate to promote cancer growth, and demonstrated that inhibition of NAAG hydrolysis by targeting glutamate carboxypeptidase II is a viable strategy for cancer therapy. Our study also suggests that NAAG concentration in plasma could be a non-invasive measurement to monitor cancer progression.
KEYWORDS: N-acetyl-aspartyl-glutamate (NAAG), glutamate carboxypeptidase II (GCPII), glutaminase inhibitor, glutamate reservoir, stable isotope-resolved metabolomics (SIRM)
Over the last few decades, creating effective therapies for advanced-stage cancers has been the main focus of many research studies. In this context, studying the metabolism of cancer cells has been explored as a new research avenue for novel treatment strategies. We, and others, have established that glutamine metabolism plays an important role in cancer proliferation.1–3 This dependency of cancer on glutamine could be exploited to develop new therapies for cancer treatment. However, the current clinical trials to block the conversion of glutamine to glutamate via pharmacological inhibition of glutaminase have provided limited clinical efficacy.4 Aiming to address these limitations by targeting other pathways, we employed mass-spectroscopy-based stable isotope-resolved metabolomics (SIRM) with 13C515N2-labeled-glutamine to explore glutamine metabolism beyond glutaminolysis. This investigation led us to discover an interesting phenomenon pertaining to the neurotransmitter metabolite, N-acetyl-aspartyl-glutamate (NAAG) in cancers. In particular, we observed a significantly higher production of NAAG from glutamine in higher-grade cancers compared to their lower-grade counterparts in three different models (in vitro, in vivo and patient samples) of three different cancer types (lymphoma, ovarian and brain tumors).5 Moreover, in brain tumors, the NAAG concentrations in patient plasma strongly reflected those in tumors. Furthermore, we discovered that NAAG concentrations in plasma mirrored tumor growth in the mice bearing human lymphoma tumors. NAAG concentration spikes were detected prior to any surges in tumor growth. The significant elevation of NAAG in higher-grade cancers and the strong mirrored relationship between NAAG in plasmas and tumor growth have shown the clinically relevant potential of NAAG, paving the path toward developing non-invasive cancer monitoring measurement. Specifically, this finding can be used for the future prospective approach of measuring the NAAG levels in peripheral blood plasma for real-time monitoring of tumor growth (Figure 1). In an effort to translate this finding into a clinically relevant application, we plan to further expand into other cancer types and use NAAG concentration in plasma in combination with current indicators of cancer (stage of cancer, grade of cancer, age, other factors) to develop a more cohesive understanding of the progression of cancer in patients.
Figure 1.
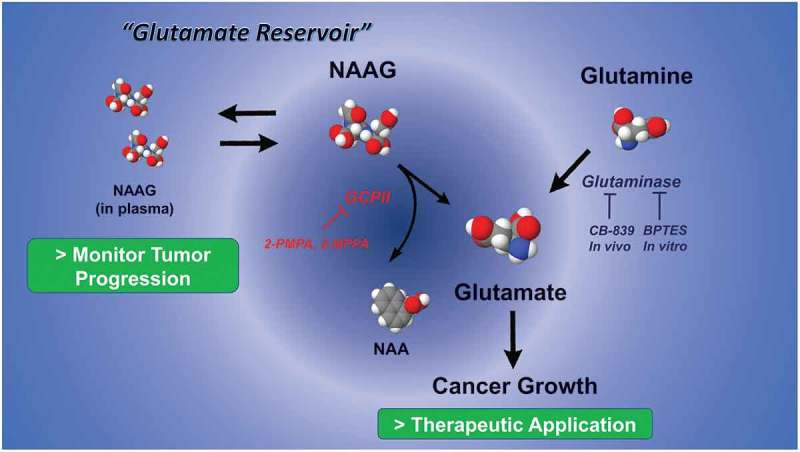
Dual role of N-acetyl-aspartyl-glutamate (NAAG) metabolism in cancer monitor and therapy. NAAG concentration in plasma can be a non-invasive measurement to monitor cancer growth. Blocking glutamate carboxypeptidase II (GCPII) in combination with glutaminase inhibition synergistically reduces production of glutamate. NAA: N-acetyl-aspartate, BPTES (bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulphide), CB-839 (2-(pyridin-2-yl)-N-(5-(4-(6-(2-(3-(trifluoromethoxy)phenyl)acetamido)pyridazin-3-yl)butyl)-1,3,4-thiadiazol-2-yl)acetamide): glutaminase inhibitor, 2-PMPA (2-(Phosphonomethyl)-pentandioic acid, 2-Phosphonomethyl pentanedioic acid), 2MPPA (2-(3-Mercaptopropyl)pentanedioic acid): GCPII inhibitor.
In parallel with the efforts to develop non-invasive measurement of NAAG concentrations for tumor progression assessment, we were dedicated to elucidating the mechanisms behind the upregulation of NAAG in promoting cancer growth as this understanding would contribute to tackling cancers from various angles to help improve therapeutic outcomes. NAAG is a well-investigated neurotransmitter in several neurological disorders.6 However, its role in cancer is still unclear. Long et al. reported a possible role of NAAG as an inhibitory factor for differentiation of glioma stem-like cells.7 Furthermore, a global metabolomics profiling of ovarian cancer (OVCA) showed that NAAG and NAA (N-acetyl-aspartate) levels were more elevated in metastatic OVCA than in primary OVCA or normal ovary, but did not provide the specific role of these metabolites.8 With the use of 15N2-labeled-NAAG, we demonstrated that the hydrolysis of NAAG directly produced 15N1-glutamate in vivo via glutamate carboxypeptidase II (GCPII). Glutamate is not only essential for bioenergy synthesis and redox homeostasis but also nucleotide synthesis precursors for DNA synthesis.9 For these reasons, the current clinical trial aims to reduce glutamate production by inhibition of glutaminase, the enzyme that converts glutamine to glutamate (Figure 1). Given our finding that NAAG can hydrolyze to glutamate in tumor expressing GCPII, we chose 2-PMPA (2-(Phosphonomethyl)-pentandioic acid, 2-Phosphonomethyl pentanedioic acid), a specific GCPII inhibitor with the greatest binding affinity (IC50 = 0.3nM)6 to stop NAAG from hydrolyzing to glutamate. We found that inhibition of GCPII reduced tumor growth in patient-derived recurrent ovarian cancer orthotopic tumors in and suppressed glutamate production. Furthermore, we believe that simultaneously targeting these glutamate-supplying pathways in cancer would yield better outcomes. Thus, we combined 2-PMPA with CB-839 (2-(pyridin-2-yl)-N-(5-(4-(6-(2-(3-(trifluoromethoxy)phenyl)acetamido)pyridazin-3-yl)butyl)-1,3,4-thiadiazol-2-yl)acetamide), a current clinical trial glutaminase inhibitor,4 and observed that tumor growth was significantly more suppressed under the combination treatment as compared to either treatment alone (Figure 1). The fundamental rationale for the significant reduction in tumor growth in the combined treatment was the synergistically-reduced amount of glutamate. The therapeutic approach of our study, including the new finding of NAAG metabolism in cancer, provides a significant improvement to the therapeutic index as they target glutamine metabolism from several angles. This encouraging result not only demonstrates the significance of NAAG in providing glutamate via a GCPII dependent pathway but also partly addresses the clinical limitation of the current clinical trial of CB-839. Of note, the oral form GCPII inhibitor, 2-MPPA (2-(3-Mercaptopropyl)pentanedioic acid), has passed a Phase I clinical trial for other diseases.10 With the availability of these two clinical-trial inhibitors, the translation of targeting two important glutamate-producing pathways into cancer therapy appears to be promising.
In conclusion, NAAG is shown to be not only a potential metabolite marker for cancer motoring but also a glutamate provider to support cancer growth. This dual role of NAAG can be translated into clinical applications. Moreover, targeting NAAG metabolism could also be employed in combination with other pharmacological approaches such as CB-839 to improve clinical outcomes. The metabolomics-based discovery of metabolic aspects of cancer provides a unique tool to identify and guide specific targeted pharmacological approaches. Our innovative metabolomics-based insights allow for the uncovering of clinically-relevant biological processes and unveil new metabolic targets in cancer.
Funding Statement
This work was supported by NIH Grants R01-CA193895, R01-CA112314, 1S10OD025226-01 and UL1 TR 001079 (to AL) and grants from Allegheny Health Network-Johns Hopkins Cancer Research Fund (to AL). This publication was also made possible, in part, by the Doris M. Weinstein Pancreatic Cancer Research Fund (to AL).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dranoff G, Elion GB, Friedman HS, Campbell GL, Bigner DD.. Influence of glutamine on the growth of human glioma and medulloblastoma in culture. Cancer Res. 1985;45:4077–4081. [PubMed] [Google Scholar]
- 2.Elgogary A, Xu Q, Poore B, Alt J, Zimmermann SC, Zhao L, Fu J, Chen B, Xia S, Liu Y, et al. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci U S A. 2016;113:E5328–36. doi: 10.1073/pnas.1611406113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding JJ, Telli ML, Munster PN, Le MH, Molineaux C, Bennett MK, Mittra E, Burris HA, Clark AS, Dunphy M, et al. Safety and tolerability of increasing doses of CB-839, a first-in-class, orally administered small molecule inhibitor of glutaminase, in solid tumors. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:abstr 2512. doi: 10.1200/jco.2015.33.15_suppl.2512. [DOI] [Google Scholar]
- 5.Nguyen T, Kirsch BJ, Asaka R, Nabi K, Quinones A, Tan J, Antonio MJ, Camelo F, Li T, Nguyen S, et al. Uncovering the role of N-acetyl-aspartyl-glutamate as a glutamate reservoir in cancer. Cell Rep. 2019;27:491–501 e6. doi: 10.1016/j.celrep.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Neale JH, Pomper MG, Kozikowski AP.. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 2005;4:1015–1026. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 7.Long PM, Moffett JR, Namboodiri AM, Viapiano MS, Lawler SE, Jaworski DM. N-acetylaspartate (NAA) and N-acetylaspartylglutamate (NAAG) promote growth and inhibit differentiation of glioma stem-like cells. J Biol Chem. 2013;288:26188–26200. doi: 10.1074/jbc.M113.487553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong MY, McDunn J, Kakar SS. Identification of metabolites in the normal ovary and their transformation in primary and metastatic ovarian cancer. PLoS One. 2011;6:e19963. doi: 10.1371/journal.pone.0019963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3(3):169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Post JP, de Visser SJ, de Kam ML, Woelfler M, Hilt DC, Vornov J, Burak ES, Bortey E, Slusher BS, Limsakun T, et al. The central nervous system effects, pharmacokinetics and safety of the NAALADase-inhibitor GPI 5693. Br J Clin Pharmacol. 2005;60:128–136. doi: 10.1111/j.1365-2125.2005.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


